Abstract
Background
The American Heart Association (AHA) has prioritized seven cardiovascular health metrics to reduce the cardiovascular burden, including: body mass index, healthy diet, physical activity, smoking status, blood pressure, HbA1c and total cholesterol. The aim of the current study was to assess the association between the AHA-defined health metrics and the risk of cardiovascular events in the EPIC-Norfolk prospective study.
Design
Prospective cohort study.
Methods
An overall cardiovascular health score was calculated based on the number of health metrics including ideal, intermediate or poor. Cox proportional hazards models were used to describe the association of the seven metrics separately and the overall health score with risk of coronary heart disease, stroke and cardiovascular disease. A total of 10,043 participants were included in the analysis (follow-up 1993-2008). For all individual health metrics a more ideal status was associated with a lower risk of cardiovascular events
Results and conclusion
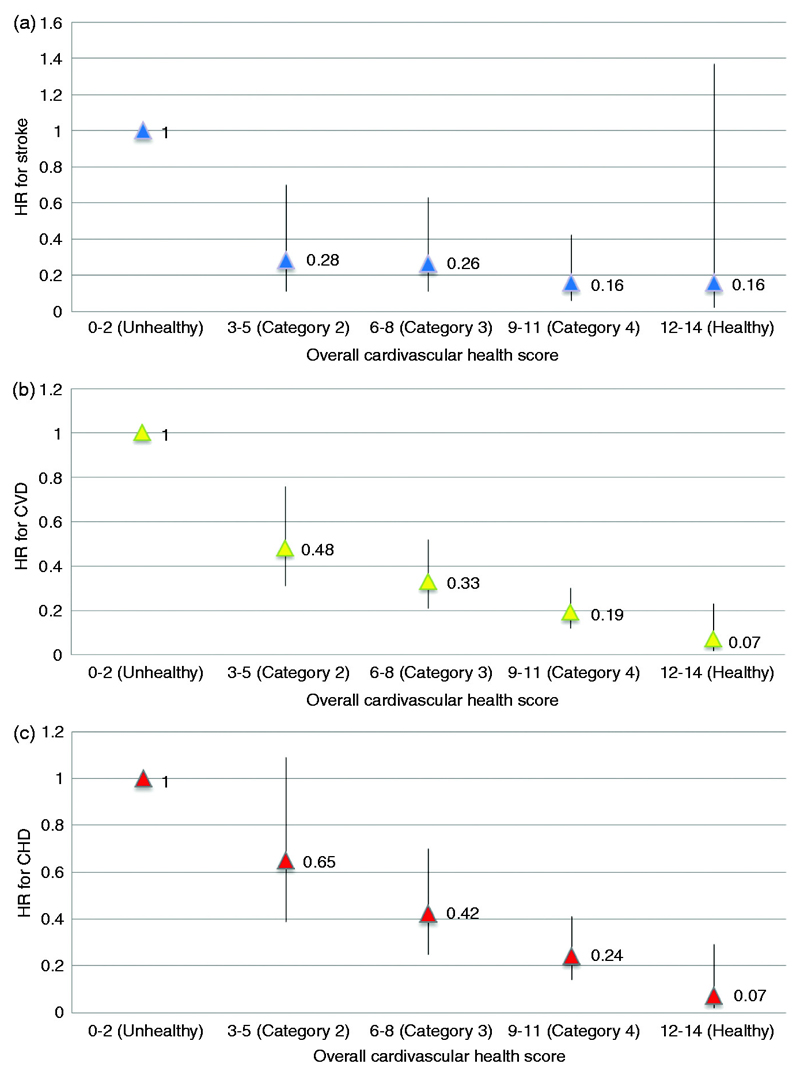
As for the overall cardiovascular health score, those in the highest (i.e. healthiest) category (score 12-14) had an adjusted hazard ratio for coronary heart disease of 0.07 (95 % CI 0.02-0.29, P<0.001), for stroke of 0.16 (95% CI 0.02-1.37, p=0.09), and for cardiovascular disease of 0.07 (CI 0.02-0.23, p<0.001), compared to people in the lowest (i.e. unhealthiest) category (score 0-2). The overall cardiovascular health score was strongly and inversely associated with risk of coronary heart disease, stroke and cardiovascular disease. Our data suggest that even small improvements in modifiable risk factors may lead to substantial reductions in the risks of cardiovascular events.
Keywords: Health metrics, risk factors, primary prevention, cardiovascular diseases
Introduction
Cardiovascular diseases (CVD) are the leading cause of mortality worldwide.(1) CVD is largely the consequence of modifiable risk factors, including lifestyle.(2) The benefits of improving modifiable risk factors are substantial. For instance, in the general population, smoking cessation, adequate physical activity and favourable dietary changes can result in mortality reduction by 50%, 20-30% and 15-40%, respectively.(3)
Clinical guidelines recognize the importance of optimizing modifiable risk factors for cardiovascular risk management worldwide.(4, 5) There is, however, a rising trend in the prevalence of unhealthy lifestyles, both in primary and secondary prevention settings.(6, 7) In 2010, the American Heart Association (AHA) has expressed the ambition to reduce cardiovascular mortality by 20% in 2020 and has defined a set of 7 cardiovascular health metrics that will be used to measure progress toward their 2020 goals for cardiovascular health in the general population: body mass index (BMI), healthy diet, physical activity, smoking behaviour, blood pressure, fasting glucose level and cholesterol level.(8) The AHA health metrics are mainly based on lifestyle related risk factors, in particular diet and physical activity, which are not routinely assessed within validated risk scores such as Framingham and SCORE. Furthermore, the association between the AHA health metrics and cardiovascular risk has not been assessed in a European population. It was therefore our objective to assess the association between the AHA-defined health metrics and the risk of cardiovascular disease in a British cohort of apparently healthy individuals.
Methods
The European Prospective Investigation into Cancer (EPIC)-Norfolk cohort is a prospective population study, which is part of the 10-country collaborative EPIC study. The design, methods and baseline characteristics of the EPIC-Norfolk study have been described previously.(9) The cohort was designed to assess dietary and other determinants of cancer. Additional data were obtained to investigate determinants of other chronic diseases. Briefly, participants were recruited from age-sex registries of general practices in the area of Norfolk. Participants completed a detailed health and lifestyle questionnaire at the baseline survey between 1993 and 1997 and underwent physical examination, blood samples were obtained and measurements were performed by trained nurses.
BMI was calculated by dividing weight in kilograms by height in meters squared. Dietary information was obtained from a 130 item food frequency questionnaire (FFQ), see supplement.(10) Physical activity was assessed using a questionnaire to quantify activity both at work and during leisure time, and categorized into four levels: active, moderately active, moderately inactive and inactive, see supplement.10 This questionnaire has been validated against energy expenditure.(11) Smoking status was self-reported, and derived from responses to the questions “Have you ever smoked as much as one cigarette a day for as long as a year?” and “Do you smoke cigarettes now?”. Blood pressure was recorded using an Accutorr sphygmomanometer (Datascope, Huntington, UK). Serum total cholesterol was measured in blood samples by colorimetry (RA 1000, Bayer Diagnostic, Basingstoke, UK).(9) HbA1c was measured in baseline blood samples by Biorad Diomat high-performance liquid chromatography (Richmond, California, USA). Funding only became available for HbA1c analyses halfway through the study and measures are therefore only available for about 10,000 participants in the second half of the recruited cohort.
Participants were identified as having been hospitalized or having died because of a cardiovascular event if the corresponding International Classification of Disease (ICD)-10 code was recorded as the underlying cause of hospitalization or mortality. Hospitalized participants were identified using their unique National Health Service number linked with the East Norfolk Health Authority (ENCORE) database. The ENCORE database identified all hospital contacts throughout England and Wales for residents of Norfolk. Death certificates were coded by trained nosologists according to the International Classification of Diseases 10 (ICD-10). Deaths or hospitalizations were attributed to coronary heart disease (CHD) if the underlying cause was coded by as ICD-10 codes 120-125, which encompass the clinical spectrum of CHD, including unstable angina, stable angina and myocardial infarction. Deaths or hospitalizations were attributed to stroke, if the underlying cause was coded as ischemic (I63) or haemorrhagic stroke (I60-62). Cardiovascular disease was defined as either a CHD or stroke. The follow-up was censored on March 31th 2008. The study protocol was approved by the Norwich District Health Authority Ethics Committee and all participants gave written informed consent.
Definition of health metrics
The AHA defined seven cardiovascular health metrics, namely BMI, healthy diet score (HDS), physical activity, smoking status, blood pressure, fasting plasma glucose and total cholesterol. These metrics were classified as ideal, intermediate or poor according to the following definitions. BMI was classified as ideal if < 25 kg/m2, as intermediate if 25-30 kg/m2 and as poor if ≥ 30 kg/m2. The HDS was based on an intake of ≥ 5.0 cups fruit and vegetables; a participant with a value ≥ 5.0 (representing ≥ 5 cups per day) was considered to meet the guidelines. The weight of the included fish items was multiplied by 7 and divided by 3.5 oz (portion size); if the value was ≥ 2, the participant was considered to consume ≥ 2 servings per week. For fibre-rich whole grains, participants consuming ≥ 3 servings per day of 1 oz each were considered to meet the guideline, as were participants with a sodium intake < 1500 mg per day and ≤ 450 kcal sugar-sweetened beverages per week. The HDS was calculated as the sum of the number of healthy food items, yielding a HDS range of 0 to 5. HDS was categorized as ideal (≥ 4), intermediate (2-3), or poor (< 2). Physical activity was defined as ideal, intermediate, and poor if the status was active, moderately active or moderately inactive, and inactive, respectively. Smoking status was classified as ideal, intermediate or poor if the study participant had never smoked, previously smoked, or was a current smoker, respectively. Blood pressure was defined as ideal if systolic pressure was < 120 mmHg and diastolic pressure was < 80 mmHg, as intermediate if systolic pressure was 120-139 mmHg or diastolic pressure was 80-89 mmHg with or without antihypertensive drug treatment, or poor if systolic pressure was ≥ 140 or diastolic pressure ≥ 90 mmHg. Total cholesterol levels were classified as ideal (<5.2 mmol/l), intermediate (5.2-6.2 mmol/l) or poor (≥ 6.2 mmol/l). In EPIC-Norfolk, HbA1c levels were used instead of fasting glucose levels which were not available. HbA1c plasma levels were classified as ideal (< 5.7 %), intermediate (5.7-6.5 %), or poor (≥ 6.5 %).
The overall cardiovascular health score (CHS) was calculated based on these 7 health metrics, giving 2 points for an ideal metric, 1 point for an intermediate metric, and 0 points for a poor metric, thus yielding an overall CHS between 0 and 14. The CHS was divided into 5 categories as follows: 0-2 (unhealthy), 3-5, 6-8, 9-11 and 12-14 (healthy).
Statistical analysis
Descriptive data were presented as percentage and number for categorical variables, mean and standard deviation for continuous variables with a normal distribution, and median with interquartile range for continuous variables not normally distributed. Study participants with missing data for any of the cardiovascular health metrics, as well as those who had prevalent CHD or stroke, were excluded from this analysis.
A Cox proportional hazards model was used to assess the association between each health metric and the risk of cardiovascular events. Hazard ratios (HR) and 95% confidence intervals (95%CI) for the risk of cardiovascular events were calculated for study participants classified as having an ideal or intermediate health metric, using those in the ‘poor’ category as reference. Hazard ratios were calculated according to an unadjusted model and a model that adjusted for sex and age. Separate analyses were performed for CHD, stroke and CVD events. HRs for CHD, stroke and CVD events were also calculated according to categories of the overall CHS using the lowest category (score range 0-2) as reference group. Given the fact that HbA1c levels were available in approximately half of the cohort, analyses were repeated without taking HbA1c levels into account as one of the health metrics. This caused the study cohort to double in size, but only 6 of the 7 AHA health metrics could be evaluated. Statistical analyses were performed in SPSS version 20. A p-value < 0.05 was considered as statistically significant.
Results
The EPIC-Norfolk cohort comprised 25,663 study participants. A total of 15,620 (61%) were excluded because of missing data for any of the cardiovascular health metrics (mostly HbA1c), and 2,160 (8.1%) were excluded because of prevalent CHD or stroke. A complete dataset on the AHA defined health metrics was available for 10,043 study participants. A total of 1,004 (10%) participants experienced a CHD event during follow-up, 171 (1.7%) experienced a stroke event, and 50 (0.5%) experienced both a CHD and a stroke event. Mean follow-up was 10 years, yielding a total of 103,961 person-years follow-up. The characteristics of the EPIC-Norfolk participants are presented in table 1. The participants’ age ranged between 39 to 79 years, and 44.1% were men. The distribution of the health metrics is presented in table 2. An ideal status for BMI, healthy diet, physical activity, and smoking status was present in 40.8%, 9.6%, 18.4% and 47.3%, respectively. An ideal status for blood pressure, HbA1c and total cholesterol was present in 18.5%, 81.3% and 19.6%, respectively.
Table 1.
| Age, years | 57.0 ± 9.6 |
| Male | 44.1 (4430) |
| Body mass index, kg/m2 | 25.8 ± 3.9 |
| Systolic blood pressure, mmHg | 133 ± 18 |
| Diastolic blood pressure, mmHg | 82 ± 11 |
| HbA1c, % | 5.3 ± 0.8 |
| Total cholesterol, mmol/l | 6.0 ± 1.1 |
| LDL cholesterol, mmol/l | 3.8 ± 1.0 |
| HDL cholesterol, mmol/l | 1.4 ± 0.5 |
| Triglycerides, mmol/l | 1.5 (1.1–2.2) |
| Healthy diet | |
| - Fruit and vegetables ≥4.5 cups per day | 68.6 (6888) |
| - Fish ≥2 servings per week | 49.1 (4932) |
| - Fiber-rich whole grains ≥3 servings per day | 43.5 (4373) |
| - Sodium < 1500 mg per day | 3.8 (381) |
| - Sugar-sweetened beverages ≤450 kcal per week | 43.5 (4371) |
| Physical activity | |
| - Inactive | 30.3 (3043) |
| - Moderately inactive | 28.2 (2830) |
| - Moderately active | 23.2 (2326) |
| - Active | 18.4 (1844) |
| Smoking behaviour | |
| - Current | 11.8 (1182) |
| - Former | 40.9 (4108) |
| - Never | 47.3 (4753) |
Data are presented as percentage (number) for categorical variables, mean ± standard deviation for continuous variables with normal distribution, or median (interquartile range) for continuous variables with a non-normal distribution. Data were available in up to 10,043 study participants. LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Table 2.
| Cardiovascular health metric | Definition | Percentage (number) |
|---|---|---|
| Body mass index | ||
| - Ideal | <25 kg/m2 | 40.8 (4097) |
| - Intermediate | 25–30 kg/m2 | 45.1 (4525) |
| - Poor | ≥30 kg/m2 | 14.1 (1421) |
| Healthy diet score | ||
| - Ideal | ≥4 healthy components | 9.6 (966) |
| - Intermediate | 2–4 healthy components | 60.1 (6034) |
| - Poor | <2 healthy components | 30.3 (3043) |
| Physical activity | ||
| - Ideal | Active | 18.4 (1844) |
| - Intermediate | Moderately active or moderately inactive | 51.3 (5156) |
| - Poor | Inactive | 30.3 (3043) |
| Smoking behaviour | ||
| - Ideal | Never | 47.3 (4753) |
| - Intermediate | Former | 40.9 (41 08) |
| - Poor | Current | 11.8 (1182) |
| Blood pressure | ||
| - Ideal | SBP < 120 and DBP <80 mmHg, without drug therapy | 18.5 (1856) |
| - Intermediate | SBP ≥ 1 20 to < 140 or DBP 80> to <90 or treated to SBP < 140 or DBP < 90 mmHg | 42.7 (4286) |
| - Poor | SBP ≥ 140 or DBP ≥90 mmHg | 38.8 (3901) |
| HbA1c | ||
| - Ideal | <5.7% | 81.3 (8167) |
| - Intermediate | 5.7–6.5% | 14.8 (1482) |
| - Poor | ≥6.5% | 3.9 (394) |
| Total cholesterol | ||
| - Ideal | <5.2 mmol/I | 19.6 (1970) |
| - Intermediate | 5.2–6.2 mmol/I | 38.6 (3874) |
| - Poor | ≥6.2 mmol/I | 41.8 (4199) |
| Total | 10,043 |
Data are presented as percentage (number). SBP, systolic blood pressure; DBP, diastolic blood pressure.
In table 3 the risk of CVD events is shown by each health metric separately. For those with an intermediate and ideal BMI the adjusted HR was 0.69 (95% CI 0.62-0.77) and 0.54 (95% CI 0.48-0.61), respectively. For those with an intermediate and ideal HDS, the adjusted HRs were 0.96 (95% CI 0.87-1.06) and 1.22 (95% CI 1.00-1.51), respectively. The adjusted HRs for the intermediate and ideal physical activity status were 0.90 (95% CI 0.82-0.98, p=0.02) and 0.88 (95% CI 0.77-1.00, p=0.04), respectively. Similar associations between more favourable health metrics and lower risk for CVD events were demonstrated for smoking, blood pressure, total cholesterol, and HbA1c. Table 4 shows the risk of CHD, stroke, and CVD events according to 5 categories of the overall CHS (i.e. 0-2, 3-5, 6-8, 9-11 and 12-14). Ideal cardiovascular health (overall CHS 12-14) was prevalent in only 2.8% of this cohort. People in the highest (healthy) category had a 93% reduced risk of CHD compared to those in the lowest (unhealthy) category (HR 0.07; 95% CI 0.02-0.29). For stroke, the HR for those in the highest versus lowest category was 0.16 (95% CI 0.02-1.37). For all CVD events, the adjusted HRs for participants in the consecutive categories were 0.48 (95% CI 0.31-0.76), 0.33 (95% CI 0.21-0.52), 0.19 (95% CI 0.12-0.30), and 0.07 (95% CI 0.02-0.23), compared to those in the lowest category (Figures 1A-C).
Table 3.
| Cardiovascular health metrics | Poor | Intermediate | Ideal | |
|---|---|---|---|---|
| Body mass index | ||||
| Number | 1421 | 4525 | 4097 | |
| Events | 201 | 549 | 339 | |
| Event rate | 14.1 | 12.1 | 8.3 | |
| Model 1 | Hazard ratio | 1.00 (ref) | 0.73 | 0.48 |
| 95% confidence interval | (0.66–0.82) | (0.43–0.54) | ||
| P value | <0.001 | <0.001 | ||
| Model 2 | Hazard ratio | 1.00 (ref) | 0.69 | 0.54 |
| 95% confidence interval | (0.62–0.77) | (0.48–0.61) | ||
| P value | <0.001 | <0.001 | ||
| Healthy diet score | ||||
| Number | 2127 | 7550 | 366 | |
| Events | 255 | 778 | 56 | |
| Event rate | 12.0 | 10.3 | 15.3 | |
| Model 1 | Hazard ratio | 1.00 (ref) | 0.96 | 1.22 |
| 95% confidence interval | (0.87–1.06) | (1.00–1.51) | ||
| P value | 0.45 | 0.06 | ||
| Model 2 | Hazard ratio | 1.00 (ref) | 0.93 | 1.08 |
| 95% confidence interval | (0.84–1.03) | (0.87–1.34) | ||
| P value | 0.15 | 0.48 | ||
| Physical activity | ||||
| Number | 3043 | 5156 | 1844 | |
| Events | 467 | 467 | 155 | |
| Event rate | 15.3 | 9.1 | 8.4 | |
| Model 1 | Hazard ratio | 1.00 (ref) | 0.63 | 0.56 |
| 95% confidence interval | (0.58–0.69) | (0.49–0.63) | ||
| P value | <0.001 | <0.001 | ||
| Model 2 | Hazard ratio | 1.00 (ref) | 0.90 | 0.88 |
| 95% confidence interval | (0.82–0.98) | (0.77–1.00) | ||
| P value | 0.02 | 0.04 | ||
| Smoking behaviour | ||||
| Number | 1182 | 4108 | 4753 | |
| Events | 158 | 529 | 402 | |
| Event rate | 13.4 | 12.9 | 8.5 | |
| Model 1 | Hazard ratio | 1.00 (ref) | 1.07 | 0.81 |
| 95% confidence interval | (0.94–1.22) | (0.71–0.92) | ||
| P value | 0.32 | 0.001 | ||
| Model 2 | Hazard ratio | 1.00 (ref) | 0.76 | 0.71 |
| 95% confidence interval | (0.66–0.86) | (0.63–0.82) | ||
| P value | <0.001 | <0.001 | ||
| Blood pressure | ||||
| Number | 3901 | 4286 | 1856 | |
| Events | 630 | 395 | 64 | |
| Event rate | 16.1 | 9.2 | 3.4 | |
| Model 1 | Hazard ratio | 1.00 (ref) | 0.45 | 0.18 |
| 95% confidence interval | (0.41–0.49) | (0.15–0.21) | ||
| P value | <0.001 | <0.001 | ||
| Model 2 | Hazard ratio | 1.00 (ref) | 0.61 | 0.33 |
| 95% confidence interval | (0.56–0.67) | (0.27–0.39) | ||
| P value | <0.001 | <0.001 | ||
| HbA1c | ||||
| Number | 394 | 1482 | 8167 | |
| Events | 118 | 234 | 737 | |
| Event rate | 29.9 | 15.8 | 9 | |
| Model 1 | Hazard ratio | 1.00 (ref) | 0.61 | 0.37 |
| 95% confidence interval | (0.51–0.72) | (0.32–0.43) | ||
| P value | <0.001 | <0.001 | ||
| Model 2 | Hazard ratio | 1.00 (ref) | 0.69 | 0.58 |
| 95% confidence interval | (0.58–0.82) | (0.50–0.68) | ||
| P value | <0.001 | <0.001 | ||
| Total cholesterol | ||||
| Number | 4199 | 3874 | 1970 | |
| Events | 571 | 384 | 134 | |
| Event rate | 13.6 | 9.9 | 6.8 | |
| Model 1 | Hazard ratio | 1.00 (ref) | 0.76 | 0.6 |
| 95% confidence interval | (0.70–0.83) | (0.53–0.68) | ||
| P value | <0.001 | <0.001 | ||
| Model 2 | Hazard ratio | 1.00 (ref) | 0.89 | 0.85 |
| 95% confidence interval | (0.81–0.97) | (0.75–0.96) | ||
| P value | 0.01 | 0.008 |
Model 1 unadjusted; Model 2 adjusted for sex and age. N = 10,043.
Table 4.
| Overall cardiovascular health score category | 1 (Unhealthy) | 2 | 3 | 4 | 5 (Healthy) | Total | ||
| Overall cardiovascular health score range | 0–2 | 3–5 | 6–8 | 9–11 | 12–14 | |||
| Number (percentage) | 55 (0.5) | 1440 (14.3) | 5017 (50.0) | 3254 (32.4) | 277 (2.8) | 10,043 (100.0) | ||
| Coronary heart disease | ||||||||
| Events | 15 | 282 | 564 | 141 | 2 | |||
| Event rate | 27.3 | 19.6 | 11.2 | 4.3 | 0.7 | |||
| Model 1 | Hazard ratio | 1.00 (ref) | 0.62 | 0.33 | 0.1 2 | 0.02 | ||
| 95% confidence interval | (0.37–1.04) | (0.20–0.54) | (0.07–0.20) | (0.004–0.08) | ||||
| P value | 0.7 | <0.001 | <0.001 | <0.001 | ||||
| Model 2 | Hazard ratio | 1.00 (ref) | 0.65 | 0.42 | 0.24 | 0.07 | ||
| 95% confidence interval | (0.39–1.09) | (0.25–0.70) | (0.14–0.41) | (0.02–0.29) | ||||
| P value | 0.1 | 0.001 | <0.001 | <0.001 | ||||
| Cerebrovascular disease | ||||||||
| Events | 5 | 38 | 103 | 24 | 1 | |||
| Event rate | 9.1 | 2.6 | 2.1 | 0.7 | 0.4 | |||
| Model 1 | Hazard ratio | 1.00 (ref) | 0.26 | 0.19 | 0.06 | 0.03 | ||
| 95% confidence i nterval | (0.10–0.65) | (0.08–0.46) | (0.02–0.17) | (0.004–0.26) | ||||
| P value | 0.004 | <0.001 | <0.001 | <0.001 | ||||
| Model 2 | Hazard ratio | 1.00 (ref) | 0.28 | 0.26 | 0.16 | 0.16 | ||
| 95% confidence i nterval | (0.11–0.70) | (0.11–0.63) | (0.06–0.42) | (0.02–1.37) | ||||
| P value | 0.007 | 0.003 | <0.001 | 0.09 | ||||
| Cardiovascular disease | ||||||||
| Events | 20 | 303 | 638 | 161 | 3 | |||
| Event rate | 36.4 | 21 | 12.7 | 4.9 | 1.1 | |||
| Model 1 | Hazard ratio | 1.00 (ref) | 0.47 | 0.26 | 0.09 | 0.020 | ||
| 95% confidence interval | (0.30–0.73) | (0.17–0.40) | (0.06–0.15) | (0.006–0.067) | ||||
| P value | 0.001 | <0.001 | <0.001 | <0.001 | ||||
| Model 2 | Hazard ratio | 1.00 (ref) | 0.48 | 0.33 | 0.19 | 0.07 | ||
| 95% confidence interval | (0.31–0.76) | (0.21–0.52) | (0.12–0.30) | (0.02–0.23) | ||||
| p-value | 0.002 | <0.001 | <0.001 | <0.0 | ||||
Model 1 unadjusted; Model 2 adjusted for sex and age
Figure 1.
A complete dataset available based on six AHA health metrics, excluding HbA1c, comprised 21,856 people. Baseline characteristics did not show any clinically relevant differences between the study populations comprising 10,043 and 21,856 people (Supplementary tables 1 and 2). The associations between the individual health metrics and CVD risk and the associations between the overall CD and risk of CVD events in the extended data set are presented in Supplementary tables 3 and 4.
Discussion
Our analysis in apparently healthy participants of the EPIC-Norfolk prospective population study shows that the prevalence of ideal cardiovascular health was low. All AHA-defined health metrics, except healthy diet, were significantly and inversely associated with the risk of CHD, stroke, and CVD events. The room for improvement in these modifiable risk factors is very large, which is in support of the approach selected by the AHA.
In the EPIC-Norfolk cohort, the association between health behaviours and overall mortality was previously addressed.(12) Non-smoking, physical activity, moderate alcohol intake and plasma vitamin C as a proxy for fruit and vegetable intake, were associated with a four-fold difference in total mortality, particularly from cardiovascular causes. In the current analysis we used the seven AHA-defined health metrics, which contains a slightly different set of modifiable risk factors, also comprising non-behavioural risk factors such as cholesterol and blood pressure. We observed a 93% lower risk of CVD events (HR 0.07; 95% CI 0.02-0.23) among people with the highest overall CHS (≥ 12 points) compared to those with the lowest score (≤ 2 points). Our findings from the EPIC-Norfolk cohort are consistent with previous validation studies performed in the Atherosclerosis Risk in Communities (ARIC) Study and the National Health and Nutrition Examination Survey (NHANES).(6, 13) In ARIC, Folsom et al. studied the AHA-defined health metrics among 12,744 healthy participants, aged 45 to 64 years and 0.1% had an ideal CHS, compared to 2.8% in the current study.(6) In NHANES, Ford et al. showed that only 1.1% met all seven health metrics. Compared to those meeting none of the health metrics, those meeting ≥ 5 health metrics had 88% reduction in the risk of cardiovascular mortality.(13) A similar trend was observed by Wu et al. in a large cohort of 101,510 apparently healthy Chinese, where 0.1% met all seven health metrics.(14) They observed similar associations between health metrics and the risk of CVD events.
Current strategies aimed at improving guidelines adherence in cardiovascular prevention still has room for improvement in the organization and there should be more focus on high risk patients (15). The AHA health metrics provides some relevant lifestyle goals in order to lower the risk of CVD and these lifestyle goals might be applied to high risk individuals as well.
Limitations
This cohort study has some limitations in the assessment of the health metrics. First, the level of physical activity was assessed by a questionnaire, which was validated against energy expenditure.(16) Nevertheless, the questionnaire referred to the past year, whereas physical activity levels may have changed over time. Second, the HDS was based on five dietary components that were quantified by FFQ. The FFQ is designed to estimate intake of foods and nutrients in the past year, which may also change over time. In addition, FFQ relies on self-reported intakes, which carry an inherent degree of inaccuracy. Also, as the AHA-defined healthy diet parameters used absolute cut-offs, we used FFQ derived absolute estimates of dietary intake. However, FFQ should ideally be used only for relative ranking of participants within cohorts. More detailed and complex instruments for assessing dietary intake are available (17), but the FFQ is commonly used because it is a feasible method for large-scale studies.
Since the EPIC-Norfolk study participants were recruited from age-sex registries from general practice, there might be potentially selection bias. However, the current analysis is based on an apparently healthy population in a very large cohort which is observed for a long time period which forms a strength of the study. Potential measurement bias was also reduced by standardized measurements of the study parameters which were assessed and conducted by trained nurses.
Our main analyses were based on a study population defined by the availability of all 7 AHA health metrics including HbA1c. In this dataset of 10,043, we did not observe an association between a healthy diet and the risk of CHD, stroke or CVD. However, when we performed a sensitivity analyses without taking HbA1c into account, the study population increased to 21,856. In this larger study population, healthy diet was significantly associated with the risk of CVD.
Conclusion
Our findings in the EPIC-Norfolk population support a strong inverse association between six of the seven AHA-defined health metrics and the risk of CVD events in this European population, and support the current AHA health metrics strategy for prevention of cardiovascular disease. Importantly, even a moderately unhealthy lifestyle was associated with a significantly lower risk of CVD events compared to those with a very unhealthy lifestyle. These data suggest that even small improvements may result in a substantial reduction of the risk of CVD events.
Supplementary Material
Acknowledgements
The authors wish to thank the participants and staff of the EPIC-Norfolk prospective population study. The EPIC-Norfolk Study is funded by Cancer Research UK grant number 14136 and the Medical Research Council grant number G1000143, SL is supported by a studentship from Unilever Corporate Research, UK. The funders had no role in the study design, data collection, analysis, decision to publish or preparation of the manuscript.
Funding sources
Cancer Research UK grant number 14136 and the Medical Research Council grant number G1000143.
Footnotes
Disclosures
None
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 3.Iestra JA, Kromhout D, van der Schouw YT, Grobbee DE, Boshuizen HC, van Staveren WA. Effect size estimates of lifestyle and dietary changes on all-cause mortality in coronary artery disease patients: a systematic review. Circulation. 2005;112(6):924–34. doi: 10.1161/CIRCULATIONAHA.104.503995. [DOI] [PubMed] [Google Scholar]
- 4.Eckel RH, Jakicic JM, Ard JD, Hubbard VS, de Jesus JM, Lee IM, et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 [Google Scholar]
- 5.Perk J, De BG, Gohlke H, Graham I, Reiner Z, Verschuren WM, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Atherosclerosis. 2012;223(1):1–68. doi: 10.1016/j.atherosclerosis.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. JAmCollCardiol. 2011;57(16):1690–6. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotseva K, Wood D, De BG, De BD, Pyorala K, Keil U. Cardiovascular prevention guidelines in daily practice: a comparison of EUROASPIRE I, II, and III surveys in eight European countries. Lancet. 2009;373(9667):929–40. doi: 10.1016/S0140-6736(09)60330-5. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van HL, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 9.Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A, et al. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. BrJCancer. 1999;80(Suppl 1):95–103. [PubMed] [Google Scholar]
- 10.Bingham SA, Welch AA, McTaggart A, Mulligan AA, Runswick SA, Luben R, et al. Nutritional methods in the European Prospective Investigation of Cancer in Norfolk. Public Health Nutr. 2001;4(3):847–58. doi: 10.1079/phn2000102. [DOI] [PubMed] [Google Scholar]
- 11.Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public health nutrition. 2003;6(4):407–13. doi: 10.1079/PHN2002439. [DOI] [PubMed] [Google Scholar]
- 12.Khaw KT, Wareham N, Bingham S, Welch A, Luben R, Day N. Combined impact of health behaviours and mortality in men and women: the EPIC-Norfolk prospective population study. PLoSMed. 2008;5(1):e12. doi: 10.1371/journal.pmed.0050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125(8):987–95. doi: 10.1161/CIRCULATIONAHA.111.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu S, Huang Z, Yang X, Zhou Y, Wang A, Chen L, et al. Prevalence of ideal cardiovascular health and its relationship with the 4-year cardiovascular events in a northern Chinese industrial city. CircCardiovascQualOutcomes. 2012;5(4):487–93. doi: 10.1161/CIRCOUTCOMES.111.963694. [DOI] [PubMed] [Google Scholar]
- 15.Ludt S, Wensing M, Campbell SM, Ose D, van Lieshout J, Rochon J, et al. The challenge of cardiovascular prevention in primary care: implications of a European observational study in 8928 patients at different risk levels. European journal of preventive cardiology. 2014;21(2):203–13. doi: 10.1177/2047487312462798. [DOI] [PubMed] [Google Scholar]
- 16.Espana-Romero V, Golubic R, Martin KR, Hardy R, Ekelund U, Kuh D, et al. Comparison of the EPIC Physical Activity Questionnaire with combined heart rate and movement sensing in a nationally representative sample of older British adults. PloS one. 2014;9(2):e87085. doi: 10.1371/journal.pone.0087085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knoops KT, Groot de LC, Fidanza F, Alberti-Fidanza A, Kromhout D, van Staveren WA. Comparison of three different dietary scores in relation to 10-year mortality in elderly European subjects: the HALE project. EurJClinNutr. 2006;60(6):746–55. doi: 10.1038/sj.ejcn.1602378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.