Abstract
Cells have evolved to dynamically respond to different types of environmental and physiological stress conditions. The information about a previous stress stimulus experience by a mother cell can be passed to its descendants, allowing them to better adapt to and survive in new environments. In recent years, live-cell imaging combined with cell-lineage tracking approaches has elucidated many important principles that guide stress inheritance at the single-cell and population level. In this review, we summarize different strategies cells can employ to pass the ‘memory’ of previous stress responses to their descendants. Among these strategies, we focus on a recent discovery of how specific features of Msn2 nucleo-cytoplasmic shuttling dynamics could be inherited across cell lineages. We also discuss how stress response can be transmitted to progenies through changes in chromatin and through partitioning of anti-stress factors and/or damaged macromolecules between mother and daughter cells during cell division. Finally, we highlight how emergent technologies will help address open questions in the field.
Keywords: Stress response, Msn2, epigenetic inheritance, single cells, yeast, mammalian cells
INTRODUCTION
All cellular life faces constant challenges of internal and external stress. Unicellular organisms such as bacteria and yeast must sense and adapt to environmental fluctuations in nutrient, temperature and osmotic pressure to ensure survival1. Exposure to toxins and high doses of radiation can cause damages to DNA, lipid and protein molecules. Cells within an embryo respond to mechanical stretch and compression during normal embryonic development. In response to oncogene activation, cellular defense mechanisms can lead to senescence or apoptosis of precancerous cells2. At the systems level, the immune system comprised of diverse cell types is a highly evolved stress response mechanism that can identify a wide variety of pathogens as well as cancerous cells and defend the organism against them.
A descendant cell’s inheritance of its ancestors’ previous stress responses in a ‘memory’-like fashion can be expected to serve as a mechanism to enhance cell survival. It is generally believed that such an inheritance allows the descendant cell to more rapidly adapt to a new environment3, or acquire immunity against a previously encountered pathogen. Inheritance of stress response can occur through both genetic and epigenetic means. For example, antibiotic treatments are known to increase genomic mutation rates in many species of bacteria, and these mutations can be passed on to descendant cells to drive drug-resistance4. Moreover, some bacteria acquire viral resistance by integrating short fragments of viral nucleic acids into CRISPR repeats5, which can be stably inherited. Contrary to genetic mutations, changes facilitated by non-genetic factors are often dynamic and reversible. Epigenetic mechanisms operating at a fast timescale can be particularly advantageous for cells growing in fluctuating environments6, because they can allow cells to rapidly switch between different gene expression or growth states in response to the dynamic changes in the external stress conditions.
In this review, we discuss different epigenetic mechanisms that cells can employ to pass their stress response histories to their descendants. We focus on the recent discoveries of the heritable features of transcription factor dynamics in response to stress. We then highlight how the emergent technologies will help address open questions in the field.
STRATEGIES for the EPIGENETIC INHERITANCE of STRESS RESPONSE
1. Inheritance of transcription factor dynamics
Cells have evolved complex signaling networks to sense and respond to different stress signals by activating specific downstream genes7. Transcription factors (TFs) are key components of the signaling cascades orchestrating a cell’s response to stress8. Curiously, many TFs exhibit dynamic behaviors in response to stress9,10. A recent genome-wide screen identified ~8% of the yeast TFs stochastically shuttle in and out of the nucleus under various conditions9. Single-cell studies in recent years have revealed that transcription factors can transmit quantitative information corresponding to distinct environmental conditions11,12. This information can in principle be encoded in the nuclear localization frequency, amplitude, and duration of the specific transcription factors11,12.
Msn2 is a major transcription factor in yeast S. cerevisiae. It regulates the expression of ~200 genes in response to a variety of stressors, including glucose starvation, oxidative stress, heat shock, and osmotic stress13,14. Using high-resolution time-lapse microscopy on single yeast cells, previous studies have found that Msn2 dynamically shuttles between cytoplasm and the nucleus11,12,15,16. In the case of stress caused by glucose limitation, the Msn2 nuclear localization was revealed to occur every 1.5–2 min on average11,16 (Fig. 1). The dynamics of Msn2 nuclear localization is controlled by the phosphorylation state of this protein. Under normal growth conditions, cAMP-dependent protein kinase A (PKA) phosphorylates the nuclear localization sequence on Msn2 and keeps it in the cytoplasm. PKA activity is downregulated upon glucose limitation, leading to dephosphorylation of Msn2 and its transport into the nucleus13 (Fig. 1). Additionally, protein phosphatase 1 (PP1) can also directly dephosphorylate Msn2, leading to its nuclear localization17. Thus, each Msn2 nuclear localization event corresponds to the simultaneous dephosphorylation of a large fraction of the ~125 copies of Msn2 molecules per cell18. Increasing the intensity of glucose limitation stress increases the frequency12,16 and amplitude16 of Msn2 nuclear localization, but does not affect its duration12,16.
Figure 1. Stress induces dynamic changes in Msn2 nuclear localization.
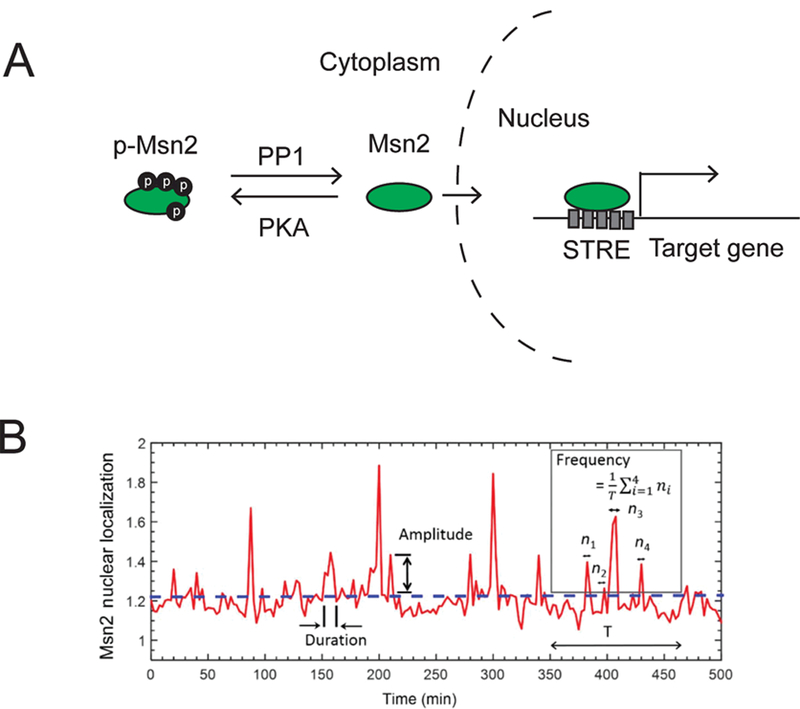
A. In response to various stress stimuli, Msn2 proteins become dephosphorylated and translocate into the nucleus to activate downstream gene expression. PP1: Protein phosphatase 1, PKA: cAMP-dependent protein kinase A, STRE: stress response element. B. Msn2 nuclear localization trajectory of a cell, showing how amplitude, frequency, and duration of Msn2 nuclear localization are quantified. The dashed horizontal line denotes the threshold level above which there would be an Msn2 nuclear localization event. ni denotes the number of above-the-threshold localization events. T denotes the length of time interval used for the calculation of frequency. Figure panel was taken from 16.
To understand if the key features of the Msn2 localization dynamics are heritable, Chatterjee & Acar (2018) used a microfluidic chip to track Msn2 nuclear localization dynamics in lineages or ‘families’ of yeast cells during long-term (15–18hrs) glucose limitation16. They found that the frequency of Msn2 nuclear localization was inherited in progenies of mother cells, whereas the amplitude and duration did not show such inheritance. At high stress levels (0.1% glucose), mother, daughter and granddaughter cells often exhibited synchronous Msn2 localization events. What can account for the inheritance of this seemingly stochastic dynamics of Msn2 between the mother cell and its descendants? One hypothesis is that the activity of either the upstream kinase PKA or the PP1 phosphatase could be passed on from mother to its descendants, leading to synchronized Msn2 phosphorylation states, and in turn, similar nuclear localization patterns across generations. Indeed, an elegant study published by Hao & O’Shea12 (2012) showed that applying a PKA inhibitor (1-NM-PP1) to yeast cells carrying mutations in all three catalytic subunits of PKA (Tpk1, Tpk2, Tpk3) is sufficient to precisely control the amplitude, frequency and duration of the Msn2 nuclear localization.
In another example, the tumor suppressor protein p53 and its negative regulator Mdm2 were shown to display heritable nuclear localization dynamics in response to DNA damage19. Geva-Zatorsky et al. (2006) tracked p53 and Mdm2 protein levels in individual breast cancer cells taken from an isogeneic clone following γ-irradiation damage19. Upon irradiation, protein levels of p53 and Mdm2 continuously oscillated in a large population of cells with a period of ~5.5hrs for at least three days. This oscillatory behavior was attributed to the presence of a negative feedback loop between p53 and Mdm220. After cell division, Mdm2 protein levels in sister cell-pairs continued to oscillate in the same phase, until the signal became unsynchronized after ~11hrs, suggesting that the information was transmitted from the mother cells to their progenies.
Similar to p53, nuclear factor κB (NFkB) also exhibits oscillatory behavior due to a negative feedback loop between NFkB and its inhibitor IκB21. NFκB is the primary TF of the innate immune system22; it also plays a role in cells’ response to mechanical stress23. Upon stimulation with TNF-α, NFκB was shown to display sustained nucleo-cytoplasmic oscillations with a period of ~100min for over 20hrs, after which the oscillations slowly dampened21. Interestingly, the period of the oscillations was highly similar (albeit slightly out of phase) in sister cell-pairs after cell division24. To find out how long this similarity could last, the authors derived multiple clonal lines from single cells and tracked them over 30 generations. Their results showed that the oscillation period distribution for each clone resembled each other. What caused the inheritance of NFkB oscillation is still unknown; the authors proposed that it may have been caused by epigenetic mechanisms acting through specific proteins or chromatin modifications24.
Despite these examples, not all transcription factors’ dynamics are heritable. In response to extracellular calcium, another transcription factor, Crz1, translocates into the nucleus in a rapid burst (synchronized among cells) followed by short (~2min), stochastic bursts11. Increased calcium concentration results in an increased frequency of Crz1 nuclear localization but does not affect its nuclear localization duration11. Unlike Msn2, however, the overall nuclear localization dynamics of Crz1 appear to operate in an asynchronous manner between related cell pairs as the burst dynamics in daughter cells do not appear to be correlated with those in the mother cells11, suggesting that Crz1 nuclear localization dynamics is not heritable.
2. Stress-induced changes in chromatin
Beyond altering nuclear localization dynamics of specific transcription factors, stress signaling can also cause changes in chromatin structure or modifications in DNA and histone marks3 (Fig 2). Several types of stressors have been shown to trigger global re-organization of chromatin structure. For example, prolonged heat stress induces decondensation of the ribosomal DNA (rDNA) region and activation of silenced repetitive elements in Arabidopsis thaliana25. Bacterial and viroid infections can cause decondensation of heterochromatin in some plants26,27. Interestingly, both glucose starvation (Xue & Acar, in revision) and rapamycin treatment28 in budding yeast induce condensation, rather than decondensation, of the rDNA chromatin. Global condensation of chromatin is also reported in HeLa cells in response to serum starvation29. The exact biological function of these stress-induced changes in chromatin structure is not well understood. Additionally, it still remains to be determined whether these changes can be stably inherited over multiple cell generations.
Figure 2. Stress can cause heritable changes in chromatin structure and biochemistry.
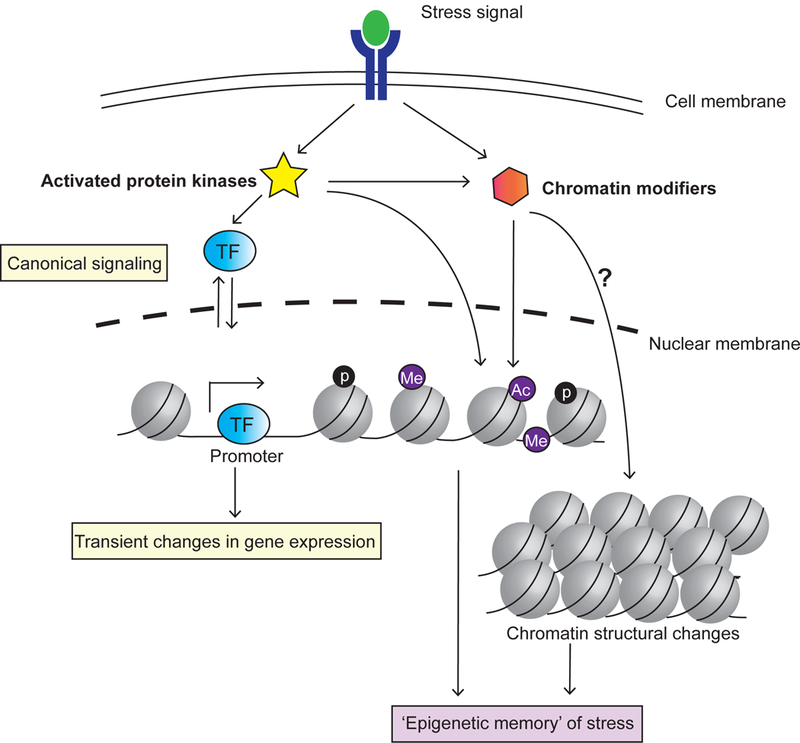
Activated protein kinases can directly or indirectly change epigenetic marks on DNA and histones. Stress response can also change the 3D structure of chromatin. If sufficiently stable, these epigenetic changes can be heritable by daughter cells, corresponding to passing an ‘epigenetic memory’ of mother’s specific transcriptional states. p: phosphorylation; Me: Methylation; Ac: Acetylation.
Numerous stress-signaling kinases, including AKT30,31, JAK232 and AMPK33, have been shown to directly or indirectly modify histone marks. In response to metabolic stress or UV damage, the mammalian AMPK kinase directly binds to promoters and open reading frames of target genes and phosphorylates histone H2B33. Oncogenic stress (e.g., due to overexpression of an oncogene) in human cells induces the expression of a histone H3K27 demethylase JMJD3, which in turn removes repressive H3K27me3 marks on tumor suppressor genes p16INK4A and p14ARF to help initiate cellular senescence34. To date, over 20 phosphorylation sites on histones regulated by upstream signaling kinases have been reported2. Another specific stress response, the DNA damage response (DDR), is intimately linked to chromatin modifications35. DNA damage can trigger phosphorylation or dephosphorylation of different histones (e.g. H2A, H2B and H3) and chromatin modifiers, with different histone phosphorylation states facilitating distinct cellular decisions, such as cell cycle arrest, DNA repair, chromatin condensation, or apoptosis36.
MicroRNAs (miRNAs), short RNAs of ~22 nucleotides, have recently been found to play a key role in regulating diverse stress responses in mammals, insects and plants37. Many miRNAs are strategically positioned as part of negative or positive feedback loops established by known transcription factors mediating stress responses. For example, miR-9, miR-155, and miR-146 have been found to be expressed as part of the NFkB-dependent signaling cascade in response to inflammation; they in turn repress the targets of pro-inflammatory pathway to help reset the inflammatory response37.
Epigenetic modifications can be either dynamically changing or relatively stable38. It has been proposed that, although some epigenetic changes are transient and can be reversed by chromatin modifiers, others may leave a lasting ‘epigenetic memory’ on chromatin, causing cells to be ‘locked’ in specific gene expression states2. Despite the appeal of this idea, experimental evidence (especially at the single cell level) in support of this hypothesis is still lacking.
3. Facilitating epigenetic inheritance via cell division and fusion
Cell division is a simple yet powerful mechanism that can allow mother cells to pass different anti-stress factors on to daughter cells (Fig. 3). In theory, this mechanism can equip daughter cells with anti-stress factors as soon as they are born into the harsh environment, hence improving their survival rate. Anti-stress factors may include transcription factors, activated protein kinases, mRNAs that encode proteins conferring stress resistance, and miRNAs. Additionally, storage carbohydrates (especially trehalose) produced in response to glucose starvation protect cells in poor nutrient conditions and contribute to chronological lifespan extension in yeast39. Some of these anti-stress factors are long-lived. For example, miRNAs can be relatively stable with a half-life of ~12 days in vivo37, implying that their activity can be passed on to descendant cells over multiple cell divisions.
Figure 3. Cell division and cell-to-cell fusion propagate stress response to descendants.
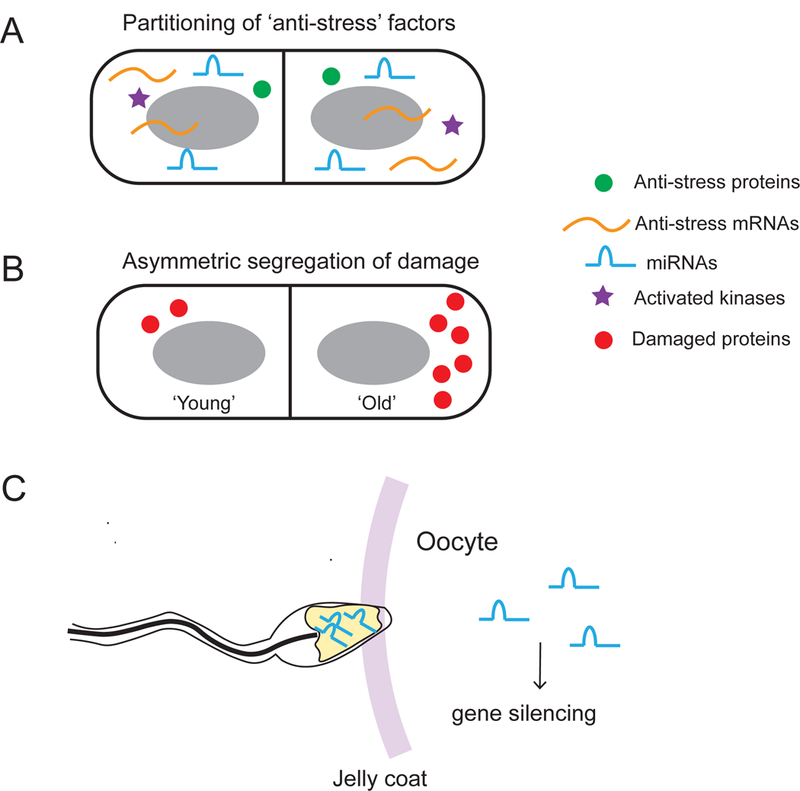
A. Anti-stress proteins, mRNAs and miRNAs are passed on to daughter cells during cell division. B. Asymmetrical segregation of damaged molecules generates a ‘damage-protected’ daughter cell at the expense of the mother cell’s being burdened with more damage. C. Sperm carrying miRNAs produced as a result of stress-induction transmits the stress signal to an oocyte during fertilization.
In addition to passing on anti-stress factors, cell division can control partitioning of damaged macromolecules and restrict them to a certain population of cells. This asymmetric damage segregation has been observed in bacteria40, yeast41,42, and stem cells43,44, and is thought to produce newborn cells that are ‘damage-protected’ at the cost of more damaged, ‘aged’ mother cells. For instance, carbonylated proteins, as a result of irreversible oxidative damage, are found to accumulate in mother cells of the budding yeast during cytokinesis41. In E. coli, damaged proteins tend to form aggregates that localize to the old pole from the previous cell division45. Recent single-cell lineage studies have shown that the degree of asymmetry in the damage partitioning process in E. coli was heritable, such that cells with more damage showed higher levels of asymmetric segregation of damaged proteins46. Continuous re-distribution of damaged proteins in individual cells was shown to be evolutionarily advantageous as it can enhance bacterial growth on a population level46.
Intriguingly, cell division can also serve as a ‘timer’ that causes mother cells and their progenies to synchronously switch between distinct phenotypic states even after cell division. Using time-lapse microscopy, Kaufmann et al. (2007) studied the phenotypic switching behavior in lineages of yeast cells containing an engineered version of the galactose utilization (GAL) network47. In the yeast strain48 they used, the endogenous negative feedback loop mediated by the Gal80 promoter had been abolished and the Gal80 expression was driven by the TET promoter. It had already been shown how Gal80 (repressor of Gal4 activity) expression and galactose concentration affected the stochastic switching frequency between the OFF and ON states of the bimodal GAL network48. The authors observed that some mother and daughter cells switched to the ON state synchronously, and this correlation in switching times lasted for several cell generations. How could this switching behavior be heritable? Quantitative modeling showed that the synchrony in phenotypic switching was predominantly dependent on Gal80. Although GAL network is not a stress-response network, stress-response networks with similar topologies would be expected to utilize the same mechanism to make related cells respond to stress in synchrony.
As a process operating in the opposite direction to cell division, cell-to-cell fusion (particularly in the context of fertilization) can be an important mechanism for trans-generational inheritance of stress responses49 (Fig. 3C). Recent studies in mice have shown that several types of miRNAs are produced in sperm cells in response to chronic stress; these miRNAs are passed on to the oocyte during fertilization and can suppress gene expression in the embryo50,51.
CONCLUSIONS and FUTURE PERSPECTIVES
In conclusion, cells can utilize multi-layered epigenetic regulatory mechanisms to pass on a ‘memory’ of previous stress responses to the next generation. These mechanisms include controlling the dynamics of the nuclear localization of transcription factors, changing chromatin structure and biochemistry, and partitioning anti-stress factors and/or damaged molecules between mother and daughter cells.
In the past decade, live-cell imaging technologies together with cell-lineage tracking approaches have uncovered many fundamental principles that guide stress-response inheritance at the single-cell and population level. Despite significant advances made, several important questions still remain unaddressed. For example, what upstream events cause the synchronous nucleo-cytoplasmic shuttling dynamics of certain transcription factors between mother and daughter cells? How are the activities of the kinases involved in stress signaling inherited across cell lineages? Which stress-induced epigenetic modifications are stably passed on to daughter cells and which are erased? What is the physiological relevance of these stable and transient modifications? What are the biological functions of the stress-induced global structural changes in chromatin? How are different anti-stress factors partitioned during the cell division and how do the different partitioning schemes impact stress response in progenies?
Some of these questions are ready to be addressed using recently developed biosensors. Live-cell biosensors for monitoring cAMP levels and PKA activity are now available for S. cerevisiae52 and mammalian cells53,54. It would be very informative to examine PKA activities in single cells and determine whether the PKA activities are similar in mother cells and their descendants. Live-cell reporters of histone H355 and H456 lysine acetylation have also been developed. More recently, a fluorescence complementation sensor has been used for real-time visualization of DNA methylation and H3K9me3 marks at major satellite repeats57. It will be exciting to apply these reporters to the tracking of the long-term inheritance of these epigenetic marks in live cells. For detection of low-abundance miRNAs in live cells, a reporter has been developed based on programmable molecular hairpins that can self-assemble to produce FRET signal58. Finally, the combination of single-cell RNA-seq technology with cell-lineage tracking methods59 can be very powerful to examine stress-induced global transcriptomic changes that occur in single cells and to track how these changes are inherited across cell lineages. Applying all these new tools to track the long-term inheritance of epigenetic changes in live cells will be instrumental in answering the questions posed earlier. We anticipate that these technological innovations will drive the discovery of new principles, expanding our understanding of the mechanisms underlying stress-response inheritance.
ACKNOWLEDGMENTS
We thank Lolahon Kadiri and Jessica Ye for critical reading of the manuscript. MA acknowledges funding from the National Institutes of Health (1DP2AG050461–01 and 1U54CA209992–01).
Footnotes
AUTHOR CONTRIBUTIONS
YX and MA designed the manuscript. YX drafted the manuscript. MA edited and revised the drafted manuscript. YX and MA read, discussed, and approved the manuscript.
REFERENCES
- 1.Estruch F Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol. Rev 24, 469–486 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Liu F, Wang L, Perna F & Nimer SD Beyond transcription factors: How oncogenic signalling reshapes the epigenetic landscape. Nat. Rev. Cancer 16, 359–372 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Urso A & Brickner JH Epigenetic transcriptional memory. Curr. Genet 63, 435–439 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodford N & Ellington MJ The emergence of antibiotic resistance by mutation. Clin. Microbiol. Infect 13, 5–18 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Brouns SJJ et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321, 960–4 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acar M, Mettetal JT & Van Oudenaarden A Stochastic switching as a survival strategy in fluctuating environments. Nat. Genet 40, 471–475 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Milisav I Cellular Stress Responses. Adv. Regen. Med. 215–232 (2011). doi: 10.1016/j.molcel.2010.09.022. [DOI] [Google Scholar]
- 8.Soontorngun N Reprogramming of nonfermentative metabolism by stress-responsive transcription factors in the yeast Saccharomyces cerevisiae. Curr. Genet 63, 1–7 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Dalal CK, Cai L, Lin Y, Rahbar K & Elowitz MB Pulsatile dynamics in the yeast proteome. Curr. Biol 24, 2189–2194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ankers JM, Spiller DG, White MR & Harper CV Spatio-temporal protein dynamics in single living cells. Curr. Opin. Biotechnol 19, 375–380 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Cai L, Dalal CK & Elowitz MB Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature 455, 485–490 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao N & O’Shea EK Signal-dependent dynamics of transcription factor translocation controls gene expression. Nat. Struct. Mol. Biol 19, 31–40 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Görner W et al. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev 12, 586–97 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitt AP & McEntee K Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A 93, 5777–82 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacquet M, Renault G, Lallet S, De Mey J & Goldbeter A Oscillatory nucleocytoplasmic shuttling of the general stress response transcriptional activators Msn2 and Msn4 in Saccharomyces cerevisiae. J. Cell Biol 161, 497–505 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee M & Acar M Heritable stress response dynamics revealed by single-cell genealogy. Sci. Adv (2018). doi: 10.1126/sciadv.1701775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Wever V, Reiter W, Ballarini A, Ammerer G & Brocard C A dual role for PP1 in shaping the Msn2-dependent transcriptional response to glucose starvation. EMBO J 24, 4115–4123 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghaemmaghami S et al. Global analysis of protein expression in yeast. Nature 425, 737–741 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Geva-Zatorsky N et al. Oscillations and variability in the p53 system. Mol. Syst. Biol 2, 1–13 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lev Bar-Or R et al. Generation of oscillations by the p53-Mdm2 feedback loop: A theoretical and experimental study. Proc. Natl. Acad. Sci 97, 11250–11255 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson DE Oscillations in NF- B Signaling Control the Dynamics of Gene Expression. Science 306, 704–708 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Hayden MS, West AP & Ghosh S NF-κB and the immune response. Oncogene 25, 6758–6780 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Mendez MG & Janmey PA Transcription factor regulation by mechanical stress. Int. J. Biochem. Cell Biol 44, 728–732 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughey JJ, Gutschow MV, Bajar BT & Covert MW Single-cell variation leads to population invariance in NF- B signaling dynamics. Mol. Biol. Cell 26, 583–590 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pecinka A et al. Epigenetic Regulation of Repetitive Elements Is Attenuated by Prolonged Heat Stress in Arabidopsis. Plant Cell 22, 3118–3129 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavet V, Quintero C, Cecchini NM, Rosa AL & Alvarez ME Arabidopsis Displays Centromeric DNA Hypomethylation and Cytological Alterations of Heterochromatin Upon Attack by Pseudomonas syringae. Mol. Plant-Microbe Interact 19, 577–587 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Annacondia ML, Magerøy MH & Martinez G Stress response regulation by epigenetic mechanisms: changing of the guards. Physiol. Plant 162, 239–250 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Tsang CK, Li H & Zheng XS Nutrient starvation promotes condensin loading to maintain rDNA stability. EMBO J 26, 448–58 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Maharana S, Wang MD & Shivashankar GV Super-resolution microscopy reveals decondensed chromatin structure at transcription sites. Sci. Rep 4, 4477 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cha T-L et al. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science 310, 306–10 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Liu Y et al. Akt Phosphorylates the Transcriptional Repressor Bmi1 to Block Its Effects on the Tumor-Suppressing Ink4a-Arf Locus. Sci. Signal 5, ra77–ra77 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu F et al. JAK2V617F-Mediated Phosphorylation of PRMT5 Downregulates Its Methyltransferase Activity and Promotes Myeloproliferation. Cancer Cell 19, 283–294 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bungard D et al. Signaling Kinase AMPK Activates Stress-Promoted Transcription via Histone H2B Phosphorylation. Science 329, 1201–1205 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agger K et al. Karl Agger, Paul A.C. Cloos, Lise Rudkjær, Kristine Williams, Gitte Andersen, Jesper Christensen, and Kristian Helin 1. Genes Dev 1171–1176 (2009). doi: 10.1101/gad.510809.GENES [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giglia-Mari G, Zotter A & Vermeulen W DNA damage response. Cold Spring Harb. Perspect. Biol 3, a000745 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossetto D, Avvakumov N & Côté J Histone phosphorylation: a chromatin modification involved in diverse nuclear events. Epigenetics 7, 1098–108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung AKL & Sharp PA MicroRNA Functions in Stress Responses. Mol. Cell 40, 205–215 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Urso A & Brickner JH Mechanisms of epigenetic memory. Trends Genet 30, 230–6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang N & Cao L Starvation signals in yeast are integrated to coordinate metabolic reprogramming and stress response to ensure longevity. Curr. Genet 63, 839–843 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winkler J et al. Quantitative and spatio-temporal features of protein aggregation in Escherichia coli and consequences on protein quality control and cellular ageing. EMBO J 29, 910–923 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aguilaniu H, Gustafsson L, Rigoulet M & Nyström T Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science 299, 1751–1753 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Coelho M et al. Fission Yeast Does Not Age under Favorable Conditions, but Does So after Stress. Curr. Biol 23, 1844–1852 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuentealba LC, Eivers E, Geissert D, Taelman V & De Robertis EM Asymmetric mitosis: Unequal segregation of proteins destined for degradation. Proc. Natl. Acad. Sci. U. S. A 105, 7732–7 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bufalino MR, DeVeale B & van der Kooy D The asymmetric segregation of damaged proteins is stem cell-type dependent. J. Cell Biol 201, 523–30 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindner AB, Madden R, Demarez A, Stewart EJ & Taddei F Asymmetric segregation of protein aggregates is associated with cellular aging and rejuvenation. Proc. Natl. Acad. Sci. U. S. A 105, 3076–81 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vedel S, Nunns H, Košmrlj A, Semsey S & Trusina A Asymmetric Damage Segregation Constitutes an Emergent Population-Level Stress Response. Cell Syst 3, 187–198 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Kaufmann BB, Yang Q, Mettetal JT & Van Oudenaarden A Heritable stochastic switching revealed by single-cell genealogy. PLoS Biol 5, 1973–1980 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acar M, Becskei A & van Oudenaarden A Enhancement of cellular memory by reducing stochastic transitions. Nature 435, 228–232 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Chen Q, Yan W & Duan E Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat. Rev. Genet 17, 733–743 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodgers AB, Morgan CP, Bronson SL, Revello S & Bale TL Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J. Neurosci 33, 9003–12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodgers AB, Morgan CP, Leu NA & Bale TL Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci 112, 13699–13704 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colombo S et al. Detection of cAMP and of PKA activity in Saccharomyces cerevisiae single cells using Fluorescence Resonance Energy Transfer (FRET) probes. Biochem. Biophys. Res. Commun 487, 594–599 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Nikolaev VO, Bünemann M, Hein L, Hannawacker A & Lohse MJ Novel single chain cAMP sensors for receptor-induced signal propagation. J. Biol. Chem 279, 37215–8 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Allen MD & Zhang J Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem. Biophys. Res. Commun 348, 716–721 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Hayashi-Takanaka Y et al. Tracking epigenetic histone modifications in single cells using Fab-based live endogenous modification labeling. Nucleic Acids Res 39, 6475–6488 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sasaki K, Ito T, Nishino N, Khochbin S & Yoshida M Real-time imaging of histone H4 hyperacetylation in living cells. Proc. Natl. Acad. Sci 106, 16257–16262 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lungu C, Pinter S, Broche J, Rathert P & Jeltsch A Modular fluorescence complementation sensors for live cell detection of epigenetic signals at endogenous genomic sites. Nat. Commun 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheglakov Z, Cronin TM, He C & Weizmann Y Live cell microRNA imaging using cascade hybridization reaction. J. Am. Chem. Soc 137, 6116–6119 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Raj B et al. Simultaneous single-cell profiling of lineages and cell types in the vertebrate brain. Nat. Biotechnol 36, 442–450 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


