Abstract
Polymeric nanoparticles have tremendous potential to improve the efficacy of therapeutic cancer treatments by facilitating targeted delivery to a desired site. The physical and chemical properties of polymers can be tuned to accomplish delivery across the multiple biological barriers required to reach diverse subsets of cells. The use of biodegradable polymers as nanocarriers is especially attractive as these materials can be designed to break down in physiological conditions as well be engineered to exhibit triggered functionality when at particular location or activated by an external source. In this review, we present how biodegradable polymers can be engineered as drug delivery systems to target the tumor microenvironment in multiple ways. These nanomedicines can target cancer cells directly, target the blood vessels that supply the nutrients and oxygen that support tumor growth, and target immune cells to promote anti-cancer immunotherapy.
Keywords: nanocarrier, drug delivery, stimuli-responsive materials, targeted delivery, angiogenesis, immunotherapy
INTRODUCTION
Novel drug delivery systems are engineered to provide new avenues for improved safety and efficacy of modern cancer therapies. Nanoparticles with proper design have a tremendous potential to increase the efficacy of active anticancer agents through increased targeting to a desired site for an efficient treatment with reduced off-target effects. To enable a systemic delivery, there are several biological barriers that need to be considered, ranging from the system level, to the organ level, and to the cellular level (1). For oral delivery, the nanocarrier should provide stability in the gastrointestinal tract and then target a site for entry. Upon entry to the blood stream, the carrier should prevent kidney filtration, uptake by phagocytes, aggregation with serum proteins, and enzymatic degradation of the cargo, and enable prolonged circulation (2). The nanoparticle design should enable sufficient diffusion in the extracellular matrix to reach the desired site, where it should either release the active agent to the extracellular microenvironment or shuttle it intracellulary within the targeted cells, depending on the cargo.
Many types of materials exist as constituent building blocks of nanocarriers, including both natural and synthetic materials. Synthetic strategies include inorganic nanoparticles such as those composed of gold (3, 4) or iron oxide (5), that can have intrinsic ability for imaging, sensing, and diagnostic applications (6, 7), but can have significant limitations for drug loading and drug release for therapeutic applications (8). Organic materials such as lipids have had a relatively long history of use for delivering biomolecules for therapeutic purposes. These include therapeutics such as DOXIL®, liposomal doxorubicin that has its surface coated with the stealth polymer poly(ethylene glycol) (PEG), that has been used to treat multiple cancers including breast cancer and ovarian cancer (9–11). An alternative class of nanocarriers is fully composed of polymers. Polymers have multiple advantages including the ability to tailor the physical, chemical, and biological properties of the nanocarrier. Biodegradable polymers are especially appealing as they break down in physiological conditions, which generally both reduces any potential toxicity of a large macromolecule as well as facilitates drug release (12, 13).
Biodegradable polymeric nanocarriers can accomplish anti-cancer targeting through multiple mechanisms. This includes delivery directly to the tumor cells facilitated by prolonged circulation in the blood stream, ability to facilitate transport across the endothelium to the tumor, and ligand-mediated targeting (14–17). In addition, polymeric nanocarriers can target other cells that make up the tumor microenvironment as well. Targeting tumor vasculature is a prime target as the tumor-associated neovasculature is key to allowing a tumor to obtain sufficient oxygen and nutrients to allow growth (18, 19). Finally, immune cells are also a critical target as the balance between an anti-cancer immune response and a tumor’s immunosuppressive microenvironment can determine whether a tumor goes into remission or expands (20, 21).
BIODEGRADABLE POLYMERIC NANOPARTICLES
Formulations
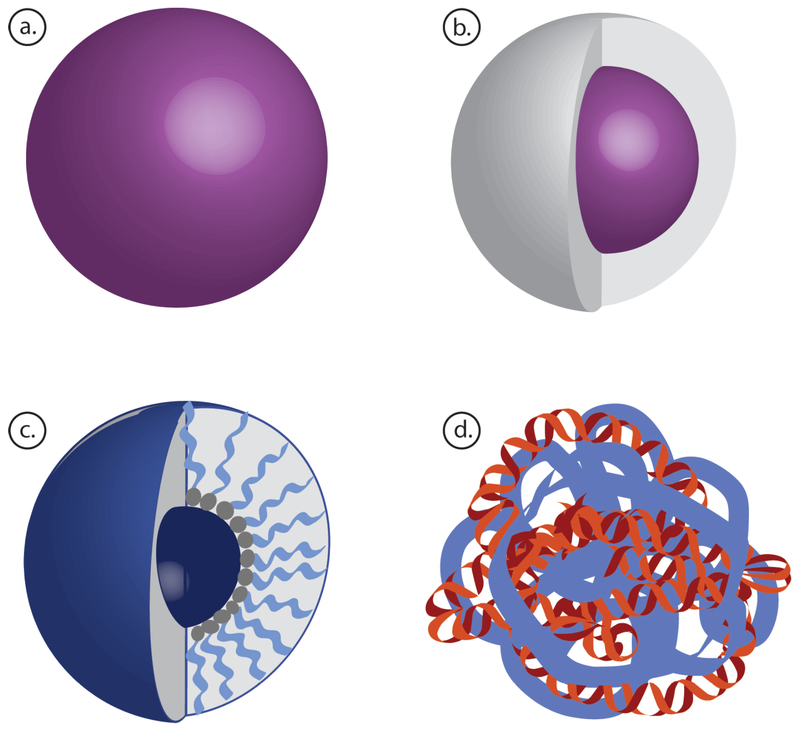
Biodegradable polymer nanoparticles can be engineered into a variety of formulations, such as solid nanoparticles, core-shell structures, polymeric micelles, and polyplexes (Figure 1). The preferred nanoparticle formulation and synthesis method depends on the properties of the chosen polymer and cargo, but polymeric nanoparticles are generally formed by either self-assembly or emulsion. Briefly, self-assembly methods include intermolecular and intramolecular interactions between the polymer itself and its cargo, such as complexation of cationic polymers with anionic nucleic acids to form polyplexes, as well as spontaneous micelle assembly, when amphiphilic block co-polymers reach a critical micelle concentration (CMC) and form particles due to hydrophobic interactions. Nanoparticles can also be formed by methods such as emulsification, where nanoparticles are formed as droplets of one phase dispersed in a second phase. Typically, the polymer is dissolved in an organic phase that is then mixed with a surfactant and sonicated in an aqueous phase with high intensity to form nano-droplets (22). The emulsion is stirred until the solvent evaporates, leaving behind hardened polymer nanoparticles. These hard nanoparticles can also be coated with another material to form core-shell nanoparticles with favorable surface properties (23, 24).
Figure 1.
Schematic of different nanoparticle fabrication strategies using biodegradable polymers as nanocarriers. (a) Solid nanoparticles. (b) Core-shell nanoparticles. (c) Polymeric micelles. (d) Polyplex nanoparticles.
Natural Polymers
Naturally derived polysaccharide and protein-based polymers (Figure 2) have already been approved for diverse food, cosmetic, and medical applications (25). They show excellent biocompatibility since they are broken down by enzymatic degradation into easily metabolized peptides or polysaccharides in the body, and this degradation rate can be tuned for a desired release profile (26). However, these polymers are more variable batch-to-batch, often require chemical modification to act as efficient nanocarriers, and must be extensively purified to avoid immunogenicity.
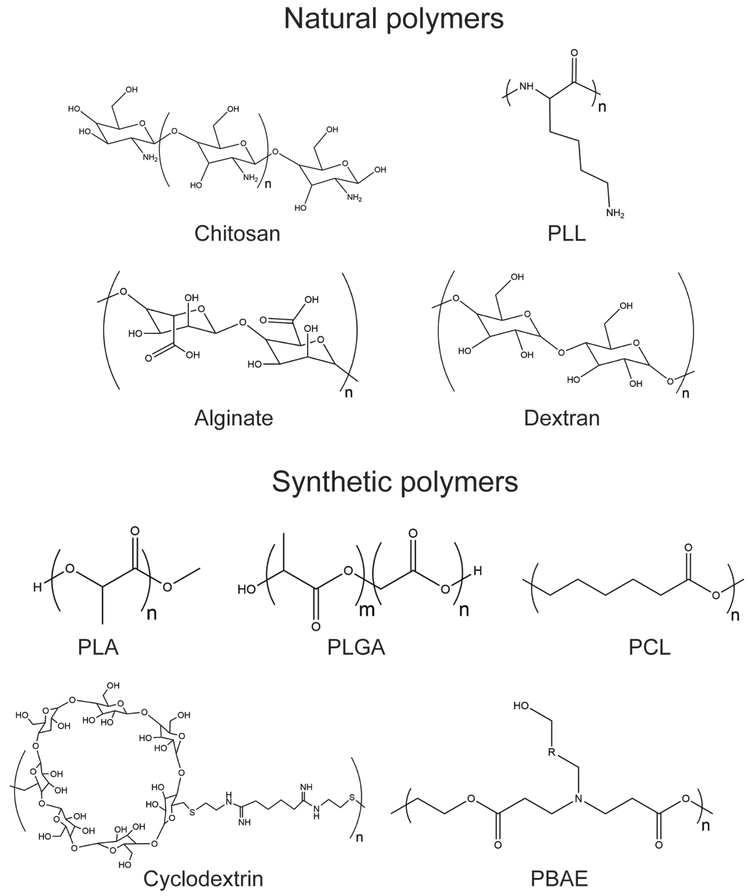
Figure 2.
Chemical structures for natural- and synthetic polymers used as drug delivery materials for anti-cancer drugs. PLL = poly(L-lysine), PLA = polylactide, PLGA = poly(lactide-co-glycolide), PCL = poly(ε-caprolactone), PBAE = poly(β-amino ester).
Chitosan is a linear polysaccharide derived from chitin, an abundant natural biopolymer found in the exoskeleton of insects and crustaceans (27). Chitosan is synthesized by the deacetylation of chitin, which forms primary amine groups, making the polymer cationic in dilute acidic solutions. As a result, chitosan electrostatically binds to and complexes with negatively charged macromolecules such as nucleic acids to form polyplexes (28, 29). Chitosan’s positive charge increases cell uptake and adhesion to negatively charged mucosal surfaces, making it well-suited for oral drug delivery (30). Degradation is highly tunable and can be optimized for biomedical applications by varying molecular weight, degree of deacetylation, and chemical modifications (31).
Dextran is a branched polysaccharide comprised of simple repeat units of α-d-glycose joined by glycosidic bonds (32). Dextran is hydrophilic and water-soluble but can be acetylated to create a hydrophobic polysaccharide (AcDex) (33). For drug delivery, it is typically combined with crosslinkers to form a hydrogel or coating. Further, dextran can be linked to a hydrophilic polymer such as PEG, Poly(ε-caprolactone) (PCL), or Polylactide (PLA) as a block co-polymer to form amphiphilic micelles, which can be loaded with hydrophobic chemotherapies (34).
Alginate is an inexpensive, naturally derived hydrophobic polymer purified from algae. It is linear and unbranched, consisting of blocks of β−1,4-linked-mannuronic acid and α-(1–4)-linked guluronic acid residues (35). Alginate is anionic, and introducing divalent cations such as calcium induces gelation (36). It is easily functionalized due to hydroxyl and carboxyl groups on the backbone, so its chemical and biological properties can be tuned (37). Alginate is biocompantible, non-immunogenic, and is used in a range of FDA approved products, from food additives to wound dressings (37).
Gelatin is a mixture of peptides and proteins derived from partial hydrolysis of animal collagen. It is biocompatible, non-immunogenic, and widely used in food and cosmetic products. Thus, gelatin nanoparticles have been investigated for both nucleic acid and small molecule drug delivery to cancer (38, 39). However, natural gelatin generally binds therapeutic cargos too loosely, so it is chemically modified for drug delivery applications. Carboxyl and hydroxyl groups allow for modification, including the introduction of thiol groups to improve binding affinity to gene cargos and form redox-responsive gelatin-based materials (38, 39). The isoelectric point of gelatin can be modified to optimize loading of charged drugs, and gelatin molecular weight and crosslinking density can be altered to control drug release (40).
Poly(L-lysine) (PLL) is the polymerized form of lysine, a cationic amino acid, with L stereochemistry for natural enzymatic degradability. High positive charge density of the polymer allows PLL to efficiently condense negatively charged molecules into nanoparticles. Further, its positive charge improves particle uptake, so PLL is often used as a coating on core-shell nanoparticles. PLL can be synthesized in linear and dendritic forms, with dendritic PLL exhibiting enhanced buffering capability and improved nucleic acid delivery (41).
Synthetic Polymers
Synthetic polymers (Figure 2) are engineered with desirable properties such as charge, hydrophobicity, and degradation profile, which are optimized for particular cargos, delivery routes, and disease targets. Synthesis is controlled for low batch-to-batch variability, and production is typically scalable for large scale manufacturing. However, unintended degradation products or metabolites can cause synthetic polymers to be cytotoxic or immunogenic.
Polyesters
Polylactide (PLA) exists in two optically active forms, since the lactide molecule is chiral: L-lactide and D-lactide. For drug delivery systems, poly(L-lactide) (PLLA) has a too slow degradation, hence poly(DL-lactide) (PDLLA) is the preferred candidate due to its faster degradation rate (42). PLA undergoes hydrolytic degradation as random scission of the ester bonds occur, releasing the particle cargo. To improve its use for gene delivery, tertiary amines are grafted onto the PLA backbone and serve as a source of positive charge to promote electrostatic interactions with nucleic acids (43). The charge density can hence be adjusted through varied degree of functional groups to the polymer structure.
Poly(Lactide-co-Glycolide) (PLGA) is a copolymer of poly-glycoic acid (PGA) and PLA. PLGA is an attractive delivery material because of its high stability, low toxicity, and a degradation rate which is easily tuned by varying the ratios of PLA and PGA monomers. PLGA nanoparticles are typically synthesized by emulsion methods and are used to encapsulate small molecule drugs. Surface modification with the addition of cationic ligands has shown to promote efficacy of gene delivery, for example using cationic lipids for siRNA delivery (44).
Poly(ε-caprolactone) (PCL) is a hydrophobic, semi-crystalline biodegradable polymer with a high capacity for drug binding and biodegradable properties due to that ester bonds break under physiological conditions (45). PCL exhibits high colloidal stability in a biological fluid, facile cellular uptake by endocytosis, low toxicity in vitro and in vivo, and controlled cargo release (46). Thus, this polymer has been used in tissue engineering scaffolds, biomedical devices, and drug delivery devices, such as implantable contraceptive Capronor® (47). PCL is often blended with other biodegradable polymers to speed degradation rate. In cancer nanomedicine, PEG-PCL block copolymers have been used to form micelles encapsulating chemotherapeutics (48, 49).
Synthetic Sugars
Cyclodextrins (CDs) are water-soluble synthetic carbohydrates comprised of 6–8 glucose units with an amphiphilic structure. CDs form a cup shape with a hydrophilic exterior and hydrophobic interior. Adamantine-PEG functionalization is used for nanoparticle stabilization without interfering the electrostatic interactions to anionic cargos. Functionalized CDs have thereby shown to form stable complexes with DNA and high transfection efficiency with low cytotoxicity (50, 51).
Synthetic Libraries
Poly(β-amino ester) (PBAE) libraries are synthesized by reacting diacrylates with amine monomers, including different structures of backbone-, side-chain-, and endcapping monomers to form polymers with diverse properties, including their size, charge, and hydrophobicity (52, 53). Using high throughput screening, PBAE formulations have been selected for high transfection efficacy, low toxicity, and cell-type specificity for targeted gene delivery to cancer cells (54, 55). For example, specific PBAE structures are able to provide a preferential DNA and siRNA delivery to patient-derived glioblastoma cells over healthy human progenitor neural cells (56, 57).
PBAEs are cationic, contain ester bonds that are hydrolytically cleavable, and can be engineered with primary-, secondary, and tertiary amines (58). These properties enable the polymers to bind anionic cargos, and facilitate endocytosis, endosomal escape, and intracellular release of cargo, enabling efficient gene delivery (55). To further promote cytoplasmic degradation, bioreducible disulfide linkages have been introduced into the polymer structure, enabling more efficient, triggered siRNA release in the reducing environment of the cytosol compared to release from ester bond hydrolysis alone (57, 59).
Chain Shattering Polymer Therapeutics (CSPTs) were developed by the Cheng group and undergo chain-shattering degradation once protective groups are removed, enabling rapid triggered release of cargos (60). A library of protecting groups has been designed with sensitivity to UV light, acid, base, hydrogen peroxide, and glutathione (60–62). Glutathione-reactive polymers degrade rapidly in the cytosol to release a small molecule chemotherapeutic, demonstrating anticancer efficacy in vitro and in vivo (61).
The Siegwart group has developed a novel polyester library for siRNA delivery, which includes 850 different functional structures that can be synthesized in a rapid and scalable manner and assemble with siRNA to form polyplexes (63). High throughput screens were used to identify formulations that would selectively transfect cancer cells in a cancer/normal matched cell line pair in vitro (63). Further, selected nanoparticle formulations showed accumulation in orthotopic lung tumors and tumor-specific gene silencing when delivered via aerosol (64).
STIMULI-RESPONSIVE POLYMERS FOR A TRIGGERED RELEASE
Materials for drug delivery should protect the active therapeutic agent from degradation and clearance during systemic blood circulation in vivo, provide transport to target tissues/cells, and release the cargo at the target site. Hence, a dual modal interaction between the polymers and drug candidate is required, with stable complexation for transport followed by readily disassembly at the target location. To meet these requirements, polymeric delivery materials are engineered to transform their physicochemical properties in response to various intra- and extracellular stimuli or external triggers (Figure 3). Stimuli-responsive materials provide targeted release at the desired site, reducing off-target delivery and adverse side effects. These stimuli can act at the cellular level for efficient cytosolic release or at the tissue level for tumor-targeted delivery.
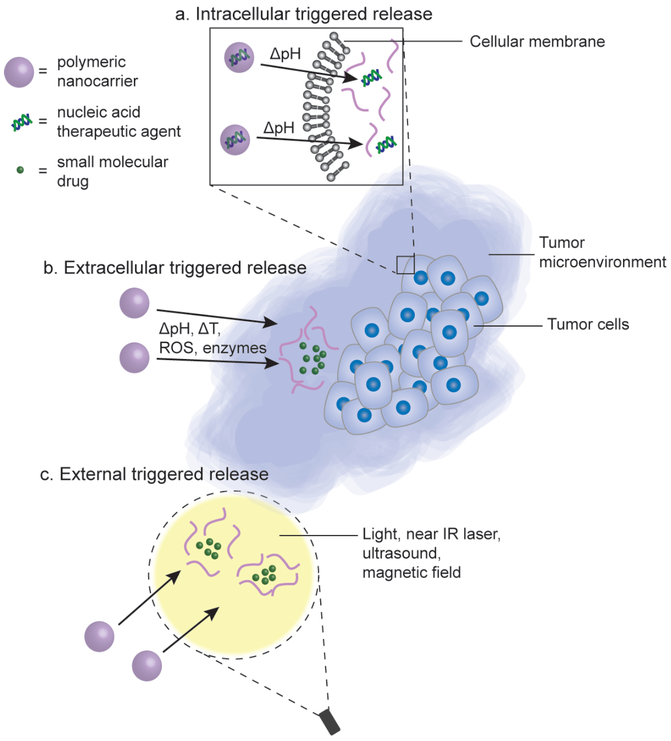
Figure 3.
Polymeric materials can be engineered to transform their properties in response to intracellular stimulus, extracellular stimulus, or external triggers to provide release of the active agent at the desired site. (a) Delivery materials for nucleic acid therapeutic agents are designed to protect the cargo during transport and subsequently provide an efficient intracellular release upon cell entry, often utilizing pH responsiveness to enable endosomal escape to the cytosol. (b) The nanocarrier can be designed to have environmentally-sensitive stimuli-responsive release of therapeutic agents based on local changes to pH, temperature (T), concentration of reactive oxygen species (ROS), and concentration of enzymes in the tumor microenvironment. (c) To obtain selective release at a specific site, external triggers, such as light, near-IR laser, ultrasound, and magnetic field can be applied to achieve spatial and temporal controlled release of anti-cancer agents from the engineered nanoparticles.
Widely used triggers for stimuli-responsive biodegradable polymeric drug delivery systems include pH (65, 66), redox potential (61, 67), temperature (68, 69), enzyme (70), light (71, 72), ultrasound (73, 74), and magnetic field (75), which cause a molecular transformation in the polymer and change interactions with protecting groups, cargo, or other polymer molecules, releasing the cargo at the desired site.
Intracellular release
In recent years, many important cancer-associated genes have been identified, underpinning new molecular therapeutics including recent cancer gene therapy efforts (76). Delivering nucleic acids, such as DNA, siRNA, miRNA, and mRNA, to cancer has tremendous therapeutic potential, allowing for sequence-specific regulation of disease-associated gene expression (77). However, without a delivery vehicle, nucleic acid molecules are unstable in the bloodstream, can be immunogenic, and cannot transverse cellular membranes due to their negative charge (78, 79). Thus, delivery systems for efficient targeted intracellular delivery are essential to bring gene therapy to a clinical setting (80, 81). Polymeric nanoparticles have been extensively used for this application due to their biocompatibility and biodegradability. These nanoparticles must be engineered to protect cargo from serum nucleases, avoid rapid hepatic and renal clearance, enter the tumor through transvascular transport, and deliver biologics intracellularly to target cells, generally via endocytosis (79, 82).
Cationic polymers are an attractive option for gene delivery as they readily self-assemble with anionic nucleic acids in aqueous solution to form electrostatically-bound polyplexes. Further, positively charged polymeric nanoparticles have been shown to efficiently penetrate the cell membrane in a process called endocytosis. Nanoparticles are taken up by endocytic vesicles, then the endosomal pH is lowered as the vesicle progresses to the late endosome stage. Polymeric nanoparticles have been engineered to respond to this acidic environment by initiating a process called endosomal escape. One commonly used approach for endosomal escape involves incorporating H+ buffering polyamines into the polymer to promote the endosomal rupture through the proton sponge effect (83, 84). The amines of the cationic polymer are protonated as the pH decreases, causing accumulation of Cl− and osmotic swelling within the endosome, which has been shown to generate a 140% increase in endosome volume (83). This volume increase facilitates endosomal rupture, releasing the nanoparticle cargo into the cytoplasm.
Cyclodextrin as nanocarrier has shown promising results for DNA and siRNA delivery (85, 86). To promote endosomal escape, cyclodextrin-based polymers are modified to include imidazole functional groups as the end-capping of the polymer termini (87). PLL has also been modified to include imidazole functional groups by grafting polyhistadine segments, generating a polymer with a pKa around 6. This modification increased the buffering capacity at endosomal pH, enhancing transfection efficiency (88)
Cyclodextrin nanoparticles are functionalized with adamantine-PEG (AD-PEG) to prevent protein aggregation in presence of serum, increasing circulation time and improving gene delivery in vivo. PEG groups reduce cellular uptake of the cyclodextrin nanoparticles, but conjugating transferrin to AD-PEG improves targeting and improves efficacy (16). Another approach to this challenge can be to use triggered PEG de-shielding when the nanoparticle reaches the tumor environment so the particle is protected in circulation, but cellular uptake in the tumor is still efficient.
Peptide and protein drugs also have been investigated as cargos for intracellular delivery vehicles (70, 89, 90). Certain protein drugs act intracellularly to interfere with dysregulated cancer pathways, but their therapeutic efficacy is limited by poor bioavailability and cell penetration in vivo. PEG-modified PLA block copolymer nanoparticles have been used to deliver anticancer peptide NuBCP-9 to solid tumors in mice, and the treatment resulted in complete tumor regression and 100% survival (89). Because protein drugs specifically interfere with cancer-related pathways, they show potential for highly targeted cell killing. Bcl-2-derived peptide delivered with polymeric micelles induced cell death in ovarian cancer cells but not in non-cancerous fibroblasts (70). Therefore, as more anticancer protein and peptide drugs are identified, biodegradable polymer nanoparticles should be further investigated for intracellular delivery.
The therapeutic cargo being delivered dictates the optimal release properties of the delivery system. Systems with siRNA, miRNA, and mRNA as the therapeutic agent should promote an endosomal release of the cargo into the cytoplasm, whereas DNA must be delivered to the nucleus. Moreover, these different cargos possess different properties, such as molecular weight, charge ratio, stability, and stiffness, and the material used for delivery must be modified accordingly.
Extracellular release
Parameters of the extracellular microenvironment, such as pH, temperature, dissolved oxygen concentration, and enzyme concentration, are influenced by disease development and progression. Stimuli-responsive polymers are engineered to respond to such changes at varying disease stages, or through external triggering, to selectively release therapeutics in the tumor. Heat-responsive polymers release small molecule cargos when exposed to the local hyperthermia (T ~ 42°C) of the tumor microenvironment (84). Many targeted nanoparticle systems are also glutathione-responsive, releasing cargo under the reducing conditions of the tumor. Both the blood and normal tissue have a pH of 7.4 whereas the tumor microenvironment generally exhibits a local pH of around 6 (91, 92). This acidic microenvironment is due to the production of lactic acid under anaerobic conditions and the high rate of glycolysis in cancer cells (93).
Certain drug delivery systems have been developed to be responsive to both the extra- and intracellular pH through a layer-by-layer design. In such an approach, one active agent is released in the slightly acidic tumor microenvironment, and another active agent is intracellularly released as pH decreases in the late endosome. This approach was shown by Wang and coworkers, in which a stepwise pH-responsive nanoparticle system with a shell comprised of a charge reversible pullulan-based (i.e., CAPL) material and a core of PBAEPLGA (94). The nanoparticle system was loaded with both paclitaxel (PTX) and combretastatin A4 (CA4) to target tumor angiogenesis and deliver chemotherapy to hepatocellular carcinoma (HCC). The CAPL-coating responded to the slightly acidic tumor microenvironment, then the proton-sponge effect was obtained from PBAE in the core, thus a sequential release of CA4 and PTX was observed (94).
Efficient and selective release can also be achieved via external triggers such as light, ultrasound, and magnetic field. Externally triggered drug delivery has spatial- and temporal control of drug release in comparison to biological stimuli-triggered drug delivery. Using light-triggered stimuli to achieve electron transfer is a non-invasive and an attractive trigger for a rapid and precise release of anticancer agents. There is a wide range of materials that are being engineered to respond to an appropriate light source, but only a few materials have been explored in clinical trials. Thermo-responsive materials have also been developed to respond to an increase in temperature, causing a change in material properties or shape to release the active agent.
Shao et al developed a light-triggered photothermal therapy system in which they encapsulated Black Phosphorous Quantum Dots (BPQD) in PLGA to form nanoparticles (95). When exposed to near-IR laser, the BPQDs release heat, killing surrounding tumor cells. This strategy exploits the enhanced permeation and retention (EPR) effect, using PLGA nanoparticles to localize BPQDs in the tumor, then triggers cancer killing by irradiating the tumor area. The combined PLGA/BPQD photothermal therapy caused tumors in mice to gradually shrink then completely disappear in 16 days with no instances of recurrence over 40 days (95). Despite promising preclinical results, these therapies require an external stimulus to be applied directly at the tumor site. Therefore, the therapies are not relevant in cases of local spread and metastasis, when the locations of tumor sites are unknown.
Ultrasound-guided microbubble enhanced delivery of nanoparticles improves tumor tissue penetration through sonoporation. Microbubbles are injected intravenously with the therapeutic, and cavitation is induced in the acoustic field of the ultrasound, resulting in oscillation and subsequent collapse of the microbubbles (96). The resulting force from this process increases the permeability of vessel walls and increases cellular uptake. A PLGA-based nanoparticle system developed by Chowdhury et al. showed 5–9 fold improved delivery to a hepatocellular carcinoma xenograft when ultrasound-guided delivery was performed (74).
APPROACHES FOR CANCER THERAPY
Therapies that deliver to the tumor microenvironment
A major hurdle to achieve therapeutic treatment for cancer patients is to enable transport across biological barriers and to deliver the active agent at the desired site to provide therapy. This has hampered the development for many cancer therapies, especially for patients with brain cancer. To facilitate therapeutic treatments of brain cancer, sufficient delivery across the blood-brain barrier (BBB) needs to be achieved. Current standard-of-care therapies for patients with glioblastoma, which is a grade IV brain tumor, involves maximal surgical resection, radiation-, and chemotherapy. Despite optimal treatment, the median survival is only one year after first diagnosis (97, 98). This poor prognosis underlies the need for new novel therapeutic treatments in glioma patients and as other cancer therapies as well. Thus, there is a need of delivery materials that would enable an efficient delivery across biological barrier to reach the target site for improved cancer therapies.
A potential cancer gene therapy strategy to treat brain cancer is the delivery of genes to the tumor cells that encode enzymes that cause the local conversion of systemically administered pro-drugs, small molecules that can cross the BBB, into active cytotoxic agents that trigger tumor cell death (99). The nanocarrier is designed to obtain accumulation at the tumor site or is locally administered and subsequently exhibits preferential uptake into the tumor cells for an efficient treatment and to avoid toxicity in healthy cells. PBAE nanocarrieras in a rat model of brain cancer can provide effective DNA delivery of the herpes simplex virus type I thymidine kinase (HSVtk) gene, that when combined with systemic administration of the prodrug ganciclovir, can cause efficient brain cancer cell killing and extension of survival (100).
Material design for tumor targeting
Biodegradable polymers can be engineered and functionalized to achieve targeted delivery to a desired site, which can be crucial to obtain the desired therapeutic effect. There are two broad classes of engineering approaches to cancer targeting, either passive- or active targeting, and both can be utilized to improve accumulation at the desired site.
Passive tumor targeting.
It has been shown that macromolecules and nanoscale drug delivery systems have the ability to accumulate in tumor tissues even without the presence of targeting ligands (101, 102). This is due to the physical properties of these nanomaterials and to the leaky vasculature that surrounds tumors. The rapid formation of tumor blood vessels in order to provide the growing tumor with essential nutrients and oxygen results in abnormalities of the endothelium, such as a relatively high proportion of proliferating endothelial cells, increased tortuosity, pericyte deficiency, and aberrant basement membrane formation (103). This causes tumor vasculature to be more permeable than regular vasculature and allows nanoparticles to more efficiently diffuse into tumor tissue. Moreover, a decreased lymphatic drainage is also present in tumors due to the defective vasculature structure, thus increasing retention of material in the tumor. This phenomenon is called the enhanced permeation and retention (EPR) effect (Figure 4), and provides a framework for the passive targeting of nanoscale drug delivery systems to tumors following administration into the bloodstream (101, 102). The cutoff size to achieve passive targeting can vary depending on tumor location and size, but particles less than ~200 nm in diameter are generally preferable (104). It is also very important that the nanocarrier be engineered to enable prolonged circulation time, hence neutrally charged non-interacting surfaces with low immunogenicity, low clearance, and high serum stability are essential to employ passive targeting.
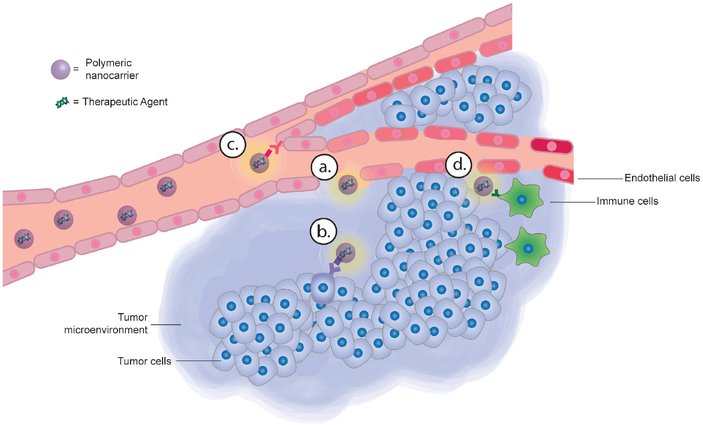
Figure 4.
Cancer targeting with polymeric nanocarriers can be achieved by targeting a number of cancer-specific features, including (a) Leaky tumor vasculature and dysfunctional lymphatic drainage (EPR effect). (b) Overexpressed protein receptors on the surface of cancer cells. (c) Dysregulated blood vessels (d) Immune cells within the cancer microenvironment.
Active tumor targeting.
As more cancer-specific surface molecules are discovered, scientists and engineers have developed active targeting methods to localize therapeutic nanoparticles to cancer cells. The nanoparticles are functionalized with targeting moieties on the surface which strongly interact with molecules that are overexpressed on the surfaces of cancer cells (Figure 4). Active targeting can be used to simply localize the nanoparticles to a tumor site or to activate downstream signaling in the cancer cell, triggering events such as apoptosis or endocytosis. Not only does the active targeting approach reduce the risk of off-target adverse side-effects, but it can also be used to provide cellular uptake through the receptor-mediated endocytosis mechanism.
Folate ligands can be used to achieve active targeting, since epithelial tumors of multiple organs, such as colon, lung, prostate, and brain all exhibit an up-regulated expression of folate receptors (105). Folate offers many advantages, it is known to be non-immunogenic, it can be rapidly taken up by tumor cells via receptor-mediated endocytosis, and it has been suggested that it may bypass cancer cells multidrug efflux-pumps (17, 106). The glycoprotein transferrin is another ligand that has shown to provide tumor targeting, since its receptor is highly upregulated in numerous cancer cell types. Transferrin is responsible for the iron supply to cells, and because tumor cells divide rapidly and have an elevated need of iron, they overexpress the transferrin receptor. Several approaches using polymeric nanoparticles functionalized with transferrin have shown cancer targeting ability. Bellocq and coworkers developed a cyclodextrin-based polymer gene delivery system functionalized with transferrin and reported that functionalization increased transfection to leukemia cells (107). Davis et al. subsequently showed that these transferrin-functionalized particles could enable systemic delivery of siRNA to melanoma patients in a phase I clinical trial (108). Hyaluronic acid (HA)-bound nanoparticles have also shown active tumor-targeting properties through interactions with CD-44 receptors, which are commonly overexpressed on cancer cells and mediate endocytosis. PLGA-HA core-shell nanoparticles have been shown to deliver docetaxel with enhanced specificity and lower systemic toxicity (99, 109). Certain integrins, such as α3β1 and αvβ3, overexpressed on cancer cells have also been targeted via functionalization with peptides containing RGD sequences (110, 111). The addition of active targeting ligands generally enhances tumor accumulation and increases therapeutic efficacy compared to non-functionalized particles which employ passive targeting alone.
Angiogenesis
Vascular networks provide tissues with essential nutrients and oxygen. In a healthy human body there is generally no significant angiogenesis, with new blood vessels only formed during embryonic development, wound healing, and in response to ovulation (103). However, when a solid tumor volume has become larger than 2 mm3, hypoxia occurs and new blood vessels are formed by tumoral angiogenesis. Increased vascularization around tumors enables disease progression by supporting tumor growth and metastasis. In healthy people, there is a balance between factors stimulating- and inhibiting blood vessel growth, but tumoral hypoxia induces upregulation of pro-angiogenic growth factors in cancer cells, including vascular endothelial growth factors (VEGF), fibroblast growth factors (FGF), epidermal growth factors (EGF), and angiogenin (112, 113). The upregulation of these factors leads to endothelial cell proliferation, differentiation, migration, and secretion of matrix metalloproteinases (MMPs), which degrade components of the extracellular matrix (ECM), allowing blood vessel formation in the tumor tissue and enabling tumor invasion (114).
Angiogenesis has become a target for cancer therapy because it is necessary for the growth of all solid tumors and generally does not occur in healthy tissues (115). Hypoxiainducible factor 1 (HIF-1) is known to be involved in crucial aspects of tumor biology, including angiogenesis, as it functions as an oxygen sensor and regulates oxygen delivery to cells and metabolic adaption to hypoxia (116, 117). Subunit HIF-1α is the oxygen- and growth factor regulator, hence upregulated HIF-1a expression promotes tumor progression and inhibition could provide a novel approach to cancer therapy (118). In seeking novel anti-angiogenesis strategies, polymeric nanocarrier mediated siRNA delivery has strong potential to target factors involved in angiogenesis. For the progression of prostate tumors, the growth factor interleukin-8 (IL-8) is known to both induce angiogenesis and regulate proliferation and survival of tumor cells (119, 120). Chen et al., showed that PLA functionalized with tertiary amine groups used as nanocarrier provided an efficient delivery of siRNA molecules targeting IL-8 in vitro (43).
Drug delivery systems have also been developed for a dual release of both anti-angiogenesis agents and conventional chemotherapeutic agents. Sengupta and coworkers designed a PLGA nanoparticle coated with PEGylated lipids, which allowed for an initial release of the anti-angiogenic agent combretastatin and then controlled release of the chemotherapeutic agent doxorubicin from the PLGA core (121). Drug agents with multimodal activity, such as a biomimetic peptide with dual action at inhibiting both angiogenesis and lymphangiogenesis in order to stop both tumor-associated blood vessel growth and cancer cell metastasis (122), are also promising agents to deliver with polymeric nanocarriers.
Immunoengineering
As a tumor forms, cancer cells develop mechanisms of immune system evasion, including immunosuppressive cytokine secretion, recruitment of suppressive immune cells, and expression of inhibitory ligands (123, 124). Anti-tumor immunotherapy strategies include amplifying positive regulators of the immune system, inhibiting negative regulators, or combining these approaches. Recently developed polymer-based immunoengineering technologies include varied approaches such as cancer vaccines, artificial antigen presenting cells, and gene delivery to immune cells. The main challenge to develop safe and effective immunotherapies is to manipulate an immune response in a predictable manner through the regulation of cellular and molecular signaling (125). To overcome this challenge, biomaterials are engineered to engender a desired immune response, control the differentiation of regulatory cells, and to regulate the spatial and temporal signals in the microenvironment.
Cytotoxic T cells have the potential to selectively identify and kill cancer cells, but many cancers can express co-inhibitory receptors to evade T cell response. Thus, therapies that can block these cancer defenses against the immune system have tremendous potential. The blocking of negative regulatory receptors on T cells to enhance an immune response to fight cancer cells is known as checkpoint blockade (126). Anti-cancer immunotherapy strategies can target the tumor microenvironment to induce killing of cancer cells by preexisting T cells or they can aid dendritic cells (DCs) to induce robust T cell priming and activation to combat the cancer cells (127). Checkpoint inhibitors, such as clinically approved antibodies targeting the PD-1 and CTLA-4 receptors, block this immunosuppressive signaling and have shown clinical promise in many types of cancers (128, 129). These groundbreaking antibody therapies can be used in synergy with novel nanoparticle systems (130, 131) or can be incorporated as cargo in nanoparticles (132). Alternatively, nanoparticles can deliver siRNA to immune cells or cancer cells for checkpoint modulation (133, 134). For example, siRNA delivery could be used to target the PD-L1 expression, which is the ligand that is inhibitory to the PD-1 receptor that activated T cells and natural killer (NK) cells express (135). Through this activation, an immune response that would combat tumor cells can be obtained. siRNA molecules targeting the STAT3 expression of cancer cells and that thereby reduce immunosuppression have shown promising results for tumor regression in vivo, where the nanocarrier was designed to target the tumor microenvironment and provide uptake into cancer cells (127, 136).
Another strategy is adoptive T cell therapy, which involves isolating patient immune cells, manipulating and stimulating them ex vivo, then reinfusing the engineered cells. These adoptive cell transfer methods have shown clinical potential in the cancer vaccine Sipuleucel-T and chimeric antigen receptor (CAR) T cell therapies for otherwise non-responsive cancers (137–140). However, culturing and engineering patient cells ex vivo is time consuming, expensive, technically challenging, and poses potential safety concerns.
To improve the availability of this approach to cancer patients and potentially reduce the cost, nanoparticles could be used to directly program T cells in circulation for tumor-recognizing capabilities. One such approach would be to design non-viral polymeric nanoparticles to deliver CAR genes to T cells in vivo without the need of adoptive cell transfer. Smith and coworkers showed that their nanoparticle design provided a DNA delivery encoding for CAR into T cells in this manner (141). The nanocarrier was made of PBAE functionalized with a ligand for T cell-targeting. Upon the DNA delivery, the reprogrammed T cells expressed CAR for weeks, resulting in tumor regression and prolonged survival in a mouse leukemia model. Thus, nanoparticle systems pose a promising alternative approach to traditional cellular immunotherapies because they can be engineered to deliver a range of immunomodulatory materials in a targeted manner in vivo with minimal off-target effects, eliminating the need to manipulate patient cells ex vivo (127).
Polymeric nanotechnology has also been employed in the development of cancer vaccines, which expose antigen-presenting cells to tumor-specific molecules in an immunostimmulatory environment, usually by co-delivering danger signals such as lipopolysaccharide or CpG dinucleotides with tumor antigen. Cancer vaccines target DCs to induce an antigen presentation, activating T cells and initiating a cytotoxic T lymphocytes (CTLs) response to recognize and kill the cancer cells. Moreover, strategies that can prevent immunosuppression in the tumor microenvironment can be key to help anti-cancer cytotoxic T cell populations overcome tumor resistance (142). It has been observed that glioblastoma tumors have a very potent immunosuppression due to upregulation of immunosuppressive protein Galectine-1 (Gal-1) (143, 144). Woensel et al. recently developed chitosan nanoparticles loaded with Gal-1 siRNA and observed 50% reduced Gal-1 expression in glioblastoma in vivo (145). Another nanoparticle design composed of PLA with crosslinked PEG provided a dual delivery of two hydrophobic drugs that inhibit TGF-β and IL-2 that both act as immunosuppression factors (146). Systemic administration of this nanoparticle design provided tumor accumulation in vivo that resulted in reduced the tumor growth and increased the survival time.
Biodegradable polymers can also be engineered as biomimetic materials that target the immune system to stimulate cancer immunotherapy. Polymer particles can be fabricated to act as artificial antigen presenting cells (aAPC) by functionalization with tumor specific peptide-MHC (pMHC) and a co-stimulatory molecule (147). Synthetic materials have the potential to serve as more effective treatments than cell-based counterparts because they can be engineered as an off-the-shelf product with well-defined characteristics for optimized T cell activation, including their size, shape, and surface presentation (148). For example, Sunshine et al. showed that ellipsoidally shaped PLGA aAPCs compared to spherically shaped PLGA aAPCs increased CD8+ T cell proliferation which led to increased survival in a melanoma mouse model (149). Kosmides et al. demonstrated that PLGA-based aAPCs stimulated cancer-specific CD8+ T cells with a synergistic effect with anti-PD-1 antibody, and the cytotoxic response increased survival time in a therapeutic melanoma model (130).
CLINICAL RESULTS
In recent years, biodegradable polymer nanoparticles have been tested in the clinic with some promising results, as outlined in Table 1.
Table 1.
Selected clinical trials of biodegradable polymeric nanoparticle cancer drug delivery systems.
| Name | Company | Material | Cargo | Phase | Cancer | Result | Reference |
|---|---|---|---|---|---|---|---|
| CALAA-01 | Calando Pharmaceuticals | PEG-Cyclodextrin | siRNA | I | Solid tumors | Significant toxicity was not observed | [151] |
| BIND-014 | BIND Therapeutics | PLGAwith PSMA targeting ligand | Docetaxel | I | Prostate | Significant toxicity was not observed, 12% overall response rate | [153] |
| NC-6004 | Nanocarrier | PEG-poly (amino acid) block co-polymer | Cisplatin | I | Pancreatic, head and neck, solid tumors | Significant toxicity was not observed | [154] |
| CRLX101 | Newlink Genetics Corporation | PEG-Cyclodextrin | Camptothecin | II | Lung, ovarian, solid tumors | Significant toxicity was not observed, 16% overall response rate | [156,157] |
| NK105 | Nippon Kayaku Co. | PEG-Polyaspartate | Paclitaxel | II | Breast, gastric | Significant toxicity was not observed, 25% overall response rate | [159,160] |
CALAA-01 (Calando Pharmaceuticals) was the first nanocarrier for siRNA delivery to reach clinical development in 2008 (101). CALAA-01 is a cyclodextrin particle decorated with PEG for biological stability and transferrin ligands to target transferrin receptors overexpressed on cancer cells (150). This carrier is used to deliver siRNA targeting the M2 subunit of ribonucleotide reductase. Tumor biopsies showed evidence of gene silencing by RNA interference, suggesting that targeted cyclodextrin nanoparticles are promising delivery vehicles for nucleic acids (108). The therapy was shown to be safe in Phase I trials, with minimal liver and kidney toxicity (151). In the Phase I trial, the most promising response was stable disease in one melanoma patient for four months (151).
Dr. Robert Langer of MIT and Dr. Omid Farokhzad of Harvard Medical School developed a promising targeted and controlled release polymeric nanoparticle tested in humans. Their company, BIND Therapeutics, was founded around their product BIND-014, a prostate-specific membrane antigen (PSMA) targeted PLGA nanoparticle containing docetaxel (152). The treatment was well-tolerated with no unanticipated toxicities in Phase 1 clinical trials, and six of 52 patients responded to the treatment, one with a complete response (153). Responses occurred in both PSMA expressing and non-expressing tumors, indicating that passive targeting played a significant role.
Nanocarrier has completed phase I clinical trials with NC-6004, a polymeric micelle made of PEG-poly (amino acid) block copolymers. These particles have been tested in clinical trials to deliver cisplatin for lung, bladder, bile duct, pancreatic, and head and neck cancers. In Phase I trials, the use of the nanocarrier increased the dose limiting toxicity of cisplatin 34-fold, and stable disease was observed for longer than four weeks in seven of 17 patients with solid tumors treated with NC-6004 (154).
CRLX101 (NewLink Genetics Corporation) particles are formed with alternating units of cyclodextrin and PEG, which improves circulation time, and camptothecin is chemically linked to the polymer for pH-dependent release (155). Phase I clinical trials showed acceptable safety and pharmacokinetics (156). In Phase II clinical trials, a measurable reduction in tumor size was observed in 74% of 22 patients with platinum-resistant ovarian cancer, and most recent results report a 16% RECIST response rate (157). Preclinical and preliminary clinical studies suggest a synergistic effect with anti-VEGF therapy bevacizumab, which will be further evaluated in future trials (110, 157).
NK105, developed by Nippon Kayaku Co., is a micellar form of paclitaxel formulated from PEG-polyaspartate block copolymers. This nanoparticle formulation demonstrated preclinical success in increasing circulation time, reducing off-target toxicity, and improving the anti-tumor effect of paclitaxel (158). Phase I trials showed a maximum tolerated dose 15 times higher than that of free paclitaxel (159). In Phase II trials, two full responses and 12 partial responses were observed for a 25% overall response rate (160).
CONCLUSIONS
The technologies highlighted in this review, biodegradable polymeric nanocarriers as drug delivery vehicles, have shown great promise at increasing the efficacy and safety of cancer therapies. Polymer properties can be customized for the delivery of specific anti-cancer agents, ranging from small-molecular drugs to biologics to ensure potent delivery. Biodegradable polymers can degrade safely in physiological conditions, and through engineering innovations, can respond to environmental and external triggers for spatial and temporal control of delivery. Despite the progress of polymeric drug delivery systems to treat cancer, improved targeting and improved drugs to deliver, are pressing concerns to further improve the clinical standard of care. Development of polymeric nanocarriers for cancer therapy has the potential to bring new combinations and multimodal avenues of therapeutic treatments to the forefront, including new anti-angiogenic therapies and immunotherapies.
ACKNOWLEDGEMENTS
HJV was supported by the NIH Cancer Nanotechnology Training Center at the JHU Institute for Nanobiotechnology. JK was supported by the Swedish Research Council International Postdoc grant (2016–06675). The authors would like to acknowledge support from the National Institutes of Health (R01EB022148) and the Johns Hopkins Bloomberg~Kimmel Institute for Cancer Immunotherapy.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERTURE CITED
- 1.Petros RA, DeSimone JM. 2010. Strategies in the design of nanoparticles for therapeutic applications. Nature Reviews Drug Discovery 9: 615–27 [DOI] [PubMed] [Google Scholar]
- 2.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. 2008. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm 5: 505–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekin A, Karatas OF, Culha M, Ozen M. 2014. Designing a gold nanoparticle-based nanocarrier for microRNA transfection into the prostate and breast cancer cells. J. Gene Med 16: 331–5 [DOI] [PubMed] [Google Scholar]
- 4.Madhusudhan A, Reddy GB, Venkatesham M, Veerabhadram G, Kumar DA, et al. 2014. Efficient pH dependent drug delivery to target cancer cells by gold nanoparticles capped with carboxymethyl chitosan. Int. J. Mol. Sci 15: 8216–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J, Zhang J, Gao W. 2014. Enhanced and selective delivery of enzyme therapy to 9L-glioma tumor via magnetic targeting of PEG-modified, beta-glucosidaseconjugated iron oxide nanoparticles. International journal of nanomedicine 9: 2905–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu J, Zheng L, Wen S, Tang Y, Shen M, et al. 2014. Targeted cancer theranostics using alpha-tocopheryl succinate-conjugated multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials 35: 7635–46 [DOI] [PubMed] [Google Scholar]
- 7.Iv M, Telischak N, Feng D, Holdsworth SJ, Yeom KW, Daldrup-Link HE. 2015. Clinical applications of iron oxide nanoparticles for magnetic resonance imaging of brain tumors. Nanomedicine (Lond) 10: 993–1018 [DOI] [PubMed] [Google Scholar]
- 8.Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, et al. 2013. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotech 31: 638–46 [DOI] [PubMed] [Google Scholar]
- 9.Xing M, Yan F, Yu S, Shen P. 2015. Efficacy and Cardiotoxicity of Liposomal Doxorubicin-Based Chemotherapy in Advanced Breast Cancer: A Meta-Analysis of Ten Randomized Controlled Trials. PLoS One 10: e0133569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien MER, Wigler N, Inbar M, Rosso R, Grischke E, et al. 2004. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol 15: 440–49 [DOI] [PubMed] [Google Scholar]
- 11.Muggia FM, Hainsworth JD, Jeffers S, Miller P, Groshen S, et al. 1997. Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. J. Clin. Oncol 15: 987–93 [DOI] [PubMed] [Google Scholar]
- 12.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. 1994. Biodegradable Long-Circulating Polymeric Nanospheres. Science 263: 1600–03 [DOI] [PubMed] [Google Scholar]
- 13.Song CX, Labhasetwar V, Murphy H, Qu X, Humphrey WR, et al. 1997. Formulation and characterization of biodegradable nanoparticles for intravascular local drug delivery. J. Control. Release 43: 197–212 [DOI] [PubMed] [Google Scholar]
- 14.Mitra S, Gaur U, Ghosh PC, Maitra AN. 2001. Tumour targeted delivery of encapsulated dextran–doxorubicin conjugate using chitosan nanoparticles as carrier. J. Control. Release 74: 317–23 [DOI] [PubMed] [Google Scholar]
- 15.Kreuter J, Hekmatara T, Dreis S, Vogel T, Gelperina S, Langer K. 2007. Covalent attachment of apolipoprotein AI and apolipoprotein B-100 to albumin nanoparticles enables drug transport into the brain. J. Control. Release 118: 54–58 [DOI] [PubMed] [Google Scholar]
- 16.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. 2007. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc. Natl. Acad. Sci. USA 104: 15549–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goren D, Horowitz AT, Tzemach D, Tarshish M, Zalipsky S, Gabizon A. 2000. Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clin. Cancer Res 6: 1949–57 [PubMed] [Google Scholar]
- 18.Kim J-H, Kim Y-S, Park K, Kang E, Lee S, et al. 2008. Self-assembled glycol chitosan nanoparticles for the sustained and prolonged delivery of antiangiogenic small peptide drugs in cancer therapy. Biomaterials 29: 1920–30 [DOI] [PubMed] [Google Scholar]
- 19.Kemp MM, Kumar A, Mousa S, Dyskin E, Yalcin M, et al. 2009. Gold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis properties. Nanotechnology 20: 455104. [DOI] [PubMed] [Google Scholar]
- 20.Cho N-H, Cheong T-C, Min JH, Wu JH, Lee SJ, et al. 2011. A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nature Nanotechnology 6: 675–82 [DOI] [PubMed] [Google Scholar]
- 21.Prasad S, Cody V, Saucier-Sawyer JK, Saltzman WM, Sasaki CT, et al. 2011. Polymer nanoparticles containing tumor lysates as antigen delivery vehicles for dendritic cell–based antitumor immunotherapy. Nanomedicine: Nanotechnology, Biology and Medicine 7: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCall RL, Sirianni RW. 2013. PLGA Nanoparticles Formed by Single- or Double-emulsion with Vitamin E-TPGS. Journal of Visualized Experiments : JoVE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Gao S, Wen-Hui Y, Yoon HS, Yi-Yan Y. 2006. Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nature Materials 5: 791. [DOI] [PubMed] [Google Scholar]
- 24.Chan JM, Zhang L, Yuet KP, Liao G, Rhee J-W, et al. 2009. PLGA–lecithin–PEG core–shell nanoparticles for controlled drug delivery. Biomaterials 30: 1627–34 [DOI] [PubMed] [Google Scholar]
- 25.Dang JM, Leong KW. 2006. Natural polymers for gene delivery and tissue engineering. Advanced Drug Delivery Reviews 58: 487–99 [DOI] [PubMed] [Google Scholar]
- 26.Nair LS, Laurencin CT. 2007. Biodegradable polymers as biomaterials. Progress in Polymer Science 32: 762–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rampino A, Borgogna M, Blasi P, Bellich B, Cesàro A. 2013. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm 455: 219–28 [DOI] [PubMed] [Google Scholar]
- 28.Hong S-C, Yoo S-Y, Kim H, Lee J. 2017. Chitosan-Based Multifunctional Platforms for Local Delivery of Therapeutics. Mar. Drugs 15: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrillo C, Sune JM, Perez-Lozano P, Garcia-Montoya E, Sarrate R, et al. 2014. Chitosan nanoparticles as non-viral gene delivery systems: determination of loading efficiency. Biomed. Pharmacother 68: 775–83 [DOI] [PubMed] [Google Scholar]
- 30.Luo Y, Teng Z, Li Y, Wang Q. 2015. Solid lipid nanoparticles for oral drug delivery: chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydrate polymers 122: 221–9 [DOI] [PubMed] [Google Scholar]
- 31.Kean T, Thanou M. 2010. Biodegradation, biodistribution and toxicity of chitosan. Advanced Drug Delivery Reviews 62: 3–11 [DOI] [PubMed] [Google Scholar]
- 32.Fonte P, Araújo F, Silva C, Pereira C, Reis S, et al. 2015. Polymer-based nanoparticles for oral insulin delivery: Revisited approaches. Biotechnology Advances 33: 1342–54 [DOI] [PubMed] [Google Scholar]
- 33.Breitenbach BB, Schmid I, Wich PR. 2017. Amphiphilic polysaccharide block copolymers for pH-responsive micellar nanoparticles. Biomacromolecules [DOI] [PubMed] [Google Scholar]
- 34.Banerjee A, Bandopadhyay R. 2016. Use of dextran nanoparticle: A paradigm shift in bacterial exopolysaccharide based biomedical applications. Int. J. of Biol. Macromol 87: 295–301 [DOI] [PubMed] [Google Scholar]
- 35.Hudson D, Margaritis A. 2014. Biopolymer nanoparticle production for controlled release of biopharmaceuticals. Crit. Rev. Biotechnol 34: 161–79 [DOI] [PubMed] [Google Scholar]
- 36.Jana S, Sen KK, Gandhi A. 2016. Alginate Based Nanocarriers for Drug Delivery Applications. Curr. Pharm. Des 22: 3399–410 [DOI] [PubMed] [Google Scholar]
- 37.Jain D, Bar-Shalom D. 2014. Alginate drug delivery systems: application in context of pharmaceutical and biomedical research. Drug. Dev. Ind. Pharm 40: 1576–84 [DOI] [PubMed] [Google Scholar]
- 38.Lee SJ, Yhee JY, Kim SH, Kwon IC, Kim K. 2013. Biocompatible gelatin nanoparticles for tumor-targeted delivery of polymerized siRNA in tumor-bearing mice. J. Control. Release 172: 358–66 [DOI] [PubMed] [Google Scholar]
- 39.Singh A, Xu J, Mattheolabakis G, Amiji M. 2016. EGFR-targeted gelatin nanoparticles for systemic administration of gemcitabine in an orthotopic pancreatic cancer model. Nanomedicine 12: 589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santoro M, Tatara AM, Mikos AG. 2014. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 0: 210–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamagata M, Kawano T, Shiba K, Mori T, Katayama Y, Niidome T. 2007. Structural advantage of dendritic poly(l-lysine) for gene delivery into cells. Bioorg. Med. Chem 15: 526–32 [DOI] [PubMed] [Google Scholar]
- 42.Acharya S, Sahoo SK. 2011. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Del. Rev 63: 170–83 [DOI] [PubMed] [Google Scholar]
- 43.Chen CK, Law WC, Aalinkeel R, Nair B, Kopwitthaya A, et al. 2012. Well-Defined Degradable Cationic Polylactide as Nanocarrier for the Delivery of siRNA to Silence Angiogenesis in Prostate Cancer. Advanced Healthcare Materials 1: 751–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasan W, Chu K, Gullapalli A, Dunn SS, Enlow EM, et al. 2012. Delivery of Multiple siRNAs Using Lipid-Coated PLGA Nanoparticles for Treatment of Prostate Cancer. Nano Letters 12: 287–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grossen P, Witzigmann D, Sieber S, Huwyler J. 2017. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 260: 46–60 [DOI] [PubMed] [Google Scholar]
- 46.Palama IE, Cortese B, D’Amone S, Gigli G. 2015. mRNA delivery using non-viral PCL nanoparticles. Biomaterials science 3: 144–51 [DOI] [PubMed] [Google Scholar]
- 47.Ulery BD, Nair LS, Laurencin CT. 2011. Biomedical Applications of Biodegradable Polymers. Journal of polymer science. Part B, Polymer physics 49: 832–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loverde SM, Klein ML, Discher DE. 2012. Nanoparticle shape improves delivery: rational coarse grain molecular dynamics (rCG-MD) of taxol in worm-like PEG-PCL micelles. Advanced materials (Deerfield Beach, Fla.) 24: 3823–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tu Y 2017. Biodegradable 11: 1957–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis ME, Pun SH, Bellocq NC, Reineke TM, Popielarski SR, et al. 2004. Self-assembling nucleic acid delivery vehicles via linear, water-soluble, cyclodextrin-containing polymers. Curr. Med. Chem 11: 179–97 [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez H, Hwang SJ, Davis ME. 1999. New class of polymers for the delivery of macromolecular therapeutics. Bioconjug. Chem 10: 1068–74 [DOI] [PubMed] [Google Scholar]
- 52.Tzeng SY, Green JJ. 2013. Subtle Changes to Polymer Structure and Degradation Mechanism Enable Highly Effective Nanoparticles for siRNA and DNA Delivery to Human Brain Cancer. Advanced Healthcare Materials 2: 468–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sunshine JC, Akanda MI, Li D, Kozielski KL, Green JJ. 2011. Effects of Base Polymer Hydrophobicity and End-Group Modification on Polymeric Gene Delivery. Biomacromolecules 12: 3592–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sunshine J, Green JJ, Mahon KP, Yang F, Eltoukhy AA, et al. 2009. Small-Molecule End-Groups of Linear Polymer Determine Cell-Type Gene-Delivery Efficacy. Advanced Materials 21: 4947–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Green JJ, Langer R, Anderson DG. 2008. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc. Chem. Res 41: 749–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guerrero-Cazares H, Tzeng SY, Young NP, Abutaleb AO, Quinones-Hinojosa A, Green JJ. 2014. Biodegradable Polymeric Nanoparticles Show High Efficacy and Specificity at DNA Delivery to Human Glioblastoma in Vitro and in Vivo. Acs Nano 8: 5141–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kozielski KL, Tzeng SY, De Mendoza BAH, Green JJ. 2014. Bioreducible Cationic Polymer-Based Nanoparticles for Efficient and Environmentally Triggered Cytoplasmic siRNA Delivery to Primary Human Brain Cancer Cells. Acs Nano 8: 3232–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lynn DM, Langer R. 2000. Degradable poly(beta-amino esters): Synthesis, characterization, and self-assembly with plasmid DNA. J. Am. Chem. Soc 122: 10761–68 [Google Scholar]
- 59.Sunshine JC, Peng DY, Green JJ. 2012. Uptake and Transfection with Polymeric Nanoparticles Are Dependent on Polymer End-Group Structure, but Largely Independent of Nanoparticle Physical and Chemical Properties. Mol. Pharm 9: 3375–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Ma L, Deng X, Cheng J. 2012. Trigger-responsive chain-shattering polymers. 224–28 pp. [Google Scholar]
- 61.Cai K, Yen J, Yin Q, Liu Y, Song Z, et al. 2015. Redox-Responsive Self-Assembled Chain-Shattering Polymeric Therapeutics. Biomaterials Sci 3: 1061–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Yin Q, Yin L, Ma L, Tang L, Cheng J. 2013. Chain-Shattering Polymeric Therapeutics with On-Demand Drug-Release Capability. Angewandte Chemie International Edition 52: 6435–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan Y, Liu L, Xiong H, Miller JB, Zhou K, et al. 2016. Functional polyesters enable selective siRNA delivery to lung cancer over matched normal cells. Proc. Natl. Acad. Sci. USA 113: E5702–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan Y, Zhou K, Xiong H, Miller JB, Motea EA, et al. 2017. Aerosol delivery of stabilized polyester-siRNA nanoparticles to silence gene expression in orthotopic lung tumors. Biomaterials 118: 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen Y, Zhan Y, Tang J, Xu P, Johnson PA, et al. 2008. Multifunctioning pH-responsive nanoparticles from hierarchical self-assembly of polymer brush for cancer drug delivery. AIChE J 54: 2979–89 [Google Scholar]
- 66.She W, Luo K, Zhang C, Wang G, Geng Y, et al. 2013. The potential of self-assembled, pH-responsive nanoparticles of mPEGylated peptide dendron–doxorubicin conjugates for cancer therapy. Biomaterials 34: 1613–23 [DOI] [PubMed] [Google Scholar]
- 67.Wang Y-C, Wang F, Sun T-M, Wang J. 2011. Redox-responsive nanoparticles from the single disulfide bond-bridged block copolymer as drug carriers for overcoming multidrug resistance in cancer cells. Bioconjug. Chem 22: 1939–45 [DOI] [PubMed] [Google Scholar]
- 68.Na K, Lee KH, Lee DH, Bae YH. 2006. Biodegradable thermo-sensitive nanoparticles from poly (L-lactic acid)/poly (ethylene glycol) alternating multi-block copolymer for potential anti-cancer drug carrier. Eur. J. Pharm. Sci 27: 115–22 [DOI] [PubMed] [Google Scholar]
- 69.Zhang L, Guo R, Yang M, Jiang X, Liu B. 2007. Thermo and pH dual-responsive nanoparticles for anti-cancer drug delivery. Advanced Materials 19: 2988–92 [Google Scholar]
- 70.Kern HB, Srinivasan S, Convertine AJ, Hockenbery D, Press OW, Stayton PS. 2017. Enzyme-Cleavable Polymeric Micelles for the Intracellular Delivery of Proapoptotic Peptides. Mol. Pharm 14: 1450–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.You J, Shao R, Wei X, Gupta S, Li C. 2010. Near-infrared light triggers release of paclitaxel from biodegradable microspheres: photothermal effect and enhanced antitumor activity. Small 6: 1022–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan NC, Cheng FY, Ho JaA, Yeh CS. 2012. Photocontrolled Targeted Drug Delivery: Photocaged Biologically Active Folic Acid as a Light-Responsive Tumor-Targeting Molecule. Angewandte Chemie International Edition 51: 8806–10 [DOI] [PubMed] [Google Scholar]
- 73.Cochran MC, Eisenbrey J, Ouma RO, Soulen M, Wheatley MA. 2011. Doxorubicin and paclitaxel loaded microbubbles for ultrasound triggered drug delivery. Int. J. Pharm 414: 161–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chowdhury SM, Wang TY, Bachawal S, Devulapally R, Choe JW, et al. 2016. Ultrasound-guided therapeutic modulation of hepatocellular carcinoma using complementary microRNAs. J. Control. Release 238: 272–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pouponneau P, Leroux J-C, Soulez G, Gaboury L, Martel S. 2011. Co-encapsulation of magnetic nanoparticles and doxorubicin into biodegradable microcarriers for deep tissue targeting by vascular MRI navigation. Biomaterials 32: 3481–86 [DOI] [PubMed] [Google Scholar]
- 76.Vogelstein B, Kinzler KW. 2004. Cancer genes and the pathways they control. Nat. Med 10: 789–99 [DOI] [PubMed] [Google Scholar]
- 77.Leung RKM, Whittaker PA. 2005. RNA interference: from gene silencing to gene-specific therapeutics. Pharmacol. Ther 107: 222–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whitehead KA, Langer R, Anderson DG. 2009. Knocking down barriers: advances in siRNA delivery. Nature Reviews Drug Discovery 8: 129–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanasty R, Dorkin JR, Vegas A, Anderson D. 2013. Delivery materials for siRNA therapeutics. Nature Materials 12: 967–77 [DOI] [PubMed] [Google Scholar]
- 80.Oh YK, Park TG. 2009. siRNA delivery systems for cancer treatment. Advanced Drug Delivery Reviews 61: 850–62 [DOI] [PubMed] [Google Scholar]
- 81.Shen H, Sun T, Ferrari M. 2012. Nanovector delivery of siRNA for cancer therapy. Cancer Gene Ther 19: 367–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aigner A 2007. Nonviral in vivo delivery of therapeutic small interfering RNAs. Curr. Opin. in Mol. Ther 9: 345–52 [PubMed] [Google Scholar]
- 83.Sonawane ND, Szoka FC, Verkman AS. 2003. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J. Biol. Chem 278: 44826–31 [DOI] [PubMed] [Google Scholar]
- 84.Shim MS, Kwon YJ. 2012. Stimuli-responsive polymers and nanomaterials for gene delivery and imaging applications. Advanced Drug Delivery Reviews 64: 1046–58 [DOI] [PubMed] [Google Scholar]
- 85.Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ. 2005. Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing’s sarcoma. Cancer Res 65: 8984–92 [DOI] [PubMed] [Google Scholar]
- 86.Bartlett DW, Davis ME. 2007. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjugate Chem 18: 456–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mishra S, Heidel JD, Webster P, Davis ME. 2006. Imidazole groups on a linear, cyclodextrin-containing polycation produce enhanced gene delivery via multiple processes. J. Control. Release 116: 179–91 [DOI] [PubMed] [Google Scholar]
- 88.Benns JM, Choi JS, Mahato RI, Park JS, Kim SW. 2000. pH-sensitive cationic polymer gene delivery vehicle: N-Ac-poly(L-histidine)-graft-poly(L-lysine) comb shaped polymer. Bioconj. Chem 11: 637–45 [DOI] [PubMed] [Google Scholar]
- 89.Kumar M, Gupta D, Singh G, Sharma S, Bhatt M, et al. 2014. Novel polymeric nanoparticles for intracellular delivery of peptide cargos: antitumor efficacy of the Bcl-2 conversion peptide NuBCP-9. Cancer Res 74: 3271–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berguig GY, Convertine AJ, Frayo S, Kern HB, Procko E, et al. 2015. Intracellular Delivery System for Antibody–Peptide Drug Conjugates. Mol. Therapy 23: 907–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, Mcfarlane JD. 1995. Extracellular Ph Distribution in Human Tumors. Int. J. Hyperthermia 11: 211–16 [DOI] [PubMed] [Google Scholar]
- 92.Chen XL, Liu LS, Jiang C. 2016. Charge-reversal nanoparticles: novel targeted drug delivery carriers. Acta Pharmaceutica Sinica B 6: 261–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tannock IF, Rotin D. 1989. Acid Ph in Tumors and Its Potential for Therapeutic Exploitation. Cancer Res 49: 4373–84 [PubMed] [Google Scholar]
- 94.Zhang C, An T, Wang D, Wan GY, Zhang MM, et al. 2016. Stepwise pH-responsive nanoparticles containing charge-reversible pullulan-based shells and poly(beta-amino ester)/poly(lactic-co-glycolic acid) cores as carriers of anticancer drugs for combination therapy on hepatocellular carcinoma. J. Control. Release 226: 193–204 [DOI] [PubMed] [Google Scholar]
- 95.Shao J, Xie H, Huang H, Li Z, Sun Z, et al. 2016. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun 7: 12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Delalande A, Kotopoulis S, Postema M, Midoux P, Pichon C. 2013. Sonoporation: Mechanistic insights and ongoing challenges for gene transfer. Gene 525: 191–99 [DOI] [PubMed] [Google Scholar]
- 97.Wen PY, Kesari S. 2008. Malignant gliomas in adults. N. Engl. J. Med 359: 492–507 [DOI] [PubMed] [Google Scholar]
- 98.Sathornsumetee S, Reardon DA, Desjardins A, Quinn JA, Vredenburgh JJ, Rich JN. 2007. Molecularly targeted therapy for malignant glioma. Cancer 110: 13–24 [DOI] [PubMed] [Google Scholar]
- 99.Zarogoulidis P, Darwiche K, Sakkas A, Yarmus L, Huang H, et al. 2013. Suicide Gene Therapy for Cancer - Current Strategies. J. Genet. Syndr. Gene Ther 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mangraviti A, Tzeng SY, Kozielski KL, Wang Y, Jin YK, et al. 2015. Polymeric Nanoparticles for Nonviral Gene Therapy Extend Brain Tumor Survival in Vivo. Acs Nano 9: 1236–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matsumura Y, Maeda H. 1986. A New Concept for Macromolecular Therapeutics in Cancer-Chemotherapy - Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res 46: 6387–92 [PubMed] [Google Scholar]
- 102.Maeda H 2010. Tumor-Selective Delivery of Macromolecular Drugs via the EPR Effect: Background and Future Prospects. Bioconjug. Chem. 21: 797–802 [DOI] [PubMed] [Google Scholar]
- 103.Park JH, Lee S, Kim JH, Park K, Kim K, Kwon IC. 2008. Polymeric nanomedicine for cancer therapy. Progress in Polymer Science 33: 113–37 [Google Scholar]
- 104.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, et al. 1998. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. U. S. A 95: 4607–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sudimack J, Lee RJ. 2000. Targeted drug delivery via the folate receptor. Advanced Drug Delivery Reviews 41: 147–62 [DOI] [PubMed] [Google Scholar]
- 106.Hilgenbrink AR, Low PS. 2005. Folate receptor-mediated drug targeting: From therapeutics to diagnostics. J. Pharm. Sci 94: 2135–46 [DOI] [PubMed] [Google Scholar]
- 107.Bellocq NC, Pun SH, Jensen GS, Davis ME. 2003. Transferrin-containing, cyclodextrin polymer-based particles for tumor-targeted gene delivery. Bioconj. Chem 14: 1122–32 [DOI] [PubMed] [Google Scholar]
- 108.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, et al. 2010. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464: 1067–U140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jeong Y-I, Kim DH, Chung C-W, Yoo JJ, Choi KH, et al. 2012. Self-assembled nanoparticles of hyaluronic acid/poly (DL-lactide-co-glycolide) block copolymer. Colloids and Surfaces B: Biointerfaces 90: 28–35 [DOI] [PubMed] [Google Scholar]
- 110.Zou Y, Fang Y, Meng H, Meng F, Deng C, et al. 2016. Self-crosslinkable and intracellularly decrosslinkable biodegradable micellar nanoparticles: A robust, simple and multifunctional nanoplatform for high-efficiency targeted cancer chemotherapy. J. Control. Release 244: 326–35 [DOI] [PubMed] [Google Scholar]
- 111.Zou Y, Meng F, Deng C, Zhong Z. 2016. Robust, tumor-homing and redox-sensitive polymersomal doxorubicin: A superior alternative to Doxil and Caelyx? J. Control. Release 239: 149–58 [DOI] [PubMed] [Google Scholar]
- 112.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, et al. 1999. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284: 1994–98 [DOI] [PubMed] [Google Scholar]
- 113.Ferrara N, Alitalo K. 1999. Clinical applications of angiogenic growth factors and their inhibitors. Nat. Med 5: 1359–64 [DOI] [PubMed] [Google Scholar]
- 114.Itoh Y, Nagase H. 2002. Matrix metalloproteinases in cancer. Proteases in Biology and Medicine 38: 21–36 [DOI] [PubMed] [Google Scholar]
- 115.Folkman J 2006. Angiogenesis. Annu. Rev. Med 57: 1–18 [DOI] [PubMed] [Google Scholar]
- 116.Semenza GL. 2003. Targeting HIF-1 for cancer therapy. Nature Reviews Cancer 3: 721–32 [DOI] [PubMed] [Google Scholar]
- 117.Semenza GL. 2002. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol. Med 8: S62–S67 [DOI] [PubMed] [Google Scholar]
- 118.Semenza GL. 2012. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci 33: 207–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Folkman J, Klagsbrun M. 1987. Angiogenic Factors. Science 235: 442–47 [DOI] [PubMed] [Google Scholar]
- 120.Wang JM, Deng XY, Gong WH, Su SB. 1998. Chemokines and their role in tumor growth and metastasis. J. Immunol. Methods 220: 1–17 [DOI] [PubMed] [Google Scholar]
- 121.Sengupta S, Eavarone D, Capila I, Zhao GL, Watson N, et al. 2005. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature 436: 568–72 [DOI] [PubMed] [Google Scholar]
- 122.Lee E, Lee SJ, Koskimaki JE, Han Z, Pandey NB, et al. 2014. Inhibition of breast cancer growth and metastasis by a biomimetic peptide. Sci. Rep 4: 7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang Y 2015. Cancer immunotherapy: harnessing the immune system to battle cancer. J. Clin. Invest 125: 3335–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Makkouk A, Weiner GJ. 2015. Cancer immunotherapy and breaking immune tolerance: new approaches to an old challenge. Cancer Res 75: 5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hotaling NA, Tang L, Irvine DJ, Babensee JE. 2015. Biomaterial Strategies for Immunomodulation. Annu Rev Biomed Eng 17: 317–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. 2015. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem. Rev 115: 11109–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cheung AS, Mooney DJ. 2015. Engineered Materials for Cancer Immunotherapy. Nano Today 10: 511–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, et al. 2012. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med 366: 2443–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, et al. 2014. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N. Engl. J. Med 371: 2189–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kosmides AK, Meyer RA, Hickey JW, Aje K, Cheung KN, et al. 2017. Biomimetic biodegradable artificial antigen presenting with PD-1 blockade to treat melanoma cells synergize. Biomaterials 118: 16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.He C, Duan X, Guo N, Chan C, Poon C, et al. 2016. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nature Communications 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. 2016. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Letters 16: 2334–40 [DOI] [PubMed] [Google Scholar]
- 133.Teo PY, Yang C, Whilding LM, Parente-Pereira AC, Maher J, et al. 2015. Ovarian Cancer Immunotherapy Using PD-L1 siRNA Targeted Delivery from Folic Acid-Functionalized Polyethylenimine: Strategies to Enhance T Cell Killing. Advanced Healthcare Materials 4: 1180–89 [DOI] [PubMed] [Google Scholar]
- 134.Li S-Y, Liu Y, Xu C-F, Shen S, Sun R, et al. 2016. Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation. J. Control. Release 231: 17–28 [DOI] [PubMed] [Google Scholar]
- 135.Iwamura K, Kato T, Miyahara Y, Naota H, Mineno J, et al. 2012. siRNA-mediated silencing of PD-1 ligands enhances tumor-specific human T-cell effector functions. Gene Ther 19: 959–66 [DOI] [PubMed] [Google Scholar]
- 136.Alshamsan A, Hamdy S, Samuel J, El-Kadi AOS, Lavasanifar A, Uludağ H. 2010. The induction of tumor apoptosis in B16 melanoma following STAT3 siRNA delivery with a lipid-substituted polyethylenimine. Biomaterials 31: 1420–28 [DOI] [PubMed] [Google Scholar]
- 137.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, et al. 2009. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 115: 3670–9 [DOI] [PubMed] [Google Scholar]
- 138.Rosenberg SA, Restifo NP. 2015. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348: 62–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, et al. 2014. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med 371: 1507–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Davila ML, Riviere I, Wang XY, Bartido S, Park J, et al. 2014. Efficacy and Toxicity Management of 19–28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci. Transl. Med 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, et al. 2017. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol 12: 813–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mellman I, Coukos G, Dranoff G. 2011. Cancer immunotherapy comes of age. Nature 480: 480–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weathers SP, Gilbert MR. 2015. Current challenges in designing GBM trials for immunotherapy. J. Neurooncol 123: 331–37 [DOI] [PubMed] [Google Scholar]
- 144.Le Mercier M, Fortin S, Mathieu V, Kiss R, Lefranc F. 2010. Galectins and Gliomas. Brain Pathol 20: 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Van Woensel M, Wauthoz N, Rosiere R, Mathieu V, Kiss R, et al. 2016. Development of siRNA-loaded chitosan nanoparticles targeting Galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J. Control. Release 227: 71–81 [DOI] [PubMed] [Google Scholar]
- 146.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, et al. 2012. Combination delivery of TGF-beta inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nature Materials 11: 895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Maus MV, Thomas AK, Leonard DGB, Allman D, Addya K, et al. 2002. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4–1BB. Nat. Biotechnol 20: 143–48 [DOI] [PubMed] [Google Scholar]
- 148.Sunshine JC, Green JJ. 2013. Nanoengineering approaches to the design of artificial antigen-presenting cells. Nanomedicine 8: 1173–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sunshine JC, Perica K, Schneck JP, Green JJ. 2014. Particle shape dependence of CD8+T cell activation by artificial antigen presenting cells. Biomaterials 35: 269–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Davis ME. 2009. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm 6: 659–68 [DOI] [PubMed] [Google Scholar]
- 151.Zuckerman JE, Gritli I, Tolcher A, Heidel JD, Lim D, et al. 2014. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. USA 111: 11449–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hrkach J, Von Hoff D, Ali MM, Andrianova E, Auer J, et al. 2012. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med 4: 12839–39 [DOI] [PubMed] [Google Scholar]
- 153.Von Hoff DD, Mita MM, Ramanathan RK, Weiss GJ, Mita AC, et al. 2016. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin. Cancer Res 22: 3157–63 [DOI] [PubMed] [Google Scholar]
- 154.Plummer R, Wilson RH, Calvert H, Boddy AV, Griffin M, et al. 2011. A Phase I clinical study of cisplatin-incorporated polymeric micelles (NC-6004) in patients with solid tumours. Br. J. Cancer 104: 593–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gaur S, Chen L, Yen T, Wang Y, Zhou B, et al. 2012. Preclinical study of the cyclodextrin-polymer conjugate of camptothecin CRLX101 for the treatment of gastric cancer. Nanomedicine: Nanotechnology, Biology and Medicine 8: 721–30 [DOI] [PubMed] [Google Scholar]
- 156.Weiss GJ, Chao J, Neidhart JD, Ramanathan RK, Bassett D, et al. 2013. First-inhuman phase 1/2a trial of CRLX101, a cyclodextrin-containing polymer-camptothecin nanopharmaceutical in patients with advanced solid tumor malignancies. Invest. New Drugs 31: 986–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pham E, Birrer MJ, Eliasof S, Garmey EG, Lazarus D, et al. 2015. Translational impact of nanoparticle-drug conjugate CRLX101 with or without bevacizumab in advanced ovarian cancer. Clin. Cancer Res 21: 808–18 [DOI] [PubMed] [Google Scholar]
- 158.Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, et al. 2005. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer 92: 1240–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hamaguchi T, Kato K, Yasui H, Morizane C, Ikeda M, et al. 2007. A phase I and pharmacokinetic study of NK105, a paclitaxel-incorporating micellar nanoparticle formulation. Br. J. Cancer 97: 170–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kato K, Chin K, Yoshikawa T, Yamaguchi K, Tsuji Y, et al. 2012. Phase II study of NK105, a paclitaxel-incorporating micellar nanoparticle, for previously treated advanced or recurrent gastric cancer. Invest. New Drugs 30: 1621–27 [DOI] [PubMed] [Google Scholar]