Abstract
The current study examined how a gene related to functioning of the dopaminergic system, catechol-O-methyltransferase (COMT) and estradiol were related to brain functioning in healthy postmenopausal women. Participants were 118 healthy, cognitively normal postmenopausal women between the ages of 50–60. All women provided a blood sample for COMT and estradiol analyses and underwent an MRI scan. Working memory performance and related brain activation were measured with BOLD fMRI during the N-back task. Results were examined across each COMT genotype and a median split was performed on the circulating estradiol levels to create high and low estradiol groups for each genotype. COMT genotype and estradiol level were hypothesized to be proxy measures for brain dopamine levels with the Met/Met and high estradiol group having the most dopamine and Val/Val and low estradiol group having the least dopamine. The fMRI results showed that the N-back task activated the expected bilateral frontal and bilateral parietal working memory network. However, no main effects of COMT genotype or estradiol group were found. There was COMT-estradiol interaction found in a small area of decreased activation in the right precentral gyrus (Brodmann Area 6) that was related to the increasing hypothesized dopamine level. Specifically, women with a Met/Met genotype in the high estradiol group had the least activation in this frontal lobe working memory region. Women with a Val/Val genotype in the low estradiol group had greater activation in this region relative to the other groups. Performance on the N-back task did not show any group differences. These data indicate that after menopause COMT genotype and potentially the menopause-related changes to the dopaminergic system are not related to cognition. Future studies should examine how the relationship between COMT, estradiol, and cognition around the menopause transition as there are appear to be differences in this relationship for pre and postmenopausal women.
1.1. Introduction
There appear to be large individual difference factors contributing to how the hormone change at menopause is related to brain functioning. Evidence for cognitive changes after menopause is equivocal with some studies showing declines in cognition after menopause (e.g. Fuh et al. 2003; Greendale et al. 2009; Halbreich et al. 1995) while other studies showed no changes from pre-menopausal levels of performance (e.g. e.g. Henderson et al. 2003; Kok et al. 2006; Luetters et al. 2007). What is clear from the prior literature on menopause and cognition is that there are large individual differences in whether or not women experience cognitive changes. In an effort to begin to disentangle the individual differences in cognition after menopause, the current study examined how a gene that influenced the functioning of the dopaminergic system was related to brain functioning in healthy postmenopausal women. The dopaminergic system may be important for cognition after menopause because age-related changes in the dopaminergic system have been implicated in normal cognitive aging (Braver and Barch, 2002). Perhaps understanding the role of dopaminergic functioning on cognition will elucidate mechanisms involved in individual differences in cognition after menopause.
Studies have shown that dopaminergic system modulates working memory performance through the striatal-frontal pathway (e.g. Cools et al. 2007) and age changes in frontal lobe dopamine systems are responsible for cognitive aging (Braver & Barch 2002). One mechanism by which this age-related decline in dopaminergic function may occur is through age-related changes in dopaminergic receptor availability that have been shown using positron emission tomography (PET; e.g. Wong et al. 1984; Volkow et al. 1998). These studies estimated a decline of 12% D2 receptor availability per decade, primarily in the frontal cortex. The age-related changes in D2 receptor availability in the frontal cortex imply that age changes in dopaminergic functioning may be related to age changes in cognition that rely on the frontal cortex such as working memory (Backman et al. 2006). In addition to age-related changes in dopaminergic systems, there are also sex differences in D2 receptor binding particularly in the frontal cortex (Wong et al. 1984). These data implicate a potential role for gonadal steroid modulation of dopaminergic functioning.
The current study “manipulated” dopaminergic functioning by examining genetic variation related to the catechol-O-methyltransferase (COMT) gene. The gene transcribes the COMT enzyme involved in degradation of released dopamine in the frontal cortex. COMT is primarily localized to the frontal cortex of the brain and has a direct effect on prefrontal cortex dopamine levels (Backman et al., 2006). The gene coding for COMT has at least five common single nucleotide polymorphisms (SNPs) that lead to different functionality of the enzyme and have effects on cognition, arousal, pain sensitivity, and stress reactivity in humans and animal models (Chen et al., 2011). The SNP most often associated with cognition has a Val to Met substitution at codon 158 (VAL158Met) and is the focus of this study. This SNP influences the stability of the enzyme in vitro and determines the enzymatic activity in vivo with the Met allele having approximately one quarter the enzymatic activity of the Val allele (Chen et al., 2004; Lachman et al., 1996; Lotta et al., 1995). Thus, individuals with the Met/Met genotype compared to Val/Met and Val/Val have greater dopamine availability and perform better on cognitive tasks that are supported by the frontal lobes like working memory and measures of executive functioning (de Frias et al., 2005; Egan et al., 2001). For example, Met homozygotes performed better than Val/Met or Val homozygotes on the N-back test of working memory (Goldberg et al., 2003), the Letter-Number Sequencing Test, and Wisconsin Card Sorting Test (Bruder et al., 2005). Additionally, aging magnifies the relationship between COMT genotype and cognitive performance with a greater performance deficit for Val carriers compared to Met homozygotes with increased age (Nagel et al., 2008; Raz et al., 2011). COMT genotype effects in aging were also magnified in frontal cortex activation on a working memory task during functional magnetic resonance imaging (fMRI; (Sambataro et al., 2009). Older Val homozygotes had increased working memory-related activation in the dorsolateral prefrontal cortex (DLPFC) and functional connectivity compared to Met homozygotes and younger participants (Sambataro et al. 2009). Critically, this study did not include an analysis by sex.
Sex also influences COMT effects on cognition. Women have 20–30% less COMT activity than men (Chen et al., 2004; Fahndrich et al., 1980). Both Raz et al. (2011) O’Hara et al. (O'Hara et al., 2006) found that sex interacted with COMT genotype such that older men who had a Val allele showed greater negative effects on cognition compared to older women (Raz et al. 2011). Thus, it is likely that gonadal steroids and the hormone change at menopause modulate COMT activity. Estrogen directly influences COMT activity by inhibiting COMT gene transcription through two estrogen response elements located on the COMT promotor (Jiang et al., 2003; Weinshilboum, 2006; Xie et al., 1999). Thus, the decline in estrogen levels after menopause may result in increased COMT transcription and decreased dopaminergic functioning, and thereby impact cognitive processes like working memory.
In addition to its role in catecholamine metabolism, COMT is involved in metabolism of estradiol and estrone. Estradiol is metabolized into catecholestrogens first by cytochrome P450 enzymes. Then catecholestrogens are then inactivated by O-methylation by COMT creating either 2-methoxyestradiol or 4-methoxyestrone (Liehr and Ricci, 1996; Liehr and Roy, 1990). There may be an important feedback relationship between the functioning of the COMT enzyme and the transcription of the COMT gene that involves estradiol and therefore may be a mechanism underlying cognitive changes after menopause.
There is one prior study that examined the relationship between COMT, estrogen level, and working memory in healthy younger premenopausal women. Jacobs and D’Esposito (2011) showed that hormone variation during the menstrual cycle in premenopausal women interacted with COMT genotype to influence working memory-related brain activation and performance. Performance measured by correct rejection of the lure items on an N-back test of working memory was best for women with a Met/Met genotype in the low estradiol phase of the menstrual cycle and while lowest performance was seen for the Val/Val genotype and the low estradiol phase of the menstrual cycle. The Met/Met group during the high estrogen phase of the cycle had the greatest decrease in prefrontal cortex activation. The group with the hypothesized least amount of dopamine (Val/Val and low estradiol) had the greatest amount of activation. Thus, activation was inversely related to the proposed level of dopamine. The decreased frontal activation as dopamine increased was interpreted as more cognitively “efficient”.
The current study was the first to examine how COMT genotype and estradiol are associated with brain functioning in healthy postmenopausal women. We hypothesized that with decreased circulating estradiol after menopause, COMT genotype relationship to cognition would remain and perhaps become more exaggerated. Specifically, we hypothesized that working memory related brain activation would decrease as the hypothesized dopamine level increased. That is, women with a Met/Met genotype would have decreased activation and better performance than women with a Val/Val genotype. In order to examine how estradiol is related to brain functioning in a similar way to Jacobs & D’Esposito (2011) we created high and low estradiol groups by doing a median split on the circulating estradiol levels. We hypothesized that the high estradiol group with the Met/Met genotype would perform the best and have least activation compared to the other groups. This group did not perform the best in the Jacobs and D’Esposito (2011) study because it was suggested that this estradiol/COMT genotype combination represented excess dopamine and less efficient performance. However, after menopause when the overall estradiol levels are lower, those postmenopausal women with higher levels of circulating estradiol and the Met/Met genotype may have an advantage in working memory and related brain functioning.
2. Materials and Method
2.1. Participants
Participants were 118 cognitively normal postmenopausal women, aged 50–60 years, M(SD) = 56.18(2.6) (See Table 1 for demographic information). Participants were recruited with media advertisements in the Burlington, VT region. Participants were required to be postmenopausal, without menses for one year, and without surgically-induced menopause. All participants passed an initial telephone screen to ensure they were post menopause and did not have any contraindications for the MRI. Medical exclusion criteria were similar to our prior studies (e.g.(Dumas et al., 2012) and included smoking, a history of breast cancer, use of hormone therapy during the last year, and medications that have CNS effects. Participants were required to not be taking any kind of postmenopausal hormone treatment currently or within the past 12 months. Women were self-reported to be physically healthy nonsmokers and have no cardiovascular disease other than mild hypertension. All participants met these criteria.
Table 1.
Demographic data (means and standard deviations) and hormone values for the postmenopausal women by COMT genotype and estradiol group. One interaction was found for the Wechsler Test of Adult Reading (WTAR; (F(2,87) = 5.08, p = .008). Abbreviations: Wechsler Test of Adult Reading (WTAR), Body Mass Index (BMI), Pittsburch Sleep Quality Inventory (PSQI), Follicule Stimulating Hormone (FSH).
| Met/Met | Met/Val | Val/Val | Met/Met | Met/Val | Val/Val | |
|---|---|---|---|---|---|---|
| Low E2 | Low E2 | Low E2 | High E2 | High E2 | High E2 | |
| N=14 | N=21 | N=13 | N=9 | N=23 | N=13 | |
| Age (y) | 57.07 (2.4) | 56.00 (2.5) | 56.33 (2.3) | 55.78 (2.5) | 56.17 (3.1) | 55.88 (2.3) |
| Years since Menopause (y) | 5.69 (2.8) | 7.14 (5.2) | 4.57 (3.5) | 5.67(4.3) | 6.26 (4.9) | 5.69 (2.5) |
| Education (y) | 15.57 (2.5) | 16.67 (2.6) | 16.77 (1.9) | 15.11 (1.8) | 15.74 (2.45) | 16.31 (2.4) |
| WTAR (IQ)* | 121.21 | 116.14 | 117.85 | 111.33 | 117.70 | 118.46 |
| (4.6) | (8.9) | (5.8) | (8.5) | (6.9) | (6.7) | |
| BMI (kg/m2) | 27.07 (5.7) | 25.07 (4.1) | 24.60 (3.6) | 27.82 (4.5) | 24.67 (4.1) | 26.65 (4.9) |
| PSQI (Total) | 4.57 (2.3) | 5.62 (3.6) | 5.46 (3.5) | 4.44 (3.1) | 6.04 (3.2) | 4.08 (3.5) |
| FSH (IU/ml) | 75.4 (20.4) | 75.22 (17.6) | 94.45 (23.7) | 76.60 (28.0) | 85.91 (26.9) | 77.95 (21.9) |
| Estradiol(pg/ml) | 4.34 (1.1) | 3.50 (1.1) | 4.08 (1.0) | 14.90 (6.3) | 14.36 (5.8) | 11.37 (5.2) |
| Range | 4.08 | 3.40 | 2.86 | 18.14 | 18.93 | 15.05 |
| Estrone (pg/ml) | 40.43 (9.4) | 34.27 (9.8) | 34.74 (7.6) | 37.81 (12.8) | 31.97 (14.3) | 31.14 (13.6) |
| Range | 33.34 | 35.30 | 26.96 | 32.38 | 66.66 | 45.84 |
interaction of COMT and estradiol group (F(2,87) = 5.08, p = .008)
Twenty-two women had a prior history of postmenopausal hormone use for an average duration of use of 1.45 years (SD=2.5 y). Seventy women were STRAW+10 Stage +1 early post menopause and 48 women were Stage +2 late post menopause based on their years since their final menstrual period. Eight participants had incidental findings on the MRI that included having more white matter hyperintensities than were expected for their age using the heuristic of more than one hypterintensity per decade of life as judged by the neuroradiologist (author JPN). They were referred for appropriate follow up care and their data were removed from the study.
All of the following assessments took place in one study day that lasted approximately three hours. After signing consent forms at the University of Vermont (UVM) Clinical Research Center (CRC), participants provided a blood sample that was used to ensure postmenopausal status of FSH > 20 IU/L and for analysis of the COMT Val158MET SNP. Participants were then cognitively evaluated using the Mini Mental State Exam (MMSE; (Folstein et al., 1975) and the Brief Cognitive Rating Scale (Reisberg and Ferris, 1988), to establish a Global Deterioration Scale score (GDS) which rated the degree of cognitive impairment (Reisberg and Ferris, 1988). Participants were required to have an MMSE score greater than or equal to 27 and a GDS score of 1 or 2.
To assess general neuropsychological functioning, all women completed the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; (Randolph et al., 1998) to assess immediate memory, language, visuospatial/constructional ability, attention, and delayed memory, as well as a global total neuropsychological functioning measure. The Letter-Number Sequencing Test was be used as a standardized measure of working memory ability. We used the Wechsler Test of Adult Reading (WTAR; (Holdnack, 2001) to assess reading and is used to estimate premorbid IQ. All participants were required to score within one standard deviation of the mean for their age on the neuropsychological functioning test to ensure they were not in the beginning stages of Mild Cognitive Impairment (MCI) or dementia. Participants were required to have a score greater than 80 on the WTAR. All participants passed the cognitive and neuropsychological screening assessments.
Behavioral screening consisted of a Structured Clinical Interview for DSM-IV-TR (SCID; (First et al., 2002) to establish the presence/absence an axis I psychiatric disorder. In addition, participants completed the Beck Depression Inventory-II (BDI-II; (Beck et al., 1996). A cut off score of 10 was used for the BDI, and participants scoring over this criterion were discontinued from further participation. No woman had current MDD or any other axis I psychiatric disorder and 20 out of 110 women had remitted major depressive disorder (MDD). Women completed the Pittsburgh Sleep Quality Index (PSQI; (Buysse et al., 1989) to assess sleep quality over a one month interval, and a menopause symptom checklist created in our laboratory (Newhouse et al., 2013).
Blood draw procedures were standard procedures performed by nurses at our CRC. Blood for the FSH assay was drawn into a 4ml SST tube and sent to the UVM Medical Center laboratory for immediate analysis. Blood for the estradiol and estrone analyses was also drawn into a 4 ml SST tube and allowed to clot for 30 minutes. It was then centrifuged in ambient temperature on the tabletop for 10 minutes at 3000 rpm. The serum was then transferred into a pre-labeled 1.8 ml aliquot tube and put in an -80C freezer for later analysis. The estradiol and estrone analyses were performed in a batch at the end of the study by Frank Stanczyk, Ph.D. at the USC Reproductive Endocrinology Clinical Laboratory. Estradiol and estrone were quantified in serum a radioimmunoassay (RIA) method as previously described (Probst-Hensch et al., 1999) by the Stanczyk Reproductive Endocrinology Laboratory at the University of Southern California. Prior to the RIA, steroids were extracted with hexane:ethyl acetate (3:2) and estradiol was separated by Celite column partition chromatography, using 40% ethyl acetate in isooctane. High specific activity tritiated estradiol (500 dpm) was added to each serum sample before the extraction step in order to follow and correct for procedural losses. Interassay coefficients of variation for measurements of low, medium, and high quality control samples in the estradiol RIA ranged from 9.0% to 12.0%, respectively. The sensitivity of the estradiol RIA is 2 pg/ml.
Blood for the genetic analysis was drawn into a 10 ml EDTA tube and was centrifuged at 4° C for 20 minutes at 3000 rpm. Using a transfer pipette, the plasma was removed from the tube without disturbing the buffy coat above the red cells. Buffy coat was placed into a 1.8 ml screw cap vial and placed into the -80 C for storage until COMT analyses were run in a batch at the end of the study. For the genetic analysis SNP rs4680 for COMT was analyzed by the UVM Cancer Center Advanced Genome Technologies Core DNA Analysis facility for qPCR using TaqMan assay materials from Applied Biosystems.
2.2. fMRI Procedure
After completion of the cognitive and behavioral assessments at the CRC, participants proceeded to the UVM MRI Center for Biomedical Imaging to complete the MRI portion of the study. All participants were scanned on a Philips 3T Achieva d-Stream scanner and received the following MR sequences as part of the imaging protocol: (1) A sagittal T1-weighted spoiled gradient volumetric sequence oriented perpendicular to the anterior commissure (AC)-posterior commissure (PC) plane using a repetition time (TR) of 9.9 ms, echo time (TE) of 4.6 ms, flip angle of 8 degrees, number signal averages (NSA) 1, field of view (FOV) of 256 mm, 256 X 256 matrix, and 1 mm slice thickness with no gap for 160 contiguous slices. (2) Fluid Attenuated Inversion Recovery (FLAIR) sequence using a TR of 4800 ms, inversion time (TI) of 1650 ms, and a TE of 260 ms. Resolution was 1.2 × 1.2 × 1.2 mm with a field of view of 250, NSA 2, and 280 slices. All images were reviewed by a board-certified neuroradiologist to exclude intracranial pathology. Eight participants had incidental findings including increased white matter hyperintensities that were abnormal for age. These data were removed and not replaced.
fMRI was performed using EpiBOLD (echoplanar blood oxygenation level dependent) imaging using a single-shot sequence (TR 2500 ms, TE 35 ms, flip angle 90 degrees, 1 NSA for 197 volumes). Resolution was 3 mm x 3 mm x 3.5 mm. Thirty-three contiguous slices 3.5 mm thick with no gap were obtained in the axial oblique plane parallel to the AC-PC plane using a FOV of 240 mm and a matrix size of 80 × 80. Field map correction for magnetic inhomogeneities was accomplished by acquiring images with offset TE at the end of the functional series.
2.3. fMRI Working Memory Task
The N-Back Test was used as the measure of verbal working memory. In this task, the participant saw a string of consonant letters (except L, W, and Y), one every three seconds during 27-second blocks. Three conditions were presented: 0-back, 1-back, 2-back, and 3-back. In each condition, the task was to decide whether the letter currently on the screen matched the target in the 0-back or the letter that had been presented 1, 2, or 3 back in the sequence. The N- back task reliably activates a bilateral frontal, parietal, and cerebellar working memory network. We have used it in a number of studies with postmenopausal women (Dumas et al., 2010; Dumas et al., 2012; Dumas et al., 2008), and Jacobs & D’Esposito (2011) used it to examine the interaction of estradiol levels and COMT genotype in younger premenopausal women. The total task time was 8.15 minutes.
2.4. fMRI Analyses
During preprocessing of the functional MRI data, the volumes were realigned to the first volume to minimize the effects of head movements. We found that 17 women had head movement on more than 50% of the frames that was greater than 2mm of movement or 2 degrees of rotation. These participants were removed from the analysis and their data were not replaced. The final N was 93. Further preprocessing included spatial (4 mm FWHM isotropic Gaussian kernel) as well as temporal filters to remove aliased signal correlated with background respiration and heart rate. Anatomical and functional images were co-registered and normalized to Talairach space. Statistical analysis was performed by multiple linear regression of the signal time course at each voxel. The hemodynamic response function was accounted for in these models.
The first level analysis attempted to model the procedures of Jacobs & D’Esposito (2011) who examined the load-related task blocks. The contrast we used was +4, +1, -5 for the 3-back, 2- back and 0-back conditions, respectively. Second level statistical analyses were performed on the first level data using a 3 (COMT genotype: MET/MET, MET/VAL, VAL/VAL) X 2 (Estradiol level: low and high) random effects ANOVA using standard ANOVA procedures in Brain Voyager (Brain Voyager QX, The Netherlands). To probe the COMT-estradiol interaction in the brain imaging data we used the following linear contrast +5, +3, +1, -1, -3, -5 for Met/Met high estradiol, Met/Val high estradiol, Val/Val high estradiol, Met/Met low estradiol, Met/Val low estradiol, Val/Val low estradiol, respectively. We utilized a linear model because Jacobs and D’Esposito (2011) found a linear relationship between the COMT-estradiol group and brain activation during the N-back task.
To correct for multiple comparisons, we used the cluster-level statistical threshold estimator from Brain Voyager QX to estimate a minimum cluster size threshold based on the approach of Forman et al. (Forman et al., 1995).This procedure estimated a minimum cluster size of 8 voxels in functional space (3×3×3) at an alpha level of 0.001 for the fMRI analyses described below.
2.5. Working Memory Performance Analysis
Working memory performance on the N-back task was analyzed using the signal detection measures of sensitivity (d’) and bias (C; (Snodgrass and Corwin, 1988). Sensitivity is a measure of how different two classes of items are as measured by d’ and is represented in standard deviation units. Larger d’s represent greater sensitivity and greater accuracy. Bias (C) is the tendency for a participant to endorse a letter as a match or mismatch also represented in standard deviation units. Liberal response bias indicates that a participant calls a large number of responses matches in contrast to conservative bias indicating that the participant makes many mismatch responses. Bias scores of greater than 0 are conservative while bias scores less than 0 are liberal.
3. Results
3.1. COMT Genotype
When we examined the COMT genotype distribution, we found 23 women were Met/Met, 44 women were Met/Val, and 26 women were Val/Val which met the Hardy-Weinberg Equilibrium (X2=0.26, df=1, n.s.). We examined differences between these three groups on the RBANS, Letter Number Sequencing, BDI, and BAI and found no group differences on any of these neuropsychological or mood measures (largest F(2,90) = 2.08, p=.13 for the BDI).
We also examined the estradiol and estrone levels. First, we probed for differences in hormone levels for the three genotypes and found no significant differences between the groups for estradiol (F(2,90)=0.43, p = .65), and a trend for a difference in estrone (F(2,90) = 2.65, p =.07) indicating that the Met/Met group had numerically higher estrone levels.
To extend the findings of Jacobs and D’Esposito (2011) in premenopausal women to our postmenopausal sample, we examined the relationship between the COMT groups, estradiol levels, and working memory brain activation and performance. We performed a median split on the estradiol levels and created six groups: Met/Met high estradiol, Met/Val high estradiol, Val/Val high estradiol, Met/Met low estradiol, Met/Val low estradiol, and Val/Val low estradiol.
3.2. Activation Data
First we examined working memory-related brain activation during the N-back across all six groups to demonstrate that the task had the expected working memory load effect. In our sample of healthy postmenopausal women, when we examined the 3-back and 2-back working memory load conditions compared to the 0-back match condition we found the expected bilateral frontal, parietal, and cerebellar working memory network (Figure 1; (Cohen et al., 1997).
Figure 1.
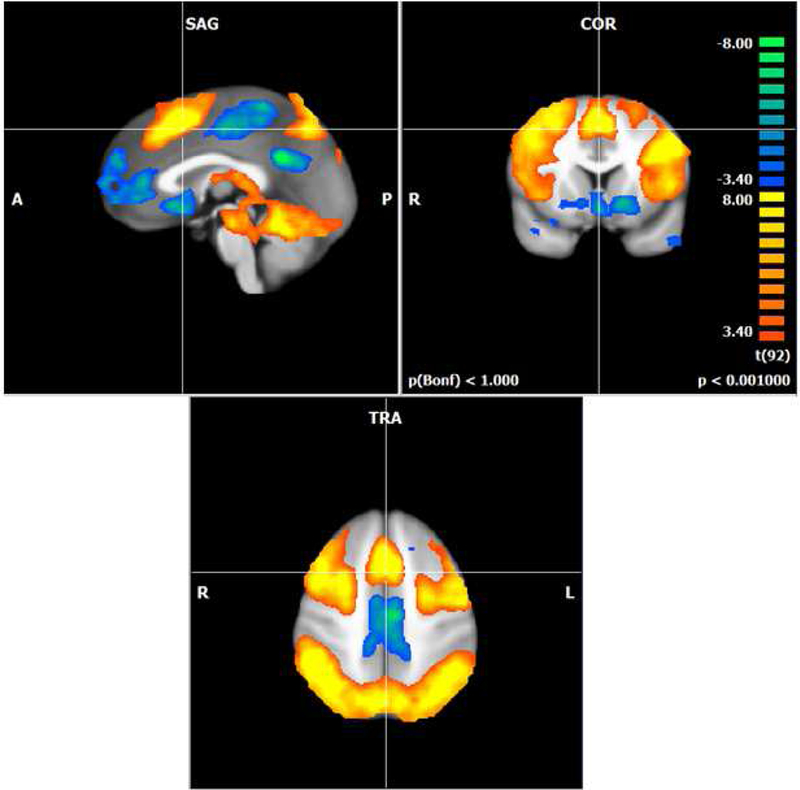
Activation map showing the N-back task effect for all participants (p < .001; cluster corrected k=8). The +4 (3-back) +1 (2-back) -5(0-back) contrast activated the expected bilateral frontal, parietal, and cerebellar regions. Orange colors represent regions where the activation is increased for the 2- and 3-back conditions compared to the 0-back. Blue colors represent activation that is decreased for the 2- and 3-back compared to the 0-back.
To test our hypothesis that higher levels of inferred dopamine would influence brain functioning, we examined main effects and the interaction of COMT genotype and estradiol group. We found no brain regions representing main effects of COMT genotype or estradiol group. When we examined the interaction of COMT and estradiol, we found one small region in the left precentral gyrus (BA 6) that had decreased activation as the hypothesized dopamine level increased (Figure 2, Table 2).
Figure 2.
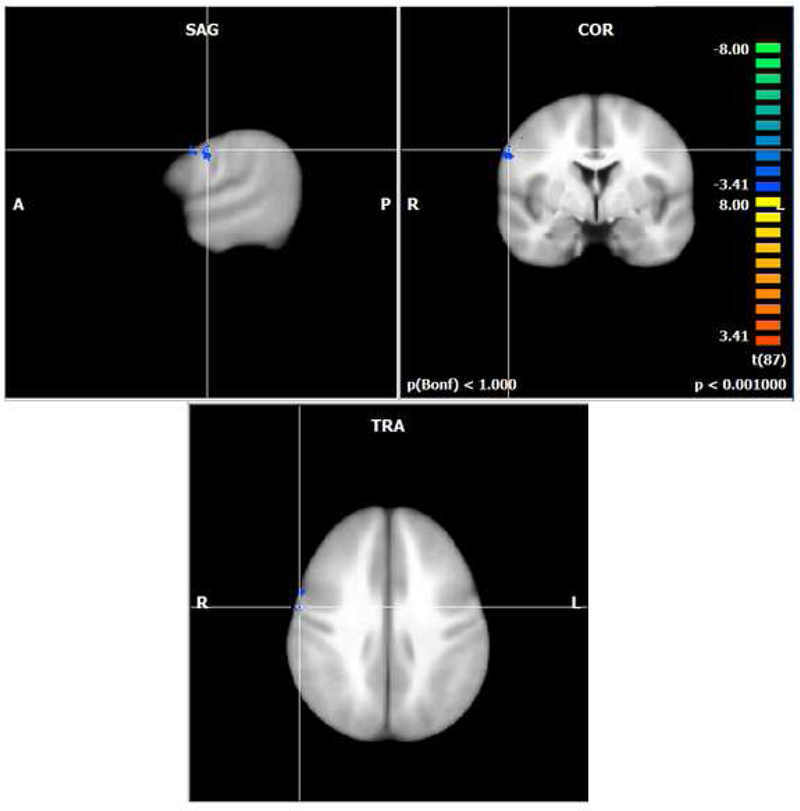
Activation map for hypothesized increase in dopamine across groups during the working memory load coditions of the N-back task (p < .001; cluster corrected k=8). Blue colors represent decreasing activation as the hypothesized dopamine level increased in the right precentral gyrus (BA 6). Greatest hypothesized dopamine in the Met/Met high estradiol group and lowest hypothesized dopamine in the Val/Val low estradiol group.
Table 2.
Activation related to the hypothesized increase in dopamine across the COMT genotype and estradiol groups during the working memory load conditions of the N-back task including Talairach coordinates, cluster size, region description (Brodmann’s area, BA), t values, and corrected voxel-level p value. Increasing dopamine refers to a linear contrast across groups from high to low inferred dopamine: Met/Met high estradiol, Met/Val high estradiol, Val/Val high estradiol, Met/Met low estradiol, Met/Val low estradiol, and Val/Val low estradiol.
| Contrast | Coordinates | Cluster Extent |
Region Description |
t value |
p value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Increasing Dopamine | |||||||
| Decreasing Activation |
57 | −4 | 31 | 155 | Right precentral gyrus (BA 6) | −4.08 | <.001 |
3.3. Working Memory Performance
Data were analyzed with a 3(COMT genotype: Met/Met, Met/Val, Val/Val) x 2(Estradiol level: high vs low) x 4(Working Memory Load: 0-back, 1-back, 2-back, 3-back) ANOVA for d’, C, and proportion correct (Figure 3). For all dependent measures there were the expected effects of working memory load which indicated the accuracy decreased as the working memory load increased (d’: F(3,261) = 85.03, p < .001, C: F(3,261) = 8.27, p < .001; proportion correct: F(3,261) = 32.44. p < .001). There were no main effects or interactions involving COMT or estradiol level.
Figure 3.
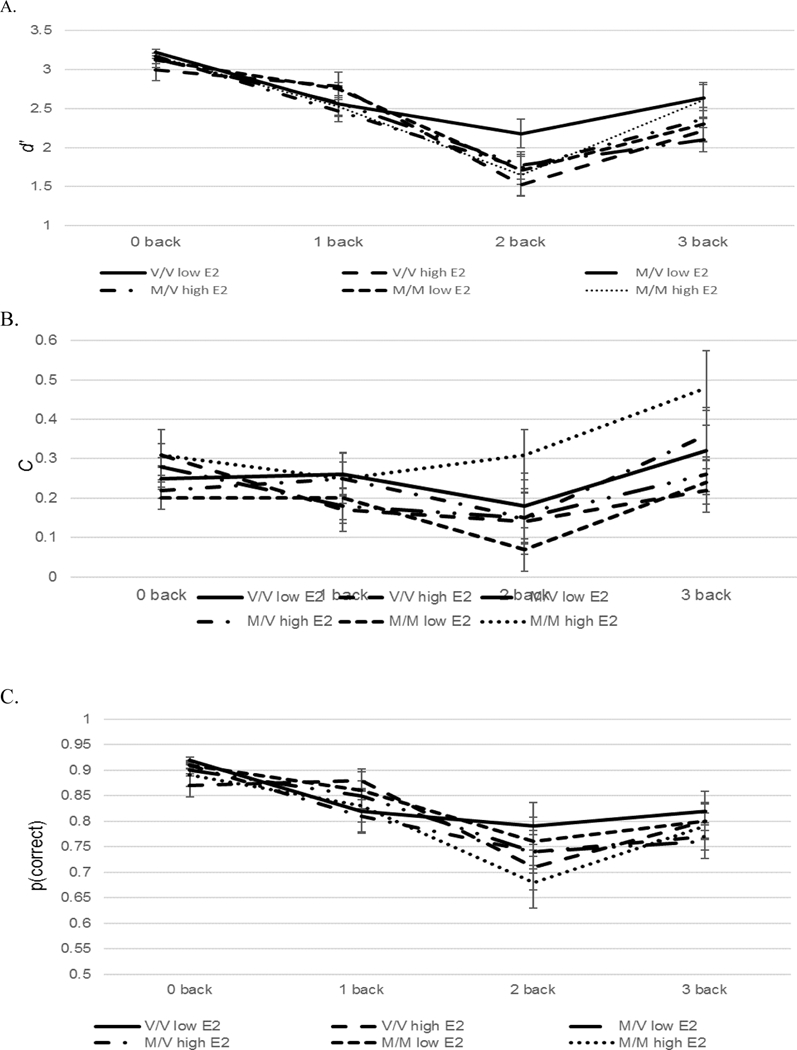
Sensitivity (d’; Figure 3a), bias (C; Figure 3b), and proportion correct (Figure 3c) with standard errors on the 0-, 1-, 2-, and 3-back conditions for the Met/Met high estradiol, Met/Val high estradiol, Val/Val high estradiol, Met/Met low estradiol, Met/Val low estradiol, and Val/Val low estradiol groups.
3.4. Neuropsychological Test Performance
When we examined the RBANS performance (Table 3) for the relationship between COMT and estradiol, we found a main effect of estradiol group for the RBANS Total score (F(1,87) = 4.83, p = .04) and the Visuospatial/Constructional score (F(1,87) = 6.21, p = .02). In both cases, the group with lower estradiol performed better than the higher estradiol group. The RBANS total score is simply a total of all of the subscales and thus, this estradiol difference is likely related to differences on the Visuospatial/Constructional score.
Table 3.
Performance (Mean(Standard Deviation)on the Repeated Battery for the Assessment of Neuropsychological Status (RBANS) for each COMT genotype and estradiol group. Group differences were found for the main effect of estradiol group on the RBANS total score and the Visuospatial/Constructional factor score that showed the low etradiol group performed better than the high estradiol group on these measures (ps < .05).
| Met/Met | Met/Val | Val/Val | Met/Met | Met/Val | Val/Val | |
|---|---|---|---|---|---|---|
| Low E2 | Low E2 | Low E2 | High E2 | High E2 | High E2 | |
| Immediate Memory |
110.36 (14.1) |
108.19 (14.7) |
104.31 (12.1) |
100.67 (11.4) |
106.17 (11.6) |
107.69 (9.4) |
| Visuospatial/ Constructional# |
115.21 (17.0) |
117.176 (8.2) |
18.38 (7.7) |
103.11 (10.2) |
114.43 (13.9) |
114.00 (12.8) |
| Language | 110.00 (15.5) |
109.43 (11.1) |
110.46 (8.8) |
106.89 (9.7) |
105.35 (11.7) |
108.08 (10.6) |
| Attention | 115.43 (13.2) |
111.00 (14.0) |
112.85 (11.4) |
107.11 (11.3) |
111.22 (10.3) |
114.46 (9.3) |
| Delayed Memory | 108.71 (13.7) |
106.86 (11.3) |
101.46 (17.3) |
97.33 (9.0) |
105.00 (15.3) |
105.08 (9.0) |
| Total# | 118.50 (16.6) |
115.38 (10.9) |
113.46 (10.7) |
104.00 (11.5) |
113.68 (11.9) |
114.15 (10.1) |
Main effect of estradiol group, Visuospatial/Constructional (F(1,87) = 6.21, p = .02), Total (F(1,87) = 4.48, p = .04).
4. Discussion
The current study was the first to examine how COMT genotype and estradiol in postmenopausal women were related to brain functioning. The results revealed that the primary outcome measures mostly showed no effects of COMT and estradiol on working memory performance and brain activation in healthy postmenopausal women. There was one small region of decreased activation as the hypothesized dopamine level increased in a frontal lobe working memory region. Specifically, women with a Met/Met genotype in the high estradiol group had the least activation in the right precentral gyrus while women with a Val/Val genotype in the low estradiol group had greater activation in this region relative to the other groups. Performance on the N-back task did not show any COMT or estradiol effects. These data extend what is known about dopaminergic functioning and cognition to women who are postmenopausal and demonstrate how the role of COMT and its interaction with estradiol might be different in women post menopause.
We hypothesized that higher levels of dopamine would be beneficial to cognition in postmenopausal women. We operationalized increased levels of dopamine with being Met/Met homozygous for the COMT gene. Women who are homozygous for Met have less transcription of the COMT enzyme than those who are Val homozygotes (Chen et al., 2004; Lachman et al., 1996; Lotta et al., 1995). COMT is also primarily located in the frontal lobes of the brain and is therefore likely to influence frontal lobe dopamine levels (Matsumoto et al., 2003). In addition, we examined levels of estradiol and operationalized higher levels of dopamine with having higher levels of estradiol. There is an estrogen response element on the COMT promotor region (Jiang et al. 2003; Xie et al. 1999; Weinshilboum 2006). Thus, when estrogen is present COMT transcription does not occur and there is more dopamine is available in the synapse. Our data showed that the hypothesized dopamine level was inversely related to brain activation during the N-back task which has been interpreted in prior studies as more efficient brain functioning (Jacobs and D'Esposito, 2011). However, performance on our task was not related to inferred dopamine level. Below we hypothesize about why this data pattern was observed, how it relates to the prior literature, and the contribution it makes to what is known about individual differences in cognition after menopause.
Jacobs and D’Esposito (2011) used COMT genotype and estradiol levels as proxy measures for dopamine in younger normal cycling premenopausal women. Their imaging data showed a linear decrease in the middle frontal gyrus associated with an increase in inferred dopamine across groups with the Met/Met high estradiol group having greatest decreased activation and the Val/Val low estradiol group having the least decrease with the other groups in the middle. For the performance analysis, they examined correct rejection of lures during the N- back task and not an overall accuracy measure like proportion correct or sensitivity. Their results showed that the Met/Met low estradiol group performed best on the lure items and the relationship between dopamine and working memory performance was an inverted-U as is often found in the relationship of neurotransmitter levels and cognitive functioning. They emphasized the importance of the COMT genotype in understanding the role of estrogen on cognition in premenopausal women.
Overall, the results in the current study from postmenopausal women were similar to the younger premenopausal women with regard to the direction of the brain imaging activation pattern, but the specific region was different and the size of the effect in postmenopausal women appears to be smaller. We did find a dopamine-sensitive decrease in the right precentral gyrus (BA 6). Jacobs and D’Esposito (2011) found activation in the bilateral MFG. In postmenopausal women this dopamine sensitive region was smaller in size, more posterior, and right lateralized. In addition, we also found no effect of COMT and estradiol on working memory accuracy performance as measured by sensitivity, bias, and proportion correct. We did not build lure items into our task so we were not able to examine these responses similar to Jacobs and D’Esposito (2011). Thus, in the presence of postmenopausal estradiol levels, COMT genotype may be less important for explaining individual differences in cognition compared to premenopausal women. As the fluctuating levels of estradiol during the menstrual cycle cease to occur, estradiol’s relationship with dopaminergically-driven cognition also appears to decline.
There have been attempts to stimulate the dopaminergic system to influence cognition in women around menopause who have subjective complaints about their cognition by Epperson and colleagues (Epperson et al., 2011; Epperson et al., 2015). In peri- and postmenopausal women, Epperson and colleagues found that atomoxetine treatment for six weeks compared to placebo improved subjective reports of memory and attention but had no effect on objective performance (Epperson et al., 2011). They also found lisdexamphetamine for four weeks compared to placebo improved subjective cognition as well as delayed recall in postmenopausal with menopause related subjective cognitive decline. Thus, studies are beginning to examine methods other than hormonal treatment after menopause to improve cognition and methods that modulate the dopaminergic system may be useful in this endeavor. It may be the case the personalization of treatments for cognition associated with the hormonal change at menopause may be possible by considering a woman’s COMT genotype and circulating estradiol. Further work is needed to examine when the COMT-estradiol influence on brain functioning and cognition changes as women age through the menopause transition.
4.1. Conclusion
The current study examined how COMT genotype and estradiol in postmenopausal women were associated with working memory-related brain functioning. The data showed that neither COMT nor postmenopausal estradiol were related to working memory-related brain functioning. There was one brain region showing an interaction between COMT and estradiol that showed decreasing activation associated with increasing dopamine. However, there were no relationships between COMT, estradiol, and working memory performance. We propose that this COMT-estradiol relationship may underlie individual differences in cognition before menopause as shown by Jacobs and D’Esposito (2011). However, in postmenopausal women, the COMT- estradiol interaction appears to be less important in explaining individual differences in cognition. The structural (Volkow et al., 1998; Wong et al., 1984) as well as functional (Backman et al., 2006) changes in the dopaminergic system will continue into old age. Further studies are needed to determine when estradiol’s relationship with working memory performance changes as its levels decrease after menopause. This information may influence the development of personalized methods for the treatment of cognitive changes after menopause.
Acknowledgements
This work was supported by NIA R21 AG047944, DoE SC 0001753. The authors wish to thank the research nursing staff of the University of Vermont Clinical Research Center for their hard work and support of this study and our volunteers for their dedication to clinical research. We would like to thank Jay Gonyea, Scott Hipko, Trevor Andrews, Ph.D., and Richard Watts, D.Phil. from the University of Vermont MRI Center for Biomedical Imaging for their help in MRI data acquisition. We would also like to thank Jessica Hoffman and Tim Hunter from the UVM Cancer Center Advanced Genome Technologies Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Backman L, Nyberg L, Lindenberger U, Li S, Farde L, 2006. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neuroscience and Biobehavioral Reviews 30, 791–807. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GT, 1996. Manual for the Beck Depression Inventory-II. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Braver TS, Barch DM, 2002. A theory of cognitive control, aging cognition, and neuromodulation. Neurosceince and Biobehavioral Reviews 26, 809–817. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Keilp JG, Xu H, Shikhman M, Schori E, Gorman JM, Gilliam TC, 2005. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry 58(11), 901–907. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ, 1989. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR, 2004. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 75(5), 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Song J, Yuan P, Tian Q, Ji Y, Ren-Patterson R, Liu G, Sei Y, Weinberger DR, 2011. Orientation and cellular distribution of membrane-bound catechol-O- methyltransferase in cortical neurons: implications for drug development. The Journal of biological chemistry 286(40), 34752–34760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE, 1997. Temporal dynamics of brain activation during a working memory task. Nature 386, 604–607. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson LG, 2005. Catechol O-methyltransferase Val158Met polymorphism is associated with cognitive performance in nondemented adults. J Cogn Neurosci 17(7), 1018–1025. [DOI] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA, 2010. Increased memory load-related frontal activation after estradiol treatment in postmenopausal women. Horm Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA, 2012. Estradiol treatment altered anticholinergic-related brain activity in postmenopausal women. Neuroimage 60, 1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Saykin AJ, McDonald BC, McAllister TW, Hynes ML, Newhouse PA, 2008. Nicotinic versus muscarinic blockade alters verbal working memory-related brain activity in older women. American Journal of Geriatric Psychiatry 16, 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR, 2001. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A 98(12), 6917–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Pittman B, Czarkowski KA, Bradley J, Quinlan DM, Brown TE, 2011. Impact of atomoxetine on subjective attention and memory difficulties in perimenopausal and postmenopausal women. Menopause 18(5), 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Shanmugan S, Kim DR, Mathews S, Czarkowski KA, Bradley J, Appleby DH, Iannelli C, Sammel MD, Brown TE, 2015. New onset executive function difficulties at menopause: a possible role for lisdexamfetamine. Psychopharmacology (Berl) 232(16), 3091–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahndrich E, Coper H, Christ W, Helmchen H, Muller-Oerlinghausen B, Pietzcker A, 1980. Erythrocyte COMT-activity in patients with affective disorders. Acta Psychiatr Scand 61(5), 427–437. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition, SCID-I/P ed Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. "Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC, 1995. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 33(5), 636–647. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS,Goldman D, Weinberger DR, 2003. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry 60(9), 889–896. [DOI] [PubMed] [Google Scholar]
- Holdnack HA, 2001. Wechsler Test of Adult Reading (WTAR). The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Jacobs E, D'Esposito M, 2011. Estrogen shapes dopamine-dependent cognitive processes: implications for women's health. J Neurosci 31(14), 5286–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Xie T, Ramsden DB, Ho SL, 2003. Human catechol-O-methyltransferase down- regulation by estradiol. Neuropharmacology 45, 1011–1018. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM, 1996. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 6(3), 243–250. [DOI] [PubMed] [Google Scholar]
- Liehr JG, Ricci MJ, 1996. 4-Hydroxylation of estrogens as marker of human mammary tumors. Proc Natl Acad Sci U S A 93(8), 3294–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liehr JG, Roy D, 1990. Free radical generation by redox cycling of estrogens. Free radical biology & medicine 8(4), 415–423. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J, 1995. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 34(13), 4202–4210. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, Kleinman JE, Weinberger DR, 2003. Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience 116(1), 127 137. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li SC, von Oertzen T, Sander T, Villringer A, Heekeren HR, Backman L, Lindenberger U, 2008. Human aging magnifies genetic effects on executive functioning and working memory. Front Hum Neurosci 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse P, Albert K, Astur R, Johnson J, Naylor M, Dumas J, 2013. Tamoxifen improves cholinergically modulated cognitive performance in postmenopausal women. Neuropsychopharmacology 38(13), 2632–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara R, Miller E, Liao CP, Way N, Lin X, Hallmayer J, 2006. COMT genotype, gender and cognition in community-dwelling, older adults. Neurosci Lett 409(3), 205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst-Hensch NM, Ingles SA, Diep AT, Haile RW, Stanczyk FZ, Kolonel LN, Henderson BE, 1999. Aromatase and breast cancer susceptibility. Endocrine-related cancer 6(2), 165–173. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN, 1998. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 20(3), 310–319. [DOI] [PubMed] [Google Scholar]
- Raz N, Dahle CL, Rodrigue KM, Kennedy KM, Land S, 2011. Effects of age, genes, and pulse pressure on executive functions in healthy adults. Neurobiol Aging 32(6), 1124–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, 1988. Brief cognitive rating scale (BCRS). Psychopharmacol Bull 24(4), 629–635. [PubMed] [Google Scholar]
- Sambataro F, Reed JD, Murty VP, Das S, Tan HY, Callicot JH, Weinberger DR, Mattay V, 2009. Catechol-O-Methrytransferase Valine158Methionine Polymorphism Modulates Brain Networks Underlying Working Memory Across Adulthood. Biological Psychiatry 66, 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass J, Corwin J, 1988. Pragmatics of measuring recognition memory: applications to dementia and amnesia. Journal of Experimental Psychology 117(1), 34–50. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang G, Fowler JS, Moberg PJ, Ding Y, Hitzemann R, Smith G, Logan J, 1998. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 155(3), 344–349. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, 2006. Pharmacogenomics: catechol O-methyltransferase to thiopurine S- methyltransferase. Cell Mol Neurobiol 26(4–6), 539–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Wagner HN Jr., Dannals RF, Links JM, Frost JJ, Ravert HT, Wilson AA, Rosenbaum AE, Gjedde A, Douglass KH, et al. , 1984. Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science 226(4681), 1393–1396. [DOI] [PubMed] [Google Scholar]
- Xie T, Ho SL, Ramsden D, 1999. Characterization and implications of estrogenic down- regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol 56(1), 31–38. [DOI] [PubMed] [Google Scholar]


