Abstract
Optic nerve astrocytes play a major role in axonal degeneration and regeneration. Astrocyte lines are an important tool to elucidate the responsible cellular mechanisms. In this study, we established a conditionally immortalized mouse optic nerve astrocyte line. Astrocytes were cultured from explants derived from postnatal day 5–7 H-2kb-tsA58 transgenic mouse optic nerves. Cells were cultured in defined astrocyte culture medium under permissive (33°C) or non-permissive (38.5°C) temperature with or without interferon-v (IFN-ɤ). Astrocytes were characterized by immunocytochemistry staining using antibodies against glial fibrillary acidic protein (GFAP) and neural cell adhesion molecule (NCAM). Cell proliferation rates were determined by cell growth curves and percentage of Ki67 positive cells. Karyotyping was performed to validate the mouse origin of established cell line. Conditional immortalization was assessed by western blot-determined expression levels of SV40 large T antigen (TAg), p53, GFAP and NCAM in non-permissive culture conditions. In addition, phagocytic activity of immortalized cells was determined by flow cytometry-based pHrodo florescence analysis. After 5 days in culture, cells migrated out from optic nerve explants. Immunocytochemistry staining showed that migrating cells expressed astrocyte makers, GFAP and NCAM. In permissive conditions, astrocytes had increased expression levels of TAg and p53, exhibited a greater cell proliferation rate as well as a higher percentage of Ki67 positive cells (n=3, p<0.05) compared to cells cultured in non-permissive conditions. One cell line (ImBlON) was further maintained through 60 generations. Karyotyping showed that ImB1ON was of mouse origin. Flow cytometry-based pHrodo florescence analysis demonstrated phagocytic activity of ImBlON cells. Quantitative PCR showed mRNA expression of trophic factors. Non- permissive culture conditions decreased expression of TAg and p53 in ImB1ON, and increased the expression of NCAM. A conditionally immortalized mouse optic nerve astrocyte line was established. This cell line provides an important tool to study astrocyte biological processes.
Keywords: optic nerve astrocyte, cell line, conditional immortalization
Introduction
Astrocytes are the major supporting cells in the central nervous system (CNS). They perform a variety of functions including regulating brain vasculature system, providing metabolic support to neurons, and modulating synaptic activities. The optic nerve, an extension of the CNS, contains axons of retinal ganglion cells that transport visual signals from the retina to the visual centers in the brain. Optic nerve astrocytes play an important role in maintaining axonal physiological activities and responding to axonal injuries.
The functions of optic nerve astrocytes are still understudied. As an in vitro model, astrocytes derived from optic nerves are an important tool to study their molecular and cellular characteristics. Primary optic nerve cells are more biologically relevant, but their study is limited by the lack of donor tissues, slow proliferation rate, and finite lifespan. Immortalized cell lines, in comparison, overcome these disadvantages, however, the number of available optic nerve astrocyte lines is limited.
Several strategies for immortalizing mammalian CNS cells have been established. One of the most commonly and successfully used methods is to introduce the simian virus 40 (SV40) T antigen into target primary cells. The large T antigen forms complexes with tumor suppressors, such as pRB-1 and p53, and induces DNA synthesis in both dividing and quiescent cells, leading to extension of lifespan of the cells [1, 2]. The SV40 T antigen transformation is usually mediated by retroviral vectors in primary cell cultures. Certain types of cells can be transfected by retroviral constructs consisting fragmented or mutated SV40 that led to immortalization. For cells difficult to culture and transfect in vitro, mice harboring SV40 transgene are useful. The expression of the immortalizing transgene in each cell of the body enables the establishment of cell lines from a variety of tissues.
The Immortomouse has been used to generate several conditionally immortalized cell lines. This transgenic mouse expresses tsA58, a SV40 temperature-sensitive (ts) mutation, driven by the mouse major histocompatibility complex (MHC) H-2kb class I promoter [3]. The transgene encodes a temperature liable large T antigen that immortalizes only at permissive temperature. The expression levels of the MHC promoter in cells derived from these transgenic mice can be induced by exposing cells to interferon Y (IFNɤ). The generation of this mouse strain has enabled the development of various conditionally immortalized cell lines [4–6].
In this study, we established and characterized a mouse optic nerve astrocyte line derived from H-2kb-tsA58 transgenic mice. Our results indicate that the cell line expresses major astrocytic markers, and exhibits astrocytic activity at non-permissive temperature. This cell line provides a useful tool to study CNS and specifically optic nerve astrocyte biological functions.
Materials and Methods
Optic nerve astrocyte culture
The H-2kb-tsA58 transgenic mice were obtained from Charles River Laboratories. Postnatal day 4–5 homozygous mice were used in this study. All animal experiments were performed in accordance with the National Institutes of Health guide for the care and use of Laboratory animals and the ARVO statement. All experimental protocols were approved by the Institutional Animal Care and Use Committee of University of North Texas Health Science Center. Optic nerves were dissected 2 mm behind the globe to the optic chiasm. Surrounding tissues and the sheath of the optic nerves were removed. Optic nerves were further cut into small explants and seeded into 60 cm cell culture dishes (BD Falcon, Franklin Lakes, NJ, USA). After 5–7 days in culture, cells migrating out of the explants were dispersed with TrypLE Express (Life Technologies, Grand Island, NY, USA) and subcultured in defined astrocyte culture medium (Am-a medium, Sciencell Research Laboratories, Carlsbad, CA, USA) at 37°C in a humidified atmosphere with 5% CO2. To study conditional immortalization, cells were further cultured under permissive (33°C) or non-permissive (37°C or 38.5°C) temperature with or without murine interferon-ɤ (IFN-ɤ) (Life Technologies) at a final concentration of 10 units/ml.
Cell growth curves
To determine the cell proliferation rate, 2 × 104 cells were plated per well of 6-well culture plates and grown for 6 days. Cell number in each well was determined daily using a hemocytometer (Fisher Scientific, Richardon, TX, USA). Two cell counts were taken/cell line/day.
Immunocytochemistry staining
For immunocytochemistry staining, cells were seeded onto glass coverslips (Fisher Scientific) and fixed at 4°C for 30 minutes with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) in phosphate-buffered- saline (PBS). After fixation, the coverslips were rinsed with PBS 3 times (5 minutes each) and pretreated with 0.5% Trixon-X (Acros Organics, NJ, USA) in PBS for 30 minutes at room temperature (RT). Following pretreatment, the coverslips were blocked with PBS-based SuperBlock (Thermo Scientific, Rockford, IL) for 2 hours at RT and incubated overnight at 4°C with primary antibodies listed in Table 1. After 3 rinses in PBS, coverslips were further incubated with Alexa488 or TRITC conjugated secondary antibodies (Life Technologies) against rabbit (for GFAP and ki67) or mouse (for NCAM) IgGs for 1 hour at RT. The coverslips were rinsed again, mounted in ProLong Gold anti-fade reagent with DAPI (Life Technologies). Non-primary control staining was performed using PBS instead of primary antibodies. Images were viewed and captured using a Nikon Ti-U Microscope containing the Nuance Multispectral imaging system (Nikon, Tokyo, Japan). For Ki67 positive cell counts, each coverslip was assessed at 9 locations (1 in the center, 2 from mid peripheral and peripheral regions of each coverslip) with approximately 200 cells counted per coverslip. Three coverslips were evaluated in a masked manner per culture condition.
Table 1.
List of antibodies
| Antigen | Host species | Supplier | Application | Dilution |
|---|---|---|---|---|
| GFAP | Rabbit | Abcam (Cambridge, MA) |
Immunocytochemistry staining | 1:300 |
| Western blotting | 1:5000 | |||
| NCAM | Mouse | Sigma (Saint Louis, MO) |
Immunocytochemistry staining | 1:100 |
| Western blotting | 1:500 | |||
| Ki67 | Rabbit | Abcam | Immunocytochemistry staining | 1:200 |
| P53 | Rabbit | Santa Cruz Biotechnology (Dallas, TX) |
Western blotting | 1:200 |
| TAg | Rabbit | Santa Cruz Biotechnology |
Western blotting | 1:200 |
| Actin | Mouse | Millipore (Burlington, MA) |
Western blotting | 1:10,000 |
Western blot analysis
Cells were collected using MPER lysis buffer (Fisher Scientific) with 1% protease inhibitor cocktail (Pierce Technology, Rockford, IL, USA). Proteins were denatured, separated by 10% SDS-PAGE and then electrophoretically transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked with blocking buffer (Licor Biosciences, Lincoln, NE, USA) for 2 hours at RT and then incubated overnight with primary antibodies listed in Table 1. After 3 rinses with TBST (10 minutes each), membranes were incubated with infrared dye conjugated secondary antibodies against rabbit or mouse (Licor Biosciences) for 1.5 hours at RT. After 3 rinses, membranes were scanned using Licor Odyssey Imager (Licor Biosciences).
Genotyping
Genotype of cultured cells was determined by polymerase chain reaction (PCR) as previously described [4]. Briefly, DNA was extracted from mouse ear punches and from cultured cells using the NucleoSpin Tissue Kit (Clontech, Mountain View, CA). The tsA58 transgene was amplified using sense and antisense primers (5’-CCATCTCCACAGTTTCACTTCTGCA-3’ and 5’-GCAGTACATTGCATCAACACCAGGA-3’), and GAPDH (sense: 5’-AGAACATCATCCCTGCATCC-3’, antisense: 5’-AGCCGTATTCATTGTCATACC-3’) was used as an endogenous control. The reaction conditions included an initial denaturation at 94 °C for 90 seconds, followed by 35 cycles of 94 °C for 30 seconds, 55 °C for 30 seconds, 72 °C for 1 minute, and a final extension at 72 °C for 5 minutes. Amplified products were electrophoresed in 2% agarose gels and stained with SYBR Safe DNA gel stain (Thermo Fisher) to confirm the expected size of the amplicons.
Karyotyping
Karyotyping was performed by KaryoLogic Inc. (Durham, NC, USA). Briefly, cells were treated in Am-a medium containing 0.45% Colcemid (Life Technologies) for 3 hours. After dissociation, standard G- banding karyotypic analysis was performed. Twenty metaphase spreads were examined using a Leica DM2500 microscope with Leica Biosystems CytoVision software (Leica, Buffalo Grove, IL). Images were taken at 1000× magnification.
Flow cytometry-based phagocytosis analysis
Synaptosomes were purified and conjugated as previously described [7–9]. Briefly, adult C57BL/6J mouse brain was dissected and the cortex isolated. Synaptosomes were purified from cortex homogenates using a discontinuous Percoll (GE Healthcare Life Sciences, Pittsburgh, PA, USA) gradient. Purified synaptosomes were further incubated with pHrodo red, succinimydyl ester (Life Technologies), in 0.1 M sodium carbonate (pH 9.0) at RT for 2 hours. After washing to remove unconjugated pHrodo, pHrodo-conjugated synaptosomes were re-suspended in Am-a medium. Astrocytes were incubated with pHrodo-conjugated synaptosomes for 24 hours. For flow cytometry analysis, cells were collected by trysinization and centrifugation, and re-suspended in Dulbecco’s PBS (Life technologies) containing 0.02% fetal bovine serum (FBS, Atlas Biologicals, Fort Collins, CO, USA). Live cells were analyzed using FC500 Flow Cytometer (Beckman Coulter Life Sciences, Indianapolis IN, USA) on the basis of pHrodo red fluorescence intensity.
Quantitative PCR
Total RNA was isolated using RNeasy mini Kit (Qiagen, Valencia CA, USA) and reverse transcription performed using the iScript™ cDNA synthesis kit (Bio-Rad Laboratories, Hercules CA, USA) following manufacturer’s instructions. The amount of total RNA used as the template was 500 ng. Gene specific primers (Table. 2) were designed using PrimerQuest Tool (Integrated DNA Technologies, Skokie IL, USA). Quantitative PCR was then performed in the BioRad CFX96 real time system (Bio-Rad Laboratories) using the SSoAdvanced™ SYBR Green master mix (Bio-Rad Laboratories). The cDNAs were amplified as follows: initial denaturation at 95°C for 3 minutes, 40 cycles of 95°C for 10 seconds, and 65°C for 30 seconds. Relative mRNA abundance was calculated by the ΔΔ cycle threshold (Ct) method. Gapdh was used as the endogenous control.
Table 2.
Primer sequences for qPCR analysis
| Gene name | Forward | Reverse |
|---|---|---|
| Bdnf | 5’-CTGGGTTAACTTTGGGAAATGC-3’ | 5’-CCTTCATGCAACCGAAGTATG-3’ |
| Ngf | 5’-CCCAATAAAGGTTTTGCCAAGG-3’ | 5’-TTGCTATCTGTGTACGGTTCTG-3’ |
| bFgf | 5’-TCT ACT G CAAG AACGGCG-3’ | 5’-CTCCCTTGATAGACACAACTCC-3’ |
| Gapdh | 5’-AGAACATCATCCCTG CATCC-3’ | 5’-AGCCGTATTCATTGTCATACC-3’ |
Data analysis
Statistical analysis was performed using Graphpad Prism 7 (GraphPad, Inc., La Jolla CA, USA). Unpaired Student t-test was used to analyze data from two experimental groups. One-way ANOVA followed by Tukey post-hoc test was performed to analyze intragroup differences. Data are presented as mean ± S.D., and a P<0.05 was considered statistically significant.
Results
Mouse optic nerve astrocyte culture
Astrocytes were cultured from explants obtained from postnatal day 4–5 H-2kb-tsA58 transgenic mouse optic nerves. After 5 days in culture, cells migrated out from the explants. To characterize the migrating cells, we performed immunocytochemistry staining, and all migrating cells were positive for GFAP, a specific astrocyte marker (Fig. 1).
Figure 1.
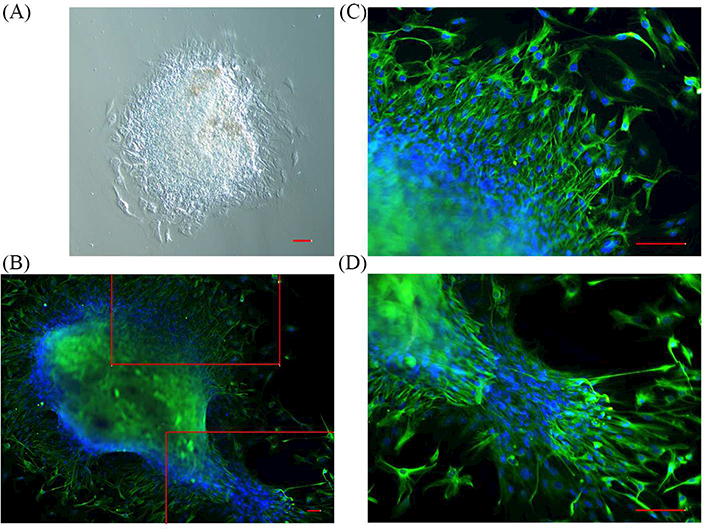
Mouse optic nerve astrocyte culture. (A) Phase-contrast imaging shows outgrowth of cells from an optic nerve explant dissected from an H-2kb-tsA58 transgenic mouse eye. (B-D) Immunocytochemistry staining shows the migrating cells were GFAP (green) positive. Nuclei were labeled with DAPI (blue). (C-D) Enlarged images of boxed areas in (B). Scale bars = 50 μm.
To optimize the immortalization conditions, we cultured the cells under permissive and non-permissive temperatures, with or without IFNɤ, and compared cell proliferation. Consistent with published literature [3], upon addition of IFNɤ to the cultures, astrocytes grown at 33oC showed the highest level of TAg expression (Fig. 2A & 2C). Astrocytes cultured under various conditions were further stained for the proliferation marker, ki67. Permissive conditions (33oC, with addition of IFNɤ) promoted cell proliferation, which correlated with the highest percentage (58 ± 2%) of cells expressing Ki67 (Fig. 2A & 2B).
Figure 2.
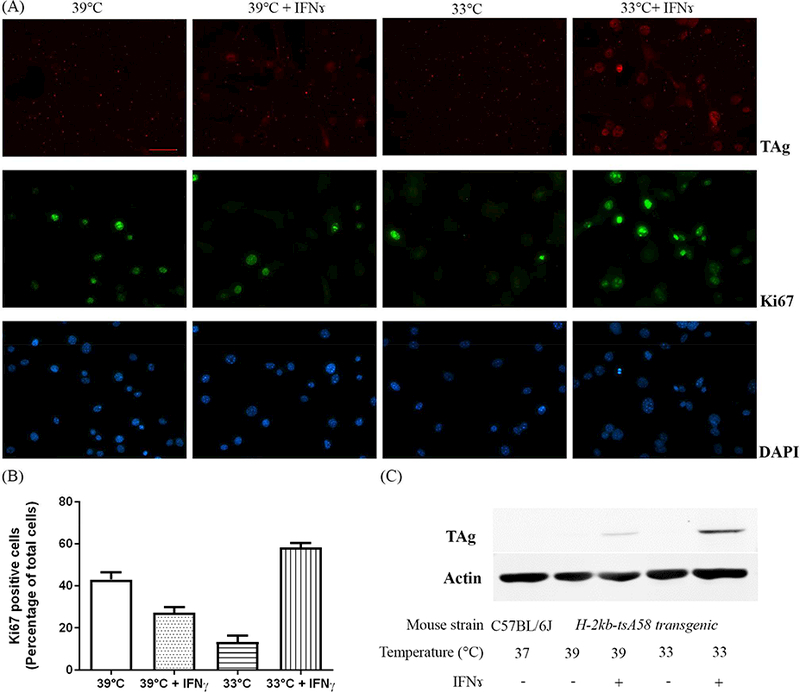
Permissive culture conditions promote cell proliferation. (A) Immunocytochemistry staining shows differentially expressed Ki67 (green) or TAg (red) levels in cells cultured under various conditions. Nuclei were labeled with DAPI (blue). Scale bars = 50 μm. (B) Quantification of percentage of Ki67 positive cells under various conditions. Data are presented as mean ± S.D., p<0.01 between each of two groups. (C) Western blot analysis of astrocytes derived from H-2kb-tsA58 transgenic mouse shows the levels of TAg correlate to the permissibility of the culture conditions.
We isolated three different ON astrocyte cultures from H-2kb-tsA58 mouse optic nerves, named ImBlON, ImB2ON, and ImB3ON. Each cell strain had been maintained under permissive conditions to induce immortalization. Although all three cell strains were derived from animals with the same genetic background, they exhibited different expression levels of TAg after 2 months in culture. The TAg forms a complex with p53 and induces cell transformation [10]. Among all three cell strains, ImBlON showed highest expression of both TAg and p53 (Fig. 3A). ImBlON cells demonstrated the fasted cell growth rate and loss of contact inhibition, which indicates successful transformation of ImBlON cells (Fig. 3B). In addition, ImBlON astrocytes were further maintained through 60 generations, attesting to the immortalization of this cell line.
Figure 3.
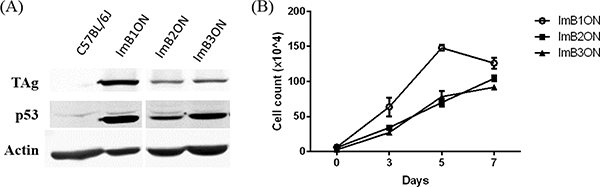
Conditionally immortalized mouse optic nerve astrocyte strains. (A) Western blot analysis of 3 different astrocyte strains derived from H-2kb-tsA58 transgenic mice shows different expression levels of TAg under permissive culture conditions. (B) Cell growth curves demonstrate that the cell strain with the highest level of TAg expression (ImB1ON) had the fasted cell growth rate.
Characterization of ImBlON astrocyte line
We examined the morphology and astrocyte maker expression of ImBlON cells at passages 2 and l7, before and after transformation, respectively. The cells on passage 2 exhibited a polygonal to fusiform soma with long processes. After transformation, cells displayed smaller cell soma size and shortened processes. Expression of GFAP and NCAM, commonly used astrocyte markers, were detected in cells both before and after transformation. As expected, GFAP showed a cytoskeletal labeling, and the pattern of NCAM indicated its cytoplasmic or cell membrane expression (Fig. 4A).
Figure 4.
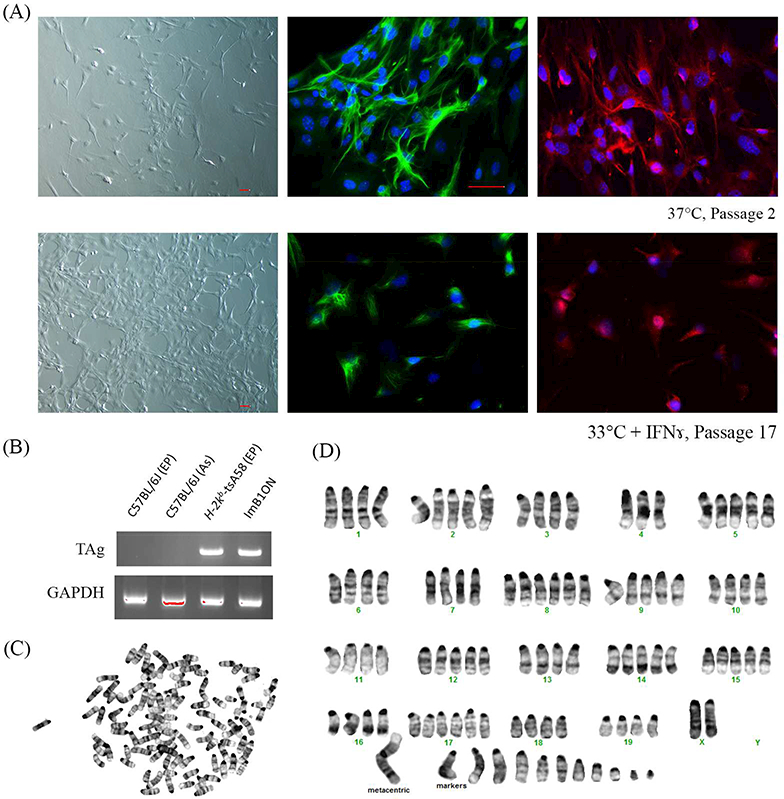
Characterization of conditionally immortalized mouse optic nerve astrocyte line. (A) Phase- contrast and immunocytochemistry images demonstrate morphology and expression patterns of GFAP (green) and NCAM (red) of ImB1ON cultured under permissive and nonpermissive conditions. Nuclei were labeled with DAPI (blue). Scale bar = 50 μm. (B) Genotyping demonstrates amplication of TAg transgene from DNA samples extracted from ImB1ON cells and H-2kb-tsA58 transgenic mice. (C) Metaphase spread shows mouse chromosome pattern. (D) Karyotyping demonstrates mouse origin of ImB1ON astrocytes.
To confirm the mouse origin of the conditionally immortalized optic nerve astrocytes, we performed genotyping and karyotyping. DNA samples extracted from wild type C57BL/6J mice were used as a negative control, as expected, the transgene could be amplified only from DNA samples extracted from ImBlON astrocytes or ear punches from transgenic mice (Fig. 4B). The banding patterns and number of chromosomes also demonstrated mouse origin of these immortalized cells (Fig. 4C-D). ImBlON cells exhibit extra copies of each chromosome, which is not unexpected in immortalized cells.
Recent studies have revealed that optic nerve astrocytes are phagocytic [8, 11, 12]. Using purified synaptosomes from the mouse cortex, we verified this activity in the immortalized astrocytes. The synaptosomes were conjugated with a pH sensitive dye, pHrodo, which fluorescents only in acidic environments such as phagosomes. Thus, the fluorescence detected from the astrocytes indicate their phagocytic activities. Compared to astrocytes derived from the C57BL/6J mice (46.4 ± 1.8 %), ImBlON demonstrated a higher percentage (64.9 ± 0.1 %) of cells with strong phagocytic fluorescence (Fig. 5).
Figure 5.
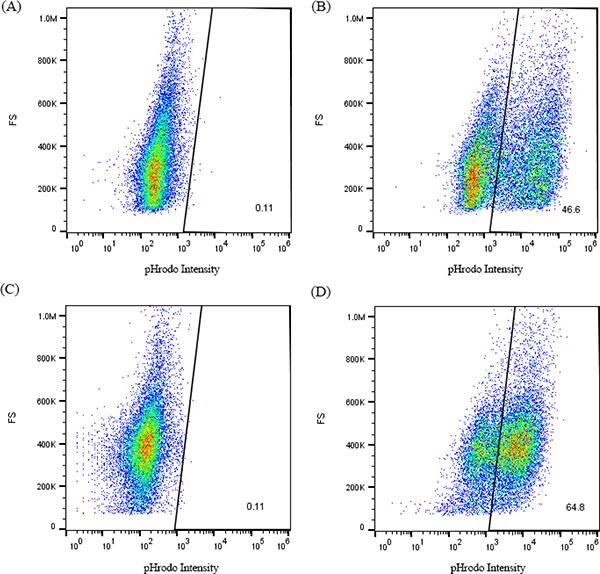
Conditionally immortalized astrocytes exhibit phagocytic activity. Astrocytes were incubated with pHrodo conjugated synaptosomes (SN) for 24 hours. FACS profiles of optic nerve astrocytes derived from C57BL/6J mice (A & B, with or without incubation of SN, respectively) and conditionally immortalized astrocytes (C & D, with or without incubation of SN, respectively) for pHrodo intensity.
Astrocytes produce trophic factors such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and basic fibroblast growth factor (bFGF) [13, 14]. We examined mRNA expression levels of these trophic factors using quantitative PCR analysis. ImB1ON cells expressed these genes. The mRNA levels of BDNF, NGF and bFGF were 20.7 ± 5.2 %, 25.8 ± 0.3 %, and 14.4 ± 1.2 % in ImB1ON cells, respectively in comparison with astrocytes derived from C57BL/6J mice (Fig. 6).
Figure 6.
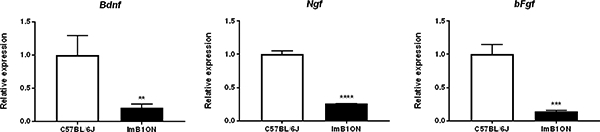
Trophic factor expression in conditionally immortalized astrocytes. Quantitative PCR shows expression of Bdnf, Ngf, and bFgf in ImB1ON cells and astrocytes derived from C57BL/6J mice. Relative expression of each gene was determined based on a 2 fold exponential using mRNA expression values normalized to Gapdh and astrocytes derived from C57BL/6J mice. Data are presented as mean ± S.D.. ** p < 0.05, *** p < 0.01, **** p < 0.005, n = 3.
To evaluate the conditionality of the immortalization, we cultured ImB1ON under non-permissive conditions, at 38.5 °C without IFNɤ for up to 4 weeks. Starting from 1 week in non-permissive conditions, the expression of TAg and p53 decreased. However, the expression of TAg was still detectable until 4 weeks in non-permissive culture. In addition, there was a dramatic increased expression of NCAM (Fig. 7).
Figure 7.
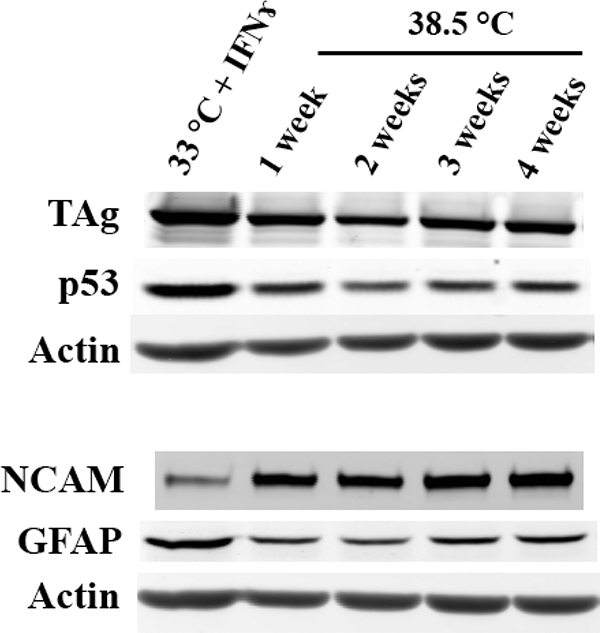
TAg and P53 expression under non-permissive culture conditions. Western analysis of ImBlON cells shows levels of TAg, p53, GFAP and NCAM under permissive and after switching to nonpermissive culture conditions.
Discussion
We have established a conditionally immortalized mouse optic nerve astrocyte line, named ImBlON. The cells exhibit astrocytic characteristics and provide an important tool to study functions of optic nerve astrocytes in vitro.
Astrocyte cell lines have been generated with SV40-TAg induced cellular transformation. For example, the A7 cell line has been derived from neonatal rat optic nerves by transduction with a murine leukemia virus containing the SV40 large TAg. This permanent immortalized cell line displays certain functions of type 1 astrocytes: the ability to secrete platelet-derived growth factor and support the growth of embryonic CNS neurons [15].
One of the major limitations of permanent immortal cell lines is the lack of differentiation that prevents the development of the full normal phenotype [16]. This may complicate the data obtained from these cell lines. To overcome this problem, a conditionally immortalized astrocyte line was generated from the cortex of a neonatal H-2kb-tsA58 transgenic mouse [17]. This cortical astrocyte line demonstrates several features associated with glial scars, including expressing a variety of cell membrane and extracellular matrix molecules present in glial scars, supporting neuronal growth, and inhibiting the migration of O-2A progenitor cells. Astrocytes are heterogeneous, and cells located in discrete regions display diverse properties and play various roles [18]. Thus, it’s necessary to establish a conditionally immortalized optic nerve astrocyte line for a precise understanding of optic nerve physiology and pathology.
The ImB1ON astrocytes grew rapidly under both permissive and non-permissive conditions. Under non- permissive conditions, ImB1ON cells still express TAg. A similar phenomenon has been reported in other cell lines immortalized with the ts TAg [19, 20]. The mechanisms for continued expression of this mutant protein are not been fully understood, it may be attributed to the mutations of nucleic acid sequences in the temperature-sensitive region of ts TAg [20]. In our study, although the expression of TAg remains under non-permissive conditions, there was decreased levels of p53, which indicates the inactivation of TAg activity. In addition, increased levels of NCAM suggest differentiation of these astrocytes. It appears that temperature shift induces differentiation and at least a partial reversion of immortalization.
Expression of SV40 tumor antigens induces polyloidy in human and rodent cells [21–23]. Large T antigen binds to DNA, functions as an ATPase and helicase, and induces chromosomal aberrations and ploidy changes [22]. Not only in ImB1ON cells, the increase in copy numbers of chromosomes have been observed also in other SV40-immortalized cell lines such as a human endometriotic cell line [24] and a fibroblast line HS74 [25]. Although with extra copies of chromosomes, ImB1ON cells showed decreased gene expression levels of trophic factors compared to wild type optic nerve astrocytes.
In vitro cell culture does not completely replicate the in vivo environment. Astrocytes in culture tend to become reactive and less representative of in vivo astrocytes [26]. In addition, the chromosomal aberrations, decreased expression of major astrocyte markers and trophic factors, along with continuous expression of TAg may limit the usefulness of the ImB1ON line. Caution needs to be taken when interpreting the results.
The ImB1ON cells were obtained by optic nerve explants. In general, explant culture may render a mixed cell population. The defined astrocyte culture medium contains low concentration fetal bovine serum, which suppresses the growth of fibroblasts. Immunofluorescent staining demonstrated almost all cells expressed GFAP and NCAM at both early and late passage numbers. Thus, we do not see any contamination with fibroblasts.
In summary, our conditionally immortalized ImB1ON astrocyte line exhibits astrocytic characteristics. This cell line provides a useful tool to study various aspects of astrocyte physiology and help delineate the pathogenesis of optic nerve diseases.
Highlights.
A conditionally immortalized optic nerve astrocyte line ImBlON has been established.
ImBlON exhibits astrocytic characteristics.
ImB1ON will be a useful tool to study astrocyte biology.
Acknowledgement
This work was supported by the NIH/NEI [EY023364; AFC] grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hubbard K and Ozer HL, Mechanism of immortalization. Age (Omaha), 1999. 22(2): p. 65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin JY and Simmons DT, The ability of large T antigen to complex with p53 is necessary for the increased life span and partial transformation of human cells by simian virus 40. J Virol, 1991. 65(12): p. 6447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jat PS, et al. , Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci U S A, 1991. 88(12): p. 5096–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamm ER, Russell P, and Piatigorsky J, Development of characterization of a immortal and differentiated murine trabecular meshwork cell line. Invest Ophthalmol Vis Sci, 1999. 40(7): p. 1392–403. [PubMed] [Google Scholar]
- 5.Whitehead RH, et al. , Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci U S A, 1993. 90(2): p. 587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen KJ, et al. , Conditionally immortalized mouse hepatocytes for use in liver gene therapy. J Gastroenterol Hepatol, 2000. 15(11): p. 1325–32. [PubMed] [Google Scholar]
- 7.Dunkley PR, Jarvie PE, and Robinson PJ, A rapid Percoll gradient procedure for preparation of synaptosomes. Nat Protoc, 2008. 3(11): p. 1718–28. [DOI] [PubMed] [Google Scholar]
- 8.Chung WS, et al. , Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature, 2013. 504(7480): p. 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westmark PR, et al. , Preparation of synaptoneurosomes from mouse cortex using a discontinuous percoll-sucrose density gradient. J Vis Exp, 2011(55). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duthu A, et al. , P53-transformation-related protein: kinetics of synthesis and accumulation in SV40-infected primary mouse kidney cell cultures. Virology, 1985. 147(2): p. 275–86. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen JV, et al. , Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc Natl Acad Sci U S A, 2011. 108(3): p. 1176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mills EA, et al. , Astrocytes phagocytose focal dystrophies from shortening myelin segments in the optic nerve of Xenopus laevis at metamorphosis. Proc Natl Acad Sci U S A, 2015. 112(33): p. 10509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kostyk SK, et al. , Optic nerve injury alters basic fibroblast growth factor localization in the retina and optic tract. J Neurosci, 1994. 14(3 Pt 2): p. 1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert W, et al. , Neurotrophin and neurotrophin receptor expression by cells of the human lamina cribrosa. Invest Ophthalmol Vis Sci, 2001. 42(10): p. 2315–23. [PubMed] [Google Scholar]
- 15.Geller HM and Dubois-Dalcq M, Antigenic and functional characterization of a rat central nervous system-derived cell line immortalized by a retroviral vector. J Cell Biol, 1988. 107(5): p. 1977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniele N, et al. , Conditionally immortalized cell lines as model systems for high-throughput biology in drug discovery. Biochem Soc Trans, 2002. 30(4): p. 800–2. [DOI] [PubMed] [Google Scholar]
- 17.Groves AK, et al. , The characterization of astrocyte cell lines that display properties of glial scar tissue. Dev Biol, 1993. 159(1): p. 87–104. [DOI] [PubMed] [Google Scholar]
- 18.Volterra A and Meldolesi J, Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci, 2005. 6(8): p. 626–40. [DOI] [PubMed] [Google Scholar]
- 19.Saenz-Robles MT, Sullivan CS, and Pipas JM, Transforming functions of Simian Virus 40. Oncogene, 2001. 20(54): p. 7899–907. [DOI] [PubMed] [Google Scholar]
- 20.Truckenmiller ME, et al. , Growth properties of neural cell lines immortalized with the tsA58 allele of SV40 large T antigen. Cell Transplant, 1997. 6(3): p. 231–8. [DOI] [PubMed] [Google Scholar]
- 21.Bloomfield M and Duesberg P, Karyotype alteration generates the neoplastic phenotypes of SV40-infected human and rodent cells. Mol Cytogenet, 2015. 8: p. 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart N and Bacchetti S, Expression of SV40 large T antigen, but not small t antigen, is required for the induction of chromosomal aberrations in transformed human cells. Virology,1991180(1): p. 49–57. [DOI] [PubMed] [Google Scholar]
- 23.Chang PL, et al. , Transformation of human cultured fibroblasts with plasmids carrying dominant selection markers and immortalizing potential. Exp Cell Res, 1986. 167(2): p. 407–16. [DOI] [PubMed] [Google Scholar]
- 24.Akoum A, et al. , Physiological and cytogenetic characterization of immortalized human endometriotic cells containing episomal simian virus 40 DNA. Am J Pathol, 1999. 154(4): p. 1245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubbard-Smith K, et al. , Altered chromosome 6 in immortal human fibroblasts. Mol Cell Biol,199112(5): p. 2273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foo LC, et al. , Development of a method for the purification and culture of rodent astrocytes. Neuron, 2011. 71(5): p. 799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]


