Abstract
Medicinal plants play a crucial role in the search for components that are capable of neutralizing the multiple mechanisms of fungal resistance. Psidium salutare (Kunth) O. Berg is a plant native to Brazil used as both food and traditional medicine to treat diseases and symptoms such as stomach ache and diarrhea, whose symptoms could be related to fungal infections from the genus Candida. The objective of this study was to investigate the influence of seasonal variability on the chemical composition of the Psidium salutare essential oil, its antifungal potential and its effect on the Candida albicans morphogenesis. The essential oils were collected in three different seasonal collection periods and isolated by the hydrodistillation process in a modified Clevenger apparatus with identification of the chemical composition determined by gas chromatography coupled to mass spectrometry (GC/MS). The antifungal assays were performed against Candida strains through the broth microdilution method to determine the minimum fungicidal concentration (MFC). Fungal growth was assessed by optical density reading and the Candida albicans dimorphic effect was evaluated by optical microscopy in microculture chambers. The chemical profile of the essential oils identified 40 substances in the different collection periods with γ-terpinene being the predominant constituent. The antifungal activity revealed an action against the C. albicans, C. krusei and C. tropicalis strains with an IC50 ranging from 345.5 to 2,754.2 µg/mL and a MFC higher than 1,024 µg/mL. When combined with essential oils at sub-inhibitory concentrations (MIC/16), fluconazole had its potentiated effect, i.e. a synergistic effect was observed in the combination of fluconazole with P.salutare oil against all Candida strains; however, for C. albicans, its effect was reinforced by the natural product in all the collection periods. The results show that the Psidium salutare oil affected the dimorphic transition capacity, significantly reducing the formation of hyphae and pseudohyphae in increasing concentrations. The results show that P. salutare oil exhibits a significant antifungal activity against three Candida species and that it can act in synergy with fluconazole. These results support the notion that this plant may have a potential use in pharmaceutical and preservative products.
Keywords: γ-terpinene, Precipitation, Micromorphology, Seasonal variation, Pathogenesis, Chemical composition, Candida sp., Morphogenesis
Introduction
Seasonality variations such as climatic conditions, water restriction, the presence of predators and soil mineral composition may alter secondary plant metabolism (Figueiredo et al., 2008) and, consequently, alter the composition of essential oils throughout the year (Prins, Vieira & Freitas, 2010). In addition, some specific constituents that present chiral chemical groups are affected by the luminosity rate (Mulas, Gardner & Craker, 2006). The isolation of plant essential oils is also influenced beyond taxonomic factors, as well as by the variety of epidermal cellular structures that are responsible for the production and storage of essential oils volatile organic compounds (Pinto et al., 2007). Therefore, understanding the seasonal events that alter the quality of the active compounds in the plant is fundamental to support pharmacological studies that contemplate and aim at the formulation of new drugs and direct collection periods in direct commercial plantations of this crop to obtain the oil with greater therapeutic potential.
Many species of the family Myrtaceae have a history of use as traditional medicines in ethnobotanical practices in both tropical and subtropical regions (Souza et al., 2014; Macêdo et al., 2016). Family members comprise the genera Eugenia, Myrcianthes, Campomanesia and Psidium. The Psidium genus has approximately 150 species and can be found in all the tropics and subtropics of America and Australia (Pino et al., 2003) with several therapeutic potentials already described, especially for Psidium guajava Linn. (Gupta, Chahal & Arora, 2011; Joseph & Priya, 2011). Antimicrobial activity has been described for several species such as Psidium cattleianum (Faleiro et al., 2016) and Psidium guineense (Fernandes et al., 2012).
Psidium salutare (Kunth) O. Berg., is popularly known in the Northeast region as a “araça preto”, often found in Cerrado areas in the Chapada do Araripe, southern Ceará state (Ribeiro-Silva et al., 2012), with five varieties of this species being recognized: var. sericeum, var. mucronatum, var. decussatum and var. pohlianum, which are also found in other countries such as Paraguay, the Caribbean and Mexico (Landrum, 2003). In the Cariri region, in addition to the fruit being edible, the leaves are used in traditional medicine to treat diseases and symptoms such as stomach ache and diarrhea, which may be related to Candida infections (Ribeiro et al., 2014; Souza et al., 2014; Macêdo et al., 2016).
Fungal infections caused by dermatophytes and yeasts of Candida spp., are a serious health problem in immunocompromised patients in particular and are aggravated by the increase in clinical resistance to the antifungal agents (Silva et al., 2012; Morais-Braga et al., 2016b). In view of this problem, the interest in the use of vegetable derivatives with therapeutic potential for antifungal action has intensified (Macêdo et al., 2015). These new substances of plant origin may represent alternative and less toxic treatments for the treatment of infections ( Vandeputte, Ferrari & Coste, 2011), synergism and inhibition of germ tube formation by compounds derived from Crocus sativus against Candida spp ( Carradori et al., 2016). Considering the medicinal importance of the genus Psidium and the absence of studies with Psidium salutare, this is the first study to describe the chemical profile of P. salutare leaf essential oil, and the influence of seasonal variation on its composition, antifungal activity and potency to inhibit the morphogenetic switch in Candida species.
Materials and Methods
Collection area of botanical material
Psidum salutare leaves were collected in an area of Cerrado sensu stricto, at Fazenda Barreiro Grande (latitude: 7°21′41.7″S and longitude 39°28′42.4″W, altitude of 909 m above sea level), located in the Chapada do Araripe, Ceará, Northeast of Brazil, presenting altitudes varying between 870 and 970 m. The region receives on average of 1.043 mm (mm) of rainfall per year (FUNCEME, 2016), where they concentrate between January and May with a dry period that lasts between five and seven months, with a critical shortage between July and September (Table 1). According to the Köppen classification system, the climate is hot humid Tropical (Aw) with an average annual of temperature between 24 and 26 °C. The collection is under the authorization of the competent ICMBio with number (no. 50362-2).
Table 1. The average annual of meteorological conditions for each collection (2016).
| Collection period | February | May | August | 2016 Average annual |
|---|---|---|---|---|
| OEFPs1/ winter | OEFPs2/ winter | OEFPs3/ summer | ||
| Precipitation (mm) | 49 | 145 | 0.0 | 968.0 |
| Yield (%) | 0.73 | 0.29 | 0.15 | |
| Temperature (°C) | 26 | 27 | 35 |
Notes.
- OEFPs
- essential oil of Psidium salutare sheets, 1, 2, 3 collection
Plant material
Fresh leaves of the species Psidum salutare were collected in the months of February, May and August in different periods, dry and rainy season, to evaluate the antifungal activity and the chemical compounds, as described in Table 1, between 8:30 am and 9:30 am. They were then transported to Laboratory of Ecology of Plants of the Regional University of Cariri—URCA. Species exsiccates were produced, identified by Dr. Marcos Sobral (specialist in the Myrtaceae family) and deposited in the Heririum of Caririense Dárdano de Andrade-Lima of the Regional University of Cariri—URCA under number 12601 HCDAL.
Obtaining and analyzing the essential oil
Approximately 500 g of fresh leaves collected were selected, washed, crushed and submitted to the hydrodistillation process for two hours in a Clevenger type apparatus. The essential oil was then dehydrated with anhydrous sodium sulfate (Na2SO4) and kept in an amber flask under refrigeration <4 °C until analyzed. The yields were determinate by volume/weight on dry weight basis.
Analysis of the oil was performed using a Shimadzu GC-17 A/MSQP5050A (GC/MS system): DB-5HT capillary column (30 m × 0.251 mm, 0.1 mm of thickness); helium carrier gas at 1.7 mL / min; injection temperature 270 °C; detector temperature 290 °C; column temperature 60 °C (2 min) −180 °C (1 min) at 4 °C/min, then 180–260 °C at 10 °C / min (10 min). The reading speed was 0.5 scan/s of m/z 40–450 with a split ratio of 1:30. The injection volume was 1 µL of 5 mg/mL of ethyl acetate solution. Avoid dead time = 3 min. The mass spectrometer was operated with ionization energy of 70 eV. The identification of the components was by comparison of their respective mass spectrum standards with those registered in the database of the Wiley Online Library and with the calculated retention indices with values in literature (McLafferty & Stauffer, 2016; Adams, 2007).
Antifungal activity evaluation
Culture media and inocula
For the antifungal activity assays, three standard strains of yeast fungi of the genus Candida were used: C. albicans (CA INCQS 40006) C. tropicalis (CT INCQS 40042) and C. krusei (CK INCQS 40095) obtained from the Oswald Cruz Cultures Collection of the National Institute of Quality Control in Health (INCQS). All strains were grown on Sabouraud Dextrose agar (SDA-KASVI) and incubated at 37 °C for 24 h. From these, suspensions of the microorganisms were prepared in tubes containing 3 ml of sterile solution (0.9% NaCl). The inoculum concentration was standardized according to the McFarland scale, comparing inoculum turbidity with the 0.5 standard on the scale equivalent to 105/106 cells per mL. The potato dextrose agar (PDA, DIFCO) was prepared by diluting it more than that recommended by the manufacturer to make it a depleted medium capable of stimulating yeast to produce hyphae. Agar was added to this diluted medium to obtain a solid medium.
Determination of the Inhibitory Concentration of 50% of the microorganisms (IC50) and obtaining the cellular viability curve
The different P. salutare essential oil samples from the periodic collections in the rainy and dry seasons were tested for their antifungal activity. Both the essential oil and antifungal fluconazole (F8929 ≥ 98% (HPLC), powder; Sigma Aldrich, St. Louis, MO, USA) was previously diluted in dimethylsulfoxide (DMSO; Dynamic, Indaiatuba, Brazil) and its final concentration was adjusted with addition of distilled water to obtain the desired concentration for (16,384 µg / ml). The oil and fluconazole solutions were posteriorly microdiluted in Sabouraud Dextrose Broth (SDB) medium in a serial concentration manner ranging from 8,192 to 8 µg/mL in 96-well plates. The penultimate well, the latter serving as a growth control (Javadpour et al., 1996). The concentration of DMSO at the oil concentrations ranged from 5 to 0.004%. Product dilutions (using saline instead of inoculum) and medium sterility controls were also achieved. The plates were then taken to an incubation chamber for 24 h at 37 °C and following this period the plates were read using an ELISA spectrophotometer (Thermoplate®) apparatus. The results obtained in the ELISA reading were used to construct the cell viability curve and to determine the IC50 of the P. salutare essential oils (Morais-Braga et al., 2016b). All test were perfomed in triplicate.
Determination of the minimal fungicidal concentration (MFC)
A small sterile rod was placed in each well of the microdilution test plate, with the exception of the sterility control. After mixing the medium in each well, the rod was taken to a large petri dish containing SDA, where by touching the surface, the solution (medium + inoculum + natural product) was transferred for yeast subculture and cell viability analysis. The plates were incubated at 37 °C for 24 h, and checked for the growth or non-growth of Candida colonies (Ernst et al., 1999). The concentration at which there was no growth of fungal colonies was considered the MFC of the natural product.
Evaluation of the Psidium salutare essential oil modulating effect on the antifungal activity of fluconazole
The solution containing the essential oil of P. salutare (OEFPs) was tested in subinhibitory concentration (MFC/16). The volume of 100 µL of a solution containing SDB (Sabouraud Dextrose Broth), 10% inoculum and natural product were distributed in each well in the alphabetical direction of the plate. Afterwards, 100 µL of the antifungal were mixed to the first well and serially microdiluted in a ratio of 1:1, the latter cavity being used as fungus growth control (Coutinho et al., 2008). The fluconazole concentrations varied gradually from 8,192 to 8 µg/mL. Dilution controls of the natural products (OEFPs) were used where the inoculum was replaced by saline/DMSO and control of sterility with the medium. The plates were incubated at 37 °C for 24 h and reading was done on a spectrophotometer, Thermoplate® ELISA, with a wavelength of 630 nm (Morais-Braga et al., 2016b).
Effect of the Psidium salutare leaf oil on Candida albicans morphogenesis
The essential oil from the three samples collected at different periods were used to observe if the natural product caused any alteration in the morphogenesis of C. albicans, the oil was tested in different concentrations such as the Superior Evaluated Concentration: SEC (8,192 µg/mL), SEC/4 (2,048 µg/mL) and SEC/16 (512 µg/mL).
The trials were performed with some modifications according to Sidrim & Rocha (2003) and Mendes Giannini et al. (2013). The medium (3 mL) were combined to the tested product, were poured into the slide of the microscope at the respective concentrations, previously homogenized by the agitator. After solidification of the medium, the yeast was seeded with the aid of a 1 µL calibrated loop and two parallel grooves were extracted. The striae were covered with sterile lamellae. Plates were incubated and after 24 h as slides were subsequently observed under a 40 × objective optical microscope. A control for yeast growth (hyphae stimulated by depleting medium) was performed, as well as a control with the conventional antifungal fluconazole for comparative purposes. Tests using DMSO as a control were previously performed (Morais-Braga et al., 2016a), demonstrating that it does not cause inhibition of hyphae at the concentrations tested.
Statistical analysis
The data obtained for each sample were checked for their normal distribution and then analyzed by one-way ANOVA followed by Tukey’s test. The IC50 values were computed by linear regression for interpolation in standard curves relating the percentage (%) growth values and the concentration of the product in µg/mL using the GraphPad Prism software, version 6.0. All analyzes were performed in triplicates (see raw data in the Supplementary Information attached).
Results
In the evaluation of the yield of the essential oil Psidium salutare in the analyzed periods, February (0.73%), May (0.29%) and August (0.15%) show that highest yields coincident with the precipitation periods and dryness in the region, however, it was not possible to obtain a statistically significant correlation. Then, when analyzing the P. salutare oil yield, the beginning of the rainy season was the ideal period for collection. In the P. salutare GC/MS analysis it was possible to identify an average of 89.13% of the constituents corresponding to 40 compounds (Table 2). When calculating the average of the compounds, a predominance of monoterpene hydrocarbons (39.79%), sesquiterpene hydrocarbons (20.94%), oxygenated monoterpenes (12.98%) and oxygenated sesquiterpenes (14.91%) can be observed.
Table 2. Determination of the percentage composition of the chemical composition of the Psidium salutare leaf essential oil by gas chromatography coupled to mass spectrometry (CG/MS) in different collection periods.
| Compounds | tR* (min) | OEFPs1 | OEFPs2 | OEFPs3 | % (media) |
|---|---|---|---|---|---|
| 1,8 Cineole | 5.5 | 0.61a | 0.51a | 1.05a | 0.72 |
| Dimethyl benzylcarbinyl acetate | 8.2 | 0.19a | 0.15a | 0.65a | 0.33 |
| Copaene | 11.2 | 3.22a | 3.53a | 1.91a | 2.89 |
| Cubenol | 14.8 | 0.63a | 0.0a | 3.42a | 1.35 |
| Espatulenol | 12.2 | 0.23a | 0.30a | 0.13a | 0.22 |
| Sabinene hydrate | 8.1 | 2.28a | 3.48a | 3.94a | 3.23 |
| Isocarofilene | 11.9 | 3.78a | 3.75a | 1.20a | 2.91 |
| Limonene | 5.4 | 1.10a | 1.15a | 1.14a | 1.13 |
| Linalool | 6.6 | 5.55a | 4.72a | 7.26a | 5.84 |
| Myrcene | 4.7 | 0.65a | 0.42a | 0.08b | 0.38 |
| Myrtenol | 7.5 | 0.21a | 0.16a | 0.09a | 0.15 |
| Ocimene | 5.7 | 2.15a | 1.93a | 1.50a | 1.86 |
| Palustrol | 14.1 | 0.05a | 0.11a | 0.10a | 0.09 |
| Patchoulane | 14.7 | 0.25a | 0.19a | 3.08a | 1.17 |
| P-Cymene | 5.3 | 5.05b | 6.37b | 17.83e | 9.75 |
| Selina-3,7 (11) -diene | 13.6 | 0.37a | 0.28a | 0.0a | 0.22 |
| Seychellene | 13.9 | 0.20a | 0.17a | 0.40a | 0.26 |
| Terpineol | 8.3 | 1.67a | 0.90a | 0.12a | 0.90 |
| Terpinolene | 6.4 | 16.99c | 14.49c | 6.90b | 12.79 |
| Valencene | 13.3 | 0.23a | 0.09a | 0.30a | 0.21 |
| Viridiflorene | 16.4 | 0.12a | 0.0a | 0.35a | 0.16 |
| Viridiflorol | 14.7 | 0.53a | 0.95a | 2.07a | 1.18 |
| α-phellandrene | 5.0 | 0.15a | 0.08a | 0.05a | 0.09 |
| α-caryophyllene | 12.4 | 0.24a | 0.29a | 1.68a | 0.74 |
| α-cubebene | 15.0 | 0.90a | 2.05a | 0.0a | 0.98 |
| α-farnesene | 13.8 | 0.03a | 0.03a | 0.24a | 0.10 |
| α-gurjunene | 11.7 | 0.21a | 0.10a | 0.08a | 0.13 |
| α-muurolene | 12.6 | 0.63a | 0.72a | 0.69a | 0.68 |
| α-pinene | 5.1 | 0.83a | 0.55a | 0.62a | 0.67 |
| β-cadinene | 5.9 | 0.96a | 1.45a | 0.83a | 1.08 |
| β-elemene | 13.7 | 0.16a | 0.12a | 0.70a | 0.33 |
| β-eudesmene | 12.8 | 0.15a | 0.11a | 0.0a | 0.09 |
| β-guaienum | 15.5 | 2.79a | 3.12a | 0.0a | 1.97 |
| γ-gurjunene | 13.6 | 0.10a | 0.21a | 0.26a | 0.19 |
| γ-muurolene | 13.2 | 2.58a | 2.42a | 3.20a | 2.73 |
| γ-terpinene | 5.9 | 13.97d | 17.09d | 10.32c | 13.79 |
| δ-cadinene | 13.3 | 5.27a | 3.88a | 3.84a | 4.33 |
| δ-cadinol | 15.3 | 1.68a | 0.0a | 0.92a | 0.87 |
| δ-guaiene | 14.9 | 0.28a | 0.28a | 3.70b | 1.42 |
| τ-cadinol | 15.2 | 12.75d | 10.51d | 10.35d | 11.20 |
| Monotherpenes hydrocarbons | 40.06 | 41.53 | 37.82 | 39.79 | |
| Sesquiterpenes hydrocarbons | 21.65 | 22.15 | 18.98 | 20.94 | |
| Oxygenated monotherpenes | 11.15 | 10.32 | 13.08 | 12.98 | |
| Oxygenated sesquiterpenes | 15.87 | 11.87 | 16.99 | 14.91 | |
| Others | 1.01 | 0.79 | 4.13 | 1.44 | |
| Total | 89.74 | 86.66 | 91.00 | 90.6 |
Notes.
- TR
- retention time
- OEFPs
- essential oil from the leaves of Psidium salutare; first collection (February), second collection (May), third collection (August). Averages followed by different letters differ by Tukey test at p < 0.05
The major constituents were linalool, p-Cymene, terpinolene, γ-terpinene and τ-cadinol. The results obtained during the collection periods showed that although several compounds presented a random composition, others remained constant. In the rainy season in February and May, the compounds that stood out were terpinolene (14.49 - 16.99%), γ-terpinene (13.97–17.09%), τ-cadinol (12.75–10.51%), p-Cymene (5.05–6.37%) and linalool (5.55–4.72%). In the dry season, represented by August, the major compounds were p-cymene (17.83%), γ-terpinene (10.32%), τ-cadinol (10.35%) and linalool (7.26%). In the dry season, represented by August, the major compounds were p-cymene (17.83%), γ-terpinene (10.32%), τ-cadinol (10.35%) and linalool (7.26%). This variation can be partly explained by the fact that environmental factors can affect certain chemical compounds while exerting any influence on the production of other chemicals.
The intrinsic P. salutare essential oil antifungal activity at different collection times, against different Candida strains showed no significant clinical activity (MIC ≥1, 024μg/mL), demonstrating that it was little influenced by changes in the chemical composition of the oil and by rainfall (Table 3). In this sense, although punctually significant, the chemical variations in the oil composition were not able to exhibit satisfactory inhibitory effect against Candida strains showing effects only in high concentrations, Candida albicans INCQS 40006 (4,096 µg/mL), Candida tropicalis INCQS 40042 (≥16,384 µg/ml) and Candida krusei INCQS 40095 (1,024 µg/ml) (Table 3).
Table 3. The inhibitory effect of association the essential oil of P. salutare with fluconazole on Candida (µg/mL).
| Tested Products | Strains | |||||
|---|---|---|---|---|---|---|
| CA INCQS 40006 | CT INCQS 40042 | CK INCQS 40095 | ||||
| CFM µg/mL | IC50 µg/mL | CFM µg/mL | IC50 µg/mL | CFM µg/mL | IC50 µg/mL | |
| Fluconazole (FCZ) | 8,192 | 16.8 | ≥16,384 | 9.3 | ≥16,384 | 271 |
| OEFPs 1+FCZ | 1,024 | 2.7 | ≥16,384 | 2.6 | 8,192 | 44.4 |
| OEFPs 2+FCZ | 8,192 | 8.0 | ≥16,384 | 5.3 | 1,024 | 32.4 |
| OEFPs 3+FCZ | 4,096 | 6.3 | ≥16,384 | 3.7 | 8,192 | 45.2 |
Notes.
- OEFPs
- essential oil of Psidium salutare leaves, 1, 2 and 3 collections
- CA
- Candida albicans
- CT
- Candida tropicalis
- CK
- Candida krusei
- INCQS
- National Institute of Health Quality Control
- IC50 (µg/mL)
- the inhibitor concentration that decreases 50% of the growth
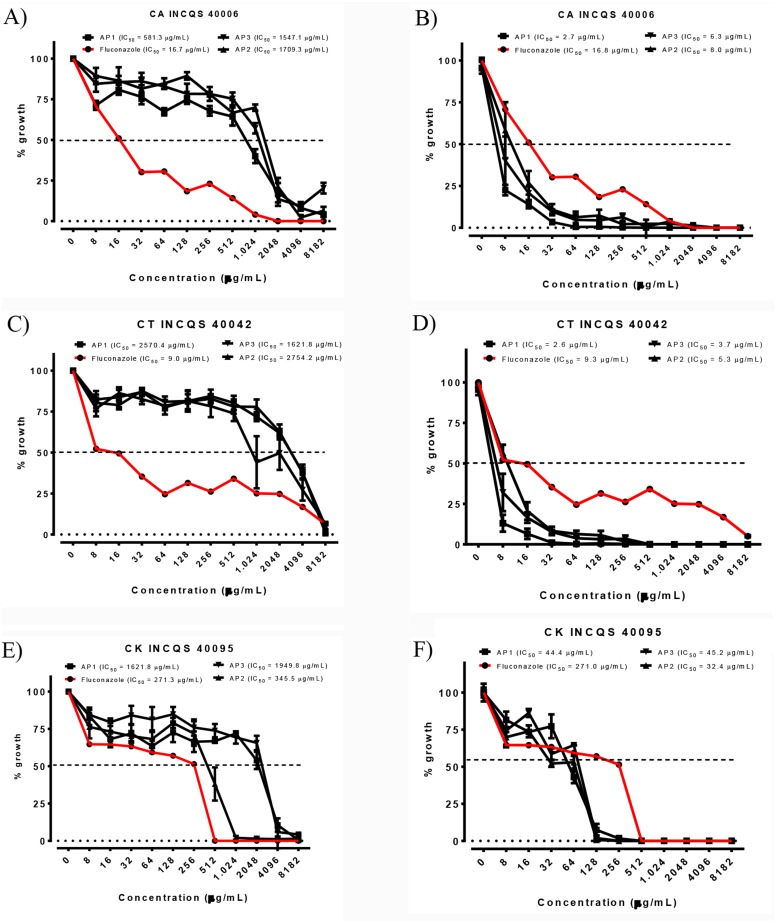
Among the analyzed periods, the IC50 (Ability to Inhibit 50% of cells), of products ranged from 345.5 to 2,754.2 µg/mL and image of the cellular viability curve in different concentrations of essential oil, the lowest value recorded for C. albicans was related to the first collection period, with an IC50 of 581.3 µg/mL (Fig. 1A), precipitation of 49 mm and elevated major compounds, such as terpinolene, τ-cadinol and γ-terpinene. For C. tropicalis, the lowest IC50 value was observed in the last collection period (1,621.8 µg/mL) (Fig. 1C), coinciding with the dry period in the region, with p-cymene, linalol, γ-terpinene and τ-cadinol in higher concentrations in the sample.
Figure 1. Cell viability curve and IC50 of the P. salutare essential oil (A, C and E) and the oil in combined with fluconazole (B, D and F) against different Candida spp. strains, at different collection periods.
Concentration of fluconazole: 2,048 µg/mL. OEFPs, Essential oil of the leaves of Psidium salutare, 1, 2 and 3 collections; CA, C. albicans; CT, C. tropicalis; CK, C. krusei; INCQS, National Institute of Quality Control in Health. (A) Cell viability curve and IC50 of Psidium salutare essential oil against Candida albicans. (B) Cell viability curve and IC50 of Psidium salutare essential oil combined with fluconazole against Candida albicans. (C) Cell viability curve and IC50 of Psidium salutare essential oil against Candida tropicalis. (D) Cell viability curve and IC50 of Psidium salutare essential oil combined with fluconazole against Candida tropicalis. (E) Cell viability curve and IC50 of Psidium salutare essential oil against Candida krusei. (F) Cell viability curve and IC50 of Psidium salutare essential oil combined with fluconazole against Candida krusei.
However, for C. krusei the antifungal activity was showed more active in second collection period (345.5 µg/mL) (Fig. 1E) with significant value when compared to fluconazole IC50 of 271.3 µg/mL (Table 4). This result corroborates with an incidence of precipitation of 145 mm and presence of the major γ-terpinene, τ-cadinol and terpinolene compounds in the sample.
Table 4. The CFM (µg/mL) of the essential oil of P. salutare on different strains of Candida in modulatory effect.
| Tested Products | ||||||
|---|---|---|---|---|---|---|
| Strains | OEFPs1 µg/mL | OEFPs1+FCZ µg/mL | OEFPs2 µg/mL | OEFPs2+FCZ µg/mL | OEFPs3 µg/mL | OEFPs3+FCZ µg/mL |
| CA INCQS 40006 | 1,024 | 581.3 | 8,192 | 1,709.3 | 4,096 | 1,547.1 |
| CT INCQS 40042 | ≥16,384 | 2,570.4 | ≥16,384 | 2,754.2 | ≥16,384 | 1,621.8 |
| CK INCQS 40095 | 8,192 | 1,621.8 | 1,024 | 345.5 | 8,192 | 1,949.8 |
Notes.
- OEFPs
- essential oil of leaves of P. salutare, 1,2 and 3 collections
- CA
- C. albicans
- CT
- C. tropicalis
- CK
- C. krusei
- INCQS
- National Institute of Quality Control in Health
For the Intrinsic Minimal Fungicide Concentration (MFC) the results showed a chemical variation in the essential oil composition between dry and rainy periods, thus influencing the concentration for C. albicans (4,096 µg/mL) and C. krusei (1,024 µg/mL), however for C. tropicalis (≥16,384 µg/mL) the concentrations remained constant.
In the verification of the potential modifier of the effect of fluconazole by the essential oil (Table 4), we can verify that there was a modulatory activity for all strains (Figs. 1B, 1D, 1F), especially for C. albicans 40006 (2.7 to 8.0 µg/mL), which exhibited lower concentrations than when compared to fluconazole alone (IC50 16.7 µg/mL), exhibiting synergism in all curves at all collection periods analyzed, promoting an inhibitory effect on microorganisms, greater than sum of the effects of individuals.
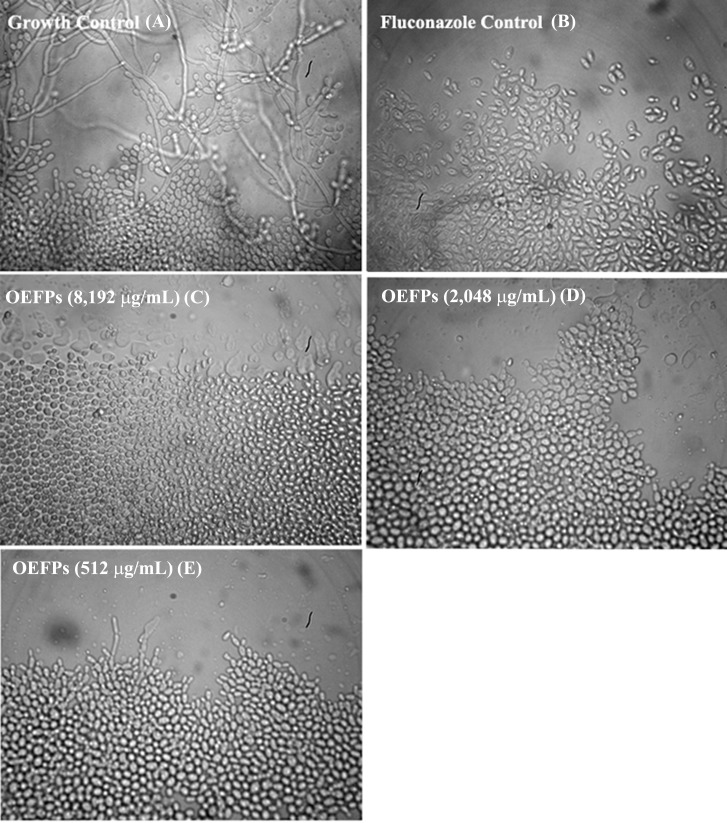
The effect of the natural product on morphological transition in C. albicans was evaluated by microcultive assay. It can be observed that the essential oil inhibited the formation of hyphae and pseudohyphae at concentrations starting from 512.0 µg/mL, resulting in the reduction of fungal virulence (Fig. 2). The microscopy results shown in Fig. 2 demonstrate that the essential oil can inhibit germinative tube formation and reduce hyphae elongation, which can be considered effective against C. albicans dimorphism, thus reducing fungal progression and the spread of infection.
Figure 2. Effect of the Psidium salutare essential oil on Candida albicans yeast micromorphological aspects.
Culture performed in depleted potato dextrose agar medium, with 40×objective visualization. (A) Growth control, (B) fluconazole antifungal effect at 2,048 µg/mL, (C) P. salutare essential oil effect at 8,192 μg/mL, (D) P. salutare essential oil effect at 2,048 µg/mL and (E) (C) P. salutare essential oil effect at 512 µg/mL; CA, C. albicans; INCQS, National Institute of Quality Control in Health.
Discussion
As expected, the chemical composition varied during the analyzed period. This result corroborates with other studies that have shown that environmental factors can affect certain chemical compounds, while in others they have no influence on their production ( Araújo et al, 2015; Estell, Fredrickson & James, 2016). In the leaves of Camellia sinensis, the main catechins (epigallocatechin gallate, epicatechin) varied during the year, and this variation was associated to the following environmental factors that can act in combination or alone: day length, sunlight and / or temperature ( Yao et al., 2016). According to studies the rainy season was favorable for the production of Copaifera langsdorffii Desff oils (Souza de Oliveira et al., 2017), Eucalyptus citriodora Hook (Castro et al., 2008) and Cymbopogon citratus (Santos et al., 2009).
In the present study, the production of the major compounds such as Terpinolene and γ-terpinene in the milder months (26 °C) was positively influenced, with an increase in the percentage of these compounds in the sample, whereas the production of P-cymene was positively influenced in the warmer period, which shows that similar compounds can be altered simultaneously by the same factor, resulting in variations throughout the year, except for the τ-cadinol compound that remained stable.
Comparing the results with available literature, the rainy season was also favorable for the yield of oils Copaifera langsdorffii Desff (Souza de Oliveira et al., 2017), Eucalyptus citriodora Hook (Castro et al., 2008) and Cymbopogon citratus (Santos et al., 2009). The presence of the major compounds as, γ-terpinene, terpinolene, τ-cadinol, p-cymene and linalool in the essential oil of the species under study, were also present in P. myrsinites Mart. (Medeiros et al., 2015), Psidium pohlianum O. Berg, Psidium guyanensis Pers (Neto et al., 1994) and Psidium caudatum McVaugh (Yáñez et al., 2002).
Variations in plant active components are important parameters to correlate biological activity, including antibacterial, antifungal and insecticide. Knowledge of the abiotic factors influencing the chemical variability and essential oil yield is important for optimizing crop conditions and harvesting time so that they are of high quality, factors essential for commercialization. In addition, a number of biotic factors such as plant/micro-organism (Stoppacher et al., 2010), plant/insects (Kessler & Baldwin, 2001) and plant/plant interactions, age and development stage, as well as abiotic factors such as luminosity (Takshak & Agrawal, 2016), temperature, rainfall, nutrition, time and harvest time (Bitu et al., 2015), may present correlations with each other, acting in conjunction, and may exert a joint influence on the chemical variability and essential oil yield.
The major compounds terpinolene, τ-cadinol and y-terpinene, have already been reported in other plants that have been studied for their antifungal activity against C. albicans (Tampieri et al., 2005); however, none of the compounds were studied to evaluate their activity against C. krusei. Moreover, studies with C. tropicalis verify that the compounds p-cymene and linalool also possess an inhibitory effect (Hsu et al., 2013; De Oliveira Lima et al., 2017).
Combination therapy using natural and antimicrobial products has been reported as an important strategy to combat the development of microbial resistance due to the production of an additive or synergistic effect. Thus, we demonstrated that the essential oil association with fluconazole may represent a therapeutic benefit in reducing the antifungal dosage, representing an improvement in toxic levels, while producing a fungicidal effect (Pemmaraju et al., 2013).
This is the first work that shows the potential modulating activity of P. salutare essential oil, so there was no way to compare the results here with respect to the species. Within the genus Psidium, some data exist; however, they are related to extracts. Morais-Braga et al. (2016a) and Morais-Braga et al. (2016b) observed fluconazole and all extracts had high inhibitor concentrations, however, when these were in association with sub-inhibitory concentrations (MIC/16), fluconazole had an improved effect, thus a synergistic effect was observed in the combination of fluconazole with extracts of Psidium brownianum against all strains of Candida (Morais-Braga et al., 2016b). According to this study, Castro et al. (2015) demonstrated that the P. cattleianum essential oil had an effect on the inhibition of important clinical fungal strains such as Trichosporon asahii (Castro et al., 2015), C. parapsilosis, C. albicans, C. lipolytica and C. guilhermondi, with concentrations ranging from 41.67 ± 18.04 to 16,670 ± 72.17 µg/mL for the tested strains.
C. albicans was selected for association study because it is the most common pathogenic agent involved in systemic infections and the main strain responsible for infections caused by Candida fungi (Romani, 2012; Yapar, 2014). In the previous studies, of our research group, with other species of this genus, P. brownianum and P. guajava extracts had their antifungal potential investigated, obtaining favorable results, where they also managed to affect the phenotypic plasticity of C. albicans and C. tropicalis, reducing the formation process of hyphae and pseudohyphae as their concentrations were increased (Morais-Braga et al., 2016b; Morais-Braga et al., 2016a; Morais-Braga et al., 2017).
C. albicans is a polymorphic fungus that can grow both in the yeast form (ovoid form), elongated ellipsoid cells with constrictions in the septa (pseudohyphae), or as true hyphae of parallel walls, as can be observed in Fig. 2A. The hyphae or pseudohyphae forms are responsible for the infectious process ranging from superficial skin infections to life-threatening systemic infections. Transition to the hyphae form can be triggered by increases in temperature to 37 °C, increases in pH, and the addition of inducers ( Shareck & Belhumeur, 2011). In this form, the germinating tube and the tip extension can generate strong pressures for tissue penetration due to the secretion of proteases, lipases and other histological enzymes. This is important since hyphae formation is central to another aspect of Candida’s virulence: development of biofilms that is associated with increased resistance to antifungal medications (Calderone & Fonzi, 2001).
Some authors have proposed that the essential oil activity may be in part related to its hydrophobicity, responsible for its partition of the cell membrane lipid bilayer, leading to a change in permeability and cell membrane damage resulting from direct damage to the membrane resulting in a reduced ability to maintain cellular functions (Braga et al., 2007; Hsu et al., 2013). Another mechanism is related to a metabolic impairment with a reduction of 3′:5″-cyclic adenosine monophosphate (cAMP) formation and, together with a mitogenic activation protein (MAP) signaling pathway, responsible for playing an important role in the formation of filamentous forms (Hollosy & Keri, 2004; Deveau et al., 2010; Dižová & Bujdáková, 2017). Several mechanisms have been tested in order to provide new and valuable means to combat Candida pathogenesis that may lead to new strategies for the development of antifungal drugs.
Conclusion
In conclusion, the essential oil of P. salutare presented, as its main components, hydrogenated monoterpenes and γ-terpinene, whose composition was influenced by the beginning of the rainy season, proving this to be the ideal period for the isolation of the oil. It is not possible to affirm that the antifungal activity of the oil was influenced by the seasonal changes in the precipitation, with the exception of C. krusei, where it presented the lower MFC and IC50 values. The essential oil demonstrated a significant effect on Candida morphogenesis, reducing the ability of morphological transitions from invasive infectious processes and resistance to C. albicans. In this way, the presented results can be a starting point for new in vivo assays for the possible development of new complementary and alternative therapies, as well as to support its popular medicinal use against diseases of fungal origin.
Supplemental Information
Funding Statement
The authors received funding for this work by CAPES, FUNCAP, CNPq and FINEP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Delmacia G. de Macêdo, Email: delmaciamacedo@yahoo.com.br.
Irwin Rose A. de Menezes, Email: irwin.alencar@urca.br.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Delmacia G. de Macêdo and Antonia Thassya L. dos Santos performed the experiments, prepared figures and/or tables, approved the final draft.
Marta Maria A. Souza, Henrique Douglas M. Coutinho and Irwin Rose A. de Menezes conceived and designed the experiments, analyzed the data, approved the final draft.
Maria Flaviana B. Morais-Braga conceived and designed the experiments, performed the experiments, prepared figures and/or tables, approved the final draft.
Rafael P. da Cruz approved the final draft.
José Galberto M. da Costa conceived and designed the experiments, contributed reagents/materials/analysis tools, approved the final draft.
Fábio Fernandes G. Rodrigues performed the experiments, approved the final draft.
Lucindo J. Quintans-junior and Jackson Roberto G. da Silva Almeida analyzed the data, authored or reviewed drafts of the paper, approved the final draft, help in the correction of the manuscript.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
The collection is under the authorization of the competent ICMBio with number (no 50362-2).
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in Data S1.
References
- Adams (2007).Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publishing Corporation; Carol Stream: 2007. [Google Scholar]
- Araújo et al (2015).Araújo TAS, Castro VTNA, Solon LGS, Silva GA, Almeida MG, Da Costa JGM, De Amorim ELC, Albuquerque UP. Does rainfall affect the antioxidant capacity and production of phenolic compounds of an important medicinal species? Industrial Crops and Products. 2015;76:550–556. doi: 10.1016/j.indcrop.2015.07.008. [DOI] [Google Scholar]
- Bitu et al. (2015).Bitu V, Da Costa J, Rogrigues F, Colares A, Coutinho H, Botelho M, Portela A, De Santana N, Menezes I. Effect of collection time on composition of essential oil of LippiagracilisSchauer (Verbenaceae) growing in Northeast Brazil. Journal of Essential Oil Bearing Plants. 2015;18(3):647–653. doi: 10.1080/0972060X.2014.935043. [DOI] [Google Scholar]
- Braga et al. (2007).Braga PC, Alfieri M, Culici M, Dal Sasso M. Inhibitory activity of thymol against the formation and viability of Candida albicans hyphae. Mycoses. 2007;50:502–506. doi: 10.1111/j.1439-0507.2007.01412.x. [DOI] [PubMed] [Google Scholar]
- Calderone & Fonzi (2001).Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends in Microbiology. 2001;9:327–335. doi: 10.1016/S0966-842X(01)02094-7. [DOI] [PubMed] [Google Scholar]
- Carradori et al. (2016).Carradori S, Chimenti P, Fazzari M, Granese A, Angiolella L. Antimicrobial activity, synergism and inhibition of germ tube formation by Crocus sativus-derived compounds against Candida spp. Journal of Enzyme Inhibition and Medicinal Chemistry. 2016;31(sup 2):189–193. doi: 10.1080/14756366.2016.1180596. [DOI] [PubMed] [Google Scholar]
- Castro et al. (2015).Castro MR, Victoria FN, Oliveira DH, Jacob RG, Savegnago L, Alves D. Essential oil of Psidium cattleianum leaves: antioxidant and antifungal activity. Pharmaceutical Biology. 2015;53:242–250. doi: 10.3109/13880209.2014.914231. [DOI] [PubMed] [Google Scholar]
- Castro et al. (2008).Castro NEA, Carvalho GJ, Cardoso MG, Pimentel FA, Correa RM, Guimarães LGL. Avaliação de rendimento e dos constituintes químicos do óleo essencial de folhas de Eucalyptus citriodora Hook. colhidas em diferentes épocas do ano em municípios de Minas Gerais. Revista Brasileira de Plantas Medicinais. 2008;10:70–75. [Google Scholar]
- Coutinho et al. (2008).Coutinho HDM, Costa JGM, Lima EO, Falcão Silva VS, Siqueira-Júnior JP. Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L., and chlorpromazine. Chemotherapy. 2008;54:328–330. doi: 10.1159/000151267. [DOI] [PubMed] [Google Scholar]
- De Oliveira Lima et al. (2017).De Oliveira Lima MI, De Medeiros ACA, Silva KVS, Cardoso GN, De Oliveira Lima E, De Oliveira Pereira F. Investigation of the antifungal potential of linalool against clinical isolates of fluconazole resistant Trichophyton rubrum. Journal de Mycologie Medicale. 2017;27:195–202. doi: 10.1016/j.mycmed.2017.01.011. [DOI] [PubMed] [Google Scholar]
- Deveau et al. (2010).Deveau A, Piispanen AE, Jackson AA, Hogan DA. Farnesol induces hydrogen peroxide resistance in Candida albicans yeast by inhibiting the Ras-cyclic AMP signaling pathway. Eukaryotic Cell. 2010;9:569–577. doi: 10.1128/EC.00321-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dižová & Bujdáková (2017).Dižová S, Bujdáková H. Properties and role of the quorum sensing molecule farnesol in relation to the yeast Candida albicans. Die Pharmazie. 2017;72:307–312. doi: 10.1691/ph.2017.6174. [DOI] [PubMed] [Google Scholar]
- Ernst et al. (1999).Ernst EJ, Klepser ME, Ernst ME, Messer SA, Pfaller MA. In vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagnostic Microbiology and Infectious Disease. 1999;33:75–80. doi: 10.1016/S0732-8893(98)00130-8. [DOI] [PubMed] [Google Scholar]
- Estell, Fredrickson & James (2016).Estell RE, Fredrickson EL, James DK. Effect of light intensity and wavelength on concentration of plant secondary metabolites in the leaves of Flourensia cernua. Biochemical Systematics and Ecology. 2016;65:108–114. doi: 10.1016/j.bse.2016.02.019. [DOI] [Google Scholar]
- Faleiro et al. (2016).Faleiro JH, Gonçalves RC, Dos Santos MNG, Da Silva DP, Naves PLF, Malafaia G. The chemical featuring, toxicity, and antimicrobial activity of Psidium cattleianum (Myrtaceae) leaves. New Journal of Science. 2016;2016:7538613. doi: 10.1155/2016/7538613. [DOI] [Google Scholar]
- Fernandes et al. (2012).Fernandes TG, De Mesquita ARC, Randau KP, Franchitti AA, Ximenes EA. In vitro synergistic effect of Psidium guineense (Swartz) in combination with antimicrobial agents against methicillin-resistant Staphylococcus aureus strains. The Scientific World Journal. 2012;2012:158237. doi: 10.1100/2012/158237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo et al. (2008).Figueiredo AC, Barroso JG, Pedro LG, Scheffer JJC. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour and Fragrance Journal. 2008;23:213–226. doi: 10.1002/ffj.1875. [DOI] [Google Scholar]
- FUNCEME (2016).Foundation Cearense for Meteorology and Water Resources (FUNCEME) 2016. Rainfall calendar in the state of Ceará. http://www.funceme.br/app/calendario/produto/macrorregioes/media/anual .
- Gupta, Chahal & Arora (2011).Gupta GK, Chahal J, Arora D. Psidium guajava Linn.: current research and future prospects. Journal of Pharmacy Research. 2011;4:42–46. [Google Scholar]
- Hollosy & Keri (2004).Hollosy F, Keri G. Plant-derived protein tyrosine kinase inhibitors as anticancer agents. Current Medicinal Chemistry. 2004;4:173–197. doi: 10.2174/1568011043482124. [DOI] [PubMed] [Google Scholar]
- Hsu et al. (2013).Hsu C-C, Lai W-L, Chuang K-C, Lee M-H, Tsai Y-C. The inhibitory activity of linalool against the filamentous growth and biofilm formation in Candida albicans. Medical Mycology. 2013;51:473–482. doi: 10.3109/13693786.2012.743051. [DOI] [PubMed] [Google Scholar]
- Javadpour et al. (1996).Javadpour MM, Juban MM, Lo W-CJ, Bishop SM, Alberty JB, Cowell SM, Becker CL, McLaughlin ML. De novo antimicrobial peptides with low mammalian cell toxicity. Journal of Medicinal Chemistry. 1996;39:3107–3113. doi: 10.1021/jm9509410. [DOI] [PubMed] [Google Scholar]
- Joseph & Priya (2011).Joseph B, Priya RM. Phytochemical and biopharmaceutical aspects of Psidium guajava (L.) essential oil: a review. Research Journal of Medicinal Plants. 2011;5:432–442. doi: 10.3923/rjmp.2011.432.442. [DOI] [Google Scholar]
- Kessler & Baldwin (2001).Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- Landrum (2003).Landrum LR. A revision of the Psidium salutare complex (Myrtaceae) SIDA, Contributions to Botany. 2003;20(4):1449–1469. [Google Scholar]
- Macêdo et al. (2016).Macêdo DG, Menezes IRA, Lacerda SR, Da Silva MAP, Ribeiro DA, Macêdo MS, Oliveira LGS, Saraiva ME, Alencar SR, Oliveira SF, Santos MO, De Almeida BV, Macedo JGF, Sousa FFS, Soares MA, De Araujo TMS, Souza MMA. Versatility and consensus of the use of medicinal plants in an area of cerrado in the Chapada do Araripe, Barbalha—CE- Brazil. Journal of Medicinal Plants Research. 2016;10:505–514. doi: 10.5897/JMPR2015.5952. [DOI] [Google Scholar]
- Macêdo et al. (2015).Macêdo DG, Ribeiro DA, Coutinho HDM, Menezes IRA, Souza MMA. Therapeutic traditional practices: usage and knowledge of cerrado plants in the State of Pernambuco (Northeastern Brazil) Boletin Latinoamericano y del Caribe de Plantas Medicinales y Aromaticas. 2015;14(6):491–508. [Google Scholar]
- McLafferty & Stauffer (2016).McLafferty FW, Stauffer DB. 11th edition Wiley; Hoboken: 2016. [Google Scholar]
- Medeiros et al. (2015).Medeiros FCM de, Del Menezzi CHS, Bizzo HR, Vieira RF. Scents from Brazilian Cerrado: Psidium myrsinites DC.(Myrtaceae) leaves and inflorescences essential oil. Journal of Essential Oil Research. 2015;27:289–292. doi: 10.1080/10412905.2015.1037020. [DOI] [Google Scholar]
- Mendes Giannini et al. (2013).Mendes Giannini MJS, Bernardi T, Scorzoni L, Sardi JCO, Fusco-Almeida AM. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. Journal of Medical Microbiology. 2013;62:10–24. doi: 10.1099/jmm.0.045054-0. [DOI] [PubMed] [Google Scholar]
- Morais-Braga et al. (2016a).Morais-Braga MFB, Carneiro JNP, Machado AJT, Sales DL, Brito DIV, Albuquerque RS, Boligon AA, Athayde ML, Calixto Júnior JT, Souza DSL, Lima EO, Menezes IRA, Costa JGM, Ferreira FS, Coutinho HDM. High-performance liquid chromatography-diodic array detector, fungistatic, and anti-morphogenical analysis of extracts from Psidium brownianum mart. ex DC. Against yeasts of the genus Candida. International Journal of Food Properties. 2016a;19:1837–1851. doi: 10.1080/10942912.2015.1079786. [DOI] [Google Scholar]
- Morais-Braga et al. (2017).Morais-Braga MFB, Carneiro JNP, Machado AJT, Sales DL, Dos Santos ATL, Boligon AA, Athayde ML, Menezes IRA, Souza DSL, Costa JGM, Coutinho HDM. Phenolic composition and medicinal usage of Psidium guajava Linn.: antifungal activity or inhibition of virulence? Saudi Journal of Biological Sciences. 2017;24(2):302–313. doi: 10.1016/j.sjbs.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-Braga et al. (2016b).Morais-Braga MFB, Sales DL, Carneiro JNP, Machado AJT, Dos Santos ATL, De Freitas MA, Martins GMDAB, Leite NF, De Matos YMLS, Tintino SR, Souza DSL, Menezes IRA, Ribeiro-Filho J, Costa JGM, Coutinho HDM. Psidium guajava L. and Psidium brownianum Mart ex DC.: chemical composition and anti—Candida effect in association with fluconazole. Microbial Pathogenesis. 2016b;95:200–207. doi: 10.1016/j.micpath.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Mulas, Gardner & Craker (2006).Mulas G, Gardner Z, Craker LE. Effect of light quality on growth and essential oil composition in rosemary. I International symposium on the labiatae: advances in production, biotechnology and utilisation, vol. 723; 2006. pp. 427–432. [Google Scholar]
- Neto et al. (1994).Neto MA, De Alencar JW, Cunha AN, Silveira ER, Batista TG. Volatile constituents of Psidium pohlianum berg, and Psidium guyanensis pers. Journal of Essential Oil Research. 1994;6:299–300. doi: 10.1080/10412905.1994.9698379. [DOI] [Google Scholar]
- Pemmaraju et al. (2013).Pemmaraju SC, Pruthi PA, Prasad R, Pruthi V. Candida albicans biofilm inhibition by synergistic action of terpenes and fluconazole. Indian Journal of Experimental Biology. 2013;51(11):1032–1037. [PubMed] [Google Scholar]
- Pino et al. (2003).Pino JA, Bello A, Urquiola A, Agüero J. Leaf oil of Psidium salutare (HBK) Berg, from Cuba. Journal of Essential Oil Research. 2003;15:19–20. doi: 10.1080/10412905.2003.9712251. [DOI] [Google Scholar]
- Pinto et al. (2007).Pinto JEBP, Cardoso JCW, De Castro EM, Bertolucci SKV, De Melo LA, Dousseau S. Morphophysiological aspects and essential oil content in Brazilian-lavender as affected by shadowing. Horticultura Brasileira. 2007;25:210–214. doi: 10.1590/S0102-05362007000200016. [DOI] [Google Scholar]
- Prins, Vieira & Freitas (2010).Prins CL, Vieira IJC, Freitas SP. Growth regulators and essential oil production. Brazilian Journal of Plant Physiology. 2010;22:91–102. doi: 10.1590/S1677-04202010000200003. [DOI] [Google Scholar]
- Ribeiro et al. (2014).Ribeiro DA, Oliveira LGS De, Macêdo DG De, Menezes IRA De, Costa JGM Da, Silva MAP Da, Lacerda SR, Souza MMDA. Promising medicinal plants for bioprospection in a Cerrado area of Chapada do Araripe, Northeastern Brazil. Journal of Ethnopharmacology. 2014;155:1522–1533. doi: 10.1016/j.jep.2014.07.042. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Silva et al. (2012).Ribeiro-Silva S, De Medeiros MB, Gomes BM, Seixas ENC, Da Silva MAP. Angiosperms from the Araripe national forest, Ceará, brazil. Check List. 2012;8:744–751. doi: 10.15560/8.4.744. [DOI] [Google Scholar]
- Romani (2012).Romani L. Candida and Candidiasis. Second edition American Society of Microbiology; Washington, D.C.: 2012. Immunology of invasive candidiasis; pp. 127–136. [Google Scholar]
- Santos et al. (2009).Santos A, Paduan RH, Gazin ZC, Jacomassi E, D’Oliveira PS, Cortez DAG, Cortez LER. Determinação do rendimento e atividade antimicrobiana do óleo essencial de Cymbopogon citratus (DC.) Stapf em função de sazonalidade e consorciamento. Revista Brasileira de Farmacognosia. 2009;19:436–441. doi: 10.1590/S0102-695X2009000300017. [DOI] [Google Scholar]
- Shareck & Belhumeur (2011).Shareck J, Belhumeur P. Modulation of morphogenesis in Candida albicans by various small molecules. Eukaryotic Cell. 2011;10(8):1004–1012. doi: 10.1128/EC.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrim & Rocha (2003).Sidrim JJC, Rocha MFG. Medical Mycology in the light of contemporary authors. 1st edition. São Paulo: Guanabara Koogan; 2003. [Google Scholar]
- Silva et al. (2012).Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiology Reviews. 2012;36:288–305. doi: 10.1111/j.1574-6976.2011.00278.x. [DOI] [PubMed] [Google Scholar]
- Souza de Oliveira et al.(2017).Souza de Oliveira LG, Alves Ribeiro D, Eufrasio Saraiva M, Gonçcalves de Macêdo D, Gonçcalves Ferreira Macedo J, GonçcalvesPinheiro P, Martins da Costa JG, De Almeida Souza MM, Alencar de Menezes IR. Chemical variability of essential oils of Copaifera langsdorffii Desf. in different phenological phases on a savannah in the Northeast, Ceará, Brazil. Industrial Crops and Products. 2017;97:455–464. doi: 10.1016/j.indcrop.2016.12.031. [DOI] [Google Scholar]
- Souza et al. (2014).Souza RKD, Da Silva MAP, De Menezes IRA, Ribeiro DA, Bezerra LR, Souza MM de A, Da Silva MAP, De Menezes IRA, Ribeiro DA, Bezerra LR, Souza MM de A. Ethnopharmacology of medicinal plants of Carrasco, northeastern Brazil. Journal of Ethnopharmacology. 2014;157:99–104. doi: 10.1016/j.jep.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Stoppacher et al. (2010).Stoppacher N, Kluger B, Zeilinger S, Krska R, Schuhmacher R. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. Journal of Microbiological Methods. 2010;81:187–193. doi: 10.1016/j.mimet.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Takshak & Agrawal (2016).Takshak S, Agrawal SB. The role of supplemental ultraviolet-B radiation in altering the metabolite profile, essential oil content and composition, and free radical scavenging activities of Coleus forskohlii, an indigenous medicinal plant. Environmental Science and Pollution Research. 2016;23:7324–7337. doi: 10.1007/s11356-015-5965-6. [DOI] [PubMed] [Google Scholar]
- Tampieri et al. (2005).Tampieri MP, Galuppi R, Macchioni F, Carelle MS, Falcioni L, Cioni PL, Morelli I. The inhibition of Candida albicans by selected essential oils and their major components. Mycopathologia. 2005;159:339–345. doi: 10.1007/s11046-003-4790-5. [DOI] [PubMed] [Google Scholar]
- Vandeputte, Ferrari & Coste (2011).Vandeputte P, Ferrari S, Coste AT. Antifungal resistance and new strategies to control fungal infections. International Journal of Microbiology. 2011;2012 doi: 10.1155/2012/713687. Article 713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez et al. (2002).Yáñez X, Pinzón ML, Solano F, Sánchez LR. Chemical composition of the essential oil of Psidium caudatum McVaugh. Molecules. 2002;7:712–716. doi: 10.3390/70900712. [DOI] [Google Scholar]
- Yao et al. (2016).Yao XH, Zhang ZB, Song P, Hao JY, Zhang DY, Zhang YF. Different harvest seasons modify bioactive compounds and antioxidant activities of Pyrola incarnata. Industrial Crops and Products. 2016;94:405–412. doi: 10.1016/j.indcrop.2016.08.033. [DOI] [Google Scholar]
- Yapar (2014).Yapar N. Epidemiology and risk factors for invasive candidiasis. Therapeutics and Clinical Risk Management. 2014;10:95–105. doi: 10.2147/TCRM.S40160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in Data S1.