Abstract
Background
Type 2 diabetes mellitus has become one of the most important public health concerns worldwide. Due to its high prevalence and morbidity, there is an avid necessity to find new therapies that slow the progression and promote the regression of the disease. Imatinib mesylate is a tyrosine kinase inhibitor that binds to the Abelson tyrosine kinase and related proteins. It enhances β-cell survival in response to toxins and pro-inflammatory cytokine. The aim of this study is to evaluate the effect of imatinib on fasting plasma glucose in subjects with normal fasting glucose, subjects with impaired fasting glucose and in subjects with type 2 diabetes mellitus.
Methods
We identified 284 subjects diagnosed with chronic myeloid leukemia or gastrointestinal stromal tumors from the Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran database. 106/284 subjects were treated with imatinib. We compared the effect of imatinib on fasting plasma glucose after 1 and 6 months of treatment. We used ANOVA test of repeated samples to determine statistical significance in fasting plasma glucose before imatinib treatment and the follow-up. Statistical analysis was performed with Statistical Package for the Social Sciences v22.
Results
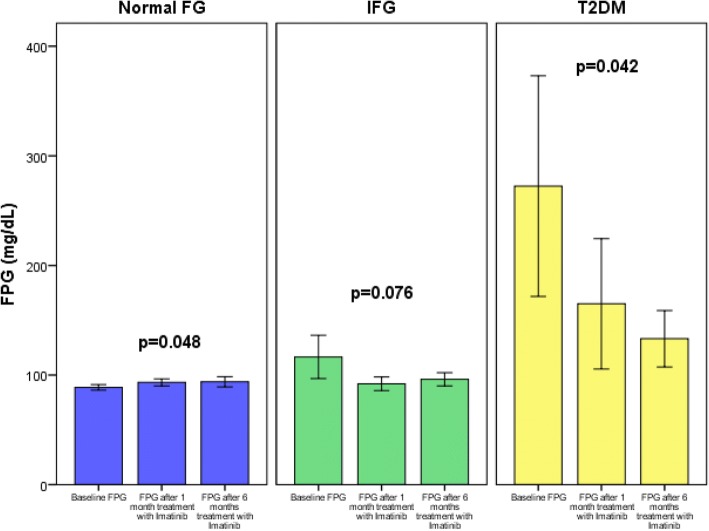
We included a total of 106 subjects: 76 with fasting plasma glucose concentrations < 100 mg/dL (normal FG), 19 subjects with fasting plasma glucose concentrations ≥100 mg/dL (impaired fasting glucose), and 11 subjects with ≥126 mg/dL (type 2 diabetes mellitus). We found a significant increase in fasting plasma glucose concentration in the normal fasting glucose group (p = 0.048), and a significant decrease in fasting plasma glucose concentration in the type 2 diabetes mellitus group (p = 0.042). In the impaired fasting glucose group, we also found a tendency towards a decrease in fasting plasma glucose (p = 0.076). We identified 11 subjects with type 2 diabetes mellitus, of whom, 7 (64%) had a reduction in their fasting plasma glucose concentrations after 6 months. A significant glycosylated hemoglobin reduction (p = 0.04) was observed.
Conclusion
Subjects with chronic myeloid leukemia or gastrointestinal stromal tumor with type 2 diabetes mellitus had a significant reduction in fasting plasma glucose and glycosylated hemoglobin at 1 and 6 months while using imatinib.
Electronic supplementary material
The online version of this article (10.1186/s12902-018-0303-x) contains supplementary material, which is available to authorized users.
Keywords: Imatinib, Fasting plasma glucose concentrations, Chronic myeloid leukemia, Gastrointestinal stromal tumor, Type 2 diabetes mellitus
Background
Type 2 diabetes mellitus (T2DM) has become one of the most important public health concerns worldwide, reaching epidemic proportions. Currently, T2DM affects over 425 million people and is estimated that the number of cases will reach 629 million by 2045 [1]. Due to its high prevalence and morbidity, there is a necessity to find new therapies that slow the progression and promote the regression of the disease. Previous publications have shown an improvement of glucose metabolism with the use of imatinib in diabetic subjects with chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GIST) [2, 3].
Imatinib mesylate is a tyrosine kinase inhibitor (TKI) that binds to the Abelson tyrosine kinase (c-Abl) and related proteins. It became the very first molecular inhibitor drug to be clinically approved [4]. Also, it inhibits the platelet-derived growth factor receptor (PDGFR) and transmembrane stem-cell factor receptor (c-Kit) [5, 6], as well as it enhances β-cell survival in response to toxins and pro-inflammatory cytokines [7, 8].
The aim of this study is to evaluate the effect of imatinib on fasting plasma glucose (FPG) concentrations in subjects with CML and GIST, classified by their FPG status: normal fasting glucose (normal FG) compared to subjects with impaired fasting glucose (IFG) and subjects with T2DM. Our hypothesis is that subjects with IFG or T2DM will show an improvement in fasting glucose concentrations.
Methods
This is a retrospective cohort study that included subjects with a diagnosis of CML or GIST that received treatment with imatinib from January 2000 to October 2016. The study was submitted and approved by the Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran Comite de etica en Investigacion/Comite de investigacion on July 11th, 2016.
Study population
We identified 284 Hispanic subjects diagnosed with CML and GIST from the Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran (INCMNSZ) database. Of these, 106 were treated with imatinib. We selected subjects aged 18 years and older. We excluded subjects who did not have enough data, such as 1-month follow-up, FPG, triglycerides (Tg), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and subjects treated with imatinib in another hospital (Fig. 1).
Fig. 1.
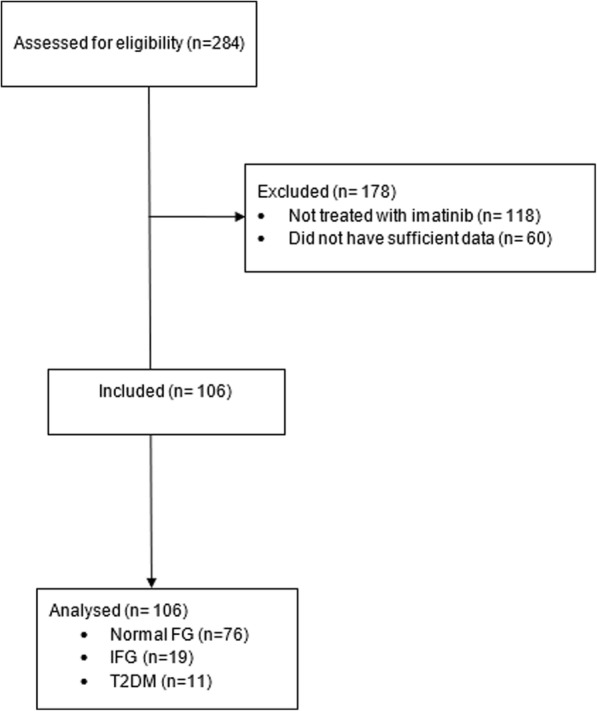
Flow chart. Normal fasting glucose; Normal FG, impaired fasting glucose; IFG, Type 2 Diabetes Mellitus; T2DM
Three study groups were formed depending on their FPG concentrations: subjects with FPG < 100 mg/dL, subjects with FPG ≥100 to < 126 mg/dL (IFG), and FPG ≥ 126 mg/dL (T2DM). Data were collected following the next criteria:
Basal information was collected with a maximum period of 3 months prior to the treatment with imatinib.
1-month follow-up: information was collected with a period of ±2 weeks of completing 1 month of treatment with imatinib.
6-month follow-up: information was collected with a period of ±1 month of completing 6 months of treatment with imatinib.
Biochemical and anthropometric measurements
We reviewed subject charts to retrieve laboratory data from the central laboratory of INCMNSZ, including fasting glucose, HbA1c, total cholesterol, LDL, HDL, triglycerides (Tg), alanine aminotransferase (ALT), magnesium (Mg), and creatinine. The measurements were carried out with commercially available standardized methods. In addition, other variables obtained from the subjects were age, sex, weight, and treatment for diabetes or other conditions.
Statistical analysis
Normally distributed data are expressed as the mean and standard deviation (±SD), whereas variables with a skewed distribution are reported as median (interquartile range). In addition, we used the Kruskal-Wallis test to determine the statistical significance between the FPG concentrations before treatment and at 1 and 6 months follow-up among the three study groups. Mauchly’s sphericity test was used to evaluate equality and homogeneity of the studied population, and Greenhouse-Geisser test to obtain the p-value to determine if the changes of FPG were statistically and clinically significant. Data were analyzed with Statistical Package for the Social Sciences (SPSS) v22.
Results
We included a total of 106 subjects that received imatinib for 6 months: 76 with normal fasting glucose concentrations < 100 mg/dL (normal FG), 19 subjects with glucose concentrations ≥100 to < 126 mg/dL (IFG), and 11 subjects with glucose concentrations ≥126 mg/dL (T2DM). Baseline characteristics of the studied subjects are shown in Table 1. Characteristics of subjects according to their FPG concentrations are shown in Table 2, there were significant differences in age (p = 0.023) and ALT concentration after 1 month of treatment with Imatinib (p = 0.008). The comparison of mean FPG concentration variations through time in each group are presented in Table 3. We analyzed the sphericity of the mean FPG within each group with the Mauchly’s sphericity test and found a p = 0.960 in the normal FG group, a p = 0.184 in the IFG group, and a p = 0.702 in the T2DM group. Due to these values, we corrected the significance with the Greenhouse-Geisser test. We found a significant increase in FPG concentration in the normal FG group, before (87.6 ± 8.3), after 1 month (93.6 ± 7.6), and after 6 months (93.4 ± 10.5), p = 0.048; and a statistically significant decrease in FPG concentration in the T2DM group, before (241 ± 120.7), after 1 month (152.7 ± 74.5), and after 6 months (128.6 ± 33.9), p = 0.042. In the IFG group, there is also a decrease in FPG concentration, at baseline (121 ± 28.7), after 1 month (97. 3 ± 12.8), and after 6 months (96. 1 ± 7.3), p = 0.076. The latter result shows imatinib’s tendency to decrease FPG concentration, even though is not statistically significant. These results are represented in Fig. 2, where we can observe an important reduction of FPG concentration in the group with T2DM and IFG; having a decrease in concentrations at one-month follow-up, and 6-month follow-up. On the other hand, we can observe that the normal FG group had a small, yet significant increase in the FPG concentrations. Also, we decided to compare all patients with normal fasting glucose against IFG plus T2DM, in the first group we obtained a p = 0.059 and in the second group a significant p value = 0.034. These results are shown in Additional file 1: Table S1 and Additional file 2: Figure S1.
Table 1.
Baseline characteristics of the studied subjects (n = 106)
| Variable | Value |
|---|---|
| Sex (male: %) | 52.5 |
| CML (%) | 81.8 |
| GIST (%) | 18.2 |
| Age (years) | 40.2 ± 16.7 |
| Weight (kg) | 68.3 ± 14.4 |
| Overweight (%) | 47.7 |
| IFG (%) | 17.9 |
| DM2 (%) | 10.4 |
| Creatinine (mg/dL) | 0.93 (0.68–1.0) |
| ALT (U/L) | 28.3 (14.2–31.0) |
| Mg (mg/dL) | 2.1 ± 0.26 |
| Glucose (mg/dL) | 109 (86–104.5) |
Variables with normal distribution are expressed as mean ± s.d. Variables with non-parametric distribution are expressed as median (interquartile range). CML chronic myeloid leukemia, GIST gastrointestinal stromal tumor, IFG impaired fasting glucose, ALT alanine aminotransferase, Mg magnesium
Table 2.
Characteristics of subjects according to fasting glucose
| Variable | Subjects with fasting glucose < 100 mg/dL (n = 76) | Subjects with fasting glucose ≥100 mg/dL and < 126 mg/dL (n = 19) | Subjects with T2DM (n = 11) | p |
|---|---|---|---|---|
| Sex (male: n, %) | 41, 53.9 | 9, 47.4 | 5, 45.5 | 0.721 |
| Cigarette (yes: n, %) | 22, 28.9 | 4, 26.7 | 3, 30 | 0.663 |
| CML (n, %) | 59, 80.8 | 13, 72.2 | 10, 90.9 | 0.747 |
| GIST (n, %) | 14, 18.4 | 5, 27.8 | 1, 9.1 | |
| Age (years) | 38.6 ± 15.7 | 41.8 ± 16.8 | 53 ± 14.2 | 0.023 |
| Weight (kg) | 70.5 ± 15.1 | 64.2 ± 14.4 | 65.3 ± 10.7 | 0.486 |
| Weight (after 1 month, kg) | 69.6 ± 14.9 | 63.7 ± 12.6 | 74.4 ± 27.5 | 0.645 |
| Weight (after 6 months, kg) | 74.9 ± 16.6 | 78.5 ± 10 | 61.6 ± 7.5 | 0.127 |
| Mg (mg/dL) | 2.2 ± 0.25 | 2 ± 0.20 | 2.1 ± 0.38 | 0.098 |
| Mg (after 1 month, mg/dL) | 2 ± 0.6 | 1.9 ± 0.18 | 2.25 ± 0.21 | 0.343 |
| Creatinine (before, mg/dL) | 0.90 (0.68–1) | 1.02 (0.62–1.13) | 1.02 (0.75–1.12) | 0.260 |
| Creatinine (after 1 month, mg/dL) | 0.81 (0.70–0.90) | 0.94 (0.68–1.05) | 0.90 (0.60–1.10) | 0.549 |
| Creatinine (after 6 months, mg/dL) | 0.86 (0.73–0.99) | 0.84 (0.58–1.06) | 0.84 (0.73–0.98) | 0.239 |
| ALT (before, U/L) | 23.6 (14–26.5) | 45.8 (14.2–48.2) | 31 (17–48.5) | 0.293 |
| ALT (after 1 month, U/L) | 24.9 (15–30) | 51.3 (19–54.7) | 17.8 (13–23.5) | 0.008 |
| ALT (after 6 months, U/L) | 22.3 (17–26) | 23.4 (20–27.5) | 16. 5 (15.2–17.7) | 0.106 |
p value was obtained with Kruskal-Wallis or chi-square test
CML chronic myeloid leukemia, GIST gastrointestinal stromal tumor, IFG impaired fasting glucose, Mg magnesium, ALT alanine aminotransferase
Table 3.
Repeated measures ANOVA
| Glucose before | Glucose after 1 month | Glucose after 6 months | Mauchly’s sphericity test | Greenhouse-Geisser p value | |
|---|---|---|---|---|---|
| Subjects with fasting glucose < 100 mg/dL (n = 76) | 87.6 ± 8.3 | 93.6 ± 7.6 | 93.4 ± 10.5 | 0.960 | 0.048 |
| Subjects with fasting glucose ≥100 mg/dL (n = 19) | 121 ± 28.7 | 97. 3 ± 12.8 | 96. 1 ± 7.3 | 0.184 | 0.076 |
| Subjects with T2DM (n = 11) | 241 ± 120.7 | 152.5 ± 74.5 | 128.6 ± 33.9 | 0.702 | 0.042 |
Database was analyzed through the ANOVA test of repeated samples to determine the statistical significance between normal FG, IFG and T2DM subjects before treatment and at 1 and 6 months follow-up. The Mauchly’s sphericity test was used to evaluate equality and homogeneity of the studied population, and the p value was obtained through the Greenhouse-Geisser test
Fig. 2.
Change in fasting plasma glucose concentration after treatment with imatinib in subjects with normal fasting glucose, impaired fasting glucose and type 2 diabetes mellitus
We identified 11 subjects with T2DM, which are represented in Table 4, from the INCMNSZ database. As shown in the table, two subjects (18.2%) had an increase in FPG concentration after 1 month of treatment with imatinib, one did not have sufficient follow-up data, and the remaining (72.7%) decreased their FPG concentration. According to the FPG concentration after 6 months of treatment, one subject (9.1%) had an elevation, seven decreased their glucose concentrations (63.6%), and three did not have sufficient data. HbA1c decreased from 7.48 (±1.82) to 6.2 (±0.57), with a significant reduction at 6 months after imatinib treatment (p = 0.04). Finally, according to the hypoglycemic treatment, four subjects (36.4%) stayed with the same treatment, two (18.2%) reduced the doses, one (9.1%) stopped taking metformin, one (9.1%) increased doses, another one (9.1%) started using insulin, and the last two subjects did not have sufficient follow-up data after 6 months of imatinib administration. With these results, we can observe that most subjects did have a reduction in the FPG concentration at 1-month follow-up (72.7%) and 6-month follow-up (63.6%), indicating a benefit of imatinib on glucose metabolism. In Table 5 we depict the studies assessing the effect of imatinib on glucose metabolism in subjects with T2DM.
Table 4.
Subjects with type 2 diabetes
| Subjects | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Glucose | 117 | 173 | 323 | 321 | 113 | 110 | 254 | 261 | 137 | 386 | 457 |
| Glucose after 1 month | 51 | 187 | 137 | 135 | 133 | 106 | – | 186 | 118 | 334 | 138 |
| Glucose after 6 months | – | 164 | 167 | 158 | – | 107 | 97 | – | 143 | 116 | 77 |
| HbA1c before | 8.6 | 10 | 8.8 | 14.6 | 6 | 6.5 | 12.1 | – | 6.3 | 8.6 | – |
| HbA1c after 1 month | – | – | 5.2 | 7.5 | – | – | – | 11 | 6 | 6.7 | – |
| HbA1c after 6 months | – | 7.1 | 6.2 | 7.6 | – | 6.3 | 6 | – | 5.7 | 7.6 | 5.7 |
| Treatment before | Glyburide 5 mg TID Metformin 500 mg TID |
Metformin 500 mg TID Glyburide 5–5 – 2.5 |
Metformin / glyburide 500 mg/5 mg TID | Glyburide 5 mg BID Metformin 850 mg QD |
Metformin 500 mg QD | Metformin 500 mg QD | Glyburide 5 mg TID Metformin 850 TID |
Insulin Glargine 30 U QD Metformin 850 mg QD |
Metformin / glyburide 500 mg/5 mg half dose TID | NPH 45–0-20 Lispro 10–10-2 |
Glyburide 5 mg TID Metformin 850 mg TID |
| Treatment after 6 months | Stayed the same | Metformin 500 mg TID Glyburide 5 mg TID |
Metformin /glyburide 500 mg/5 mg BID | Stayed the same | – | Stayed the same | NPH insulin 25 U QD Metformin 850 mg TID |
– | Stayed the same | NPH 25–0-20 Lispro 10–10-3 |
Suspended Metformin Glyburide 5 mg TID |
HbA1c Glycated hemoglobin, TID three times a day, BID two times a day, QD once a day, NPH neutral protamine Hagedorn
Table 5.
Studies assessing effect of imatinib on glucose metabolism of subjects with T2DM
| Author | Year | N | Effect | Reference |
|---|---|---|---|---|
| Agostino et al. | 2011 | 17 | 47% of the subjects could discontinue their medications. All the subjects had a reduction in FPG. | [2] |
| Breccia et al. | 2004 | 7 | 85.7% of the subjects reduced FPG concentration allowing dose decrease of oral hypoglycemic agents. | [3] |
| Iurlo et al.a | 2015 | 27 | Improve FPG levels and reduction of the dosage of antihyperglycemic drugs. | [22] |
| Dingli et al. b | 2007 | 7 | There was no reduction of FG, HbA1c or antidiabetic treatment in any of the patients. | [9] |
FPG fasting plasma glucose
aIn this study, the authors did not separate subjects with IFG and T2DM
bTwo patients of the group with normal fasting glucose developed diabetes during the treatment with imatinib
Discussion
We observed that imatinib reduced the FPG and HbA1c concentration in subjects with T2DM. However, due to the design of our study, we cannot establish a real therapeutic effect of imatinib in subjects with T2DM. As described previously, only three of the eleven subjects with T2DM reduced their hypoglycemic therapy, but four remained with the same anti-diabetic treatment. HbA1c reduction indicates that FG was reduced, especially in subjects with high-starting HbA1c values; as in most diabetes clinical trials the magnitude of improvement in HbA1c is related to baseline A1c, the higher the A1c the greater the drop, so when A1c is normal you cannot expect a lot of improvement.
Imatinib inhibits the phosphorylation of proteins which may result in better signaling, better function of effectors, or both, with improvement in insulin sensitivity; thus, decreasing HbA1c levels in patients with high-starting values. Therefore, we can assume there is a therapeutic benefit in patients with T2DM. We cannot attribute this effect entirely to the imatinib treatment, but data in our study suggests there is a relationship between its administration and the lowering of FPG concentration.
Due to our study population, formed by Hispanic subjects with cancer, many confounding factors can alter the results. Nausea, vomiting, weight loss, hydroxyurea, and redistribution of adipose tissue [9] could influence glucose metabolism and insulin resistance. Even though there are animal trials that confirm the effect of imatinib on insulin resistance and glucose metabolism, these confounding variables need to be evaluated in prospective studies. We could not assess the effect after discontinuing therapy as Agostino et al. [2].
Ethnicity could affect imatinib’s treatment response. Several studies compared the difference of imatinib therapy response between different ethnic groups, but few studies have enough Hispanic subjects to compare against other ethnic groups. Lee et al. performed a study with more Hispanic subjects with CML compared to non-Hispanics [60.9% vs 39.1%, respectively], and concluded that Hispanic subjects achieved better treatment responses to imatinib when compared to non-Hispanic subjects [10].
We reviewed the pathophysiology of T2DM and several animal and human studies that aimed to establish the mechanism by which imatinib lowers FPG concentration. T2DM derives from the abnormal metabolism of carbohydrates, fats, and proteins which leads to hyperglycemia and hyperlipidemia. Within time, high levels of glucose and lipids induce changes in the metabolic pathways of insulin causing impaired insulin secretion from the β-cells of pancreatic islets, insulin resistance and decreased glucose use in peripheral tissues, and abnormal hepatic glucose production. Imatinib has shown to interfere in these pathways [11].
Mice models with type 1 diabetes mellitus (T1DM) and T2DM treated with TKIs have shown beneficial effects, improving several aspects of the disease. A study by Louvet et al. reported non-obese diabetic mice with new-onset T1DM experienced the regression of the disease when treated with imatinib [7]. Additionally, Chang Qing et al. established that there is an increase in the production of insulin in residual β-cells, with or without glucose stimulation, through an indirect control of the genetic expression of insulin in response to glucose, and through the promotion of the expression of glucose transporter-2 (GLUT-2) in β-cells [12]. Furthermore, it has been proven that TKIs prevent β-cell apoptosis via activation of antiapoptotic nuclear factor kappa-light-chain-enhancer of activated B cells (NF-Kb) and/or inhibition of the proapoptotic mitogen-activated protein kinase/c-jun N-terminal kinase (MAPK/JNK) [13, 14]. Moreover, Wijesekara et al. found that adiponectin promotes the phosphorylation of the protein kinase B (Akt/pkB) and the extracellular signal-regulated kinase (ERK) which leads to protection against apoptosis and stimulation of gene expression and secretion in pancreatic beta cells [15]. It has been proven that adiponectin concentration rises three times in plasma after three months of treatment with imatinib [16].
The hypoglycemic effect of this drug might be due to the inhibition of the multiple tyrosine kinases, such as c-Abl [12], PDGFR, Akt/pkB [4], and the extracellular regulatory kinases ERK1 and ERK2 which are crucial to the control and signaling activity of cellular effectors in the insulin pathway [17]. Phosphorylation of ERK by imatinib could result in better signaling, better functioning of the effectors or both [18], and it could also have an antiapoptotic effect [17]. In addition, inhibition of vascular endothelial growth factor receptor 2 (VEGFR2) reduces the degree of islet cell inflammation (insulitis) [19]. Likewise, the tyrosine phosphorylation of insulin receptor and phosphorylation of Akt/pkB after insulin administration was dose-dependent [20]. It is noteworthy that c-kit inhibition is not required for the reversal of hyperglycemia [5]. Markers of endoplasmic reticulum (ER) stress, protein kinase RNA-like endoplasmic reticulum kinase (PERK), eukaryotic initiation factor 2α (eIF2α), phosphorylated tribbles homolog 3 protein (TRB3), C/EBP homologous protein (CHOP), and phosphorylated JNK, decreased with imatinib [21].
Several case reports and retrospective human studies have been published assessing the effect of imatinib on glucose metabolism. Salalori et al. reported a subject with T1DM with the translocation-ets-leukemia/platelet-derived growth factor receptor β (TEL/PDGFRβ) rearrangement mutation and symptomatic hypoglycemia that had a reduction in the insulin dosage after treatment with imatinib [22]. Breccia et al. performed a study of 7 diabetic subjects with CML treated with imatinib, 6 showed improvements in fasting glucose concentrations, allowing a dose decrease of oral hypoglycemic agents and insulin. Before starting imatinib, they had a mean glucose of 220 mg/dL, after 3 months of treatment the mean FPG concentration was 110 mg/dL and after 12 months 108 mg/dL. The subject who was resistant to imatinib had also a decrease in FPG concentrations [3]. A similar study by Agostino et al. analyzed the effect of multiple TKIs in glucose metabolism, and a subgroup of diabetic subjects (8 of 17, 47%) could discontinue their medications, including insulin in some of them. The mean FPG decreased in all individuals associated with treatment with TKI [2]. Additionally, other case series not only found beneficial effects of imatinib on FPG concentrations, but also in the lipid profile, lowering total, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol concentrations [16, 23]. It has been proven that imatinib’s effect on glucose concentrations is stable after the end of treatment in comparison with dasatinib, which also lowered glucose concentrations [23, 24]. It is important to recall that this study has the biggest number of subjects, compared with others with the same topic.
For future studies in this topic, it would be very important to adjust the effect of imatinib on glucose metabolism for important confounders such as weight changes.
Conclusion
We conclude that subjects with CML or GIST with T2DM had a statistically and clinically reduction in mean FPG and HbA1c at 1 and 6 months of imatinib therapy. Clinicians should consider the hypoglycemic effect of this drug when treating CML or GIST subjects with T2DM, and investigations on this drug need to continue to discover its potential use in diabetes therapy.
Additional files
Table S1. Repeated measures ANOVA. (DOCX 13 kb)
Figure S1. Fasting plasma glucose concentrations follow up. FPG, fasting plasma glucose; FG, fasting glucose; IFG, impaired fasting glucose; T2DM, type 2 diabetes mellitus. (JPG 20 kb)
Acknowledgements
MAGS would like to acknowledge Luz del Carmen Abascal-Olascoaga for her support.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets generated and/or analysed during the current study are available from Alfonso Gulias-Herrero, MD, PhD via e-mail (alfonso.tiranicida@gmail.com) on reasonable request.
Authors’ contribution
MAGS, JEBB, MCC, BDWC, FVM, DMB, JAAR, LPB, DCR, FJGP, AZD, AAG, and AGH recollected the patients’ information from the hospital database. MAGS and DCR made the statistical analysis, MAGS, JEBB, MCC, BDWC, FVM, DMB, JAAR, LPB, DCR, FJGP, AZD, AAG, and AGH interpreted the results obtained from the statistical analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was submitted and approved by the Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran Comite de Etica en Investigacion/Comite de investigacion on 11th of July 2016. REF 1974. The ethics committee waivered the need for consent from each patient since we do not include personal information.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Miguel Ángel Gómez-Sámano, Phone: 52 55 54870900, Email: miguelangelgomezsamano@gmail.com.
Jorge Enrique Baquerizo-Burgos, Phone: +593 989627111, Email: jorgebaquerizoburgos@gmail.com.
Melissa Fabiola Coronel Coronel, Phone: +593 989946032, Email: melissacoronelc@gmail.com.
Buileng Daniela Wong-Campoverde, Phone: +593 997951301, Email: builengwong@gmail.com.
Fernando Villanueva-Martinez, Phone: 52 55 11620200, Email: ferdbgt@yahoo.com.
Diego Molina-Botello, Phone: 52 55 51065308, Email: diegombotello@gmail.com.
Jose Alonso Avila-Rojo, Phone: 52 1 6862478853, Email: alonso_2694@hotmail.com.
Lucía Palacios-Báez, Phone: 52 55 54870900, Email: lucypbaez@hotmail.com.
Daniel Cuevas-Ramos, Phone: 52 55 54870900, Email: ceptamim@gmail.com.
Francisco Javier Gomez-Perez, Phone: 52 55 54870900, Email: gomezperezfco@gmail.com.
Alejandro Zentella-Dehesa, Phone: 52 55 54870900, Email: azentell@iibiomedicas.unam.mx.
Álvaro Aguayo-González, Phone: 52 55 38880857, Email: alvaroaguayo@hotmail.com.
Alfonso Gulias-Herrero, Phone: 52 55 54870900, Email: alfonso.tiranicida@gmail.com.
References
- 1.International Diabetes Federation . IDF Diabetes Atlas. 8. Brussels: International Diabetes Federation; 2017. [PubMed] [Google Scholar]
- 2.Agostino NM, Chinchilli VM, Lynch CJ, Koszyk-Szewczyk A, Gingrich R, Sivik J, et al. Effect of the tyrosine kinase inhibitors (sunitinib, sorafenib, dasatinib, and imatinib) on blood glucose levels in diabetic and nondiabetic patients in general clinical practice. J Oncol Pharm Pract. 2011;17:197–202. doi: 10.1177/1078155210378913. [DOI] [PubMed] [Google Scholar]
- 3.Breccia M, Muscaritoli M, Aversa Z, Mandelli F, Alimena G. Imatinib mesylate may improve fasting blood glucose in diabetic Ph+ chronic myelogenous leukemia patients responsive to treatment. J Clin Oncol. 2004;22:4653–4655. doi: 10.1200/JCO.2004.04.217. [DOI] [PubMed] [Google Scholar]
- 4.Mokhtari D, Welsh N. Potential utility of small tyrosine kinase inhibitors in the treatment of diabetes. Clin Sci (Lond) 2010;118:241–247. doi: 10.1042/CS20090348. [DOI] [PubMed] [Google Scholar]
- 5.Lau J, Zhou Q, Sutton SE, Herman AE, Schmedt C, Glynne R. Inhibition of c-kit is not required for reversal of hyperglycemia by imatinib in NOD mice. PLoS One. 2014;9:1–5. doi: 10.1371/journal.pone.0084900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuveson DA, Willis NA, Jacks T, Griffin JD, Singer S, Fletcher CD, et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20:5054–5058. doi: 10.1038/sj.onc.1204704. [DOI] [PubMed] [Google Scholar]
- 7.Louvet C, Szot GL, Lang J, Lee MR, Martinier N, Bollag G, et al. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2008;105:18895–18900. doi: 10.1073/pnas.0810246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hägerkvist R, Sandler S, Mokhtari D, Welsh N. Amelioration of diabetes by imatinib mesylate (Gleevec): role of beta-cell NF-kappaB activation and anti-apoptotic preconditioning. FASEB J. 2007;21:618–628. doi: 10.1096/fj.06-6910com. [DOI] [PubMed] [Google Scholar]
- 9.Dingli D, Wolf RC, Vella A. Imatinib and type 2 diabetes. Endocr Pract. 2007;13:126–130. doi: 10.4158/EP.13.2.126. [DOI] [PubMed] [Google Scholar]
- 10.Lee JP, Birnstein E, Masiello D, Yang D, Yang AS. Gender and ethnic differences in chronic myelogenous leukemia prognosis and treatment response: a single-institution retrospective study. J Hematol Oncol. 2009;2:30. doi: 10.1186/1756-8722-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akash MSH, Rehman K, Chen S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2013;114:525–531. doi: 10.1002/jcb.24402. [DOI] [PubMed] [Google Scholar]
- 12.Xia CQ, Zhang P, Li S, Yuan L, Xia T, Xie C, et al. C-Abl inhibitor imatinib enhances insulin production by β cells: C-Abl negatively regulates insulin production via interfering with the expression of NKx2.2 and GLUT-2. PLoS One. 2014;9:1–11. doi: 10.1371/journal.pone.0097694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf AM, Wolf D, Rumpold H, Ludwiczek S, Enrich B, Gastl G, et al. The kinase inhibitor imatinib mesylate inhibits TNF-α production in vitro and prevents TNF-dependent acute hepatic inflammation. Proc Natl Acad Sci U S A. 2005;102:13622–13627. doi: 10.1073/pnas.0501758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hägerkvist R, Makeeva N, Elliman S, Welsh N. Imatinib mesylate (Gleevec) protects against streptozotocin-induced diabetes and islet cell death in vitro. Cell Biol Int. 2006;30:1013–1017. doi: 10.1016/j.cellbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Wijesekara N, Krishnamurthy M, Bhattacharjee A, Suhail A, Sweeney G, Wheeler MB. Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J Biol Chem. 2010;285:33623–33631. doi: 10.1074/jbc.M109.085084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitter S, Vandyke K, Schultz CG, White D, Hughes TP, Zannettino ACW. Plasma adiponectin levels are markedly elevated in imatinib-treated chronic myeloid leukemia (CML) patients: a mechanism for improved insulin sensitivity in type 2 diabetic CML patients? J Clin Endocrinol Metab. 2010;95:3763–3767. doi: 10.1210/jc.2010-0086. [DOI] [PubMed] [Google Scholar]
- 17.Fred RG, Boddeti SK, Lundberg M, Welsh N. Imatinib mesylate stimulates low-density lipoprotein receptor-related protein 1-mediated ERK phosphorylation in insulin-producing cells. Clin Sci (Lond) 2015;128:17–28. doi: 10.1042/CS20130560. [DOI] [PubMed] [Google Scholar]
- 18.Mokhtari D, Al-Amin A, Turpaev K, Li T, Idevall-Hagren O, Li J, et al. Imatinib mesilate-induced phosphatidylinositol 3-kinase signalling and improved survival in insulin-producing cells: role of Src homology 2-containing inositol 5′-phosphatase interaction with c-Abl. Diabetologia. 2013;56:1327–1338. doi: 10.1007/s00125-013-2868-2. [DOI] [PubMed] [Google Scholar]
- 19.Fountas A, Diamantopoulos LN, Tsatsoulis A. Tyrosine kinase inhibitors and diabetes: a novel treatment paradigm? Trends Endocrinol Metab. 2015;26:643–656. doi: 10.1016/j.tem.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Prada PO, Ropelle ER, Moura RH, de Souza CT, Pauli JR, Rocco SA, et al. EGFR tyrosine kinase inhibitor (PD153035) improves glucose tolerance and insulin action in high-fat diet-fed mice. Diabetes. 2009;58:2910–2919. doi: 10.2337/db08-0506.P.O.P. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Han MS, Chung KW, Cheon HG, Rhee SD, Yoon C-H, Lee M-K, et al. Imatinib mesylate reduces endoplasmic reticulum stress and induces remission of diabetes in db/db mice. Diabetes. 2009;58:329–336. doi: 10.2337/db08-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salaroli A, Loglisci G, Serrao A, Alimena G, Breccia M. Fasting glucose level reduction induced by imatinib in chronic myeloproliferative disease with TEL-PDGFRβ rearrangement and type 1 diabetes. Ann Hematol. 2012;91:1823–1824. doi: 10.1007/s00277-012-1493-3. [DOI] [PubMed] [Google Scholar]
- 23.Iurlo A, Orsi E, Cattaneo D, Resi V, Orofino N, Sciumè M, et al. Effects of first- and second-generation tyrosine kinase inhibitor therapy on glucose and lipid metabolism in chronic myeloid leukemia patients : a real clinical problem ? Oncotarget. 2015;6:33944–33951. doi: 10.18632/oncotarget.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breccia M, Muscaritoli M, Cannella L, Stefanizzi C, Frustaci A, Alimena G. Fasting glucose improvement under dasatinib treatment in an accelerated phase chronic myeloid leukemia patient unresponsive to imatinib and nilotinib. Leuk Res. 2008;32:1626–1628. doi: 10.1016/j.leukres.2008.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Repeated measures ANOVA. (DOCX 13 kb)
Figure S1. Fasting plasma glucose concentrations follow up. FPG, fasting plasma glucose; FG, fasting glucose; IFG, impaired fasting glucose; T2DM, type 2 diabetes mellitus. (JPG 20 kb)
Data Availability Statement
The datasets generated and/or analysed during the current study are available from Alfonso Gulias-Herrero, MD, PhD via e-mail (alfonso.tiranicida@gmail.com) on reasonable request.