Abstract
Objectives
The role of radiation therapy (RT) in resected pancreatic cancer (PC) remains incompletely defined. We sought to determine clinical variables which predict for local-regional recurrence to help select patients for adjuvant RT.
Methods
We identified 73 patients with PC who underwent resection and adjuvant gemcitabine-based chemotherapy alone. We performed detailed radiologic analysis of first patterns of failure. Local-regional recurrence was defined as recurrence of PC within standard post-operative radiation volumes. Univariate analyses (UVA) were conducted using the Kaplan-Meier method and multivariate analyses (MVA) utilized the Cox proportional hazard ratio model. Factors significant on UVA were used for MVA.
Results
At median follow up of 20 months, rates of local-regional recurrence only (LRRO) were 24.7%, local-regional recurrence as a component of any failure (LRR) 68.5%, metastatic recurrence as a component of any failure (MR) 65.8%, and overall disease recurrence (OR) 90.5%. On UVA, elevated post-operative CA 19-9 (>90 U/mL), pathological lymph node positive (pLN+) disease, and higher tumor grade were associated with increased LRR, MR, and OR. On MVA, elevated post-operative CA 19-9 and pLN+ were associated with increased MR and OR. Additionally, positive resection margin was associated with increased LRRO on both UVA and MVA.
Conclusions
About 25% of patients with PC treated without adjuvant RT develop LRRO as initial failure. The only independent predictor of LRRO was positive margin, while elevated post-operative CA 19-9 and pLN+ were associated with predicting MR and OS. These data may help determine which patients benefit from intensification of local therapy with radiation.
Keywords: pancreatic cancer, radiation therapy, patterns of failure, gemcitabine, local-regional recurrence
INTRODUCTION
An estimated 53,670 patients will be diagnosed with pancreatic adenocarcinoma in 2017 and 43,090 will die of their disease [1]. The prognosis remains dismal with five-year overall survival of 8% [1]. Surgical resection continues to be the mainstay for management and is the only option considered to be potentially curative. However, only about 20% of patients will present with disease amenable to resection and the best survival rates at five years following surgery are only 25–30% [2]. Overall, more than 90% of patients will ultimately succumb to their disease with about 70% having extensive metastatic disease and 30% with more limited metastatic disease but with locally-advanced tumors [3]. As a result of high rates of disease recurrence, multiple studies have evaluated adjuvant therapy strategies, including adjuvant chemoradiation therapy (CRT) [4–10].
Early randomized trials investigating CRT concluded mixed results [4, 5]. Although these trials utilized outdated split-course radiation techniques, a disease-free survival and overall survival (OS) benefit was seen in the GITSG 9173 trial, while there was a trend towards improved survival seen in the EORTC 40891 trial when focusing only on pancreatic head tumors. The CONKO-001 trial evaluated the role of adjuvant gemcitabine (without CRT) and found that adjuvant gemcitabine results in improved disease-free survival and overall survival when compared with observation [8, 11]. Aside from gemcitabine, other chemotherapy regimens tested in the adjuvant setting that have shown some benefit include 5-fluorouracil (5FU) and S-1 [7, 10]. The only prospective clinical trial that attempted to compare chemotherapy with CRT was the ESPAC-1 trial, which employed a 2×2 design accruing patients to observation, chemotherapy, CRT, or CRT followed by chemotherapy [6]. The results of this trial suggested that chemotherapy was beneficial while CRT was detrimental to OS. However, many practitioners do not deem this data practice changing because of the numerous criticisms including outdated radiation technique without quality assurance, questionable study design, high rate of non-adherence, physician bias in patient accrual, and allowance of previous chemotherapy or CRT [12–14]. The only trial using modern radiation therapy with quality assurance was RTOG-9704 which showed no difference between adjuvant 5-FU or gemcitabine given both before and after 50.4 Gy of 5-FU based CRT [9]. Remarkably, local failure in the previously mentioned ESPAC-1, EORTC, and CONKO-001 trials was high, ranging from 37–63%. In contrast, patients treated with modern radiation therapy on RTOG 9704 only experienced a 25% local failure rate despite higher rates of positive/unknown margins, suggesting post-operative chemoradiation is attenuating local recurrence. The importance of local control in pancreatic cancer was also suggested by a rapid autopsy study which showed that ~30% of patients die as a direct result of local disease progression [3].
Interestingly, multiple large retrospective studies comparing patients who received additional local-regional therapy after resection in the form of modern chemoradiation versus those that did not, suggest a survival improvement with chemoradiation [15]. However, these studies do not provide level 1 evidence supporting a benefit with chemoradiation. An ongoing multi-center U.S. trial seeks to directly address the role of adjuvant CRT (RTOG 0848) wherein patients are randomized to CRT versus additional gemcitabine after completion of 5 cycles of gemcitabine.
Given these uncertainties, recommendations for adjuvant radiation therapy remain incompletely defined (NCCN 2017). Ideally, the use of clinical and molecular biomarkers would enable practitioners to predict patterns of disease failure and assist with clinical guidance regarding adjuvant therapy. Because overall survival is improved with adjuvant chemotherapy, this is the current standard-of-care and patients receiving adjuvant chemotherapy would be an ideal cohort to reveal factors that increase local-regional disease recurrence in spite of chemotherapy administration. Several studies have attempted to elucidate the underlying clinical factors contributing to local-regional disease failure, including the presence of lymph node metastases, positive margins, increased pre-operative CA 19-9, and perineural invasion [16–21]. However, there is some inconsistency with the results of these studies and the patient populations included various neoadjuvant or adjuvant approaches, including radiation [16–21]. Molecular biomarkers such as SMAD4 have also been evaluated, but there have been mixed results and no clinically useful molecular biomarker has been validated for routine clinical practice [3, 22, 23]. Many of these clinical and molecular biomarkers were elucidated in groups of patients who received a combination of observation, neoadjuvant, and/or adjuvant therapies, which creates a difficult dataset for interpreting the significance of the clinical or molecular biomarkers identified in such studies.
Because there is a lack of definitive data regarding the role for adjuvant radiation therapy following resection, practice patterns vary and selecting which patients will benefit from the addition of RT remains controversial. In this study, we sought to determine clinical factors associated with local-regional recurrence in patients not treated with CRT based on our institutional experience. Additionally, our cohort consists of patients who uniformly received adjuvant chemotherapy only, and thus represents the most relevant group of patients for discovering which clinical factors predict early local-regional recurrence following resection and chemotherapy alone. Identification of such factors predisposing to local-regional failure could allow better selection of patients for adjuvant chemoradiation.
MATERIALS AND METHODS
Patient Selection and Clinical Outcomes
We reviewed the medical records of 73 patients with pancreatic adenocarcinoma who underwent curative resection followed by adjuvant gemcitabine at the Ohio State University Wexner Medical Center between 2007–2012. This retrospective review was approved by the Institutional Review Board. Tumors were staged according to American Joint Committee on Cancer (AJCC) guidelines 7th edition. Those patients staged initially with 6th edition were re-staged using the 7th edition guidelines. Numerous characteristics were analyzed consisting of age, post-operative CA 19-9, pathologic tumor stage (pT), pathologic nodal stage (pN), number of positive lymph nodes (LN), ratio of positive LNs to number of LNs resected, histologic grade, lympho-vascular space invasion, perineural invasion, margin status, pathologic tumor size, and chemotherapy use and duration. Frequency distributions for the evaluated characteristics were calculated. For analysis of survival outcomes, the variables were dichotomized at the median value. For patterns of failure, we defined overall recurrence (OR) as having any type of recurrence (whether local-regional or distant) at some point during the patient’s course. Then, we sub-classified first failure as either (1) local-regional recurrence only (LRRO) if the first failure was a local-regional recurrence in the absence of metastatic disease; (2) local-regional recurrence (LRR) if the first failure was a local-regional recurrence with concomitant presence or absence of metastatic failure (and therefore included all LRRO patients); and (3) metastatic recurrence (MR) if their first failure was a metastatic recurrence with concomitant presence or absence of local-regional failure (and therefore did not include LRRO patients). Patterns of failure were determined using cross-sectional imaging and radiologist review with a board-certified radiologist specializing in gastrointestinal diseases (C.G.), with local-regional recurrence defined as evidence for disease recurrence in the resection bed and/or regional lymph nodes that would be encompassed within a typical post-operative radiation field, as described by RTOG 9704 [9, 24]. Target lesions were evaluated with RECIST criteria 1.1, such that recurrence was defined as at least a 20% increase in the sum of diameters of target lesions while also achieving an absolute increase of 5 mm, or presence of new lesions.
Statistical Analysis
Univariate analyses (UVA) were conducted using the Kaplan-Meier method, and the log-rank test was used to identify factors associated with improved clinical outcomes, with p<0.05 being considered significant. For multivariate analyses (MVA), the Cox proportional hazard ratio model was utilized. Factors significant on univariate analysis were used in the multivariate analysis. Both UVA and MVA were completed for all four clinical recurrence endpoints. All statistical analysis was completed using SPSS, version 22 (SPSS Inc., Chicago, IL).
RESULTS
Seventy-three patients were identified for this study, with a median follow up of 20 months. During the time interval in which these patients were treated, the preferred practice at our institution for adjuvant therapy included gemcitabine-based chemotherapy with omission of adjuvant CRT. The majority of patients received gemcitabine alone (n=68) and other chemotherapy regimens included gemcitabine/oxaliplatin (n=3), gemcitabine/cisplatin (n=1), and gemcitabine/capecitabine (n=1), and the median number of cycles given was 6.
Median age at diagnosis was 61 years, with 63% of our patients being male. Patient characteristics for our cohort are displayed in Table 1. At diagnosis, 68% (n=50) of patients had an elevated pre-operative CA 19-9. Of these, only 12 (16.4%) had a persistently elevated post-operative CA 19-9 > 90 U/mL, while 38 (52.1%) patients had CA 19-9 ≤ 90 U/mL. Of note, 93.2% had a T3 pathologic tumor stage, 75.3% had pathologically positive nodal disease, 52.1% demonstrated lymphovascular space invasion, 93.2% showed perineural invasion, and 19.2% of resection margins were positive. The median tumor size was 3.6 cm and the median ratio of positive (pathologically involved) to total resected lymph nodes was 0.154.
Table 1.
Demographic, Pathologic, and Recurrence Data.
| Variable | n (%) | n (%) | n (%) |
|---|---|---|---|
| Gender (male vs female) | 46 (63) | 27 (37) | NA |
| Post-operative CA19-9 (≤ 90 vs > 90 U/mL) | 38 (52.1) | 12 (16.4) | NA |
| pT Stage (2 vs 3 vs 4) | 4 (5.5) | 68 (93.2) | 1 (1.4) |
| pN Stage (0 vs 1) | 18 (24.7) | 55 (75.3) | NA |
| Grade (1 vs 2 vs 3) | 4 (5.5) | 57 (78.1) | 12 (16.4) |
| LVSI (present vs absent vs unknown) | 38 (52.1) | 26 (35.6) | 9 (12.3) |
| PNI (present vs absent vs unknown) | 68 (93.2) | 4 (5.5) | 1 (1.4) |
| Margin (positive vs negative) | 14 (19.2) | 59 (80.8) | NA |
| LRR (yes vs no) | 50 (68.5) | 23 (31.5) | NA |
| Location of LRR (tumor bed only vs LN only vs both) | 30 (60.0) | 13 (26.0) | 7 (14.0) |
| LRRO (yes vs no) | 18 (24.7) | 55 (75.3) | NA |
| MR (yes vs no) | 48 (65.8) | 25 (34.2) | NA |
| OR (yes vs no) | 66 (90.4) | 7 (9.6) | NA |
Abbreviations: pT = pathologic tumor, pN = pathologic nodal, LVSI = lymph vascular space invasion, PNI = perineural invasion, LRR = Local regional recurrence, LN = lymph node, LRRO = local regional recurrence only, MR = metastatic recurrence, OR = overall recurrence.
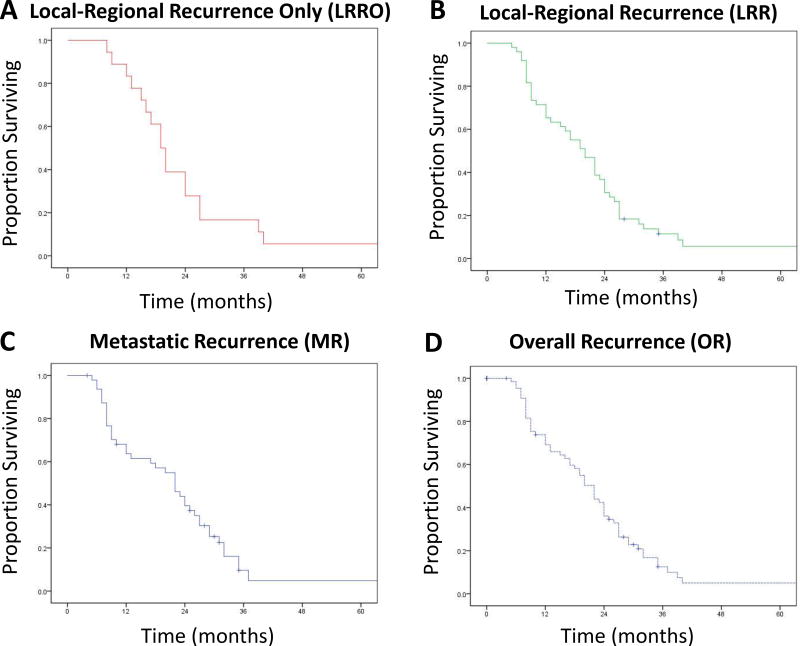
In conjunction with a radiologist, we determined patterns of first failure including local-regional recurrence only (LRRO), any local regional recurrence as a component of failure (LRR), metastatic recurrence as a component of failure (MR), and overall recurrence (OR) as defined in the Methods section. In this cohort of patients, 90.4% developed a recurrence (overall recurrence, OR) during the follow-up period. Of these, we sub-classified patterns of first failure and found the following frequencies: LRRO 24.7%, LRR 67.1%, and MR 65.8%. Median time to failure for patients with LRRO, LRR, MR, and OR was 8.5, 8.0, 8.0, and 8.0 months, respectively. Overall survival for patients with LRRO and LRR is shown in Figure 1A–B, while overall survival for patients with MR and OR is shown in Figure 1C–D. Median overall survival for patients with LRRO, LRR, MR, and OR were 19.5, 20.0, 22.0, and 20.0 months respectively.
Figure 1.
Kaplan-Maier plots showing overall survival in patients who experienced local-regional recurrence only (A), local-regional recurrence as a component of disease recurrence (B), metastatic recurrence as a component of disease recurrence (C), and any disease recurrence (D).
Next, we performed univariate analysis (UVA) of the clinical and pathologic factors in Table 1 and their correlation with recurrence patterns (Table 2). On UVA, improved OR was significantly associated with post-operative CA 19-9 ≤90 U/mL, pN0 status, grade 1–2 histology, and negative margins. Likewise, reduced metastatic recurrence correlated with post-operative CA 19-9 ≤90 U/mL, pN0 status, reduced LNs positivity, lower LN positive ratio (below the median), histologic grade 1–2, and smaller tumor size (below the median). With regards to local-regional recurrence as any component of first failure, reduced LRR was significantly correlated with post-operative CA 19-9 ≤90 U/mL, pN0 status, grade 1–2 histology, and smaller tumor size. For LRRO, only positive margin status was found to significantly correlate with increased failure.
Table 2.
Univariate Analysis(only displaying factors which were significant for one of the 4 recurrence outcomes).
| Variable | LRRO, p-value | LRR, p-value | MR, p-value | OR, p-value |
|---|---|---|---|---|
| Post-operative CA19-9 (≤ 90 vs > 90 U/mL) | NS | <0.001 | 0.003 | 0.041 |
| pN Stage (0 vs 1) | NS | 0.02 | 0.001 | 0.008 |
| LN ratio positive/resected (below median vs above median) | NS | NS | 0.005 | 0.08 |
| Grade (1–2 vs 3) | 0.092 | 0.001 | 0.002 | 0.009 |
| Margin (positive vs negative) | 0.006 | 0.059 | NS | 0.047 |
| Tumor Size (below median vs above median) | 0.096 | 0.001 | 0.021 | 0.08 |
Abbreviations: LRRO = local regional recurrence only, LRR = Local regional recurrence, MR = metastatic recurrence, OR = overall recurrence, NS = not significant, pN = pathologic nodal, LN = lymph node.
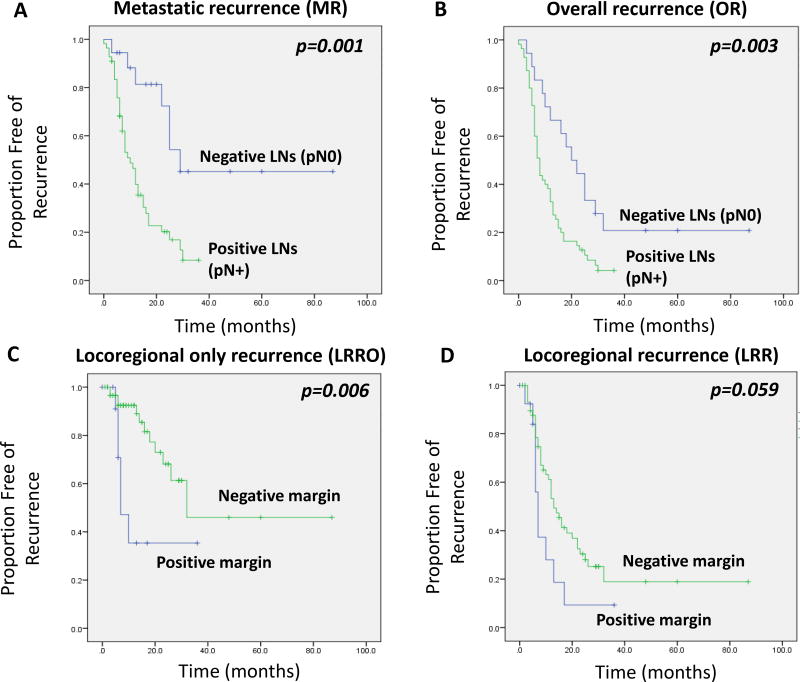
Next, we performed multivariate analysis (MVA) to determine whether the factors identified in the UVA remained independent predictors of recurrence outcomes (Table 3). For OR, pN0 status (p=0.03) was significantly and independently associated with reduced OR, and CA 19-9 ≤90 U/mL was borderline associated with reduced OR (p=0.052). Similarly, pN0 status was significantly associated with reduced metastatic recurrence (HR 0.21, 95% CI 0.06–0.68, p=0.01), but not CA 19-9 ≤90, which was borderline significant (p=0.08). As shown in Figure 2, patients with pathologically node negative (pN0) disease had substantially reduced MR (Figure 2A) and OR (Figure 2B). For LRR, no clinical variables remained significant in the MVA (although tumor size was borderline significant). However, for patients with LRRO, margin status remained a strongly independent predictor of LRRO. Specifically, positive margins were associated with a 7.2-fold increased risk of a LRRO (95% CI 1.57–33.4; p=0.01), after adjusting for other clinical variables. Indeed, positive margin status was strongly correlated with an increased LRRO failure pattern, as shown in Figure 2C (log rank p=0.006). The association between positive margin status and a LRR failure as a component of first failure was also noted, but of borderline significance (Figure 2D, log rank p=0.059).
Table 3.
Multivariate Analysis(from significant factors identified in univariate analysis; NS= not significant).
| Multivariate Analysis | LRRO, HR (p- value) |
LRR, HR (p- value) |
MR, HR (p- value) |
OR, HR (p- value) |
OS, HR (p- value) |
|---|---|---|---|---|---|
| Post-operative CA19-9 (≤ 90 vs > 90 U/mL) | NS | NS | 0.40, [0.14–1.12] (p=0.081) | 0.41, [0.17–1.01] (p=0.052) | 0.35 [0.16–0.75] (p=0.007) |
| pN Stage (0 vs 1) | NS | NS | 0.21, [0.06–0.68] (p=0.010) | 0.37, 0.148–0.913 (p=0.031) | NS |
| Margin (positive vs negative) | 7.24, [1.57–33.38] (p=0.011) | NS | NS | NS | 2.03 [0.96–4.29] (p=0.063) |
| Tumor Size (below median vs above median) | NS | 0.48, [0.22–1.07] (p=0.072) | NS | NS | NS |
Abbreviations: LRRO = local regional recurrence only, LRR = Local regional recurrence, MR = metastatic recurrence, OR = overall recurrence, NS = not significant, pN = pathologic nodal.
Figure 2.
Kaplan Maier plots showing recurrence rates for metastatic recurrence (A) and overall recurrence (B) depending on the presence or absence of lymph node positivity in the surgical specimen. Kaplan Maier plots showing recurrence rates for LRRO (C) and LRR (D) depending on the presence or absence of a positive margin in the surgical specimen. Log-rank p-values shown.
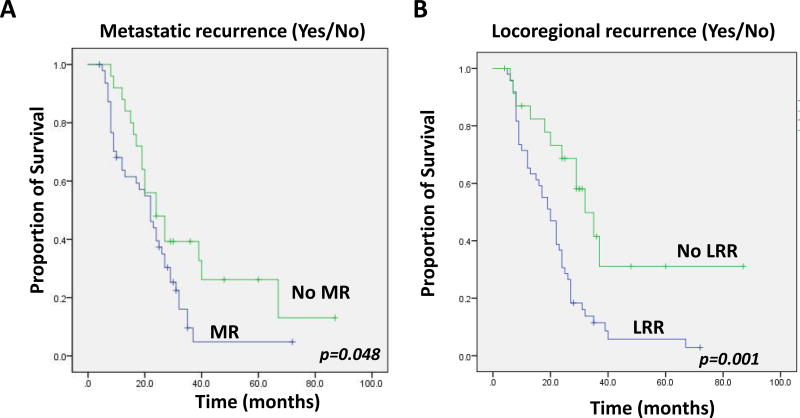
It is commonly believed that metastatic recurrence drives poor survival in pancreatic cancer. Indeed, in our study, patients who developed MR had worse overall survival than those who did not experience metastases (Figure 3A, log-rank p=0.048). However, as previously mentioned, some studies have also suggested that local progression can also drive mortality, such as the rapid autopsy series from Iacobuzio-Donahue et al.[3] In order to assess the impact of local recurrence on survival in our cohort, we examined all patients and determined survival based on whether they had any local-regional recurrence (LRR) event or not. Interestingly, patients who developed LRR as any component of first failure (including patients with LRRO) had a significantly worse overall survival compared to those who didn’t (Figure 3B, log-rank p=0.001). Those patients who developed a LRRO as the first site of failure did not have a significant difference in survival compared to the rest of the group (not shown). Next, we performed multivariate analysis of traditional factors impacting overall survival (OS) in patients with pancreatic cancer, including CA 19-9, pN stage, and margin status (these factors were also significant for failure patterns in our multivariate analysis in Table 3). As shown in the last column of Table 3, we found post-operative CA 19-9 ≤90 U/mL was the most significant predictor of OS (HR 0.35, p=0.007) with a trend for positive margin also being significantly associated with worse OS (HR 2.03, p=0.06).
Figure 3.
Comparison of overall survival in patients who experience (A) metastatic disease failure versus those who did not, and (B) who experience local-regional recurrence versus those who did not. Log-rank p-values shown.
DISCUSSION
A more reliable algorithm for predicting patterns of initial failure following resection for pancreatic adenocarcinoma might facilitate judicious use of additional local-regional therapy and improve the delivery of personalized care. This would also allow a more appropriate allocation of resources that are likely to benefit certain patients and perhaps avoid toxicity in those less likely to achieve clinical gains from intensification of local therapy. Secondary to improving patient outcomes, it might also increase the value of healthcare spending. Our study, showed that the most reliable clinical factor to predict for local-regional recurrence as the first site of recurrence was the presence of a positive margin. By coupling this finding with our data showing that lymph node positive disease and higher post-operative CA 19-9 predict for a more metastatic predominant failure pattern, we believe this will aid physicians by allowing them to focus on delivering therapy that will target the most likely sites of disease failure (i.e. post-operative chemoradiation for patients with increased local-regional recurrence risk, or intensive systemic therapy for high distant recurrence/low local-regional recurrence risks).
Distant metastatic failure, which very often occurs in the liver, is the most common outcome following surgery with or without adjuvant therapy and contributes greatly to death from pancreatic cancer. Rates of distant disease recurrence as a component of recurrence range from 50–75% in the major randomized trials [4–6, 8, 9]. Our group had a similar rate with 65.8% having distant failure as the initial site of failure. The second major recurrence following surgery is local-regional recurrence, which is generally defined as disease failure within the retroperitoneum around the superior mesenteric and celiac arteries, including the tumor bed, remnant pancreas, hepatic hilum, or its regional lymph nodes [25]. Rates of local-regional disease recurrence in the major clinical trials ranged between 30–63% with ~15–20% having local-regional failure without concurrent distant failure [4–6, 8, 9]. In comparison, our rate of LRR as a component of first failure was fairly high at 68.5% and LRRO was also slightly higher at 24.7%. We hypothesize that this relatively higher rate of local failure results from the lack of radiation therapy in our cohort, but may also be due to other causes, such as lack of proper radiologic review in those former studies. Indeed, a strength of our study was rigorous radiologic review of cases for recurrence patterns. While local-regional failure is less often directly linked to patient mortality, it is a significant factor for patient morbidity and could lead to further systemic spread of disease [25]. Furthermore, autopsy studies show persistent local-regional disease in many patients who do not yet have macroscopic disease detectable on imaging; therefore, it is likely that many studies underestimate the true prevalence of local-regional recurrence [25]. In addition, a rapid autopsy series has directly provided evidence that local-regional failure can be a predominant pattern of failure that drives patient mortality in ~30% of patients [3]. It is interesting that in our study, having a LRR as a component of first failure seemed to have a larger effect on survival compared to having a metastatic recurrence (compare Figure 3A to 3B).
Several studies have highlighted clinical and pathologic factors that contribute to local-regional disease recurrence, including positive margins, abnormal pre-op and/or post-op CA 19-9 level, lymph node stage, and tumor size. However, many of these studies did not control for adjuvant or neoadjuvant therapy, and didn’t report “local-regional only” first failure pattern [16]. It is widely accepted that, at a minimum, adjuvant chemotherapy alone following resection is recommended based on level 1 evidence established from multiple trials (e.g. ESPAC-1, CONKO-001, etc.). Therefore, our population, who received adjuvant gemcitabine after surgery, is ideal to determine if certain clinical risk factors can help guide the use of additional local-regional therapy, with chemoradiation. Our cohort received a median of 6 cycles of gemcitabine with about 50% receiving exactly 6 cycles. Similarly, the CONKO-001 trial reported a median of 6 cycles with 63% receiving 6 cycles [8]. It is likely that several of our patients wouldn’t have been eligible for the CONKO-001 trial due to the restriction of CA 19-9 ≤ twice the upper limit of normal, and the majority of our patients who didn’t receive the planned chemotherapy had early disease failure prior to completing 6 cycles. However, we believe our cohort does approximate the expected clinical experience with adjuvant gemcitabine alone, similar to the CONKO-001 trial.
It is well accepted that a positive resection margin has a higher likelihood of local disease recurrence, and in some series, worse DFS and/or OS [16–18, 21, 26, 27]. The rates of positive margin in major randomized trials are between 19–35%, and modern large retrospective studies demonstrate similar rates between 15–33% [9, 15, 28]. The rate of positive margin in our study of 19% was consistent with the literature. However, our rate of LRR at 68.5% and LRRO at 24.7% are at the higher end when comparing with randomized trials. Out of all these randomized trials, the group most similar to our patient cohort is the gemcitabine arm from the CONKO-001 trial. While the positive margin rate in this group was similar at 19%, the LRR was reported as 37%, which is much lower than the LRR rate appreciated in our study. It is possible that the eligibility criteria of CA 19-9 ≤ twice the upper limit of normal in the CONKO-001 trial engendered accrual of a lower risk population than is typically encountered in general clinical practice. The recently reported ESPAC-4 study, which compared adjuvant gemcitabine versus gemcitabine and capecitabine following complete resection showed that more intensive chemotherapy significantly improved median survival by 2.5 months (25.5 vs 28.0 months) [27]. It is interesting to note that despite this survival improvement, the more intense chemotherapy regimen did not significantly alter disease relapse rates (65–66%) or patterns of failure. In both arms, patients had a positive margin rate of 60% and a local failure rate of ~ 50%. However, this was the first study to define R1 as a margin 1 mm or less, which may account for the higher rates of margin positivity. Indeed, other studies have highlighted the importance of using a more generous definition of positive margin (e.g. 1–2 mm) [29, 30]. They did not specifically report if the R1 group had higher rates of local recurrence, but did show that regardless of chemotherapy group, R1 resection portended worse median OS of 23 vs 27.9 months (gemcitabine arm) and 23.7 vs 39.5 months (gemcitabine and capecitabine arm). It is interesting to note that much of the benefit of gemcitabine-capecitabine was in the R0 resection group. As a result of studies suggesting that location and quantity of margin are important, the AJCC 8th edition now considers R1 positivity to be a surgical margin of ≤ 1 mm. The authors concluded that gemcitabine and capecitabine should be adopted as a standard adjuvant treatment but with a median survival of 23–24 months in each R1 group, the more intense chemotherapy regimen doesn’t appear to mitigate the risk of positive margin even with the new R1 definition of ≤ 1 mm. A retrospective study conducted by Merrell et al. reported a positive margin rate of 16.2% and a LRR rate of 17% at a median follow up of 84 months, which is much lower than the rate of 68.5% seen in our study [31]. Of the 458 patients included in the study, 378 (82.5%) received radiation therapy which could explain the lower rate of LRR. They also found that positive margin did not contribute to LRR. However, they showed that not receiving adjuvant radiation therapy was the strongest predictor of LRR, despite that those who received radiation had a positive margin rate of 18%, while those who did not receive radiation had a rate of 6% [31]. One potential explanation is that the use of radiation therapy was able to prevent patients with positive margins from developing a LRR. Corroborating this study is another recently published study which analyzed the National Cancer Database and showed that the use of chemoradiation therapy improved OS when compared with chemotherapy alone and that those with an R1 resection had a 21% reduction in death if they received chemoradiation [15]. Although this study is not able to evaluate LRR and has other limitations due to the more limited data provided by the National Cancer Database, it does suggest improved disease outcome with adjuvant CRT rather than chemotherapy alone [15]. The only completed phase III randomized trial to utilize modern radiation therapy and enforce quality assurance was RTOG 97-04. Although both randomized groups received chemoradiation, one can garner several important observations [9, 24]. First, this trial carried the highest rate of positive resection margin at ~35% (also with unknown margins in 25%) than any of the other previously-mentioned randomized controlled trials, which ranged from 0–28% [4–6, 8, 9]. Consequently, there is an expectation of higher rates of local disease progression. However, the trial showed the lowest rates of local recurrence, with 23% in the 5-FU arm and 28% in the gemcitabine arm, compared to the aforementioned randomized trials that demonstrated rates of 37–63% in the various treatment arms [4–6, 8, 9, 24]. Furthermore, when directly comparing patients in the 2 more modern trials, RTOG 97-04 and CONKO-001, positive margins were detected in 35% of patients in RTOG 97-04 and only 17% of patients in CONKO-001, while LRR was observed in 25% and 34% of patients, respectively. While hypothesis-generating, this does seem to suggest that chemoradiation may improve local-regional control, in part by mitigating local-regional disease failure in the setting of positive margins [8, 11, 24].
Because of the competing patterns of disease failure, it is reasonable to consider the factors which are likely to produce early distant relapse, since patients with these risk factors are unlikely to benefit from chemoradiation and should likely be treated with intensified systemic therapy. In our analysis, we found that the strongest independent predictor of metastatic disease failure was lymph node involvement with those displaying an elevated post-operative CA 19-9 also at a heightened risk. Rates of pathologic lymph node positivity in the randomized trials evaluating adjuvant therapy for resected pancreatic adenocarcinoma ranged from 30–71%, with modern trials showing 50–71%, and two large modern retrospective trials showing rates of 66% and 67% [4–6, 8, 9, 15, 28]. Pathologic lymph node involvement occurred in 75% of the patients in our cohort with the median number of positive LNs being 3 and the median resected being 20 (ratio ~15%). The CONKO-001 study had a similar rate of node positivity at 71% and node positive patients had worse DFS and OS regardless of being enrolled to gemcitabine or observation; however, the authors did not evaluate ratio of LN positivity with patterns of disease failure [8]. ESPAC-4 had 80% LN positivity and this risk factor had a major detrimental effect on survival, but the authors did not analyze the effect of LN positivity on patterns of recurrence. Furthermore, neither of these studies attempted to analyze ratio of LN positivity with patterns of disease failure. Several recent retrospective trials have attempted to answer this question. Merrell et al. concluded that a LN positive to resected ratio ≥ 0.2 was significantly correlated with LRR, but metastatic failure was not specifically studied and the patients had a mixture of adjuvant therapy [31]. Another recent retrospective study evaluated factors that contribute to local-regional disease failure and determined that LN status was a significant predictor of this outcome [16]. However, it is unclear if LN positive disease also predicted metastatic recurrence as this was not studied and this group of patients included a mixture of patients who received either observation (38.9%) or adjuvant chemotherapy (61.1%, which was split between gemcitabine and 5FU-containing regimens), thus making the conclusions confounded by treatment heterogeneity [16]. Another recent retrospective study determined that LN involvement did significantly correlate with LRR, but they determined that only patients with a positive margin had significantly poorer local disease control compared with negative margin regardless of LN involvement, suggesting that margin status was the strongest predictor of LRR [18]. However, no adjuvant treatment data was presented in this publication [18]. One study evaluating the recurrence pattern in 127 patients showed that LN metastases was the strongest predictor for distant failure [23]. Furthermore, those patients with LN positive disease were much more likely to have distant recurrence as the only site of recurrence (79% vs 21%) and those without LN metastases were more likely to have local recurrence as the only site of recurrence (60% vs 40%) [23]. Our results support that LN involvement predicts for distant recurrence in both univariate and multivariate models, but also predicts for LRR (although this did not remain significant on multivariate analysis). Overall, it appears that LN involvement predicts for any disease recurrence which could be local-regional, distant, or both. However, with the strong competing risk for metastatic disease recurrence, this may be a group of patients that would most benefit from local therapy intensification if they also have a positive margin.
CA 19-9 (carbohydrate antigen 19-9) is a glycoprotein that is often overexpressed in patients with pancreatic adenocarcinoma [32]. While pre-operative CA 19-9 can be useful for prognostication [16, 19], it has been shown that post-operative CA 19-9 is more useful to determine clinical outcomes, including survival, and the degree of post-operative normalization can be useful for detecting residual disease or occult metastatic disease [33, 34]. Although post-op CA 19-9 was not significant on MVA in our study, it produced a high hazard ratio for distant failure which did trend toward significance (p=0.081). In a study by Sugiura et al, CA 19-9 level ≥100 U/mL was strongly associated with risk of disease failure within 6 months of surgery, with 53% of patients with high CA 19-9 values having early failure versus 11% of those with low values [19]. Our data corroborated this with a mean time to disease recurrence for those with CA 19-9 >90 U/mL versus ≤90 U/mL of 6.2 months and 14.0 months, respectively. Thus, it is unlikely that patients with high post-op CA 19-9 levels would benefit from intensification of local therapy because these distant failures tend to occur early. Taken together, we conclude post-operative CA 19-9 has repeatedly shown value in predicting more rapid and widespread disease failure, and should certainly be considered when determining the need for additional adjuvant therapy.
Overall, our study is consistent with the majority of previously reported randomized and retrospective trials evaluating patterns of failure from resected pancreatic adenocarcinoma. The advantages of our study are that we studied a homogeneous population of patients treated with modern surgery and adjuvant gemcitabine alone (i.e. no chemoradiation), which during the years of our study was the standard recommendation at our institution. In addition, our study had a well-annotated database with extensive patterns of failure analysis, which was confirmed by radiologic review with a board-certified radiologist specializing in gastrointestinal cancers. Our results also suggest that patients with positive resection margins are at a high risk of LRR and LRRO, and that those with lymph node involvement and/or post-operative CA 19-9 >90 U/mL are at high risk for metastatic disease failure and were more likely to have a rapid disease failure. Taken together, these data suggest that LN positivity and elevated CA 19-9 are very poor prognostic features that predict for a shorter disease free interval and a pattern of failure which will most likely include development of metastatic disease. To optimize medical resources and individualize patient treatment recommendations, these patients should likely receive multi-agent chemotherapy primarily, since they are likely to benefit less from intensification of local-regional therapy (unless their distant risk can be mitigated). However, patients with positive (≤ 1 mm) margins are at a heightened risk of local-regional disease failure and this is likely the group of patients where chemoradiation would be of most benefit. In patients with a mixture of these risk factors, it may be reasonable to use chemotherapy intensification initially, and re-consider chemoradiation after completion of all systemic therapy if no evidence of disease progression has occurred on restaging scans. Ultimately, the development of molecular risk-based stratification groups will further complement clinical risk factors and aid in the decision-making process. This data suggests that intensification of local therapy through adjuvant chemoradiation might be most likely to benefit patients with risk factors associated with an LRRO pattern of failure (not patients at high risk of distant metastatic disease), and may guide development of future trials, or analysis of ongoing prospective trials (e.g. RTOG 0848).
Acknowledgments
This work was presented at the American Society of Radiation Oncology (ASTRO) annual meeting in 2015 (San Antonio, TX), and is supported by the following grants: Award Number Grant KL2TR001068 from the National Center for Advancing Translational Sciences, and NIH R01 CA198128 (TW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Katz MH, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16(4):836–47. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iacobuzio-Donahue CA, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27(11):1806–13. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120(8):899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 5.Klinkenbijl JH, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230(6):776–82. doi: 10.1097/00000658-199912000-00006. discussion 782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neoptolemos JP, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 7.Neoptolemos JP, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304(10):1073–81. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 8.Oettle H, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–77. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 9.Regine WF, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299(9):1019–26. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 10.Maeda A, et al. Randomized phase III trial of adjuvant chemotherapy with gemcitabine versus S-1 in patients with resected pancreatic cancer: Japan Adjuvant Study Group of Pancreatic Cancer (JASPAC-01) Jpn J Clin Oncol. 2008;38(3):227–9. doi: 10.1093/jjco/hym178. [DOI] [PubMed] [Google Scholar]
- 11.Oettle H, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473–81. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 12.Bydder S, Spry N. Chemotherapy for pancreatic cancer. N Engl J Med. 2004;350(26):2713–5. author reply 2713-5. [PubMed] [Google Scholar]
- 13.Crane CH, Ben-Josef E, Small W., Jr Chemotherapy for pancreatic cancer. N Engl J Med. 2004;350(26):2713–5. author reply 2713-5. [PubMed] [Google Scholar]
- 14.Morris SL, Beasley M, Leslie M. Chemotherapy for pancreatic cancer. N Engl J Med. 2004;350(26):2713–5. doi: 10.1056/NEJM200406243502617. author reply 2713-5. [DOI] [PubMed] [Google Scholar]
- 15.Rutter CE, et al. Addition of radiotherapy to adjuvant chemotherapy is associated with improved overall survival in resected pancreatic adenocarcinoma: An analysis of the National Cancer Data Base. Cancer. 2015;121(23):4141–9. doi: 10.1002/cncr.29652. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, et al. Risk Factors Associated with Loco-Regional Failure after Surgical Resection in Patients with Resectable Pancreatic Cancer. PLoS One. 2016;11(6):e0157196. doi: 10.1371/journal.pone.0157196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van den Broeck A, et al. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009;35(6):600–4. doi: 10.1016/j.ejso.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Gnerlich JL, et al. Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg. 2012;147(8):753–60. doi: 10.1001/archsurg.2012.1126. [DOI] [PubMed] [Google Scholar]
- 19.Sugiura T, et al. Serum CA19-9 is a significant predictor among preoperative parameters for early recurrence after resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16(5):977–85. doi: 10.1007/s11605-012-1859-9. [DOI] [PubMed] [Google Scholar]
- 20.Raut CP, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246(1):52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcea G, et al. Tumour characteristics predictive of survival following resection for ductal adenocarcinoma of the head of pancreas. Eur J Surg Oncol. 2007;33(7):892–7. doi: 10.1016/j.ejso.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Crane CH, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol. 2011;29(22):3037–43. doi: 10.1200/JCO.2010.33.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter JM, et al. Failure patterns in resected pancreas adenocarcinoma: lack of predicted benefit to SMAD4 expression. Ann Surg. 2013;258(2):331–5. doi: 10.1097/SLA.0b013e31827fe9ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger AC, et al. Five year results of US intergroup/RTOG 9704 with postoperative CA 19-9</=90 U/mL and comparison to the CONKO-001 trial. Int J Radiat Oncol Biol Phys. 2012;84(3):e291–7. doi: 10.1016/j.ijrobp.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 25.Hishinuma S, et al. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006;10(4):511–8. doi: 10.1016/j.gassur.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Parikh AA, et al. Adjuvant Therapy in Pancreas Cancer: Does It Influence Patterns of Recurrence? J Am Coll Surg. 2016;222(4):448–56. doi: 10.1016/j.jamcollsurg.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neoptolemos JP, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 28.Hsu CC, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17(4):981–90. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghaneh P, et al. The Impact of Positive Resection Margins on Survival and Recurrence Following Resection and Adjuvant Chemotherapy for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2017 doi: 10.1097/SLA.0000000000002557. [DOI] [PubMed] [Google Scholar]
- 30.Osipov A, et al. Redefining the Positive Margin in Pancreatic Cancer: Impact on Patterns of Failure, Long-Term Survival and Adjuvant Therapy. Ann Surg Oncol. 2017;24(12):3674–3682. doi: 10.1245/s10434-017-6076-z. [DOI] [PubMed] [Google Scholar]
- 31.Merrell KW, et al. Predictors of Locoregional Failure and Impact on Overall Survival in Patients With Resected Exocrine Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2016;94(3):561–70. doi: 10.1016/j.ijrobp.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Duffy MJ, et al. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 2010;21(3):441–7. doi: 10.1093/annonc/mdp332. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery RC, et al. Prediction of recurrence and survival by post-resection CA 19-9 values in patients with adenocarcinoma of the pancreas. Ann Surg Oncol. 1997;4(7):551–6. doi: 10.1007/BF02305535. [DOI] [PubMed] [Google Scholar]
- 34.Kondo N, et al. Prognostic impact of perioperative serum CA 19-9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol. 2010;17(9):2321–9. doi: 10.1245/s10434-010-1033-0. [DOI] [PubMed] [Google Scholar]