Abstract
Data on cognitive aging in chimpanzees are extremely sparse, yet can provide an invaluable phylogenetic perspective, especially since Alzheimer Disease-like (AD) neuropathology has recently been described in the oldest chimpanzee brains. This finding underscores the importance of data on cognitive aging in this fellow hominin, our closest biological relative. We tested 30 female chimpanzees, 12 to 56 years old, on a computerized analog of the Wisconsin Card Sort test. This test assesses cognitive flexibility, which is severely impaired in normal aging and AD. Subjects selected stimuli according to color or shape; the rewarded dimension (i.e. color or shape) switched without warning and the chimpanzee had to adapt her responses accordingly. We found that increasing age was associated with an increased number of perseverative errors and an increased number of trials to reach criterion in each switching dimension. The number of aborted trials was similar across age groups. These data show that like humans, chimpanzees show a clear age-related decline in cognitive flexibility that is already observed at middle-age.
Keywords: Aging, Ape, Cognition, Cognitive Flexibility, Prefrontal Cortex
INTRODUCTION
As the population ages, the burden that cognitive impairment and dementia will pose to the population looms larger (Blazer et al., 2015). Because of limitations of human studies and the need to understand age-related decline at a mechanistic level, research in animal models is essential to uncovering interventions to alleviate age-related cognitive decline. Although numerous animal species have been used as models of human cognitive aging (Arey and Murphy, 2017; Bizon and Woods, 2009; Cotman and Head, 2008; Gallagher et al., 2011; Park and Reuter-Lorenz, 2009), most studies have been conducted in rats and mice (Gallagher et al., 2011). The short life span of rodents permits researchers to observe the aging process and neural hallmarks of decline within individual animals. Developments in transgene technology have also allowed the design of mice with mutant forms of amyloid precursor protein, presenilins, Tau and other genes important for Alzheimer’s disease (AD) (Onos et al., 2016). Although mouse models of AD have furthered our understanding of several aspects of the disease, including the formation of amyloid plaques and neurofibrillary tangles, their repeated failure as preclinical models (Franco and Cedazo-Minguez, 2014) has highlighted a critical need for aging studies in species more closely related to humans.
Research in nonhuman primates (NHPs), permits study of cognitive aging in model systems with neural, physiological and behavioral characteristics that resemble those of our species. Most cognitive aging research in NHPs has been conducted in the rhesus monkey, a species with a maximum lifespan of 40 years, which shares many features of cognitive and brain aging with humans. Several laboratory groups, including our own, have developed batteries of cognitive tests for NHPs designed to assess normal function and decline in a large range of cognitive domains, including recognition memory, working memory and executive function (Bartus et al., 1978; Moss et al., 1988; Rapp, 1988; Voytko, 1993). Overall, studies on cognitive aging in rhesus monkeys have revealed age-related decline in most tasks (Herndon et al., 1997), some starting at middle-age (Moore et al., 2006), that follow a pattern very similar to the human pattern (Park and Reuter-Lorenz, 2009; Salthouse, 2010). It is worth noting that alternative NHP models of human cognitive aging are also being used (Lacreuse and Herndon, 2009), often based on species-specific characteristics advantageous for aging research, such as a shorter life span (e.g., the common marmoset, Callithrix jacchus, Lacreuse et al., 2014a; Tardif et al., 2011; the mouse lemur, Microcebus murinus, Picq, 2006).
The chimpanzee (Pan troglodytes) is the primate species most closely related to humans. It also possesses one of the richest cognitive repertoires among NHPs (Lonsdorf et al., 2010), a brain organization that greatly overlaps with that of the human brain (Rilling, 2014) and a long maximum lifespan of 62 years in captivity (Dyke et al, 1995). Nevertheless, very few studies of chimpanzee aging have been conducted, perhaps because few older chimpanzees were available in the early years of primate research. Two studies conducted in the 1960s were inconclusive with regards to age-related cognitive decline in this species. Bernstein (1961) compared the performance of 8 young (11–19 years of age) and 8 old (28–40 years of age) chimpanzees of unspecified sex in a series of object discriminations and a wheel-rotating task. No difference between the age groups was found in any of the tasks. Riopelle and Rogers (1965) studied 19 chimpanzees (7 to 41 years) of unspecified sex. Confirming the Bernstein study, no effect of age was found on two different object discrimination tasks. An age-related decline was observed in the Spatial Delayed Response task, a classic task of working memory, but the age differences were unexpectedly observed at the short delays of 0s or of 5 s, and not at the longer delay of 10s. A significant decline with age was also noted in a four-choice oddity task, in which chimpanzees had to select the odd object among three identical stimuli. A later study attempted to replicate the findings of Riopelle and Rogers, but revealed no differences in performance as a function of age in two chimpanzees (Bloomstrand and Maple, 1985).
A renewed interest for cognitive aging in the chimpanzee came about only recently. Using a battery of 12 tasks initially designed to compare social and physical cognition in apes and humans (Herrmann et al., 2007), as well as a task of fine motor function, we tested a group of 38 female chimpanzees ranging in age from young adulthood (10 years) through very old age (52 years), for a period of 3 years. Poorer performance with age was found in 2 tasks of social cognition, an attention-getting task and a gaze-following task. Minimal age differences were observed in tasks of physical cognition, which involved cognitive processing of physical stimuli presented to the subject. An exception among physical cognition tasks was a spatial memory test in which subjects attempted to locate and retrieve food items which were hidden as they observed. Performance on this task declined significantly in the 4 individuals older than 50 years old (Lacreuse et al., 2014b). In another study, 5 species of apes, including chimpanzees, ranging from pre-adolescence to old age, were tested in a motor task requiring sliding a horizontal door to the left or to the right in order to obtain a reward. Following learning, the response requirement was reversed. Both young and old apes made more errors in the reversal phase than the middle-aged ones, suggesting an age-related impairment in executive function (Manrique and Call, 2015).
Decreases in executive function, the ability to modify strategies for solving a complex task, is one salient feature of human age-related cognitive decline. Executive function encompasses several cognitive processes that can be conceptualized as planning, updating, inhibiting and set or task switching (Chudasama and Robbins, 2006). One benchmark measure of set switching in humans is provided by the Wisconsin Card Sorting Test (WCST). In this task, the participant is asked to sort cards based on one of 3 dimensions - color, number or shape- and only receives a yes/no feedback following his/her choice. During the task, the sorting rule is changed from color to shape or number without awareness of the participant, who has to shift response patterns according to the feedback received. The ability to shift from one dimension to another in the WCST is profoundly impaired in human patients by lesions of the prefrontal cortex (PFC; Milner, 1963). In the NHP version of this task, the Conceptual Set Shifting Task (CSST; Moore et al., 2003), the stimuli are presented on a computer touchscreen. The sets or dimensions of stimuli in the test are shape (triangle, circle or star) and color (red, blue or green). Once the subject responds reliably in one of the dimensions (e.g. color) the response contingency switches to the other (shape). Old and middle-aged rhesus monkeys require more time to learn the shifts in dimension than do young ones (Moore et al., 2003; Moore et al., 2006; Voytko et al., 2009). In addition, the decline in performance correlates with monoamine receptor binding in the PFC (Moore et al., 2005), the same brain region involved in age-related declines in WCST performance in humans (Esposito et al., 1999; Head et al., 2009; Nagahama et al., 1997).
We designed the present study to investigate whether aged chimpanzees show a decline in the set-shifting component of executive function as measured by the CSST. This question is of renewed interest in view of recent findings suggesting the occurrence of neurological hallmarks of AD in this species (Edler et al., 2017). Until recently, it was thought that NHPs could develop β-amyloid plaques, but not the neurofibrillary tangles (NFTs) associated with the disease. However, two recent reports describe the presence of NFTs, in addition to β-amyloid plaques, in aged macaques (Paspalas et al, 2018) and aged chimpanzees (Edler et al, 2017), suggesting that AD pathology is not unique to human brains. Interestingly, in macaques, mature tangles were found at the extreme end of the lifespan (>35 years old). The chimpanzee study showed that 4 (aged 45, 49, 57, and 58 years) of the 20 older chimpanzees examined (aged 37–62) had NFT lesions. In the absence of cognitive assessments, however, it is unknown whether brain pathology in either species is associated with a behavioral phenotype resembling AD. The first step towards addressing this question is to conduct comprehensive assessments of age-related cognitive decline in these species. Age-related cognitive decline in aged macaques has been well characterized. Although behavioral signs of AD have never been documented in this species, individuals over 35, coincident with the development of mature NFTs, are typically not included in cognitive aging studies. In the chimpanzee, comprehensive assessments of age-related cognitive decline are lacking. As this great ape most closely matches humans in terms of higher cognitive processes, complex neural organization and longer lifespan, it is imperative to characterize normal age-related decline in this species. This study addresses whether one cognitive domain that is significantly affected by age in humans, cognitive flexibility, is also impaired in aging in our closest relative.
MATERIAL AND METHODS
Subjects
Thirty-six female chimpanzees were initially included in the study. Chimpanzees were maintained in social groups at the Yerkes National Primate Research Center (YNPRC) of Emory University in Atlanta Georgia. Animal care and housing were provided according to standard procedures at the YNPRC. Housing units for each group provided access to both indoor and outdoor areas. The specific procedures of this study were approved by the Institutional Animal Care and Use Committee of Emory University.
The chimpanzees ranged in age from 12 years to 56 years of age (YOA), a range covering nearly the entire life span from young adulthood to very old age. The original sample of subjects included equal numbers of Young, Middle-aged Adult, and Old Adult chimpanzees. Three Young Adult and 3 Old Adult chimpanzees did not reliably perform the touch screen task, and were removed from the study. Thus, 9 Young (12–17, mean 15.6 YOA), 12 Middle-aged (17–31, mean 22.5 YOA), and 9 Old (33–56, mean 45.5 YOA) Adult chimpanzees participated in the study. All subjects had participated in a wide variety of studies of social and cognitive behavior over previous decades, and all had participated in the study reported in Lacreuse et al (2014b).
Executive Function Testing
Chimpanzees in the study had a history of cognitive testing in which they responded manually to a touch or joystick-based sensitive screen for food rewards (such as pieces of fruit or candy) given by researchers when a desired response was produced. For the present study we used the CSST, a version of the Wisconsin Card Sorting test modified by Moore et al (2003) to be performed by NHPs using a computerized touchscreen. The chimpanzee was presented with 3 sets of shapes (triangle, disk, and star) of 3 different colors (red, blue, and green) on a touchscreen. At each trial, the chimpanzee had to touch one of the 3 stimuli. The correct choice was indicated by an auditory cue and the provision of a food reward (squirt of juice or preferred food item) by the experimenter. Food items were supplementary to the normal feeding regimen, which was not altered during the study. An incorrect answer was accompanied by a different sound and not rewarded. Each day of testing consisted of 80 trials, separated by an inter-trial interval of 15s. Testing continued on successive days, resuming at the point left off in the prior session, until a learning criterion of 10 consecutive correct answers was achieved. This learning criterion was also employed in earlier studies using the CSST (Moore et al, 2003, 2005, 2006). Following attainment of criterion, the correct dimension was switched to another dimension without any warning. The chimpanzee had to adapt her response pattern to the new rule based on reward feedback. The test began within the color dimension, or concept, with “Red” as the correct response. This initial discrimination was followed by 3 discriminations in which the rewarded dimension was switched. The same sequence of dimensions was used for all subjects; after the initial concept of “Red” was learned, 2nd, 3rd, and 4th concepts were “Triangle”, “Blue”, and “Star”. There were thus 4 Levels or concepts, in the study. A schematic diagram of the CSST is presented in Figure 1.
Figure 1.

Schematic diagram of the Conceptual Set-Shifting Task (CSST). Each panel represents the touchscreen for a single trial, and contains 3 stimuli differing in shape (triangle, disc, star) and in color (red, blue, green). The first row of panels presents trials on the initial concept (“red”), during which the red stimuli (triangle, star, and disc, respectively) are scored as correct. The second row of panels show three trials for second concept presented (“triangle”). For this concept the triangle is always correct, regardless of color. Correct responses are indicated by “*” (which is not visible to the chimpanzees).
The primary dependent measure for the study was the number of trials each chimpanzee required to learn each discrimination; that is, the number of trials completed before the first correct response of the 10 successive correct responses required to reach criterion (trials to criterion, TTC). We also analyzed the number of errors and the number of trials on which the chimpanzee refused to respond. Performance on specific tasks, including tasks dependent upon the PFC, has been shown to fluctuate as a function of the menstrual cycle in women (Hampson and Morley, 2013). Female chimpanzees may continue to experience ovarian cycles and to remain capable of producing viable offspring until near the end of the natural lifespan (Lacreuse et al, 2008). However, a substantial proportion fail to show menstrual cycles even at young ages (Herndon et al, 2012). In the present study, 4 (of 9) Young, 5 (of 12) Middle-aged, and 3 (of 6) Old subjects did not show menstrual cycles. For this reason, we also considered Cycling Status (cycling versus non-cycling) as a factor in statistical analysis of cognitive data in the present study. The data on trials, errors, and refusals were analyzed by means of a Linear Mixed Effects Model (LME), in which Age (in years) and Level (i.e. discriminations 1–4) were considered as Fixed Effects and the Individual Subjects were considered as Random Effects. In a subsequent phase of the analysis, Cycling Status and the interaction of Age x Level were added to this initial model.
We also tabulated perseverative errors, defined as incorrect responses following a shift in concept or correct dimension, that would have been correct under the previous response contingency. We employed a 1-way ANOVA to compare the perseverative errors in the 3 age groups.
RESULTS
As shown in Figure 2, the number of trials required to master the discriminations generally increased as the chimpanzees progressed from the initial (Level 1) discrimination through the 3 dimension-shifts (Levels 2 – 4) of the CSST. This increase was strongly significant, as indicated by the LME model (t = 4.90, df = 72, P < 0.0001; Table 1). The same model also indicated that increasing Age was associated with an increasing number of trials required to reach criterion, across all 4 Levels of the study (t = 2.44, df = 28, P = 0.02, Table 1). The presence or absence of menstrual cycles and the Age x Level interaction were not significant and these terms were not included in the final LME model. A 1-way ANOVA revealed no difference in the number of trials required to acquire the initial discrimination (F = 0.81, df = 2,27, P = 0.48; Figure 2). Essentially identical results were obtained in the LME model for the number of errors made by the 3 age groups during the initial discrimination and the 3 shifts (Table 1). Chimpanzees failed to select any of the stimuli on about 2% of the trials. This rate of refusals did not change significantly across the four discrimination levels (t= 1.29, df = 75, P = 0.77) or differ among the three age groups (t = 0.30, df = 28, P = 0.77).
Figure 2.
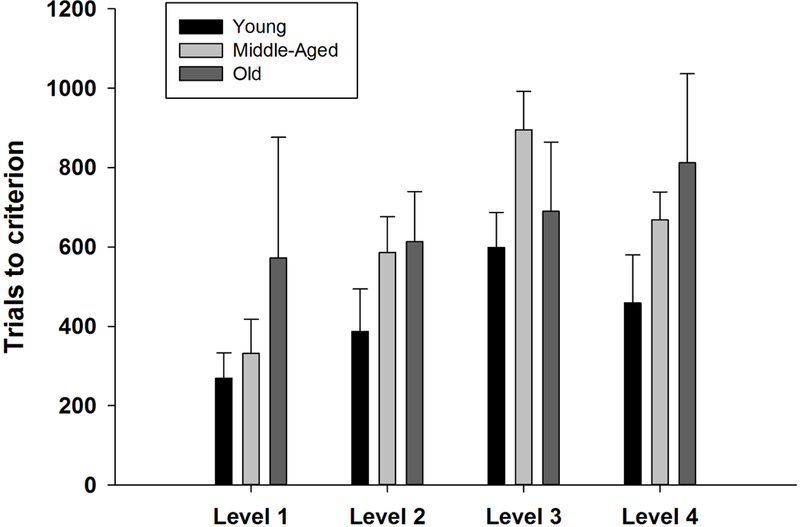
Mean (± SEM) number of trials required for chimpanzees in each Age group to reach learning criterion of 10 consecutive correct responses for each of the 4 levels, or concepts, in the study. More trials were required with increasing subject age (P = 0.018) and with increasing Level number (P < 0.0001)
Table 1.
Linear Mixed Effect Models for trails to criterion, errors to criterion, and refusals to respond of chimpanzees tested on the Cognitive Set Shifting Task.
| Value | SE | DF | t | P-value | ||
|---|---|---|---|---|---|---|
| Trials | Intercept | −52.4 | 174.1 | 72 | −0.30 | 0.76 |
| Age | 13.1 | 5.4 | 28 | 2.44 | 0.02 | |
| Level | 135.2 | 27.6 | 72 | 4.90 | <0.0001 | |
| Errors | Intercept | −202.5 | 142.7 | 75 | −1.42 | 0.16 |
| Age | 13.2 | 4.4 | 28 | 3.03 | 0.01 | |
| Level | 94.6 | 21.6 | 75 | 4.39 | <0.0001 | |
| Refusals | Intercept | 3.3 | 9.0 | 75 | 0.37 | 0.71 |
| Age | 0.1 | 0.3 | 28 | 0.30 | 0.77 | |
| Level | 2.3 | 1.8 | 75 | 1.29 | 0.20 | |
We also analyzed the total number of perseverative errors made by each age group.Total perseverative errors differed significantly among the three age groups (Figure 3; 1-Way ANOVA; F = 3.75, df = 2,25, p=0.037). Post hoc tests (with false discovery rate correction) revealed that the Old and Middle-aged groups did not differ in number of perseverative errors (P = 0.63). The Young group had significantly fewer perseverative errors than both the Old (P = 0.047) and the Middle-aged groups (P = 0.047).
Figure 3.
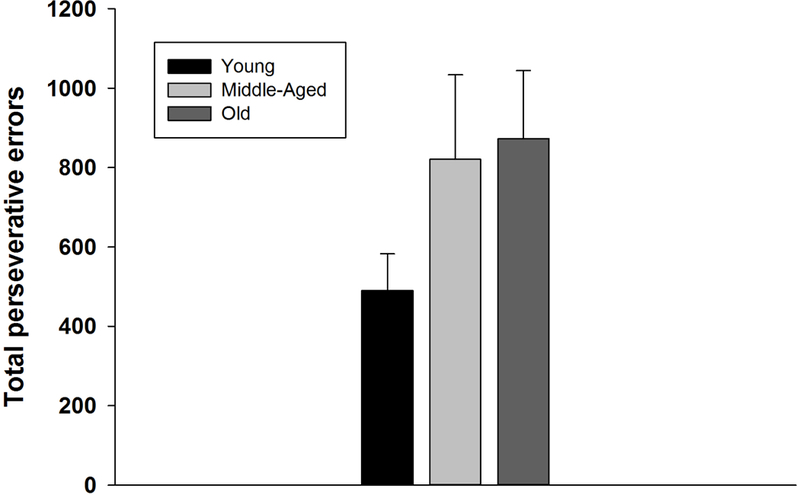
Mean (± SEM) number of perseverative errors committed during the learning of the 3 set shift conditions. The Age groups differed significantly (P=0.04).
DISCUSSION
Thirty female chimpanzees ranging in age from young adulthood (12 years) through very old age (56 years) were tested in the CSST, a computerized version of the Wisconsin Card Sorting Test, a benchmark measure of executive function. A significant age-related decline in performance on this set-shifting task was found, indicating impaired cognitive flexibility with increasing age in our closest relative.
Executive function, including set-switching, is markedly impaired with age in humans (Head et al., 2009), monkeys (Moore, 2003), and rats (Barense et al., 2002). Neuropsychological investigations in humans (Milner, 1963), and lesion studies in rats (Dias et al., 1996) and monkeys (Moore et al., 2009), indicate that performance on the WCST or its analogs is dependent on the PFC. In monkeys, the role of the PFC in set-shifting task has also been demonstrated by means of functional MRI (Nakahara et al., 2002). In human neuroimaging studies, consistent activation of the inferior frontal cortex (Konishi et al., 1998; Berman et al., 1995; Nagahama et al., 1998) and/or dorsolateral PFC (Berman et al., 1995; Monchi et al., 2001; Nagahama et al., 2001) is observed during WCST performance.
Several observations indicate that the PFC is a region particularly vulnerable to aging. First, the lateral PFC is the region that undergoes the largest volumetric reduction with age in humans (Raz et al., 2005). There are also substantial age-related alterations of white matter within the PFC, as assessed by atrophy (Raz, 1997; Salat et al., 1999a), increases in white matter intensities (Prins and Scheltens, 2015) and reductions in the micro-structure integrity of fiber tracts measured by Diffusion Tensor Imaging (Madden et al., 2007; Madden et al., 2004; O’Sullivan et al., 2001; Raz et al., 2005; Salat et al., 1999b). In addition to these anatomical changes, there are reductions in dopamine receptor density and dopamine transporter availability in the PFC (Volkow, 1998). Data from macaque models of human aging have confirmed the loss of subcortical white matter with age (Peters and Sethares, 2002; Peters and Rosene, 2003), showed reductions in dopamine concentration in the PFC (Goldman-Rakic and Brown, 1981) and revealed an age-related loss of dendritic spines (Duan et al., 2003), and of synapses (Peters et al., 2008; Peters et al., 1998) in area 46 Furthermore, electrophysiological investigations in aged NHPs have highlighted some of the molecular mechanisms contributing to impaired PFC functioning with age. With advancing age, monkeys show reduced PFC neuron firing during the delay of working memory tasks which correlates with impaired performance (Wang et al, 2011). Age-related reduction in neuronal firing in the PFC is related to increased cAMP-K+ channel signaling with age, a mechanism also involved in the vulnerability to degeneration, as assessed by increased phosphorylated Tau, in the aged macaque PFC (Carlyle et al., 2014).
Not surprisingly, age-related decrements in WCST performance have been attributed to some of these PFC alterations. For example, significant associations between age-related increases in perseveration in the WCST and smaller PFC volume were reported in several MRI studies in humans (Gunning-Dixon and Raz, 2003; Head et al., 2009; Raz et al., 1998; reviewed in the meta-analysis by Yuan and Raz, 2014). In addition, functional neuroimaging studies have found associations between age-related decreases in the activation of the left dorsolateral PFC and left inferior lobule and poor WCST performance in older adults (Nagahama et al., 1997; d’Esposito, 1999). Other studies have found perseveration errors on the WCST to be related to the volume of white matter intensities within the PFC (Gunning-Dixon and Raz, 2003).
Unlike in humans, no overt alterations of the PFC with age, as assessed by MRI, have been observed in the chimpanzee (Sherwood et al., 2011). It should be cautioned, however, that these data only included structural MRIs from individuals aged 10 to 45 years old and therefore did not capture volumetric measures in oldest old chimpanzee brains. Herndon et al (1999) reported significant age-related brain shrinkage in chimpanzees aged 7 to 59 years old, suggesting that significant brain atrophy may occur in this species in the last decade of life. Given these findings, it remains possible that the chimpanzee PFC as well undergoes some of the age-related changes observed in humans but late in their lifetime (see Chen, 2013 for similar conclusions). In addition, cellular and molecular analyses of age-related brain changes have not been conducted in the chimpanzee PFC, leaving open the possibility of more subtle changes (in receptor densities, dendritic spines, cAMP channel signaling) not detectable by MRI that could significantly impact PFC function.
To conclude, we found evidence for age-related changes in PFC function in the chimpanzee that resemble the age-related decline in cognitive flexibility observed in humans, monkeys and rodents. These changes can already be detected in middle-age, supporting the idea that executive function is the domain that undergoes the earliest age-related cognitive decline, as shown in rhesus monkeys (Moore et al., 2006). Available data on cognitive aging in the chimpanzee are extremely sparse, leaving the question of age-related decline in other cognitive domains (i.e, declarative memory) unanswered. As reviewed in the introduction, early research on cognitive aging in the chimpanzee provided inconsistent results. The more recent research from Lacreuse et al (2014) assessed cognitive function in a battery tasks that did not strongly tax memory. Thus, it remains unclear whether chimpanzees show a similar pattern of age-related cognitive impairment in multiple domains as established in humans and macaque models of human aging. Additional research in aging chimpanzees, other great apes and monkeys is critically needed, as comparative studies of age-related cognitive decline and neuropathology in humans vs. other primates with different brain architectures, life histories and lifespans are likely to provide new key findings for understanding how aging impacts cortical circuitry and cognition, and increases vulnerability to AD.
Highlights (MS 18–512).
A set-shifting task was used to assess cognitive flexibility in female chimpanzees
Middle-aged and Old chimpanzees made more perseverative errors than the Young
Cognitive flexibility significantly declines with age in female chimpanzees
Acknowledgements
This study was funded by the National Center for Research Resources P51RR000165, Office of Research Infrastructure Programs P51OD011132, and NIA P01AG02642.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors declare no conflict of interest.
The chimpanzees were humanely treated in accordance with the Animal Welfare Act and the US Department of Health and Human Services “Guide for the Care and Use of Laboratory Animals.” All research reported in this manuscript complied with the protocols approved by the Animal Care and Use Committee of Emory University. The YNPRC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
LITERATURE CITED
- Arey RN, Murphy CT 2017. Conserved regulators of cognitive aging: From worms to humans. Behavioural Brain Research 322, 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Fox MT, Baxter MG 2002. Aged Rats Are Impaired on an Attentional Set-Shifting Task Sensitive to Medial Frontal Cortex Damage in Young Rats. Learning & Memory 9(4), 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus JM, Fleming D, Johnson HR 1978. Aging in the rhesus monkey: Debilitating effects on short-term memory. J Gerontol 34, 209–19. [DOI] [PubMed] [Google Scholar]
- Berman KF, Ostrem JL, Randolph C, Gold J, Goldberg TE, Coppola R, Carson RE, Herscovitch P, Weinberger DR 1995. Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: a positron emission tomography study. Neuropsychologia 33, 1027–46. [DOI] [PubMed] [Google Scholar]
- Bernstein IS 1961. Response variability and rigidity in the adult chimpanzee. J Gerontol 16, 381–6. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Woods AG 2009. Animal models of human cognitive aging Springer. [Google Scholar]
- Blazer DG, Yaffe K, Karlawish J 2015. Cognitive aging: A report from the institute of medicine. JAMA 313(21), 2121–2. [DOI] [PubMed] [Google Scholar]
- Bloomstrand M, Maple TL 1985. An analysis of learning in two 50-year old chimpanzees. Am J Primatol 8, 333. [Google Scholar]
- Carlyle BC, Nairn AC, Wang M, Yang Y, Jin LE, Simen AA, Ramos BP, Bordner KA, Craft GE, Davies P, Pletikos M, Sestan N, Arnsten AF, Paspalas CD 2014. cAMP-PKA phosphorylation of tau confers risk for degeneration in aging association cortex. Proc Natl Acad Sci U S A 111(13), 5036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Errangi B, Li L, Glasser MF, Westlye LT, Fjell AM, Walhovd KB, Hu X, Herndon JG, Preuss TM, Rilling JK, 2013. Brain aging in humans, chimpanzees (Pan troglodytes), and rhesus macaques (Macaca mulatta): magnetic resonance imaging studies of macro- and microstructural changes. Neurobiol Aging 34(10), 2248–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW 2006. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol 73(1), 19–38. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Head E 2008. The canine (dog) model of human aging and disease: dietary, environmental and immunotherapy approaches. J Alzheimers Dis 15(4), 685–707. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC 1996. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci 110(5), 872–86. [DOI] [PubMed] [Google Scholar]
- Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR 2003. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb Cortex 13(9), 950–61. [DOI] [PubMed] [Google Scholar]
- Dyke B, Gage T, Alford P, Swenson B, Willams-Blangero S, 1995. Model life table for captive chimpanzees. Am. J. Primatol 37 (1), 25–37. [DOI] [PubMed] [Google Scholar]
- Edler MK, Sherwood CC, Meindl RS, Hopkins WD, Ely JJ, Erwin JM, Mufson EJ, Hof PR, Raghanti MA 2017. Aged chimpanzees exhibit pathologic hallmarks of Alzheimer’s disease. Neurobiology of Aging 59(Supplement C), 107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Kirkby BS, Van Horn JD, Ellmore TM, Berman KF 1999. Context-dependent, neural system-specific neurophysiological concomitants of ageing: mapping PET correlates during cognitive activation. Brain 122(Pt 5), 963–79. [DOI] [PubMed] [Google Scholar]
- Franco R, Cedazo-Minguez A 2014. Successful therapies for Alzheimer’s disease: why so many in animal models and none in humans? Frontiers in Pharmacology 5(146). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Stocker AM, Koh MT 2011. Mindspan: lessons from rat models of neurocognitive aging. ILAR J 52(1), 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM, 1981. Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience 6(2), 177–187. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N 2003. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia 41(14), 1929–41. [DOI] [PubMed] [Google Scholar]
- Hampson E, Morley EE 2013. Estradiol concentrations and working memory performance in women of reproductive age. Psychoneuroendocrinology 38(12), 2897–904. [DOI] [PubMed] [Google Scholar]
- Head D, Kennedy KM, Rodrigue KM, Raz N 2009. Age differences in perseveration: cognitive and neuroanatomical mediators of performance on the Wisconsin Card Sorting Test. Neuropsychologia 47(4), 1200–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ 1997. Patterns of cognitive decline in aged rhesus monkeys. Behavioural Brain Research 87(1), 25–34. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Paredes J, Wilson ME, Bloomsmith MA, Chennareddi L, Walker ML, 2012. Menopause occurs late in life in the captive chimpanzee (Pan troglodytes). Age (Dordr) 34(5), 1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Preuss TM, Rilling JK, 2013. Brain aging in humans, chimpanzees (Pan troglodytes), and rhesus macaques (Macaca mulatta): magnetic resonance imaging studies of macro- and microstructural changes. Neurobiol Aging 34(10), 2248–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Tigges J, Anderson DC, Klumpp SA, McClure HM 1999. Brain weight throughout the life span of the chimpanzee. Journal of Comparative Neurology 409(4), 567–72. [PubMed] [Google Scholar]
- Herrmann E, Call J, Hernandez-Lloreda MV, Hare B, Tomasello M 2007. Humans have evolved specialized skills of social cognition: the cultural intelligence hypothesis. Science 317(5843), 1360–6. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kameyama M, Nakahara K, Sekihara K, Miyashita Y 1998. Transient activation of inferior prefrontal cortex during cognitive set shifting. Nat Neurosci 1(1), 80–4. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Chang J, Metevier CM, Laclair M, Meyer JS, Ferris CM 2014a. Oestradiol modulation of cognition in adult female marmosets (Callithrix jacchus). J Neuroendocrinol 26(5), 296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Chennareddi L, Gould KG, Hawkes K, Wijayawardana SR, Chen J, Easley KA, Herndon JG, 2008. Menstrual cycles continue into advanced old age in the common chimpanzee (Pan troglodytes). Biol Reprod 79(3), 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Herndon JG 2009. Nonhuman primate models of cognitive aging. Animal Models of Human Cognitive Aging Humana Press, pp 1–30. [Google Scholar]
- Lacreuse A, Russell JL, Hopkins WD, Herndon JG 2014b. Cognitive and motor aging in female chimpanzees. Neurobiol Aging 35(3), 623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf EV, Ross SR, Matsuzawa T 2010. The mind of the chimpanzee: Ecological and experimental perspectives The University of Chicago press, Chicago. [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA 2007. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol Aging 28(3), 459–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM 2004. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage 21(3), 1174–81. [DOI] [PubMed] [Google Scholar]
- Manrique HM, Call J 2015. Age-dependent cognitive inflexibility in great apes. Animal Behaviour 102(0), 1–6. [Google Scholar]
- Milner B 1963. Effects of different brain lesions on card sorting: The role of the frontal lobes. Archives of Neurology 9(1), 90–100. [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A 2001. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. Journal of Neuroscience 21(19), 7733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB 2003. Impairment in abstraction and set shifting in aged rhesus monkeys. Neurobiol Aging 24(1), 125–34. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB 2005. A non-human primate test of abstraction and set shifting: an automated adaptation of the Wisconsin Card Sorting Test. Journal of Neuroscience Methods 146(2), 165–73. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB 2006. Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiology of Aging 27(10), 1484. [DOI] [PubMed] [Google Scholar]
- Moore TL, Schettler SP, Killiany RJ, Rosene DL, Moss MB 2009. Effects on executive function following damage to the prefrontal cortex in the rhesus monkey (Macaca mulatta). Behavioral Neuroscience 123(2), 231–41. [DOI] [PubMed] [Google Scholar]
- Moss MB, Rosene DL, Peters A 1988. Effects of aging on visual recognition memory in the rhesus monkey. Neurobiol Aging 9, 495–502. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Fukuyama H, Yamauchi H, Katsumi Y, Magata Y, Shibasaki H, Kimura J 1997. Age-related changes in cerebral blood flow activation during a Card Sorting Test. Exp Brain Res 114, 571–7. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, Konishi J, Fukuyama H, Shibasaki H 2001. Dissociable mechanisms of attentional control within the human prefrontal cortex. Cerebral Cortex 11(1), 85–92. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Sadato N, Yamauchi H, Katsumi Y, Hayashi T, Fukuyama H, Kimura J, Shibasaki H, Yonekura Y 1998. Neural activity during attention shifts between object features. Neuroreport 9(11), 2633–8. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Hayashi T, Konishi S, Miyashita Y 2002. Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science 295(5559), 1532–6. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SCR, Markus HS 2001. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology 57(4), 632–8. [DOI] [PubMed] [Google Scholar]
- Onos KD, Sukoff Rizzo SJ, Howell GR, Sasner M 2016. Toward more predictive genetic mouse models of Alzheimer’s disease. Brain Research Bulletin 122(Supplement C), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P 2009. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol 60, 173–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas CD, Carlyle BC, Leslie S, Preuss TM, Crimins JL, Huttner AJ, van Dyck CH, Rosene DL, Nairn AC, Arnsten AFT 2018. The aged rhesus macaque manifests Braak stage III/IV Alzheimer’s-like pathology. Alzheimers Dement 14(5), 680–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Rosene DL, 2003. In aging, is it gray or white? J Comp Neurol 462(2), 139–143. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C 2002. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol 442(3), 277–91. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Luebke JI 2008. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience 152(4), 970–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Sethares C, Moss MB 1998. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. CerebCortex 8(8), 671–84. [DOI] [PubMed] [Google Scholar]
- Picq JL. Aging affects executive functions and memory in mouse lemur primates. Exp Gerontol. 2006 doi: 10.1016/j.exger.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Prins ND, Scheltens P 2015. White matter hyperintensities, cognitive impairment and dementia: an update. Nature Reviews Neurology 11(3), 157. [DOI] [PubMed] [Google Scholar]
- Rapp PR 1988. Toward a nonhuman primate model of age-dependent cognitive dysfunction. Neurobiol Aging 9(5–6), 503–5. [DOI] [PubMed] [Google Scholar]
- Raz N 1997. Selective aging of human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex 7, 268–82. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD 1998. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology 12(1), 95–114. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD 2005. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 15(11), 1676–89. [DOI] [PubMed] [Google Scholar]
- Rilling JK 2014. Comparative primate neuroimaging: insights into human brain evolution. Trends in Cognitive Sciences 18(1), 46–55. [DOI] [PubMed] [Google Scholar]
- Riopelle AJ, Rogers CM 1965. in: Schrier AM, et al. (Ed.). Behavior of Nonhuman Primates: Modern Research Trends Academic Press, New York/London. [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS 1999a. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol 56(3), 338–44. [DOI] [PubMed] [Google Scholar]
- Salat DH, Stangl PA, Kaye JA, Janowsky JS 1999b. Sex differences in prefrontal volume with aging and Alzheimer’s disease. Neurobiol Aging 20(6), 591–6. [DOI] [PubMed] [Google Scholar]
- Salthouse T 2010. Major issues in cognitive aging Oxford University Press, New York, NY. [Google Scholar]
- Sherwood CC, Gordon AD, Allen JS, Phillips KA, Erwin JM, Hof PR, Hopkins WD 2011. Aging of the cerebral cortex differs between humans and chimpanzees. Proc Natl Acad Sci U S A 108(32), 13029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif SD, Mansfield KG, Ratnam R, Ross CN, Ziegler TE 2011. The marmoset as a model of aging and age-related diseases. ILAR J 52(1), 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J 1998. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. American Journal of Psychiatry 155(3), 344–9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Logan J, Fowler JS, Wang G-J, Gur RC, Wong C, Felder C, Gatley SJ, Ding Y-S, Hitzemann R, Pappas N 2000. Association Between Age-Related Decline in Brain Dopamine Activity and Impairment in Frontal and Cingulate Metabolism. Am J Psychiatry 157(1), 75–80. [DOI] [PubMed] [Google Scholar]
- Voytko ML 1993. Cognitive changes during normal aging in monkeys assessed with an automated test apparatus. Neurobiology of Aging 14(6), 643–4. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Murray R, Higgs CJ 2009. Executive Function and Attention Are Preserved in Older Surgically Menopausal Monkeys Receiving Estrogen or Estrogen Plus Progesterone. J Neurosci 29(33), 10362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Raz N 2014. Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neuroscience & Biobehavioral Reviews 42, 180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


