Graphical Abstract
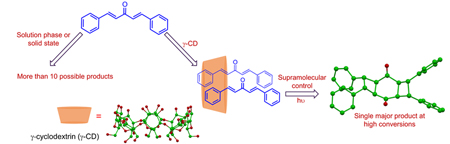
Photoexcitation of the dibenzalacetones (1a-d) in homogenous media and solid state yields a mixture of products with poor conversions. Irradiation of the reactants complexed to γ-cyclodextrin affords a single dimer (syn adduct 6) predominantly despite possibility for several monomeric and dimeric products. High selectivity in the cavitand-mediated reaction along with structural characterization of the inclusion complex provides insight into the supramolecular interactions that drive the self-assembly of the host-guest system.
Photocycloaddition [2+2] is one of the most studied and practically useful chemical reactions.1 Its utility in applied fields such as photopolymerization,2, 3 material sciences,4–6 photolithography,7, 8 organic synthesis,9, 10 and medicinal chemistry11, 12 is immense. Product of [2+2] photocycloaddition (PCA), the tetrasubstituted cyclobutene core, is part of several natural compounds.13, 14 Molecular memory aspect of PCA – product structure corresponding to reactants’ orientation – has been of great value to supramolecular chemists in predicting relative-orientation of molecules, and deducing strengths and directionality of supramolecular interactions.15 Two main approaches, host-guest chemistry16 and crystal engineering,17, 18 have been pursued by researchers to improve reaction efficiency. Generality and reliability of the host-guest approach have been employed by several groups to affect stereo- and regioselective PCA; this approach (cavitand-mediated PCA) takes advantage of spatial restriction imposed by a macrocyclic host to pre-dispose encapsulated reactants to improve reaction efficiency and selectivity.16, 19 However, such works has been limited to short mono alkenes only; PCA of cinnamic acids,20, 21 coumarins,22, 23 1,2-diaryl ethylenes,24, 25 acenaphthylenes,26 and styryl ketones have been explored. There have been no examples of such studies in cavitand-mediated PCA with high degree of reaction selectivity, which is much needed for realizing the applied potential of PCA. Herein we report successful PCA of a diene system, dibenzalacetones (symmetric chalcones), with high degree of product selectivity by confining and pre-orienting the reactants within the macrocyclic host γ-cyclodextrin (γ-CD). While several products are possible and observed in solution and solid-state reactivity, encapsulation of the reactants within γ-CD affords a single dimer as the predominant product in high yields. Time-dependent photoreactivity, thermal profile of host-guest complexation, and computational analysis of the complex structures provide insight into the nature of weak interactions that govern self-assembly. In addition to fundamental and applied photochemistry significance, the work presented herein is expected to be of value in: (a) synthetic organic chemistry as a reliable method for producing rigid and stereospecific tricyclic framework, and (b) in medicinal chemistry due to structural resemblance of reactants to curcumin (antineoplastic agent), and that of the product to phytochemicals such as incarvillateine,27pipercyclobutanamide A,28 and others.13, 29 In addition, this work also reports ITC and computational chemistry study of ternary complex of cyclodextrin, which have very limited precedence in host-guest chemistry literature.
Photochemical excitation of chalcone 1a (1,3-diphenyl-1,4-pentadienone) in homogeneous solutions results in a reaction mixture that contains at least six products as observed by NMR spectroscopy. Considering the two reactive sites in the alkene and based on unimolecular and bimolecular reaction pathways at least eight products from primary reaction, and additional products from subsequent reactivity of photoproducts is predicted (ESI, Scheme S1). Considering the cross-conjugation between the two alkenes through the carbonyl group, it is expected that a single-photon excitation would affect the excited state reactivity at both alkenes in the substrate, similar to the PCA of aromatic 1,4-butadienes. Irradiation of homogeneous solution of 1a resulted in formation of isomerization products and no photodimerization (Table 1). Solid-state reaction of compound recrystallized from ethyl acetate yielded a mixture of isomerization and several photodimerization products with no selectivity for any specific product at low conversion despite prolonged irradiation; 1H NMR signal suggests presence of polymeric/oligomeric products as well as indicated by broad 1H NMR signals, which could result from multiple end-to-end alkene PCA. The number of photoproducts in the reaction mixture obtained denotes high degree of molecular freedom available to the reactants and hence the need for proportionately high degree of reaction control necessary if reaction selectivity is to be realized.
Table 1.
Photoproducts from irradiation of 1a in different media
| Medium | H:G equiv. | % 6a | % conv. | Time (h) |
|---|---|---|---|---|
| Solution | - | - | 65 | 12 |
| Solid state | - | 8 | 24 | 48 |
| γ-CD slurry | 1:2 | 72 | 90 | 36 |
| 1:1 (or 2:2) | 78 | 90 | ||
| 1:4 | 42 | 71 |
(i) Average of three independent experiments. (ii) The remaining percent corresponds to sum of all other products. (iii) percentages are based on relative proportion of 1H NMR signals of 6 cyclobutene signals with respect to characteristic signal of other compounds.
Cyclodextrins are a family of water-soluble macrocyclic cavitands with hydrophobic cavities that can encapsulate water-insoluble organic compounds. Propensity of the cavitands to include organic compounds in aqueous media, driven by hydrophobic forces, has been utilized to manipulate physicochemical properties of the compound.30 The larger and less-studied member of the cyclodextrin host family, γ-cyclodextrin (γ-CD, figure 1), is known for its ability to form ternary (1:2 or 1:1:1) host-guest complexes because of its wide cavity.
Figure 1.
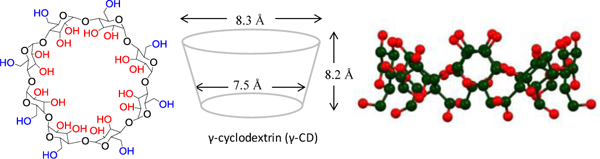
(Left) Chemical structure of γ-cyclodextrin (γ-CD). (Middle) The dimensions of the cavitand is conducive for forming ternary (one host two guests) complexes. (Right) Ball and stick representation of the cavitand.
We envisioned that the cavity of macrocyclic cavitand, which has been previously demonstrated to efficiently direct photodimerization of a wide range of mono-alkenes,21, 23–25 can direct the photoreaction of extended alkenes as well. Aqueous solution of one equivalent of γ-CD stirred with two equivalents of powdered chalcone 1a forms a chalky precipitate over few hours indicating complexation, which was washed with cold water and methanol to removed uncomplexed components. Irradiation of 1a2@γ-CD over 36 hrs results in more than 85% conversion and affords more than 70% of a single dimeric product (6a) as indicated by presence of quasi-doublets in 1H NMR spectrum characteristic of the diphenyldicarboxycyclobutane signals. Considering the presence of two reactive sites and extended length of the guest, the observed selectivity was intriguing. Selectivity in favour on one product over several isomeric and monomeric products suggests a high degree of self-organization in the complex.
As the reactant alkene is an extended guest that is longer than the controlling (encapsulating) host, it was expected that two hosts might lead to a more stable complex and higher degree of selectivity. Reaction with one equivalent of host and guest (1:1) was performed under similar condition. However, only a slight increase in selectivity of the predominant dimer (by 6%) was observed suggesting that 1:2 host:guest stoichiometry exerts a high degree of selectivity already. It is also curious to note that despite the opportunity to form an isolated 1:1 complex, which would predominantly yield isomerization product(s), the same dimeric product 6a was observed. This suggests that a ‘double occupancy’ complex structure is preferred over single occupancy: i.e. either a 1:2 host:guest complex (with uncomplexed excess host) or a 2:2 complex is preferred over a 1:1 complex. To distinguish between the two possibilities (1:2 vs 2:2) further experiments were performed to ascertain the more stable complex stoichiometry (Job’s plot and ITC vide infra). On the other hand, less than half equivalent of host (1:4 H:G) stoichiometry yielded significantly lesser amount of the dimer 6a with increase in proportion of other products, as might be predicted. Monitoring reaction over time showed steady increase in proportion of all products over time without significant variation in their relative proportions (ESI, Figure S10). This suggests that γ-CD acts as a reaction mediator that yields dimer 6a in a single step, as opposed to a supramolecular catalyst that converts trans- or cis- monomers through a catalytic cycle through a secondary reaction sequence.31
Despite several possible dimeric products from photochemistry of chalcone 1a (scheme 1), a single product resulted when irradiated in presence of γ-CD. As unambiguous resolution of product stereochemistry through spectroscopic methods would be challenging due to lack of literature precedence for the structure and the closely-related NMR of the possible dimers. Hence X-ray crystallography of the product were sought for characterization. Major product isolated through preparative-HPLC was verified for purity through NMR spectroscopy and single crystals of the compound was obtained from a solution of ethyl acetate. The product structure was that of the fully dimerized syn photoadduct 6a (figure 2).
Scheme 1.
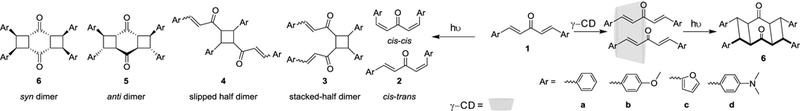
Structure of symmetric chalcones used in this study, and possible and observed photoproducts in the experiments.
Figure 2.

Crystal structures of 6a and 6c, major products from γ-cyclodextrin-mediated photodimerization of the respective chalcones. Hydrogens have been omitted for clarity.
Intrigued by the remarkable selectivity in the reaction, we tested the generality of this approach in mediating dimerization of three more reactants: 4-methoxy chalcone (1b), 2-furyl chalcone (1c), and 4-N,N-dimethyl chalcone (1d). In case of 1a and 1b the reactants yielded one dimer as the major product respectively (higher than 75%) with trace amounts of alkene products. The products in both reactions were isolated using preparative HPLC and characterized through 1H and 13C NMR spectroscopy. The spectral characteristics of the compounds 6b and 6c were similar to that of 6a. We were able to grow single crystals of dimer from 1c (the furyl dimer, from ethyl acetate), which revealed the structure of the dimer to be the syn adduct 6c as well.
While we were not able to obtain single crystal for 6b (the 4-methoxy dimer), the known structure of 6a established using crystallography and spectral characteristics of were used to deduce the structure of dimer from 6b. NOE spectrum of 6b (figure 3) showed intramolecular through-space correlations between cylobutane protons and the downfield (ortho-) aromatic protons, which were identical to that observed for 6a. Similarly, the 1H NMR signals of the characteristic cyclobutane signals of 6b were similar in shape (quazi doublets) to that of 6a (ESI), and the chemical shifts of the cyclobutene of 6b signals appeared upfield by 0.15 ppm as would be expected based on the electron-donating methoxy groups. This confirmed the syn stacked geometry of all three dimers. However, in case of chalcone 1d no dimers formed. However, significant degree of photoisomerization was observed, which implies that the substrate is photoactive and that the lack of dimerization is due to due to 1:1 stoichiometry. Job’s plot of 1d with γ-CD performed at 5 × 10−4 M yielded maxima at around 0.5 mol fraction of the host, reinforces the inference that lack of dimerization is due to complex stoichiometry.
Figure 3.

NOESY spectrum for 4-methoxy chalcone dimer showing signals corresponding to the proposed structure 6b.
Structure of the major product obtained in the reactions possess three common spatial features that allude to the inclusion complex structure: (a) extent of dimerization (partial vs. complete dimerization), (b) slipped vs. end-to-end stacking, and (c) syn vs. anti stereoselectivity. Dimerization of both alkene bonds (factor a) in the molecule suggests that the confining effect of γ-CD is exerted over the entire length of the alkene as opposed to just one (aromatic) end. The high degree of selectivity was achieved with just half equivalent of the host and there was no significant difference in selectivity between 1:1 and 1:2 (host:guest). This suggests that despite the opportunity to form a 2:2 complex with slipped arrangement, the chalcones self-assemble in a compact stacking arrangement. This can be deduced to be the result of stabilizing π-π stacking interaction (factor b). Finally, the syn geometry indicates the effect of confinement restricting product formation to the more compact structure over the spatially demanding anti geometry.
Product selectivity, time-dependence, guest-variation, and stoichiometry studies afforded specific insights regarding the inclusion complex structure and non-covalent interactions in it. To further understand the characteristics of the complex ITC, Job’s plot, and ab-initio computational studies were undertaken. ITC of 0.5 mM host in 100 μL syringe added to 1000 μL 0.05mM guest showed exothermic events for each addition of titrant to the guest throughout the experiment (figure 4). Isotherm of heat evolved per injection (after background subtraction) vs. moles of injected host was subject to various binding models available in Nanoanalyze® (TA instruments nano ITC) yielded the best fit for independent binding model. The n value of 0.525 suggested that the binding stoichiometry of the host-guest in solution phase is 1:2, which is consistent with that deduced from slurry experiments. While this finding was not surprising, it is curious to note the correlation between the complexes in two different phases; there were no significant differences in product selectivity between 1:2 and 1:1 (or 2:2) equivalents suggesting that one γ-CD is capable of encapsulating two guests. Consistent with the exothermic binding events driven by hydrophobic forces and the loss of entropy due to complexation, ΔH value of −65.4 kj/mol, and ΔS value of −0.128 kJ/mol were obtained from the fit. The binding constant for the complexation processes is in the order of 105 M−1, which indicates moderate binding strength. The binding stoichiometry was further corroborated by Job’s plot continuous variation method. Plot of ΔP vs. Xγ-CD showed highest variation in observable property (absorbance) at 0.38 (ESI). This corresponds to a host-guest ratio that is between a stoichiometry of 1:1 and 1:2
Figure 4.

Isothermal titration calorimetry of aqueous 5×10−4 M γ-CD (syringe) titrated into an aqueous solution of 5 × 10−5 M 1b (well) at 25 Cο.
It must be noted that there were two inflection points in the isotherm that slightly deviate from the simple independent binding model. The inflection points in the isotherm might be attributed to of step-wise (1:1 followed by 1:2) binding event, whereas the independent binding model presumes a completely one-step binding event. However, the binding isotherm is well-represented by the chosen model and both a step-wise and one-step model present the same binding stoichiometry and overall binding constant. We also tried to fit the ITC data to a multiple site binding algorithm available in NanoAnalyze®, representing a step-wise binding model to obtain individual binding constants for each step in which an empty γ-CD and a singly occupied γ-CD (binary complex) being the two different binding sites. However, the fitting parameters in multiple-site binding model was still predominantly represented by the second binding even than the first one, essentially resulting in a dominant one-step binding equilibrium. Preference for 1:2 (host:guest) ternary complex over 1:1 binary complex despite presence of excess host is a supramolecular characteristic of γ-CD complexes that we had observed in our earlier work.21 It is also important to note that surprisingly there is no notable literature precedence for 1:2 (H:G) complex that has been studied using ITC. This study is expected to spur interest in thermodynamic study of ternary complexes formed by γ-CD.
Computational chemistry analysis of 1a2@γCD complex was performed to gain perspective of complex structure and interplay of weak forces (figure 5). In addition to the strong dipole-dipole interaction between the components, influence of π-π interaction was presumed. Hence, this required use of higher level theory with that would account for dispersive supramolecular interactions. Considering the large complex structure and the lack of π-electrons in γ-CD, two-layer ONIOM calculation was performed in which the lower level basis set (SE-PM6) was applied to the host, and higher-level theory (wB97XD/631++G(2d,2p))32 was applied to the guests. Geometry-minimized structure of the complex in the gas phase showed that both guests were well-encapsulated by the cavitand. In addition, a well-stacked arrangement of the two guests was observed in which the aromatic rings of the guests were in close proximity suggesting operation of potential π-π interaction stabilizing the complex structure. This also explains the inability of the guest to form a 2:2 complex: the pi-stacked structure (involving both rings) is not long-enough to bind to two γ-CD simultaneously, while a slipped arrangement that would enable binding to two γ-CD would involve loss of stabilization from one π-stacking interaction. Computational analysis of the complex structure reinforces the empirical inferences.
Figure 5.

Geometry-optimized structure in the gas phase obtained using Gaussian ‘09 software for 1a2@γ-CD using two level ONIOM (wB97XD/631++G(2d,2p)/Auto:PM6) calculation. Hydrogens have been omitted for clarity.
Conclusions
The 2+2 photocycloaddition of dibenzalacetones was affected stereo- and regioselectively using the macrocyclic cavitand γ-CD. The high degree of reaction control achieved in the reaction for a substrate that produces multitude of products provides insight into the nature of supramolecular interactions sustaining the host-guest complex. Product structures were ascertained using x-ray crystallography and NOESY spectroscopy. Understanding the supramolecular aspects of this cavitand-mediated reaction involved calorimetry and computational chemistry experiments, which are less explored for ternary complexes in cyclodextrin literature. This method can serve as a synthetic tool for producing stereospecific tricyclic structure. The efficient photodimerization with high degree of selectivity should attract interest of supramolecular photochemists to explore diene reactions analogous to those which have been explored for mono-alkenes till date. We anticipate that this will renew interest in cyclodextrin as an efficient container for controlling molecular behaviour in general.
Supplementary Material
Table 2.
Photoproducts from irradiation of 2a–4a in different media
| Medium | H:G equiv. | % 6 | % Conv. | Time (h) |
|---|---|---|---|---|
| 1b | ||||
| Solid state | - | - | 15 | 50 |
| γ-CD slurry | 1:2 | 78 | >90 | 40 |
| 1:1 (or 2:2) | 81 | >90 | ||
| 1c | ||||
| Solid state | - | 8 | 25 | |
| γ-CD slurry | 1:1 (or 2:2) | 87 | >90 | 36 |
| 1d | ||||
| γ-CD slurry | 1:2 | - | 50 | 16 |
| Cucurbit[8]uril | 1:2 | - | 100 | |
(i) Average of three independent experiments. (ii) The remaining percent corresponds to sum of all other products. (iii) percentages are based on relative proportion of 1H NMR signals of 6 cyclobutene signals with respect to characteristic signal of other compounds.
Acknowledgements
This research was supported by ACS-Petroleum Research Fund (#54862-UR4) and NIGMS (1U54GM115458–01). MP thanks Dr. Richard Mocarski (Director of Office for Sponsored Programs) for his support and encouragement.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Notes and references
- 1.Poplata S, Troester A, Zou Y-Q and Bach T, Chem. Rev. (Washington, DC, U. S.), 2016, 116, 9748–9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medishetty R, Park I-H, Lee SS and Vittal JJ, Chem. Commun. (Cambridge, U. K.), 2016, 52, 3989–4001. [DOI] [PubMed] [Google Scholar]
- 3.Hasegawa M, Adv. Phys. Org. Chem, 1995, 30, 117–171. [Google Scholar]
- 4.Truong VX, Li F, Ercole F and Forsythe JS, ACS Macro Lett, 2018, 7, 464–469. [DOI] [PubMed] [Google Scholar]
- 5.Ramanujam PS, Lohse B and Berg RH, Proc. SPIE-Int. Soc. Opt. Eng, 2004, 5351, 144–149. [Google Scholar]
- 6.Trouts TD, Tyson DS, Pohl R, Kozlov DV, Waldron AG and Castellano FN, Adv. Funct. Mater, 2003, 13, 398–402. [Google Scholar]
- 7.Ghorai S, Sumrak JC, Hutchins KM, Bucar D-K, Tivanski AV and MacGillivray LR, Chem. Sci, 2013, 4, 4304–4308. [Google Scholar]
- 8.Ye X, Jiang X, Yu B, Yin J and Vana P, Biomacromolecules, 2012, 13, 535–541. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann N, Chem. Rev. (Washington, DC, U. S.), 2008, 108, 1052–1103. [DOI] [PubMed] [Google Scholar]
- 10.Lee-Ruff E and Mladenova G, Chem. Rev. (Washington, DC, U. S.), 2003, 103, 1449–1483. [DOI] [PubMed] [Google Scholar]
- 11.Priebe A, Hunke M, Tonello R, Sonawane Y, Berta T, Natarajan A, Bhuvanesh N, Pattabiraman M and Chandra S, J Pain Res, 2018, 11, 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunke M, Martinez W, Kashyap A, Bokoskie T, Pattabiraman M and Chandra S, Anticancer Res, 2018, 38, 4469–4474. [DOI] [PubMed] [Google Scholar]
- 13.Dembitsky VM, Phytomedicine, 2014, 21, 1559–1581. [DOI] [PubMed] [Google Scholar]
- 14.Sergeiko A, Poroikov VV, Hanus LO and Dembitsky VM, Open Med Chem J, 2008, 2, 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonoda Y, Molecules, 2010, 16, 119–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pattabiraman M, Sivaguru J and Ramamurthy V, Isr. J. Chem, 2018, 58, 264–275. [Google Scholar]
- 17.T. Friscic, D. B. Varshney, E. Elacqua, J. C. Sumrak, A. N. Sokolov and L. R. MacGillivray, 2013.
- 18.Yang S-Y, Naumov P and Fukuzumi S, J. Am. Chem. Soc, 2009, 131, 7247–7249. [DOI] [PubMed] [Google Scholar]
- 19.Ramamurthy V and Parthasarathy A, Isr. J. Chem, 2011, 51, 817–829. [Google Scholar]
- 20.Pattabiraman M, Natarajan A, Kaanumalle LS and Ramamurthy V, Org. Lett, 2005, 7, 529–532. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen N, Clements AR and Pattabiraman M, New J. Chem, 2016, 40, 2433–2443. [Google Scholar]
- 22.Zhang Q, Qu D-H, Wu J, Ma X, Wang Q and Tian H, Langmuir, 2013, 29, 5345–5350. [DOI] [PubMed] [Google Scholar]
- 23.Moorthy JN, Venkatesan K and Weiss RG, J. Org. Chem, 1992, 57, 3292–3297. [Google Scholar]
- 24.Rao KSSP, Hubig SM, Moorthy JN and Kochi JK, J. Org. Chem, 1999, 64, 8098–8104. [DOI] [PubMed] [Google Scholar]
- 25.Kaliappan R, Maddipatla MVSN, Kaanumalle LS and Ramamurthy V, Photochem. Photobiol. Sci, 2007, 6, 737–740. [DOI] [PubMed] [Google Scholar]
- 26.Wang HM and Wenz G, Beilstein J Org Chem, 2013, 9, 1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang ML, Yu G, Yi SP, Zhang FY, Wang ZT, Huang B, Su RB, Jia YX and Gong ZH, Sci Rep, 2015, 5, 16107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu R, Zhang M, Wyche TP, Winston-McPherson GN, Bugni TS and Tang W, Angew. Chem., Int. Ed, 2012, 51, 7503–7506, S7503/7501-S7503/7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dembitsky VM, J Nat Med, 2008, 62, 1–33. [DOI] [PubMed] [Google Scholar]
- 30.Chen G and Jiang M, Chem Soc Rev, 2011, 40, 2254–2266. [DOI] [PubMed] [Google Scholar]
- 31.Kang Y, Tang X, Yu H, Cai Z, Huang Z, Wang D, Xu JF and Zhang X, Chem Sci, 2017, 8, 8357–8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Costa LM, Stoyanov SR, Gusarov S, Tan X, Gray MR, Stryker JM, Tykwinski R, J. W. d. M. Carneiro, P. R. Seidl and A. Kovalenko, Energy Fuels, 2012, 26, 2727–2735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


