Abstract
We have previously published that miR15a can reduce inflammatory cytokines, which could be key to diabetic retinal pathology. In this work, we wanted to investigate whether miR15a altered NLR pyrin domain 3 (NLRP3) proteins. Whole retinal lysates from both miR15a overexpressing mice and endothelial cell specific miR15a/16 knockout mice were used to investigate protein levels of forkhead box protein O1 (Foxo1), NLRP3, cleaved caspase 1 and interleukin-1 beta (IL-1β). Primary human retinal endothelial cells (REC) were cultured in normal and high glucose followed by transfection with a miR15a mimic for protein analyses. MiR15a expression was verified by quantitative PCR, and a luciferase binding assay was used to examine whether miR15a directly bound Foxo1. In mouse retinal lysates, loss of miR15a increased Foxo1, IL-1β, NLRP3, and cleaved caspase 1 levels. REC grown in high glucose transfected with the miR15a mimic had decreased levels of Foxo1 and NLRP3. miR15a directly binds to Foxo1. miR15a regulates NLRP3 actions in the retinal vasculature. Work in mice showed that loss of miR15a increased NLRP3 pathway signaling and Foxo1. miR15a mimics decreased levels of Foxo1 and NLRP3. Taken together, miR15a reduced inflammasome proteins and Foxo1 levels in the retinal vasculature.
Keywords: miR15a, Retinal endothelial cells, NLRP3 inflammasome proteins, FOXO1a
1. Introduction
Diabetic retinopathy is the leading cause of blindness in working-age adults and is associated with chronic, low-grade inflammation. Activation of pro-inflammatory signals is a major contributor to retinal endothelial cell dysfunction and diabetic retinal pathology (Joussen et al., 2004). Work on elucidating potential inflammatory signaling pathways in the diabetic retina is needed to provide therapeutic strategies in preventing disease initiation and/or progression. One such regulatory pathway is microRNA. In a population-based cohort study by Zampetaki et al. (2010), decreased levels of microRNA-15a (miR15a) in bone marrow cells were associated with type 2 diabetes (Zampetaki et al., 2010). miR15a has been shown to inhibit inflammatory cytokines in the retina, leading to reduced retinal leukostasis, permeability, and angiogenesis in knockout and overexpressing transgenic miR15a mice (Wang et al., 2016; Ye et al., 2016). miR15a is implicated in the pathogenesis of acute coronary syndrome, developing from inflammatory processes and leading to plaque progression and thrombosis (Schroeder and Falk, 1996). In this study, miR15a was shown to be decreased in patients with acute coronary syndrome, as well as regulate coactivator-associated arginine methyltransferase 1 (CARM1), which activates inflammatory chemokines (Liu et al., 2014).
Previous work in our lab demonstrated that miR15a regulated insulin signaling. miR15a decreased tumor necrosis factor alpha (TNFα) levels, which can help to restore normal insulin signaling (Jiang et al., 2012, 2018). In addition to TNFα, other inflammatory cytokines, such as IL-1β, are also upregulated in diabetic retinopathy and indicated in diabetic complications (Liu et al., 2012). One study found that increased retinal expression of IL-1β begins in the vascular endothelium as a result of hyperglycemia. In this study, IL-lβ secretion occurs in an autocrine and paracrine manner in endothelial and macroglia cells, increasing the features of neuroinflammation (Liu et al., 2012). IL-1β is also released following inflammasome formation, resulting from the cleavage of pro-caspase 1 into caspase 1 (Schroder and Tschopp, 2010; Ting et al., 2008). Both TNFα and IL-1β levels can be regulated through toll-like receptor (TLR) signaling, which is reduced by miR-15a/16 in LPS-treated macrophages (Wang et al., 2015). In addition, a decrease in the rise of high glucose-induced TLR4 levels reduced inflammasome proteins, such as cleaved caspase-1 and IL-1β in REC (Jiang et al., 2017).
Studies suggest that activation of NLR family pyrin domain containing 3 inflammasome (NLRP3) by danger associated molecular pattern receptors (DAMPs) occurs in diabetes (Rheinheimer et al., 2017). Work by Kim et al. (2017) showed that Foxo1 stimulated the NLRP3 inflammasome, causing the release of IL-1β in mouse liver and HepG2 cells (Kim et al., 2017). Foxo1 is a transcription factor regulating apoptosis, proliferation, cell stress, and differentiation (Sanchez et al., 2014), and has been implicated in the regulation of IL-1β auto-stimulation in retinal endothelial cells (REC) and diabetic mice retinas (Wu et al., 2017). Foxo1 is activated in early stage diabetic retinopathy (Behl et al., 2009).
In this study, we hypothesized that miR15a could regulate NLRP3 inflammasome proteins, which could be regulated by Foxo1. We used REC in culture and endothelial cell specific knockout mice for miR15a/ 16 and miR15a overexpressing mice to test this hypothesis.
2. Materials and methods
2.1. Mice
Experiments were done using miR15a endothelial cell specific knockout mice (Ye et al., 2016) and overexpressing mice (Ye et al., 2017). Mice of both sexes at 3 months of age were used. Animal procedures were reviewed and approved by the Institutional Animal Care and Use Committees of Wayne State University School of Medicine (17– 07-301) and conform to NIH guidelines.
2.2. Retinal endothelial cell culture (REC)
Primary REC were purchased from Cell Systems Corporation (CSC, Kirkland, WA). Cells were grown in normal (5 mM) or high glucose (25 mM) Cell Systems Medium. The media was augmented with microvascular growth supplement (Invitrogen), 10 μg/mL gentamycin, and 0.25 μg/mL amphotericin B. For experiments, cells were exposed to high glucose medium for a minimum of 3 days. Only cells prior to passage 5 were used.
2.3. Cell transfection with miR15a mimic
REC grown in high glucose were transfected with miR15a mimic (hsa-miR15a-5p) and a negative control (Ye et al., 2016). In addition, some normal glucose and high glucose control cell groups were treated with oligofectamine only. The levels of miRNA expression were verified using real-time quantitative reverse transcription polymerase chain reaction.
2.4. qPCR
Total RNA was isolated and purified using Trizol (Ye et al., 2016). Purity and quantity of RNA was measured. RNA were processed for polyA tailing reverse-transcriptase, purified, and reverse-transcribed into cDNA using Superscript IV reverse transcriptase with an oligo-dT adapter primer (Ye et al., 2016). PCR was performed and miRNA expression calculated using the double delta Ct method.
2.5. Luciferase binding assay
Primary human REC were purchased from Cell Systems Corporation (CSC, Kirkland, WA). The cells were grown in Cell Systems Medium augmented with microvascular growth supplement (Invitrogen), 10 μg/ mL gentamycin, and 0.25 μg/mL amphotericin B. Cells were grown in normal glucose (5 mM) medium in 60 mm dishes. At 80–90% con-fluency, cells were transferred to a 96 well plate within 24 h of transfection. Only cells prior to passage 5 were used.
The 3′ UTR of Foxo1a tagged with luciferase was purchased from Genecopoeia (Rockville, MD). The 3′ UTR was transfected into REC using Dharmafect duo (Dharmacon, Lafayette, CO). Another group of REC were co-transfected with the Foxo1 3′UTR and miR15a mimic (hsa-miR-15a-5p) (Invitrogen, Carlsbad, CA). Additional cells were transfected with the luciferase receptor and a scrambled miR (negative control) or empty vector. Cells were assessed for luciferase activity using the LightSwitch Assay kit (SwitchGear Genomics, Inc), following manufacturer’s instructions. Luciferase activity was measured using a SpectraMax L Luminometer.
2.6. Western blotting
REC or whole retinal lysates were collected into lysis buffer containing protease and phosphatase inhibitors. Equal amounts of protein were loaded and separated onto precast tris-glycine gels (Invitrogen, Carlsbad, CA) and then blotted onto a nitrocellulose membrane. The membranes were blocked in TBST (10 mM Tris-HCl buffer, pH 8.0, 150 mM NaCl, 0.1% Tween 20) and 5% BSA, and then incubated with corresponding primary antibodies. Incubation with secondary antibodies labeled with horseradish peroxidase followed. The primary antibodies used were Foxo1a, IL-1β, NLRP3 (Abcam, Cambridge, MA), and cleaved caspase 1 (Cell Signaling, Danvers, MA). Each primary antibody was compared to beta actin (Santa Cruz, Santa Cruz, CA) for analysis. Antigen-antibody complexes were identified using a chemillumiminescence reagent kit (Thermo Scientific, Pittsburgh, PA). Imaging was performed using a C500 (Azure Biosystems, Dublin, CA).
2.7. ELISA
A cleaved caspase 1 ELISA (R &D systems, Minneapolis, MN) was performed according to the manufacturer’s instructions on mouse whole retinal lysates. An IL-1β ELISA (R&D Systems, Minneapolis, MN) was performed based upon the manufacturer’s instructions with the following exceptions: 100 μg protein of each sample was added into wells, and the incubation of samples and antibody reagents occurred overnight at 4 °C.
2.8. Statistical analyses
Prism software 6.0 (GraphPad, La Jolla, CA) was used for statistical analyses. An unpaired Student t-test with two-tailed p value was used for mice data. A one-way ANOVA with Student Newman Keuls post-hoc test was used for cell culture work. P < 0.05 was considered to be significant.
3. Results
3.1. Loss of miR15a increases Foxo1, NLRP3, cleaved caspase 1 and IL-1β levels in endothelial cell specific knockout mice
We analyzed the effects of the loss of miR15a in whole retinal lysates from miR15a/16 floxed and CreLox mice and found that Foxo1 and NLRP3 levels were significantly increased in miR15a CreLox mice compared to their floxed littermates (Fig. 1a and b). ELISA analyses showed significantly increased cleaved caspase 1 and IL-1β levels with the loss of miR15a in the mouse retina (Fig. 1c and d). The results suggest the loss of miR15a in the retinal vasculature increased levels of Foxo1 and NLRP3 inflammasome proteins.
Fig. 1.
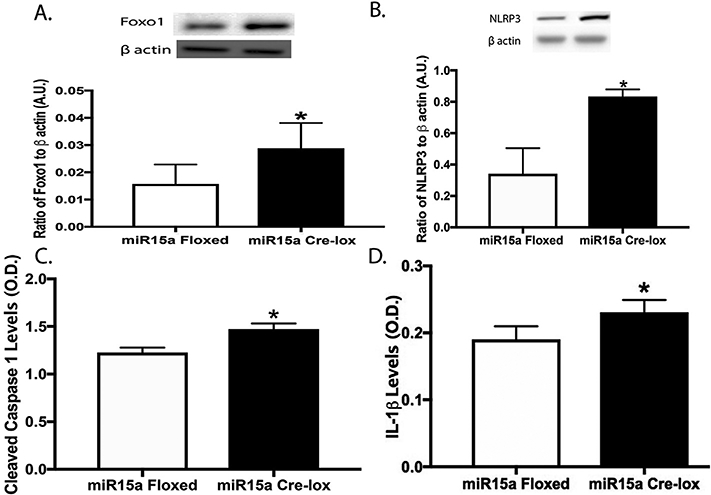
Western blotting for the ratio of Foxo1 levels (A) and NLRP3 (B) to β actin in whole retinal lysates from miR15a floxed mice or cdh5-miR15a-CreLox mice. Panels C and D are ELISA results for cleaved caspase 1 (C) and IL-1β (D) levels in whole retinal lysates from miR15a floxed mice or cdh5-miR15a-CreLox mice. P < 0.05 vs. miR15a floxed mice. N = 5 for all mice.
3.2. Overexpression of miR15a decreases Foxo1, NLRP3, cleaved caspase 1 and IL-1β levels
To further elucidate the protective actions of miR15a in the mouse retinal vasculature, we used endothelial cell-specific miR15a over-expressing mice. When miR15a was overexpressed in the mouse retinal vasculature, there was a significant reduction in Foxo1, NLRP3, cleaved caspase 1 and IL-1β levels. (Fig. 2a-d). Overall, these data strongly support our hypothesis that miR15a can reduce NLRP3 inflammasome proteins.
Fig. 2.
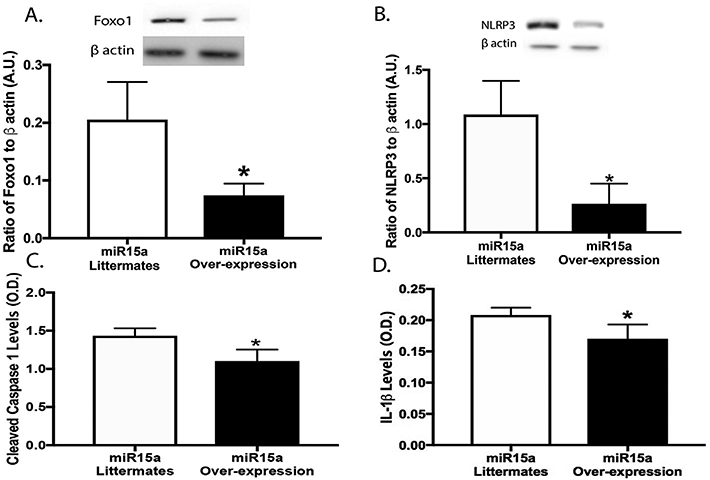
Western blotting for the ratio of Foxo1 levels (A) and NLRP3 (B) to β actin in whole retinal lysates from miR15a control littermates or miR15a overexpressing mice. Panels C and D are ELISA results of cleaved caspase 1 and IL-1β levels in whole retinal lysates from miR15a control littermates or miR15a overexpressing mice. *P < 0.05 vs. control littermates. N = 5 for all mice.
3.3. miR15a transfection increased miR15a expression and decreased Foxo1, NLRP3, cleaved caspase 1 and IL-1β levels
To test our work in vitro, we used REC transfected with miR15a mimics. We used qPCR to ensure that transfection with the miR15a mimic significantly increased miR15a expression (Fig. 3a). miR15a mimic transfection led to a significant reduction in Foxo1, NLRP3, cleaved caspase 1, and IL-1β levels when compared to the high glucose only group and the negative control transfection group (Fig. 3b-e).
Fig. 3.
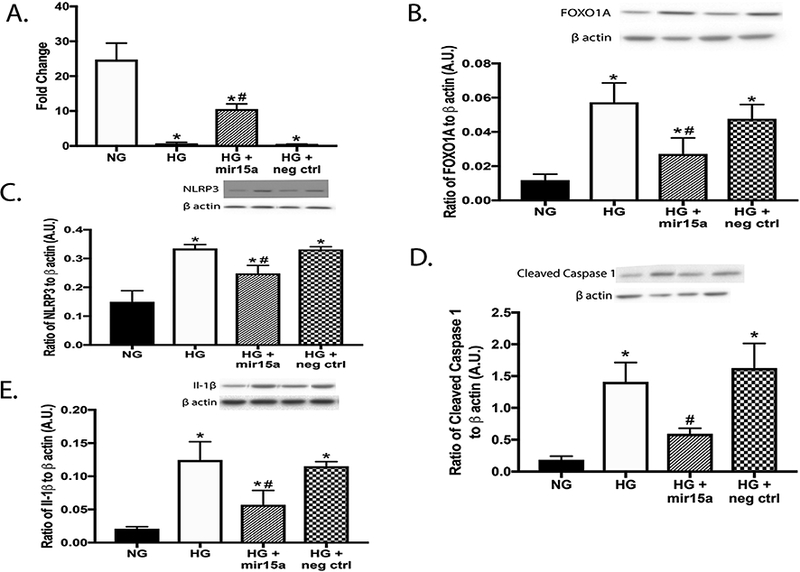
Panel A shows qPCR results of miR15a expression levels in REC grown in normal glucose (NG) and high glucose (HG). Some REC grown in HG were transfected with miR15a mimic or a negative control. Panel B-E is Western blotting for the ratio of Foxo1 levels (B), NLRP3 (C), cleaved caspase 1 (D) and IL-1β (E) to β actin. *P < 0.05 vs. NG, #P < 0.05 vs. HG. N = 3–5 for all groups.
3.4. miR15a directly binds Foxo1
Since we observed that miR15a regulates both Foxo1 and NLRP3 inflammasome proteins, we wanted to determine if a link existed between Foxo1 and miR15a. Foxo1 is a potential target of miR15a based upon targetscan. org. We verified the in silico program using REC grown in normal glucose transfected with the 3′ UTR of Foxo1 (Reporter) and a miR15a mimic. Fig. 4 shows decreased luciferase activity when the 3’ UTR of Foxo1 is bound to miR15a. There is no significant change in luciferase activity when the reporter is used alone, when the reporter is used with a scrambled miRNA, or when the reporter is used with an empty vector. This data indicates miR15a directly binds Foxo1. Taken together, data suggest miR15a can reduce NLRP3 proteins, potentially through binding to Foxo1 and decreasing its actions.
Fig. 4.
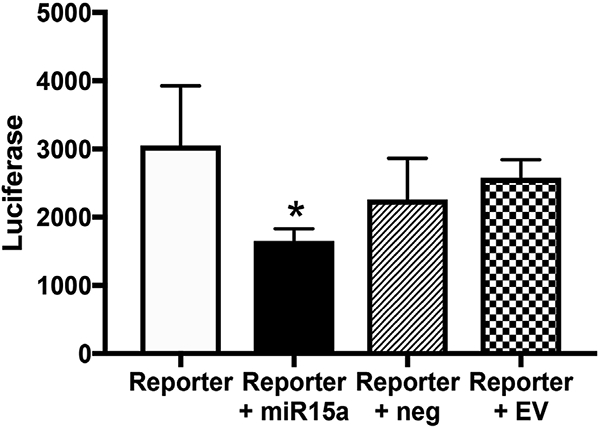
Luciferase activity of retinal endothelial cells (REC) transfected with the 3′UTR for Foxo1 receptor (reporter), the reporter and a miR15a mimic (reporter + miR15a), the reporter with a negative control mimic (reporter + neg Ctrl), or the reporter with an empty vector. *P < 0.05 vs. reporter only. N = 4–5 for all groups.
4. Discussion
Inflammation has been implicated in the pathogenesis of diabetes and is a major contributor to complications in the diabetic retina (Chiazza et al., 2015). Low grade inflammation causes the upregulation of endothelial cell adhesion molecules e.g. ICAM-1 and VCAM-1 (Joussen et al., 2001; Miyamoto et al., 1999). These markers lead to increased leukostasis, as well as continuous inflammation and vascular wall injuries (Blake and Ridker, 2001; Pepys and Hirschfield, 2003). Ultimately, endothelial dysfunction contributes to the alteration of blood flow, oxidative stress, and angiogenesis characteristic of diabetic retinopathy (Fukumura et al., 2001; Hartnett et al., 2000; Poston and Taylor, 1995). miRNAs have been shown to be involved in several cellular mechanisms, including inflammatory pathways, which could be key to retinal pathology (Wang et al., 2016). In this study, we focused on the role of miR15a and its potential role in reducing NLRP3 in-flammasome proteins in retinal endothelial cells. Previous work in our lab recently demonstrated that miR15a regulated insulin signaling (Jiang et al., 2018), and miR15a maintained the retinal endothelial barrier in REC and mice (Ye et al., 2017). Furthermore, miR15a has been shown to decrease inflammatory cytokines (Wang et al., 2016). When miR15a was overexpressed in LPS-murine macrophages, TLR4 and IL-1 receptor-associated kinase (IRAK-1) levels were decreased, allowing for decreased activation of inflammatory pathways (Heyn et al., 2012; Wang et al., 2015).
We therefore wanted to investigate other inflammatory proteins regulated by miR15a. We initially investigated NLRP3 inflammasome proteins, as we have shown that Epac1 can reduce NLRP3 actions in REC (Jiang et al., 2017). To determine if miR15a may regulate these proteins similar to Epac1, we used endothelial cell specific miR15a/16 knockout mice. We found that the loss of miR15a in the whole retinal lysates increased levels of Foxo1, NLRP3, cleaved caspase 1, and IL-1β in the CreLox mice compared to floxed littermates. In support of these findings, miR15a overexpressing mice had decreased levels of Foxo1, NLRP3, cleaved caspase 1, and IL-1β levels. Work in myeloid cells where miR15a was deleted showed increased phagocytic and bactericidal capability of bone marrow-derived macrophages through an upregulation of TLR4 (Moon et al., 2014), which is in agreement with our findings. TLR4 has been shown to prime the NLRP3 inflammasome in high-fat derived dendritic cells compared to chow-derived cells, leading to increased sensitivity to inflammatory stimuli (Reynolds et al., 2012).
When we used miR15a mimics to increase miR15a expression in vitro, we found a decrease in Foxo1, NLRP3, cleaved caspase 1, and IL-1β levels. These results suggest that miR15a decreases activation of NLRP3 inflammasome proteins. To investigate pathways by which miR15a could regulate NLRP3 proteins, we used targetscan. org to find other targets potentially involved in retinal vascular inflammation. Targetscan.org suggested that miR15a could directly bind to the 3′UTR of Foxo1. Dong et al. (2014) found that over-expression of miR15a/b interacted with the target region of Foxo1 mRNA in porcine pre-adi-pocytes (Dong et al., 2014). Using a luciferase assay, we confirmed that miR15a directly binds Foxo1 in REC. Foxo1 may represent a key link in miR15a regulation of inflammasome actions, as we showed that miR15a directly binds Foxo1. In agreement with this idea is work showing that betaine (a Foxo1 inhibitor) reduced NLRP3 inflammasome formation and IL-1β production (Kim et al., 2017). Future studies will focus on investigating whether Foxo1 is required for miR15a to inhibit NLRP3 inflammasome proteins, and potential mechanisms by which Foxo1 regulates NLRP3 inflammasome proteins in the retina.
5. Conclusion
Taken together, our data suggest miR15a decreases NLRP3 inflammasome proteins. Using conditional knockout and transgenic mice for miR15a in endothelial cells, we found that miR15a targets Foxo1, NLRP3, cleaved caspase 1, and IL-1β. In cell culture work, increased miR15a expression reduced Foxo1, NLRP3, cleaved caspase 1, and IL-1β pathways. In addition, miR15a directly binds Foxo1 in REC. Overall, our data offer new targets that can be used to block inflammatory cascades in the retinal vasculature.
Acknowledgments
Funding
This work was supported by the National Institutes of Health R01EY022330 (JJS), P30EY04068 (Hazlett), and an Unrestricted Grant to the Department of Ophthalmology from Research to Prevent Blindness (Kresge Eye Institute).
List of abbreviations
- miR15a
microRNA-15a
- Foxo1
Forkhead box O1
- IL-1β
Interleukin-1 beta
- REC
Human retinal endothelial cells
- NLRP3
NLR family pyrin domain containing 3 inflammasome
- (DAMPs)
Danger associated molecular pattern receptors
- rhFoxo1
Recombinant human Foxo1 agonist
- NG
Normal glucose
- HG
High glucose
References
- Behl Y, Krothapalli P, Desta T, Roy S, Graves DT, 2009. FOXO1 plays an important role in enhanced microvascular cell apoptosis and microvascular cell loss in type 1 and type 2 diabetic rats. Diabetes 58, 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake GJ, Ridker PM, 2001. Novel clinical markers of vascular wall inflammation. Circ. Res 89, 763–771. [DOI] [PubMed] [Google Scholar]
- Chiazza F, Couturier-Maillard A, Benetti E, Mastrocola R, Nigro D, Cutrin JC, Serpe L, Aragno M, Fantozzi R, Ryffel B, Thiemermann C, Collino M, 2015. Targeting the NLRP3 inflammasome to reduce diet-induced metabolic abnormalities in mice. Mol. Med 21, 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong P, Mai Y, Zhang Z, Mi L, Wu G, Chu G, Yang G, Sun S, 2014. MiR-15a/b promote adipogenesis in porcine pre-adipocyte via repressing FoxO1. Acta Biochim. Biophys. Sin 46, 565–571. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK, 2001. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. U.S.A 98, 2604–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnett ME, Stratton RD, Browne RW, Rosner BA, Lanham RJ, Armstrong D, 2000. Serum markers of oxidative stress and severity of diabetic retinopathy. Diabetes Care 23, 234–240. [DOI] [PubMed] [Google Scholar]
- Heyn J, Ledderose C, Hinske LC, Limbeck E, Mohnle P, Lindner HA, Kreth S, 2012. Adenosine A2A receptor upregulation in human PMNs is controlled by miRNA-214, miRNA-15, and miRNA-16. Shock 37, 156–163. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Liu L, Curtiss E, Steinle JJ, 2017. Epac1 blocks NLRP3 inflammasome to reduce IL-1beta in retinal endothelial cells and mouse retinal vasculature. Mediat. Inflamm 2017 2860956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liu L, Steinle JJ, 2018. miRNA15a regulates insulin signal transduction in the retinal vasculature. Cell. Signal 44, 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Zhang Q, Soderland C, Steinle JJ, 2012. TNFalpha and SOCS3 regulate IRS-1 to increase retinal endothelial cell apoptosis. Cell. Signal 24, 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP, 2001. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am. J. Pathol 158, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP, 2004. A central role for inflammation in the pathogenesis of diabetic retinopathy. Faseb. J. Official Pub. Fed. Am. Soc. Exp. Biol 18, 1450–1452. [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim SM, Lee B, Lee EK, Chung KW, Moon KM, An HJ, Kim KM, Yu BP, Chung HY, 2017. Effect of betaine on hepatic insulin resistance through FOXO1-induced NLRP3 inflammasome. J. Nutr. Biochem 45, 104–114. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang L, Li H, Lu X, Hu Y, Yang X, Huang C, Gu D, 2014. Coactivatorassociated arginine methyltransferase 1 targeted by miR-15a regulates inflammation in acute coronary syndrome. Atherosclerosis 233, 349–356. [DOI] [PubMed] [Google Scholar]
- Liu Y, Biarnes Costa M, Gerhardinger C, 2012. IL-1beta is upregulated in the diabetic retina and retinal vessels: cell-specific effect of high glucose and IL-1beta autostimulation. PLoS One 7, e36949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, Aiello LP, Ogura Y, Adamis AP, 1999. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc. Natl. Acad. Sci. U.S.A 96, 10836–10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HG, Yang J, Zheng Y, Jin Y, 2014. miR-15a/16 regulates macrophage phagocytosis after bacterial infection. J. Immunol 193, 4558–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys MB, Hirschfield GM, 2003. C-reactive protein: a critical update. J. Clin. Invest 111, 1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston L, Taylor PD, 1995. Glaxo/MRS Young Investigator Prize. Endothelium-mediated vascular function in insulin-dependent diabetes mellitus. Clin. Sci. (Lond.) 88, 245–255. [DOI] [PubMed] [Google Scholar]
- Reynolds CM, McGillicuddy FC, Harford KA, Finucane OM, Mills KH, Roche HM, 2012. Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells-implications for diet-induced insulin resistance. Mol. Nutr. Food Res 56, 1212–1222. [DOI] [PubMed] [Google Scholar]
- Rheinheimer J, de Souza BM, Cardoso NS, Bauer AC, Crispim D, 2017. Current role of the NLRP3 inflammasome on obesity and insulin resistance: a systematic review. Metabolism 74, 1–9. [DOI] [PubMed] [Google Scholar]
- Sanchez AM, Candau RB, Bernardi H, 2014. FoxO transcription factors: their roles in the maintenance of skeletal muscle homeostasis. Cell. Mol. Life Sci. 71, 1657–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J, 2010. The inflammasomes. Cell 140, 821–832. [DOI] [PubMed] [Google Scholar]
- Schroeder AP, Falk E, 1996. Pathophysiology and inflammatory aspects of plaque rupture. Cardiol. Clin 14, 211–220. [DOI] [PubMed] [Google Scholar]
- Ting JP, Willingham SB, Bergstralh DT, 2008. NLRs at the intersection of cell death and immunity. Nat. Rev. Immunol 8, 372–379. [DOI] [PubMed] [Google Scholar]
- Wang Q, Navitskaya S, Chakravarthy H, Huang C, Kady N, Lydic TA, Chen YE, Yin KJ, Powell FL, Martin PM, Grant MB, Busik JV, 2016. Dual anti-inflammatory and anti-angiogenic action of miR-15a in diabetic retinopathy. EBioMedicine 11, 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang X, Liu X, Wang X, Xu J, Hou S, Zhang X, Ding Y, 2015. miR-15a/ 16 are upreuglated in the serum of neonatal sepsis patients and inhibit the LPS-induced inflammatory pathway. Int. J. Clin. Exp. Med 8, 5683–5690. [PMC free article] [PubMed] [Google Scholar]
- Wu L, Guo F, Wu Y, Wang Q, Ma X, Zhao Y, Qin G, 2017. The role of FoxO1 in interleukin-1beta-induced autostimulation in retina endothelial cells and retinas of diabetic rats. Microvasc. Res 112, 93–100. [DOI] [PubMed] [Google Scholar]
- Ye EA, Liu L, Jiang Y, Jan J, Gaddipati S, Suvas S, Steinle JJ, 2016. miR-15a/16 reduces retinal leukostasis through decreased pro-inflammatory signaling. J. Neuroinflammation 13, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye EA, Liu L, Steinle JJ, 2017. miR-15a/16 inhibits TGF-beta3/VEGF signaling and increases retinal endothelial cell barrier proteins. Vis. Res 139, 23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M, 2010. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res 107, 810–817. [DOI] [PubMed] [Google Scholar]


