Summary
Aim
To explore risk factors that may influence knee pain (KP) through central or peripheral mechanisms.
Methods
A questionnaire-based prospective community cohort study with KP defined as pain in or around a knee on most days for at least a month. Baseline prevalence, and one year incidence and progression (KP worsening) were examined. Central (e.g., Pain Catastrophizing Scale (PCS)) and peripheral (e.g., significant injury) risk factors were examined. Adjusted odds ratio (OR) and 95% confidence interval (CI) were calculated using logistic regression. Proportional risk contribution (PRC) was estimated using receiver-operator-characteristic (ROC) analysis.
Results
Of 9506 baseline participants, 4288 (45%) had KP (men 1826; women, 2462). KP incidence was 12% (men 11%, women 13%), and KP progression 19% (men 16%, women 21%) at one year. While both central and peripheral factors contributed to prevalence, central factors contributed more to progression, and peripheral factors more to incidence of KP. For example, although PCS (OR 2.06, 95% CI 1.88–2.25) and injury (5.62, 4.92–6.42) associated with KP prevalence, PCS associated with progression (2.27, 1.83–2.83) but not incidence (1.14, 0.86–1.52), whereas injury more strongly associated with incidence (69.27, 24.15–198.7) than progression (2.52, 1.48–4.30). The PRC of central and peripheral factors were 19% and 23% for prevalence, 14% and 29% for incidence, and 29% and 5% for progression, respectively.
Conclusions
Both central and peripheral risk factors influence KP but relative contributions may differ in terms of development (mainly peripheral) and progression (mainly central). Further study of such relative contributions may inform primary and secondary prevention strategies.
Keywords: Knee pain, Risk factors, Cohort, Incidence, Osteoarthritis
Key messages.
-
•
Knee pain (KP) is associated with both central and peripheral risk factors
-
•
Peripheral risk factors such as joint injury contribute more to the development of KP
-
•
Central factors such as pain catastrophising and depression appear the main drivers for the progression of KP
Introduction
Approximately 25% of people aged over 55 have chronic knee pain (KP)1. KP is multifactorial and may be caused predominantly by peripheral risk factors such as knee osteoarthritis (OA)2, or by alteration in central pain modulatory pathways as in fibromyalgia3. KP is a clinical malady related to but not fully explained by knee OA in middle-aged and older adults4, 5, 6. This may explain in part the common discordance between KP and structural knee OA7. Risk factors for KP and knee OA may differ, and consideration of both peripheral and central risk factors is important in clinical assessment and to inform the management plan8.
KP affects quality of life9, causes disability10 and associates with increased mortality11. Despite treatment attempts an increasing number of people undergo total joint replacement (TJR). However, 15–30% of those undergoing TJR subsequently still experience chronic KP12 suggesting that central pain modulation is an important driver of their pain.
The contribution of individual risk factors has been examined previously. However, systematic reviews emphasise that many risk factors have been examined predominantly in cross-sectional studies, and persuasive evidence for causal relationships from cohort studies examining incidence and progression of KP is limited13, 14, 15, 16. For example, only a few studies report an association between depression/anxiety and incident KP, and between widespread pain (WSP), previous knee injury and progression of KP14, 16, 17, 18, 19.
Understanding mechanisms of KP has important implications for targeted management of chronic KP and for predicting response to existing therapies20, 21, 22. The current study aimed to explore risk factors that may influence prevalent KP, incident KP and progression of KP through either peripheral or central mechanisms in a community-based cohort study.
Methods
This study was approved by Nottingham University Hospitals NHS Trust and the Nottingham Research Ethics Committee 1 (Ref 14/EM/0015).
Study design and participant selection
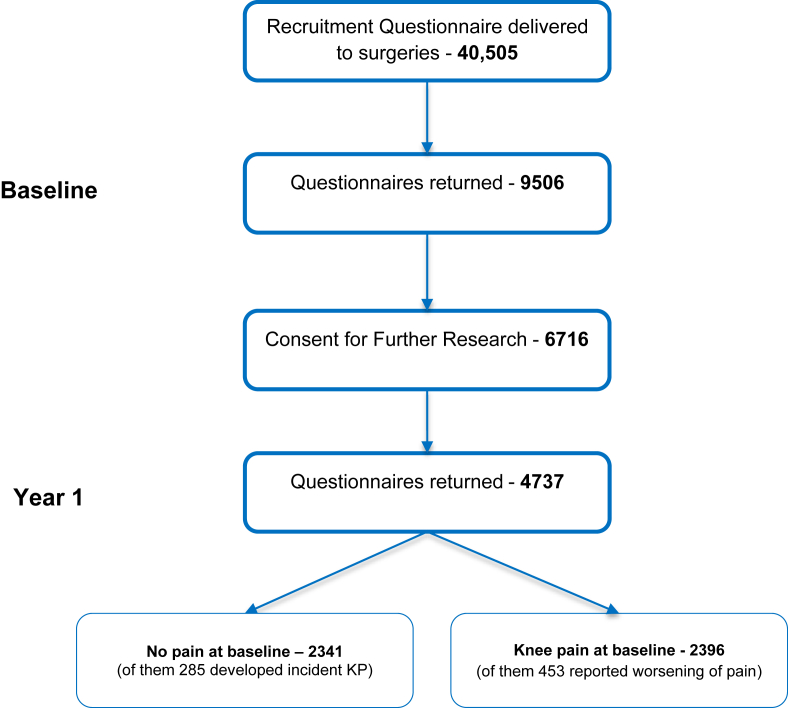
Participants were selected from the KP and Related Health in the Community Study (KPIC), an ongoing prospective cohort study aimed to determine the natural history of KP including prevalence, incidence, progression and associated risk factors in community-derived adults23. At baseline 40,505 questionnaires were mailed and 9506 men and women aged ≥40 years replied. One year later another questionnaire was posted to the 6716 live participants who indicated willingness to receive further questionnaires. Responders to the Year 1 questionnaire (n = 4737) reported a higher frequency of KP and pain severity at baseline compared to non-responders (Appendix 1). Exclusion criteria at baseline were known terminal illness, severe psychiatric illness or dementia, or any other condition or circumstance considered by their General Practice to make them unsuitable to receive the questionnaire.
For the current study we constructed two sub-cohorts from the Year-1 questionnaire responders1: an incidence cohort comprising participants with no KP at baseline and therefore at risk of developing new-onset KP (n = 2341) during follow-up; and2 a progression cohort comprising participants with KP at baseline and therefore at risk for progression (n = 2396). An overview of recruitment is presented in Appendix 2.
Pain measures
KP was defined as pain in or around a knee on most days for at least a month ever24. Current KP was defined as pain on most days of the past month. Prevalence of KP was defined by satisfying the KP criterion at baseline. Incident KP was defined as no KP at baseline but KP reported at follow-up. Worsening of KP was defined using the question “Since it has started, do you think the severity of your KP has overall greatly improved/slightly improved/remained the same/worsened” (Patient Global Assessment (PGA)). Average pain intensity in the past month was assessed on a 0–10 numerical rating scale (NRS).
Neuropathic-like KP was determined using the Pain-DETECT questionnaire modified for use in knee OA (mPDQ score ≥19)25. KP was also classified as intermittent and/or constant using the Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP)26. A summative score for each sub-scale was calculated and standardised into 0–100 scale as recommended. Tertiles were calculated for baseline scores of people with KP.
GP-diagnosed OA was self-reported and defined using the question “Have you ever been diagnosed by your doctor as having osteoarthritis of the knee?”
Risk factors
Age, gender, weight, height and data on other risk factors were included in the questionnaire. Risk factors were divided into three main groups interpreted by the authors as central (related to pain perception and pain physiology), peripheral (related to structural changes in the knee joint) and others (mixed influence).
Central risk factors
-
•
WSP was identified using a diagrammatic manikin and defined as concurrent pain experienced within the past 4 weeks axially, above and below the waist, and on both sides of the body27. Current KP was not counted for this.
-
•
The Hospital Anxiety and Depression Scale (HADS) and Pain Catastrophizing Scale (PCS) scores were each summated as recommended28, 29. A score on HADS-A or HADS-D ≥8 was used to indicate anxiety or depression28. Tertiles of the PCS score were calculated from the whole sample including those with and without KP.
Peripheral
-
•
A major/significant knee injury was defined as a history of knee injury that required seeing a doctor (leg fracture was recorded separately and not included in this definition).
-
•
Self-reported frontal plane knee alignment was assessed in both knees using a validated line diagram instrument30. Participants separately reported their current and early adult life (in their 20's) alignment. Constitutional malalignment was defined as bilateral varus/valgus malalignment (mild or severe) in their 20's.
-
•
High risk occupation was classified based on published evidence31 (Appendix 3). Each listed occupation per individual was analysed and the data dichotomised into high- or low-risk groups.
-
•
2D:4D (index:ring finger length) ratio was self-reported using a validated line-drawing instrument32. Type 3 pattern (index shorter than ring finger) is a risk factor for knee OA32.
Other factors
-
•
Nodal OA, which has high heritability and increases risk of knee OA, was determined using a validated line diagram and classified as present in those reporting nodes on at least two rays of each hand33.
-
•
Comorbidities: History or current evidence of comorbidities was recorded for the following: cardiovascular disease (high cholesterol*, heart attack*, angina*, hypertension*), lung disease (chronic obstructive pulmonary disease (COPD), asthma, idiopathic pulmonary fibrosis), endocrine disease (diabetes*, underactive/overactive thyroid, thyroiditis), non-restorative pain disorders (irritable bowel syndrome*, fibromyalgia*, chronic fatigue syndrome*), liver disease (cirrhosis, hepatitis, non-alcoholic fatty liver disease), chronic kidney disease/failure (CKD), central nervous system disorders (stroke*, multiple sclerosis), chronic rheumatic conditions (rheumatoid arthritis, lupus, psoriatic arthritis, ankylosing spondylitis), and gout. In the questionnaire a list of specific conditions (marked *above) and an open “others” question were provided. All conditions were then grouped according to the system as listed above. A comorbidity count was calculated as a total number of affected systems (0–9).
-
•
Analgesics: Use of prescribed and/or over-the-counter analgesics (e.g., paracetamol; non-steroidal anti-inflammatory drugs (NSAIDs), including COX-2 selective inhibitors; opioids) was self-reported.
Statistical analysis
Sample size
Sample size was calculated based on a multivariable logistic regression model34, considering 80% power with 5% type I error and the following assumptions1: an odds ratio (OR) of 1.4 for a weak but significant association (because a weak association requires larger sample size)34; and2 a moderate correlation between covariates (r = 0.5)35. Based on the above assumptions, 452 participants were required for a cross sectional study for the association given a 25% prevalence of KP in the general population1, 2548 participants were required for an incidence cohort study according to an annual KP incidence of 3%, and 676 participants were required for a progression cohort study according to an annual rate of worsening of KP of 14%36.
Analysis
The primary focus was to determine multiple risk factors associated with KP. At baseline potential risk factors for prevalent KP were examined using multivariable logistic regression analysis. OR and 95% confidence interval (CI) were estimated with adjustment for age, gender, body mass index (BMI). Adjusting all exposures by the same group of known confounding factors makes adjusted ORs comparable between exposures and would minimize bias related to “Table II Fallacy”37. At follow-up risk factors for incident KP or worsening KP were examined using multivariable logistic regression analysis, all participants having the same one year follow-up period.
Table II.
Risk factors associated with prevalent, incident and worsening knee pain (KP)
| Age, gender, BMI-adjusted ORs (95% CI) |
|||
|---|---|---|---|
| Prevalence | Incidence | Worsening | |
| Central risk factors | |||
| Widespread pain | 3.02 (2.71; 3.37) | 2.23 (1.63; 3.06) | 1.68 (1.35; 2.10) |
| HADS anxiety score ≥8 | 1.87 (1.71; 2.06) | 1.27 (0.95; 1.70) | 1.68 (1.35; 2.08) |
| HADS depression score ≥8 | 2.40 (2.14; 2.70) | 1.99 (1.38; 2.87) | 1.85 (1.46; 2.34) |
| PCS in the highest tertile ≥9) | 2.06 (1.88; 2.25) | 1.14 (0.86; 1.52) | 2.27 (1.83; 2.83) |
| mPDQ ≥ 19 | n/a§ | n/a§ | 2.65 (1.92; 3.65) |
| Peripheral risk factors | |||
| Significant injury | 5.62 (4.92; 6.42) | 1.08 (0.68; 1.72) | 0.89 (0.70; 1.13) |
| New injury (past 12 months) | n/a | 69.27 (24.15; 198.7) | 2.52 (1.48; 4.30) |
| Early varus malalignment | 1.49 (1.18; 1.88) | 1.28 (0.64; 2.57) | 1.60 (0.99; 2.58) |
| Early valgus malalignment | 1.72 (1.25; 2.36) | 1.05 (0.36; 3.12) | 1.64 (0.89; 3.03) |
| Varus malalignment | 5.43 (3.44; 8.58) | n/a | 2.28 (1.30; 4.02) |
| Valgus malalignment | 4.18 (2.74; 6.38) | n/a | 0.71 (0.37; 1.37) |
| High risk occupation | 1.43 (1.31; 1.56) | 0.99 (0.76; 1.30) | 1.29 (1.04; 1.60) |
| 2D4D ratio (type 3) | 1.04 (0.95; 1.14) | 1.18 (0.90; 1.54) | 0.86 (0.69; 1.07) |
| Other risk factors | |||
| Age, mean (SD)∗ | 1.00 (0.99; 1.00) | 1.00 (0.99; 1.01) | 1.01 (1.00; 1.02) |
| Age categories (reference age group 40–49) | |||
| 50–59 | 1.10 (0.95; 1.26) | 1.23 (0.80; 1.90) | 1.29 (0.88; 1.87) |
| 60–69 | 1.06 (0.93; 1.21) | 1.01 (0.67; 1.53) | 1.19 (0.83; 1.71) |
| 70–79 | 0.93 (0.81; 1.07) | 1.20 (0.78; 1.84) | 1.47 (1.01; 2.14) |
| ≥80 | 1.10 (1.01; 1.20) | 0.63 (0.21; 1.84) | 1.63 (0.80; 3.35) |
| Women, n (%)† | 1.10 (1.01; 1.20) | 1.15 (0.89; 1.50) | 1.34 (1.07; 1.66) |
| BMI (kg/m2), mean (SD)‡ | 1.08 (1.07; 1.09) | 1.06 (1.03; 1.09) | 1.05 (1.04; 1.07) |
| Nodal OA | 1.89 (1.64; 2.17) | 1.49 (0.99; 2.23) | 1.62 (1.23; 2.14) |
| ICOAP intermittent in the highest tertile (≥7) | n/a§ | n/a§ | 3.61 (2.89; 4.50) |
| ICOAP constant in the highest tertile (≥7) | n/a§ | n/a§ | 3.70 (2.96; 4.63) |
| N of comorbidities | 1.21 (1.15; 1.28) | 1.31 (1.12; 1.53) | 1.13 (1.00; 1.27) |
| Any comorbidities | 1.29 (1.18; 1.42) | 1.48 (1.12; 1.95) | 1.29 (1.02; 1.64) |
| Any comorbidities ≥ 2 | 1.37 (1.22; 1.53) | 1.26 (0.90; 1.78) | 1.26 (0.98; 1.61) |
| Any comorbidities ≥ 3 | 1.68 (1.34; 2.11) | 2.55 (1.41; 4.60) | 0.98 (0.62; 1.54) |
| Any comorbidities ≥ 4 | 1.51 (0.80; 2.84) | 8.85 (1.95; 40.16) | 0.70 (0.15; 3.18) |
In bold - If a 95% confidence interval does not include the null value (i.e. there is a statistically meaningful or statistically significant difference between the groups).
Abbreviations: BMI - body mass index; KP – knee pain; OR – odds ratio; CI –confidence interval; HADS - Hospital Anxiety and Depression Scale; PCS - Pain Catastrophizing Scale; SD - standard deviation; PDQ – Pain DETECT questionnaire; ICOAP - Measure of Intermittent and Constant Osteoarthritis Pain.
Adjusted for gender and BMI only.
Adjusted for age and BMI only.
Adjusted for age and gender only.
mPDQ and ICOAP scores (intermittent and constant) were knee-specific, so if there was no KP at baseline, the score was 0 and therefore could not be a risk factor for prevalent or incident KP.
Sensitivity analysis was undertaken using an alternative definition of KP worsening, defined by a significant increase from baseline in KP severity on NRS. A significant change from the baseline was calculated using the least significant criterion (LSC)38.
We used receiver-operator-characteristic (ROC) curves and areas under the curve (AUC) to examine proportional risk contribution (PRC) of central and peripheral risk factors. ROC curves were based on the multivariable logistic regression model with prevalent/incident/worsening KP as an outcome. Firstly, we built the full risk model for each outcome with a ROC curve (AUCf). Secondly, we removed the exposure(s) of interest to examine the contribution of the exposure (s) removed through the reduction of the ROC curve, i.e., the partial AUC (AUCp). Thirdly we calculated the PRC using the following formula: , where 0.5 is the AUC under the diagonal line of the ROC curve indicating no discrimination at all by all included risk factors. The full model included central (WSP, HADs, PCS), peripheral (history of significant injury, early life mal-alignment, current mal-alignment, high risk occupation) and other risk factors (age, gender, BMI, number of comorbidities, nodal hand osteoarthritis). In addition, the full model for incident and worsening KP included new knee injury (past 12 months) as a peripheral risk factor.
The described models for PRC are different from the models used to estimate associations (age, gender and BMI adjusted) because of the different purposes of the two models. While the latter model aimed to make a comparison between different risk factors, the models used to estimate PRC aimed to examine the contribution of central and peripheral risk factors in the context of all possible risk factors.
All analyses were undertaken using SAS 9.4.
Results
Demographics of participants
Characteristics of the study population are presented in Table I. The baseline mean age was 62.1 (range 40–86) years, 57% were women, and the mean BMI was 27.31 (SD 5.30, range 13–74). Prevalence of KP was 45% (men, 44%; women, 46%), prevalence of current KP was 28% (men 27%, women 29%), and 14% had GP-diagnosed OA (men 12%, women 15%). People with KP used more NSAIDs and opioids than those without (6% vs 2% for prescribed NSAIDs, 22% vs 9% for over-the-counter NSAIDs, 15% vs 5% for opioids). More than half the study population reported one or more comorbidities (61% of people with KP, 53% of those without). The most prevalent conditions in KP participants were cardiovascular diseases (54%), non-restorative pain disorders (16%), and endocrine diseases (15%). Detailed comorbidity results are presented in Appendix 4.
Table I.
Characteristics of the study population (questionnaire survey)
| Baseline |
Year-1 |
|||||
|---|---|---|---|---|---|---|
| Total | No KP | KP | Total | Incident KP | Worsening KP | |
| N | 9506 | 5218 | 4288 | 4737 | 285 | 453 |
| Age (years), mean (SD) | 62.10 (10.56) | 62.33 (10.69) | 61.82 (10.40) | 63.95 (10.13) | 62.38 (10.09) | 63.03 (10.19) |
| Age categories | ||||||
| 40–49 | 1442 (15.17) | 793 (15.20) | 649 (15.14) | 640 (13.51) | 39 (13.68) | 53 (11.70) |
| 50–59 | 2297 (24.16) | 1210 (23.19) | 1087 (25.35) | 1112 (23.47) | 70 (24.56) | 115 (25.39) |
| 60–69 | 3094 (32.55) | 1658 (31.77) | 1436 (33.49) | 1697 (35.82) | 96 (33.68) | 157 (34.66) |
| 70–79 | 2339 (24.61) | 1359 (26.04) | 980 (22.85) | 1167 (24.63) | 76 (26.67) | 115 (25.39) |
| ≥80 | 276 (2.90) | 162 (3.10) | 114 (2.66) | 122 (2.57) | 4 (1.40) | 13 (2.87) |
| Women, n (%) | 5371 (56.50) | 2909 (56.03) | 2462 (57.62) | 2733 (57.69) | 173 (60.70) | 290 (64.02) |
| BMI (kg/m2), mean (SD) | 27.31 (5.30) | 26.36 (4.63) | 28.47 (5.80) | 27.26 (5.30) | 27.36 (4.65) | 29.83 (6.28) |
| Knee pain, n (%) | 4288 (45.10) | – | 4288 (100) | 2681 (56.60) | 285 (100) | 453 (100) |
| Current KP, n (%) | 2681 (28.20) | – | 2681 (62.52) | 1372 (28.96) | 175 (61.40) | 396 (87.42) |
| Current KP severity (NRS 0–10), mean (SD) | 1.97 (2.96) | 0.09 (0.60) | 4.25 (3.07) | 2.06 (2.98) | 3.54 (2.61) | 6.26 (2.64) |
| GP-diagnosed knee OA (%) | 1279 (13.45) | 94 (1.80) | 1185 (27.64) | 722 (15.24) | 45 (15.79) | 269 (59.38) |
| Use of analgesics | ||||||
| Prescribed NSAIDs, n (%) | 351 (3.69) | 103 (1.97) | 248 (5.78) | 195 (4.12) | 14 (4.91) | 44 (9.71) |
| Opioids, n (%) | 1438 (15.13) | 241 (4.62) | 646 (15.07) | 1077 (22.73) | 30 (10.53) | 127 (28.04) |
| Over-the-counter NSAIDs, n (%) | 887 (9.33) | 492 (9.43) | 946 (22.06) | 514 (10.85) | 75 (26.32) | 211 (46.58) |
Abbreviations: SD – standard deviation; NRS – numerical rating scale 0–10; BMI – body mass index; OA – osteoarthritis; NSAIDs – non-steroidal anti-inflammatory drugs; GP -general practitioner.
Incidence of KP during the 1 year follow-up was 12% (285/2341). This was similar in men (11%) and women (13%). During the one year follow-up, 19% (453/2396) reported worsening of KP (men 16%, women 21%).
Risk factors for prevalent KP
Both central and peripheral risk factors associated with KP (Table II, Appendix 5). Among central factors, WSP was the strongest (OR 3.02, 95% CI 2.71, 3.37), followed by depression (OR 2.40, 95% CI 2.14, 2.70 for HAD-D≥8), pain catastrophizing (OR 2.06, 95% CI 1.88, 2.25 for PCS≥9) and anxiety (OR 1.87, 95% CI 1.71, 2.06 for HAD-A≥8). Among peripheral factors, previous knee injury was the strongest (OR 5.62, 95% CI 4.92, 6.42), followed by constitutional mal-alignment (OR 1.49, 95%CI 1.18, 1.88 for varus, and OR 1.72, 95%CI 1.25, 2.36 for valgus) and high risk occupation (OR 1.43, 95% CI 1.31, 1.56). Among other risk factors nodal hand OA (OR 1.89, 95% CI 1.64, 2.17), comorbidity (OR 1.29, 95% CI 1.18, 1.42 for any comorbidity), gender and BMI associated with KP.
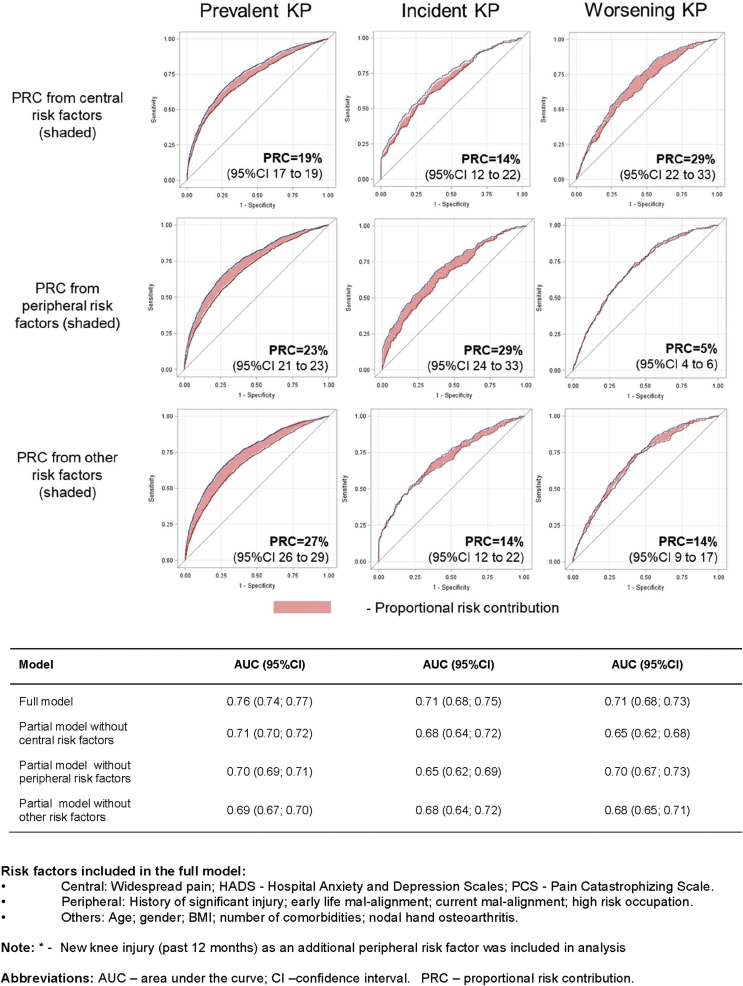
The AUC for the full model including central, peripheral and other factors was 0.76 (0.74, 0.77). The PRC of central and peripheral factors to the full model was 19% and 23%, respectively, and contribution of other factors was 27% (Fig. 1). Detailed ROC analyses and relative contributions of central and peripheral risk factors are shown in Appendix 6.
Fig. 1.
Proportional risk contribution (PRC) to prevalence, incidence and progression of knee pain (KP).
Risk factors for incident KP
The strongest central factors associated with incident KP were WSP (OR 2.23, 95% CI 1.63, 3.06) and depression (OR 1.99, 95% CI 1.38, 2.87 for HAD-D≥8). Peripheral risk factors (knee injury reported at baseline and constitutional mal-alignment) had no significant effect on risk of incident KP. However, new knee injury during follow-up was a strong risk factor for KP onset (OR 69.27, 95%CI 24.15, 198.7). BMI and presence of any comorbidity were also risk factors for incident KP. The relationship of each baseline risk factor to development of incident KP is presented in Table II and Appendix 7.
The PRC of central and peripheral risk factors to incident KP was 14% and 29% respectively (Fig. 1).
Risk factors for KP worsening
In people with KP both central and peripheral risk factors contributed to KP worsening during 1 year. The following central factors associated with worsening: pain catastrophising (OR 2.27, 95%CI 1.83, 2.83 PCS≥9), depression (OR 1.85, 95% CI 1.46, 2.34), anxiety (OR 1.68, 95% CI 1.35, 2.08) and WSP (OR 1.68, 95% CI 1.35, 2.10). In addition, those with neuropathic-like pain and higher ICOAP scores (both intermittent and constant) were more likely to report worsening (Table II, Appendix 8). With respect to peripheral factors, significant injury before baseline did not associate with worsening KP, whereas injury during follow-up) did (OR 2.52, 95% CI 1.48, 4.30). Current varus mal-alignment (OR 2.28, 95% CI 1.30, 4.02) and high risk occupation also associated. Other risk factors such as nodal hand OA (OR 1.62, 95% CI 1.23, 2.14), presence of any comorbidity (OR 1.29, 95% CI 1.02, 1.64) and BMI associated with KP worsening.
The sensitivity analysis using an alternative definition of KP worsening showed that only nodal OA and high risk occupation predicted increase in KP severity from baseline (Appendix 9).
Overall, the contribution from peripheral factors to KP worsening was much smaller (PRC = 5%) than that from central factors (PRC = 29%) (Fig. 1).
Discussion
This is the first community-based cohort study to investigate the contribution of central and peripheral risk factors associated with KP prevalence, incidence and progression. Central factors were those related to pain perception, psychological and behavioural response that could affect normal pain physiology39, while peripheral factors were those related to structural changes in the knee. Some risk factors such as comorbidities and nodal hand OA could influence KP through both central and peripheral mechanisms so were classified as “other” risk factors. The main findings are1: both central and peripheral risk factors associated with prevalence of KP2; peripheral risk factors such as knee injury contributed more to incidence of KP; and3 central risk factors such as PCS contributed more to KP progression.
We examined central (e.g., WSP, anxiety, depression, catastrophizing), peripheral (e.g., injury and mal-alignment) and other risk factors and confirm that many of these associate with KP (Table II). Some, such as WSP and comorbidities appear to act particularly as risk factors for developing KP, whereas others such as neuropathic-like pain, pain catastrophizing and current varus mal-alignment may in part result from KP and particularly associate with pain progression. Furthermore, localised KP or OA might have reciprocal effects on incident depression or WSP which later contribute to worsening of symptoms40, 41, 42. For each outcome (prevalent, incident and worsening of KP) we performed a two-stage analysis. Firstly, we examined the association between independent risk factors and KP using OR as a measure of association. Secondly, we explored the relative contribution of risk factors in composite divided into three main groups (central, peripheral and others) using PRC.
The positive association between depression and KP worsening accords with two large longitudinal studies of people with KP and knee OA43, 44. The contribution of anxiety to KP worsening has been reported previously by Mallen et al. (2007)45. In a study by Jinks et al. (2008) WSP (two or more pain sites) was associated with onset but not progression of KP17. However, our study showed a strong association with KP for WSP and depression in the cross-sectional analysis, and also convincing evidence of a causal relationship from the two sub-cohort studies of incidence and progression of KP in one year. A consistent association between prevalence, incidence or worsening of KP and central risk factors highlights the important contribution of centrally-mediated mechanisms to the natural history of KP, supporting the concept that KP shares risk factors in common with other chronic pain disorders such as fibromyalgia and low back pain46, 47.
Major knee trauma is a recognised important risk factor for knee OA onset14. In our study, previous knee injury was a strong risk factor for KP in the cross-sectional analysis, and new injury associated with both incidence and progression of KP (Table II). However, the association between some peripheral factors and KP in our study did not always replicate findings from some previous cohort (mainly knee OA) studies. For example, although constitutional (early life) varus/valgus mal-alignment was a risk factor for incident KP in a study by Ingham et al. (2011)48, we found no association between constitutional malalignment and incidence or progression of KP. The literature about effect of constitutional mal-alignment on KP is limited15. However, our findings that current knee mal-alignment, which is more likely a consequence of knee OA, is a stronger predictor for progression of KP accords with other literature15, 49.
We additionally examined the relative contribution of central and peripheral risk factors in a full risk model using a proportion derived from ROC curve analysis (PRC). For incident KP the contribution of peripheral factors was larger than that of central factors (29% vs 14%), whereas for worsening KP the contribution of central factors was larger than that of peripheral factors (29% vs 5%). This suggests that the relative contribution of central and peripheral risk factors to KP may differ at different stages of the condition (development or progression). Incident KP is not often centrally driven, however the influence of central risk factors (existing before or developing later due to chronic pain) increases as the condition progresses50, 51.
There are a number of caveats to this study. Firstly, the overall prevalence of KP in this population (45%) was slightly higher than most previous studies (e.g., 28% in a previous Nottingham study24). This may reflect response bias in that people with KP may more likely respond to a KP questionnaire. Indeed comparison between responders and non-responders to the Year-1 questionnaire showed that people with KP and those with higher pain severity were more likely to respond (Appendix 2). Secondly, previous studies showed that the question we used to define KP is the most sensitive (58.7%) but least specific (59.1%) predictor of grade ≥1 osteophytes24 and applying a different KP definition might give different results. Thirdly, pain and risk factors were measured at just two time points and repeated measures over a longer period might reveal different trajectories of KP progression and better determination of associations. Fourthly, both definitions for worsening were self-reported and may have limitations. PGA reflects the perception of worsening (i.e., retrospective perspective by the participant at the time of Y1 assessment) for the full course of the disease but the risk factors captured at baseline may have changed since the start of the disease (Table II). In contrast, NRS was measured at only two time points during a short time period - one year, which cannot reflect the actual pain trajectory. Fifthly, we interpret our data as showing risk from central and peripheral factors, but cannot exclude possible confounding by other factors. Furthermore, the number of peripheral factors studied was limited. The use of imaging and clinical assessments would have allowed more detailed assessment of abnormal joint features and changes in knee alignment over time. However, this would not affect the examination of the relative contribution of the questionnaire based surrogate measures of peripheral risk factors in different stages of KP. Finally, this is the first time that we have used this measure for PRC of central or peripheral risk factors. A caveat of this measure is that it increases with number of risk factors collected. However, when we compare the same group of risk factors across different stages of a disease, e.g., early (incidence) and later (progression) stages of KP, relative PRCs can be determined. As with the original measure of AUC, the derived measure of PRC is also open to bias due to model overfitting especially when the sample size is small. However, this study involved a large sample of 9506 community-derived participants so overfitting is unlikely to be an important issue52.
In conclusion, this study confirms that a number of peripheral and central risk factors associate with prevalence of KP. While peripheral risk factors such as joint injury appear the main drivers for development of KP, central factors such as PCS appear the main drivers for KP progression. Further study is needed to examine the contribution of other peripheral risk factors such as radiographic change, synovial changes and muscle strength, as well as more objective central risk factor measures such as quantitative sensory testing and neuroimaging.
Authors' contributions
AS, WZ and MD made substantial contributions to the conception and design of the study. All authors contributed to the acquisition of questionnaire data. AS, MD and WZ conducted the data analysis and interpretation. AS wrote the first draft. WZ has full access to the data and takes responsibility for the content and guarantees the integrity and accuracy of the work undertaken. All authors have read, provided critical feedback on intellectual content and approved the final manuscript.
Declaration of Helsinki
This study complies with the Declaration of Helsinki and was approved by Nottingham University Hospitals NHS Trust and the Nottingham Research Ethics Committee 1 (Ref 14/EM/0015).
Conflict of interest disclosures
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Funding/support
This study was supported financially by the Arthritis Research UK Pain Centre (Centre Initiative grant number 20777) and Arthritis Research UK Centre for Sport, Exercise and Osteoarthritis (Grant reference 20194). The authors would also like to thank the Bolashak scholarship programme, offered by the Ministry of Education and Science of the Republic of Kazakhstan, for financially supporting the PhD research programme (AS) and the University of Nottingham as sponsor and host institution of this project.
Role of the funder/sponsor
The sponsor did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript and the decision to submit the manuscript for publication.
Disclaimer
The opinions, results and conclusions reported in this article are those of the authors and are independent from the funding sources.
Acknowledgements
The research team would like to thank the individuals who generously shared their time and participated in this study. The authors would also like to acknowledge the wider KPIC team for their time and contribution to the study set up and data collection, Nadia Frowd, Laura Marshall, Joann Stocks, Hollie Harvey, Sophie Warner, Jane Healy, Rose Farrands-Bentley, Ivy Leech, Amanda Broniewski, Fraser Kesteven and Danielle Sinclair. WZ had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
A. Sarmanova, Email: aliyasarmanova@gmail.com.
G.S. Fernandes, Email: gwen.fernandes@nottingham.ac.uk.
H. Richardson, Email: helen.richardson@nottingham.ac.uk.
A.M. Valdes, Email: ana.valdes@nottingham.ac.uk.
D.A. Walsh, Email: david.walsh@nottingham.ac.uk.
W. Zhang, Email: weiya.zhang@nottingham.ac.uk.
M. Doherty, Email: michael.doherty@nottingham.ac.uk.
Appendix 1. Clinical characteristics of the responders and non-responders to the Year 1 questionnaire
| Non-Responders (N = 4769) | Responders (N = 4737) | P-value∗ | |
|---|---|---|---|
| Age (years), mean (SD) | 61.73 (10.96) | 62.47 (10.14) | 0.0011 |
| Women, n (%) | 2631 (55.17) | 2740 (57.84) | 0.0291 |
| BMI, mean (SD) | 27.37 (5.30) | 27.26 (5.30) | 0.3164 |
| Pain severity (NRS 0–10)†, mean (SD) | 1.78 (2.90) | 2.15 (3.00) | <0.0001 |
| Knee pain, n (%) | 1892 (39.67) | 2396 (50.58) | <0.0001 |
| GP-diagnosed knee OA, n (%) | 557 (11.68) | 722 (15.24) | <0.0001 |
| Widespread pain | 960 (20.13) | 1110 (23.43) | 0.0001 |
| HADS anxiety score ≥8 | 1623 (34.04) | 1460 (30.81) | 0.0008 |
| HADS depression score ≥8 | 940 (19.71) | 767 (16.19) | <0.0001 |
| PCS in the highest tertile (≥9) | 1647 (34.56) | 1609 (33.97) | 0.5441 |
| mPDQ ≥ 19 | 184 (3.86) | 202 (4.26) | 0.3180 |
| Significant injury | 662 (14.48) | 889 (19.18) | <0.0001 |
| Early varus malalignment | 141 (3.40) | 174 (3.98) | 0.1594 |
| Early valgus malalignment | 95 (2.31) | 87 (2.02) | 0.3596 |
| Varus malalignment | 72 (1.68) | 72 (1.60) | 0.7605 |
| Valgus malalignment | 77 (1.80) | 87 (1.93) | 0.6434 |
| High risk occupation | 2053 (43.06) | 1926 (40.65) | 0.0173 |
| 2D4D ratio (type 3) | 2124 (48.59) | 2360 (51.93) | 0.0017 |
| Nodal OA | 471 (10.40) | 589 (12.92) | 0.0002 |
| ICOAP intermittent in the highest tertile (≥7) | 664 (13.93) | 835 (17.62) | <0.0001 |
| ICOAP constant in the highest tertile (≥7) | 699 (14.66) | 792 (16.72) | 0.0059 |
Abbreviations: SD - standard deviation; BMI - body mass index; NRS – numerical rating scale (range 0–10).
t-test for continuous and chi-square test for categorical.
Average pain severity in the past month.
Appendix 2. Recruitment of participants in the KPIC study.
Appendix 3. High risk occupation category, filter searches and links to supporting literature
| Agreed high risk category | Filter searches | References |
|---|---|---|
| Auto-industry/mechanic | Car Fitter, Car Seat Assembly, Paint Sprayer/Car Repair (seat assembler, reconditioning, painting), car mechanic, car repairer, Panel beater | 1 |
| Tradesmen: construction | Brick, Build, Property, Construction, Scaffold, Damp, Ground worker, Quarry worker/operative, roof, Joiner, Carpenter, Wood, Fitter, Sign maker, Furniture design and manufacture, Upholsterer, Cabinet Maker, Shop Fitter, Window, Glazing, Glazier, Double Glazier | 1,2,3 |
| Tradesmen: other | Electric, Jointer Electrical, Lineman, Panel wirer/wireman, Carpet, Floor, Slab, Paint, Plaster, Cabinet Sprayer, Plumber, Pipe Fitter | 1,2,3 |
| Emergency services | Police, Firefighter, Army, Soldier, National Service, RAF, Marines, Navy, Naval, Paratrooper, Royal (air force etc.), Ambulance, Paramedic, Military, WRNS (Women's Royal Navy Service) | |
| Factory/warehouse worker | Factory, Workshop, Worker, Assembly, Production, Warehouse, Picker, Packer | 4 |
| Land management/cultivation | Agriculture, Agricultural, Farm, Farmer, “Picker (fruits)”, Herdsman, Dairy man, Gardener, Green, Landscape, Lawn, Horticulture, Groundsman, Greenkeeper, Horticulturalist, Timber yard worker, forest worker, forester, forestry | 1,2 |
| Manual workers engineering | Engineer, Millwright, Lagger (Insulators)/Lagging | 3 |
| Health Services | Nurse, Nursing, carer, care worker, care assistant, support worker, Home help, RGN (registered general nurse), RNMH (registered nurse mental health), Ward Orderly, SRN (State Registered Nurse), RMN (Registered Mental Nurse), RNLD | 2,5 |
| Coal miner | Coal, Mine, Colliery, Down pit, Mining, Collier | 3 |
| Metal worker | Steel, Weld, Metal, Blacksmith, Foundry, Moulder, Plater, Fabricator | 1 |
| PCM (Porters, cleaners, maintenance) | Porter, Clean Window, Cleaner, Valeter, Wash (Car), Janitor, Domestic Assistant, Chambermaid, Hospital domestic, domestic, caretaker, building maintenance, maintenance, handyman, building assistant | 1,3 |
| PE teacher | PE, Physical | 3 |
| Postman | Post, mail | Agreed in Consensus Meeting due to prolongued walking, bending and lifting. |
| Labourer | Labourer, Pipe yard Worker, Concrete, Installer, Mason, Fibre glass, Asphalt(er), Digging trenches, Fencer, Manual | 4,6 |
| Road worker | Roadworks, Road, Highway, Platelayer, Tarmac layer, Tarmacer | 4 |
| Shipyard worker | Shipwright | 1,3 |
| Sports-other | Any, Climbing instructor, Parachute instructor |
1 Coggon D, Croft P, Kellingray S, et al. Occupational physical activities and osteoarthritis of the knee. Arthritis and rheumatism 2000;43(7):1443-9. doi: 10.1002/1529-0131(200007)43:7<1443::aid-anr5>3.0.co;2-1 [published Online First: 2000/07/21]
2 Andersen S, Thygesen LC, Davidsen M, et al. Cumulative years in occupation and the risk of hip or knee osteoarthritis in men and women: a register-based follow-up study. Occup Environ Med 2012;69(5):325-30. doi: 10.1136/oemed-2011-100033 [published Online First: 2012/01/14]
3 Pensions IIACaDfWa. Disorders of the knee: report by the Industrial Injuries Advisory Council, 1995.
4 Palmer KT. Occupational activities and osteoarthritis of the knee. British medical bulletin 2012;102:147-70. doi: 10.1093/bmb/lds012
5 Companies DS. Hardest Working Knees: Occupational Activities That Can Cause or Aggravate Knee Osteoarthritis. Information Sheet 2015 [Available from: https://www.depuysynthes.com/binary/org/DPY_SYN/knee/campaigns/HWK_Backgrounder_12_02_15FINAL.pdf.]
6 Lindberg H, Montgomery F. Heavy labor and the occurrence of gonarthrosis. Clinical orthopaedics and related research 1987(214):235-36.
Appendix 4. The presence of comorbidity at baseline
| Total | No KP (Q11) | Knee pain (Q11) | P-value | |
|---|---|---|---|---|
| N | 9506 | 5218 | 4288 | |
| Conditions | ||||
| High cholesterol, n (%) | 2634 (27.71) | 1356 (26.00) | 1278 (29.80) | <0.0001 |
| Heart attack/angina, n (%) | 604 (6.35) | 287 (5.50) | 317 (7.39) | 0.0002 |
| Hypertension, n (%) | 2845 (29.93) | 1400 (26.85) | 1445 (33.70) | <0.0001 |
| Diabetes, n (%) | 899 (9.46) | 423 (8.11) | 476 (11.10) | <0.0001 |
| Underactive/overactive thyroid, thyroiditis, n (%) | 369 (3.88) | 185 (3.54) | 184 (4.29) | 0.0608 |
| Stroke, n (%) | 271 (2.85) | 142 (2.72) | 129 (3.01) | 0.4045 |
| Multiple sclerosis, n (%) | 18 (0.19) | 10 (0.19) | 8 (0.19) | 0.9551 |
| Irritable bowel syndrome, n (%) | 963 (10.13) | 427 (8.19) | 536 (12.50) | <0.0001 |
| Fibromyalgia, n (%) | 246 (2.59) | 68 (1.30) | 178 (4.15) | <0.0001 |
| Chronic fatigue syndrome, n (%) | 133 (1.40) | 55 (1.05) | 78 (1.82) | 0.0016 |
| Chronic obstructive pulmonary disease (COPD), n (%) | 103 (1.08) | 54 (1.03) | 49 (1.14) | 0.6126 |
| Asthma, n (%) | 412 (4.33) | 212 (4.06) | 200 (4.66) | 0.1514 |
| Idiopathic pulmonary fibrosis, n (%) | 3 (0.03) | 3 (0.06) | 0 (0.00) | 0.1164 |
| Liver cirrhosis, n (%) | 6 (0.06) | 4 (0.08) | 2 (0.05) | 0.5622 |
| Hepatitis, n (%) | 5 (0.05) | 2 (0.04) | 3 (0.07) | 0.5031 |
| Non-alcoholic fatty liver disease, n (%) | 6 (0.06) | 4 (0.08) | 2 (0.05) | 0.5622 |
| Rheumatoid arthritis, n (%) | 92 (0.97) | 25 (0.48) | 67 (1.56) | <0.0001 |
| Ankylosing spondylitis, n (%) | 19 (0.20) | 3 (0.06) | 16 (0.37) | 0.0006 |
| Lupus, n (%) | 12 (0.13) | 4 (0.08) | 8 (0.19) | 0.1331 |
| Psoriatic arthritis, n (%) | 17 (0.18) | 4 (0.08) | 13 (0.30) | 0.0093 |
| Systems affected | ||||
| Cardiovascular disease, n (%) | 4124 (43.38) | 2105 (40.34) | 2019 (47.08) | <0.0001 |
| Endocrine disease, n (%) | 1229 (12.93) | 595 (11.40) | 634 (14.79) | <0.0001 |
| Non-restorative pain disorders, n (%) | 1164 (12.24) | 497 (9.52) | 667 (15.56) | <0.0001 |
| Lung disease, n (%) | 497 (5.23) | 263 (5.04) | 234 (5.46) | 0.3624 |
| Liver disease, n (%) | 17 (0.18) | 10 (0.19) | 7 (0.16) | 0.7447 |
| Chronic kidney disease/failure (CKD), n (%) | 23 (0.24) | 8 (0.15) | 15 (0.35) | 0.0523 |
| Central nervous system disorders, n (%) | 289 (3.04) | 152 (2.91) | 137 (3.19) | 0.4246 |
| Gout, n (%) | 94 (0.99) | 54 (1.03) | 40 (0.93) | 0.6175 |
| Chronic rheumatic conditions, n (%) | 138 (1.45) | 35 (0.67) | 103 (2.40) | <0.0001 |
| Comorbidity count | ||||
| N of comorbidities, mean (SD), n (%) | 0.80 (0.85) | 0.71 (0.81) | 0.90 (0.90) | <0.0001 |
| Any comorbidities, n (%) | 5355 (56.33) | 2740 (52.51) | 2615 (60.98) | <0.0001 |
| Any comorbidities ≥ 2, n (%) | 1804 (18.98) | 822 (15.75) | 982 (22.90) | <0.0001 |
| Any comorbidities ≥ 3, n (%) | 367 (3.86) | 137 (2.63) | 230 (5.36) | <0.0001 |
| Any comorbidities ≥ 4, n (%) | 45 (0.47) | 19 (0.36) | 26 (0.61) | 0.0868 |
Appendix 5. Risk factors associated with prevalent knee pain
| Descriptive |
ORs (95% CI) |
||||
|---|---|---|---|---|---|
| Total | No KP | KP | Crude | Age, gender, BMI-adjusted | |
| N | 9506 | 5218 | 4288 | ||
| Central risk factors | |||||
| Widespread pain, n (%) | 2070 (21.77) | 683 (13.09) | 1387 (32.35) | 3.17 (2.86; 3.52) | 3.02 (2.71; 3.37) |
| HADS anxiety score ≥8, n (%) | 3083 (32.43) | 1349 (25.85) | 1734 (40.44) | 1.95 (1.79; 2.12) | 1.87 (1.71; 2.06) |
| HADS depression score ≥8, n (%) | 1707 (17.96) | 593 (11.36) | 1114 (25.98) | 2.74 (2.45; 3.05) | 2.40 (2.14; 2.70) |
| PCS in the highest tertile (≥9), n (%) | 3256 (34.25) | 1354 (25.96) | 1902 (44.37) | 2.27 (2.09; 2.48) | 2.06 (1.88; 2.25) |
| Peripheral risk factors | |||||
| Significant injury, n (%) | 1551 (16.31) | 352 (6.97) | 1199 (28.83) | 5.40 (4.76; 6.14) | 5.62 (4.92; 6.42) |
| Early varus malalignment, n (%) | 315 (3.31) | 147 (3.18) | 168 (4.31) | 1.37 (1.09; 1.72) | 1.49 (1.18; 1.88) |
| Early valgus malalignment, n (%) | 182 (1.91) | 71 (1.56) | 111 (2.88) | 1.87 (1.38; 2.53) | 1.72 (1.25; 2.36) |
| Varus malalignment, n (%) | 144 (1.51) | 23 (0.49) | 121 (2.99) | 6.31 (4.03; 9.88) | 5.43 (3.44; 8.58) |
| Valgus malalignment, n (%) | 164 (1.73) | 35 (0.74) | 129 (3.19) | 4.42 (3.03; 6.44) | 4.18 (2.74; 6.38) |
| High risk occupation, n (%) | 3979 (41.85) | 1973 (37.80) | 2006 (46.78) | 1.45 (1.33; 1.57) | 1.43 (1.31; 1.56) |
| 2D4D ratio (type 3), n (%) | 4484 (47.17) | 2439 (49.86) | 2045 (50.81) | 1.04 (0.96; 1.13) | 1.04 (0.95; 1.14) |
| Other risk factors | |||||
| Age, mean (SD)∗ | 62.10 (10.56) | 62.33 (10.69) | 61.82 (10.40) | 1.00 (0.99; 1.00) | 1.00 (0.99; 1.00) |
| Women, n (%)† | 5371 (56.50) | 2909 (56.03) | 2462 (57.62) | 1.07 (0.98; 1.16) | 1.10 (1.01; 1.20) |
| BMI (kg/m2), mean (SD)‡ | 27.31 (5.30) | 26.36 (4.63) | 28.47 (5.80) | 1.08 (1.07; 1.09) | 1.08 (1.07; 1.09) |
| Nodal OA, n (%) | 1060 (11.15) | 445 (8.86) | 615 (15.11) | 1.83 (1.61; 2.08) | 1.89 (1.64; 2.17) |
| N of comorbidities, mean (SD), n (%) | 0.80 (0.85) | 0.71 (0.81) | 0.90 (0.90) | 1.29 (1.23; 1.36) | 1.21 (1.15; 1.28) |
| Any comorbidities, n (%) | 5355 (56.33) | 2740 (52.51) | 2615 (60.98) | 1.41 (1.30; 1.53) | 1.29 (1.18; 1.42) |
| Any comorbidities ≥ 2, n (%) | 1804 (18.98) | 822 (15.75) | 982 (22.90) | 1.59 (1.43; 1.76) | 1.37 (1.22; 1.53) |
| Any comorbidities ≥ 3, n (%) | 367 (3.86) | 137 (2.63) | 230 (5.36) | 2.10 (1.69; 2.61) | 1.68 (1.34; 2.11) |
| Any comorbidities ≥ 4, n (%) | 45 (0.47) | 19 (0.36) | 26 (0.61) | 1.67 (0.92; 3.03) | 1.51 (0.80; 2.84) |
In bold - If a 95% confidence interval does not include the null value (i.e. there is a statistically meaningful or statistically significant difference between the groups).
Abbreviations: BMI - body mass index; KP – knee pain; OR – odds ratio; CI –confidence interval; HADS - Hospital Anxiety and Depression Scale; PCS - Pain Catastrophizing Scale; SD – standard deviation.
Adjusted for gender and BMI only.
Adjusted for age and BMI only.
Adjusted for age and gender only.
Appendix 6. Contribution of central, peripheral and other risk factors to knee pain
| Outcome | Model | AUC (95%CI) | PRC |
|---|---|---|---|
| Prevalent KP | Full | 0.76 (0.74; 0.77) | 100% |
| without central | 0.71 (0.70; 0.72) | 19% | |
| without peripheral | 0.70 (0.69; 0.71) | 23% | |
| without others | 0.69 (0.67; 0.70) | 27% | |
| Incident KP | Full∗ | 0.71 (0.68; 0.75) | 100% |
| without central | 0.68 (0.64; 0.72) | 14% | |
| without peripheral | 0.65 (0.62; 0.69) | 29% | |
| without others | 0.68 (0.64; 0.72) | 14% | |
| Worsening KP | Full∗ | 0.71 (0.68; 0.73) | 100% |
| without central | 0.65 (0.62; 0.68) | 29% | |
| without peripheral | 0.70 (0.67; 0.73) | 5% | |
| without others | 0.68 (0.65; 0.71) | 14% |
Risk factors included in the full model:
• Central: Widespread pain; HADS - Hospital Anxiety and Depression Scales; PCS - Pain Catastrophizing Scale.
• Peripheral: History of significant injury; early life mal-alignment; current mal-alignment; high risk occupation.
• Others: Age; gender; BMI; number of comorbidities; nodal hand osteoarthritis.
Abbreviations: AUC – area under the curve; CI –confidence interval. PRC – proportional risk contribution.
New knee injury (past 12 months) as an additional peripheral risk factor was included in analysis.
Appendix 7. Risk factors associated with incident knee pain
| Descriptive |
OR (95% CI) |
||||
|---|---|---|---|---|---|
| Total | No KP | Incident KP | Crude | Age, gender, BMI-adjusted | |
| N | 2341 | 2056 | 285 | ||
| Central risk factors | |||||
| Widespread pain at baseline, n (%) | 316 (13.50) | 247 (12.01) | 69 (24.21) | 2.34 (1.73; 3.16) | 2.23 (1.63; 3.06) |
| HADS anxiety score ≥8 at baseline, n (%) | 559 (23.88) | 477 (23.20) | 82 (28.77) | 1.34 (1.02; 1.76) | 1.27 (0.95; 1.70) |
| HADS depression score ≥8 at baseline, n (%) | 216 (9.23) | 169 (8.22) | 47 (16.49) | 2.21 (1.55; 3.13) | 1.99 (1.38; 2.87) |
| PCS in the highest tertile (≥9) at baseline, n (%) | 611 (26.1) | 524 (25.50) | 87 (30.63) | 1.29 (0.98; 1.69) | 1.14 (0.86; 1.52) |
| Peripheral risk factors | |||||
| Significant injury at baseline, n (%) | 183 (7.82) | 158 (7.85) | 25 (8.90) | 1.15 (0.74; 1.78) | 1.08 (0.68; 1.72) |
| New injury (past 12 months), n (%) | 38 (1.62) | 4 (0.19) | 34 (11.93) | 69.49 (24.46; 197.4) | 69.27 (24.15; 198.7) |
| Early varus malalignment, n (%) | 72 (3.07) | 62 (3.25) | 10 (3.86) | 1.20 (0.61; 2.36) | 1.28 (0.64; 2.57) |
| Early valgus malalignment, n (%) | 28 (1.20) | 23 (1.23) | 5 (1.96) | 1.60 (0.60; 4.26) | 1.05 (0.36; 3.12) |
| High risk occupation, n (%) | 833 (35.57) | 732 (35.59) | 101 (35.44) | 0.99 (0.77; 1.29) | 0.99 (0.76; 1.30) |
| 2D4D ratio (type 3), n (%) | 1171 (50.02) | 1023 (51.10) | 148 (54.81) | 1.16 (0.90; 1.50) | 1.18 (0.90; 1.54) |
| Other risk factors | |||||
| Age (years), mean (SD)∗ | 62.54 (10.25) | 62.56 (10.28) | 62.38 (10.09) | 1.00 (0.99; 1.01) | 1.00 (0.99; 1.01) |
| Women, n (%)† | 1343 (57.37) | 1170 (56.93) | 173 (60.70) | 1.17 (0.91; 1.51) | 1.15 (0.89; 1.50) |
| BMI (kg/m2), mean (SD)‡ | 26.20 (4.50) | 26.04 (4.46) | 27.36 (4.65) | 1.06 (1.03; 1.09) | 1.06 (1.03; 1.09) |
| Nodal OA, n (%) | 225 (9.61) | 188 (9.41) | 37 (13.60) | 1.52 (1.04; 2.21) | 1.49 (0.99; 2.23) |
| N of comorbidities, mean (SD), n (%) | 0.69 (0.79) | 0.67 (0.77) | 0.86 (0.90) | 1.32 (1.14; 1.53) | 1.31 (1.12; 1.53) |
| Any comorbidities, n (%) | 1213 (51.82) | 1039 (50.54) | 174 (61.05) | 1.53 (1.19; 1.98) | 1.48 (1.13; 1.95) |
| Any comorbidities ≥ 2, n (%) | 335 (14.30) | 285 (13.86) | 50 (17.54) | 1.32 (0.95; 1.84) | 1.26 (0.90; 1.78) |
| Any comorbidities ≥ 3, n (%) | 62 (2.65) | 46 (2.24) | 16 (5.61) | 2.60 (1.45; 4.66) | 2.54 (1.41; 4.60) |
| Any comorbidities ≥ 4, n (%) | 8 (0.34) | 4 (0.19) | 4 (1.40) | 7.30 (1.82; 29.36) | 8.85 (1.95; 40.16) |
In bold - If a 95% confidence interval does not include the null value (i.e. there is a statistically meaningful or statistically significant difference between the groups).
Abbreviations: BMI - body mass index; KP – knee pain; OR – odds ratio; CI –confidence interval; HADS - Hospital Anxiety and Depression Scale; PCS - Pain Catastrophizing Scale; SD - standard deviation.
Adjusted for gender and BMI only.
Adjusted for age and BMI only.
Adjusted for age and gender only.
Appendix 8. Risk factors associated with worsening of knee pain at 1 year
| Descriptive |
OR (95% CI) |
||||
|---|---|---|---|---|---|
| Total | Stable/improved | Worsened | Crude | Age, gender, BMI-adjusted | |
| N | 2396 | 1943 | 453 | ||
| Central risk factors | |||||
| Widespread pain, n (%) | 794 (33.14) | 594 (30.57) | 200 (44.15) | 1.80 (1.46; 2.21) | 1.68 (1.35; 2.10) |
| HADS anxiety score ≥8, n (%) | 900 (37.56) | 678 (34.89) | 222 (49.01) | 1.79 (1.46; 2.20) | 1.68 (1.35; 2.08) |
| HADS depression score ≥8, n (%) | 551 (23.00) | 394 (20.28) | 157 (34.66) | 2.09 (1.67; 2.61) | 1.85 (1.46; 2.34) |
| PCS in the highest tertile (>=9), n (%) | 998 (41.65) | 726 (37.36) | 272 (60.04) | 2.52 (2.04; 3.11) | 2.27 (1.83; 2.83) |
| mPDQ ≥ 19, n (%) | 202 (8.43) | 123 (6.33) | 79 (17.44) | 3.13 (2.31; 4.23) | 2.65 (1.92; 3.65) |
| ICOAP intermittent in the highest tertile (>=7), n (%) | 826 (34.47) | 551 (28.36) | 275 (60.71) | 3.90 (3.15; 4.83) | 3.61 (2.89; 4.50) |
| ICOAP constant in the highest tertile (>=7), n (%) | 788 (32.89) | 518 (26.66) | 270 (59.60) | 4.06 (3.28; 5.02) | 3.70 (2.96; 4.63) |
| Peripheral risk factors | |||||
| Significant injury at baseline, n (%) | 706 (29.47) | 583 (30.80) | 123 (28.21) | 0.88 (0.70; 1.11) | 0.89 (0.70; 1.13) |
| Significant injury in the past 12 months, n (%) | 63 (2.63) | 39 (2.01) | 24 (5.31) | 2.73 (1.62; 4.59) | 2.52 (1.48; 4.30) |
| Early varus malalignment, n (%) | 102 (4.26) | 76 (4.23) | 26 (6.31) | 1.53 (0.96; 2.41) | 1.60 (0.99; 2.58) |
| Early valgus malalignment, n (%) | 59 (2.46) | 44 (2.48) | 15 (3.75) | 1.54 (0.85; 2.79) | 1.64 (0.89; 3.03) |
| Varus malalignment, n (%) | 61 (2.55) | 41 (2.21) | 20 (4.67) | 2.17 (1.26; 3.75) | 2.28 (1.30; 4.02) |
| Valgus malalignment, n (%) | 68 (2.84) | 55 (2.96) | 13 (3.04) | 1.03 (0.56; 1.90) | 0.71 (0.37; 1.37) |
| High risk occupation, n (%) | 1093 (45.62) | 867 (44.62) | 226 (49.89) | 1.24 (1.01; 1.52) | 1.29 (1.04; 1.60) |
| 2D4D ratio (type 3), n (%) | 1188 (49.58) | 979 (52.89) | 209 (49.64) | 0.88 (0.71; 1.09) | 0.86 (0.69; 1.07) |
| Other risk factors | |||||
| Age (years), mean (SD)∗ | 62.41 (10.02) | 62.26 (9.98) | 63.03 (10.19) | 1.01 (1.00; 1.02) | 1.01 (1.00; 1.02) |
| Women, n (%)† | 1397 (58.31) | 1107 (56.97) | 290 (64.02) | 1.34 (1.09; 1.66) | 1.34 (1.07; 1.66) |
| BMI (kg/m2), mean (SD)‡ | 28.29 (5.80) | 27.93 (5.62) | 29.83 (6.28) | 1.05 (1.03; 1.07) | 1.05 (1.04; 1.07) |
| Nodal OA, n (%) | 364 (15.19) | 269 (14.44) | 95 (22.35) | 1.71 (1.31; 2.22) | 1.62 (1.23; 2.14) |
| N of comorbidities, mean (SD), n (%) | 0.90 (0.90) | 0.87 (0.89) | 1.05 (0.91) | 1.24 (1.11; 1.39) | 1.13 (1.00; 1.27) |
| Any comorbidities, n (%) | 1461 (60.98) | 1152 (59.29) | 309 (68.21) | 1.47 (1.19; 1.83) | 1.29 (1.02; 1.64) |
| Any comorbidities ≥ 2, n (%) | 553 (23.08) | 419 (21.56) | 134 (29.58) | 1.53 (1.22; 1.92) | 1.26 (0.98; 1.61) |
| Any comorbidities ≥ 3, n (%) | 127 (5.30) | 99 (5.10) | 28 (6.18) | 1.23 (0.80; 1.89) | 0.98 (0.62; 1.54) |
| Any comorbidities ≥ 4, n (%) | 14 (0.58) | 11 (0.57) | 3 (0.66) | 1.17 (0.33; 4.21) | 0.70 (0.15; 3.18) |
In bold - If a 95% confidence interval does not include the null value (i.e. there is a statistically meaningful or statistically significant difference between the groups).
Abbreviations: BMI - body mass index; KP – knee pain; OR – odds ratio; CI –confidence interval; HADS - Hospital Anxiety and Depression Scale; PCS - Pain Catastrophizing Scale; SD - standard deviation; mPDQ – modified Pain Detect Questionnaire; ICOAP - Measure of Intermittent and Constant Osteoarthritis Pain.
Adjusted for gender and BMI only.
Adjusted for age and BMI only.
Adjusted for age and gender only.
Appendix 9. Risk factors associated with increase in severity of knee pain on NRS (≥1∗) at 1 year
| Descriptive |
OR (95% CI) |
||||
|---|---|---|---|---|---|
| Total | Stable/improved | Worsened | Crude | Age, gender, BMI-adjusted | |
| N | 2396 | 1738 | 658 | ||
| Central risk factors | |||||
| Widespread pain, n (%) | 794 (33.14) | 561 (32.28) | 233 (35.41) | 1.15 (0.95; 1.39) | 1.11 (0.92; 1.35) |
| HADS anxiety score ≥8, n (%) | 900 (37.56) | 642 (36.94) | 258 (39.21) | 1.10 (0.92; 1.32) | 1.09 (0.90; 1.31) |
| HADS depression score ≥8, n (%) | 551 (23.00) | 398 (22.90) | 153 (23.25) | 1.02 (0.82; 1.26) | 1.00 (0.80; 1.24) |
| PCS in the highest tertile (≥9), n (%) | 998 (41.65) | 722 (41.54) | 276 (41.95) | 1.02 (0.85; 1.22) | 1.01 (0.84; 1.22) |
| mPDQ ≥ 19, n (%) | 202 (8.43) | 160 (9.21) | 42 (6.38) | 0.67 (0.47; 0.96) | 0.63 (0.44; 0.91) |
| ICOAP intermittent in the highest tertile (≥7), n (%) | 826 (34.47) | 616 (35.44) | 210 (31.91) | 0.85 (0.71; 1.03) | 0.81 (0.66; 0.99) |
| ICOAP constant in the highest tertile (≥7), n (%) | 788 (32.89) | 584 (33.60) | 204 (31.00) | 0.89 (0.73; 1.08) | 0.82 (0.67; 1.00) |
| Peripheral risk factors | |||||
| Significant injury at baseline, n (%) | 706 (29.47) | 497 (29.30) | 209 (32.45) | 1.16 (0.95; 1.41) | 1.18 (0.96; 1.44) |
| Significant injury in the past 12 months, n (%) | 63 (2.63) | 40 (2.31) | 23 (3.51) | 1.54 (0.91; 2.59) | 1.43 (0.84; 2.44) |
| Early varus malalignment, n (%) | 102 (4.26) | 76 (4.73) | 26 (4.32) | 0.91 (0.58; 1.43) | 0.89 (0.56; 1.42) |
| Early valgus malalignment, n (%) | 59 (2.46) | 42 (2.66) | 17 (2.85) | 1.08 (0.61; 1.91) | 1.02 (0.57; 1.85) |
| Varus malalignment, n (%) | 61 (2.55) | 48 (2.89) | 13 (2.08) | 0.71 (0.38; 1.32) | 0.66 (0.35; 1.27) |
| Valgus malalignment, n (%) | 68 (2.84) | 52 (3.13) | 16 (2.56) | 0.81 (0.46; 1.43) | 0.80 (0.45; 1.43) |
| High risk occupation, n (%) | 1093 (45.62) | 764 (43.96) | 329 (50.00) | 1.27 (1.07; 1.53) | 1.30 (1.07; 1.56) |
| 2D4D ratio (type 3), n (%) | 1188 (49.58) | 854 (51.66) | 334 (53.96) | 1.10 (0.91; 1.32) | 1.11 (0.92; 1.34) |
| Other risk factors | |||||
| Age (years), mean (SD)† | 62.41 (10.02) | 62.48 (9.96) | 62.22 (10.20) | 1.00 (0.99; 1.01) | 1.00 (0.99; 1.01) |
| Women, n (%)‡ | 1397 (58.31) | 997 (57.36) | 400 (60.79) | 1.15 (0.96; 1.38) | 1.15 (0.96; 1.39) |
| BMI (kg/m2), mean (SD)§ | 28.29 (5.80) | 28.21 (5.62) | 28.50 (6.25) | 1.01 (0.99; 1.02) | 1.01 (0.99; 1.02) |
| Nodal OA, n (%) | 364 (15.19) | 248 (14.87) | 116 (18.71) | 1.32 (1.03; 1.68) | 1.34 (1.04; 1.72) |
| N of comorbidities, mean (SD), n (%) | 0.90 (0.90) | 0.90 (0.91) | 0.89 (0.86) | 0.98 (0.89; 1.09) | 0.95 (0.85; 1.06) |
| Any comorbidities, n (%) | 1461 (60.98) | 1053 (60.59) | 408 (62.01) | 1.06 (0.88; 1.28) | 1.05 (0.86; 1.28) |
| Any comorbidities ≥ 2, n (%) | 553 (23.08) | 407 (23.42) | 146 (22.19) | 0.93 (0.75; 1.16) | 0.88 (0.70; 1.10) |
| Any comorbidities ≥ 3, n (%) | 127 (5.30) | 99 (5.70) | 28 (4.26) | 0.74 (0.48; 1.13) | 0.66 (0.42; 1.03) |
| Any comorbidities ≥ 4, n (%) | 14 (0.58) | 11 (0.63) | 3 (0.46) | 0.72 (0.20; 2.59) | 0.46 (0.10; 2.07) |
In bold - If a 95% confidence interval does not include the null value (i.e. there is a statistically meaningful or statistically significant difference between the groups).
Abbreviations: BMI - body mass index; KP – knee pain; OR – odds ratio; CI –confidence interval; HADS - Hospital Anxiety and Depression Scale; PCS - Pain Catastrophizing Scale; SD – standard deviation; mPDQ – modified Pain Detect Questionnaire; ICOAP - Measure of Intermittent and Constant Osteoarthritis Pain.
A significant change from baseline was calculated using he least significant criterion (LSC) (Nguyen and Eisman, 2000, Wang et al., 2016).
Adjusted for gender and BMI only.
adjuSted for age and BMI only.
Adjusted for age and gender only.
References
- 1.Peat G., McCarney R., Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91–97. doi: 10.1136/ard.60.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neogi T., Felson D., Niu J., Nevitt M., Lewis C.E., Aliabadi P. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staud R. Evidence for shared pain mechanisms in osteoarthritis, low back pain, and fibromyalgia. Curr Rheumatol Rep. 2011;13:513–520. doi: 10.1007/s11926-011-0206-6. [DOI] [PubMed] [Google Scholar]
- 4.Peat G., Greig J., Wood L., Wilkie R., Thomas E., Croft P. Diagnostic discordance: we cannot agree when to call knee pain ‘osteoarthritis’. Fam Pract. 2005;22:96–102. doi: 10.1093/fampra/cmh702. [DOI] [PubMed] [Google Scholar]
- 5.Hadler N.M. KNee pain is the malady—not osteoarthritis. Ann Intern Med. 1992;116:598–599. doi: 10.7326/0003-4819-116-7-598. [DOI] [PubMed] [Google Scholar]
- 6.Hannan M.T., Felson D.T., Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513–1517. [PubMed] [Google Scholar]
- 7.Bedson J., Croft P.R. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Muscoskel Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conaghan P.G., Dickson J., Grant R.L. Care and management of osteoarthritis in adults: summary of NICE guidance. BMJ. 2008;336:502–503. doi: 10.1136/bmj.39490.608009.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laslett L.L., Quinn S.J., Winzenberg T.M., Sanderson K., Cicuttini F., Jones G. A prospective study of the impact of musculoskeletal pain and radiographic osteoarthritis on health related quality of life in community dwelling older people. BMC Muscoskel Disord. 2012;13:168. doi: 10.1186/1471-2474-13-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross M., Smith E., Hoy D., Nolte S., Ackerman I., Fransen M. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 11.Kluzek S., Sanchez-Santos M.T., Leyland K.M., Judge A., Spector T.D., Hart D. Painful knee but not hand osteoarthritis is an independent predictor of mortality over 23 years follow-up of a population-based cohort of middle-aged women. Ann Rheum Dis. 2016;75:1749–1756. doi: 10.1136/annrheumdis-2015-208056. [DOI] [PubMed] [Google Scholar]
- 12.Wylde V., Palmer S., Learmonth I.D., Dieppe P. The association between pre-operative pain sensitisation and chronic pain after knee replacement: an exploratory study. Osteoarthritis Cartilage. 2013;21:1253–1256. doi: 10.1016/j.joca.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Silverwood V., Blagojevic-Bucknall M., Jinks C., Jordan J.L., Protheroe J., Jordan K.P. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:507–515. doi: 10.1016/j.joca.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Blagojevic M., Jinks C., Jeffery A., Jordan K.P. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Tanamas S., Hanna F.S., Cicuttini F.M., Wluka A.E., Berry P., Urquhart D.M. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Care Res. 2009;61:459–467. doi: 10.1002/art.24336. [DOI] [PubMed] [Google Scholar]
- 16.Bastick A.N., Belo J.N., Runhaar J., Bierma-Zeinstra S.M. What are the prognostic factors for radiographic progression of knee osteoarthritis? A meta-analysis. Clin Orthop Relat Res. 2015;473:2969–2989. doi: 10.1007/s11999-015-4349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jinks C., Jordan K.P., Blagojevic M., Croft P. Predictors of onset and progression of knee pain in adults living in the community. A prospective study. Rheumatology. 2008;47:368–374. doi: 10.1093/rheumatology/kem374. [DOI] [PubMed] [Google Scholar]
- 18.Phyomaung P.P., Dubowitz J., Cicuttini F.M., Fernando S., Wluka A.E., Raaijmaakers P. Are depression, anxiety and poor mental health risk factors for knee pain? A systematic review. BMC Muscoskel Disord. 2014;15:10. doi: 10.1186/1471-2474-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muraki S., Akune T., Oka H., Ishimoto Y., Nagata K., Yoshida M. Incidence and risk factors for radiographic knee osteoarthritis and knee pain in Japanese men and women: a longitudinal population-based cohort study. Arthritis Rheum. 2012;64:1447–1456. doi: 10.1002/art.33508. [DOI] [PubMed] [Google Scholar]
- 20.Conaghan P.G., Kloppenburg M., Schett G., Bijlsma J.W. Osteoarthritis research priorities: a report from a EULAR ad hoc expert committee. Ann Rheum Dis. 2014;73:1442–1445. doi: 10.1136/annrheumdis-2013-204660. [DOI] [PubMed] [Google Scholar]
- 21.Malfait A.-M., Schnitzer T.J. Towards a mechanism-based approach to pain management in osteoarthritis. Nat Rev Rheumatol. 2013;9:654–664. doi: 10.1038/nrrheum.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen E., Lee Y.C. A mechanism-based approach to the management of osteoarthritis pain. Curr Osteoporos Rep. 2015;13:399–406. doi: 10.1007/s11914-015-0291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandes G.S., Sarmanova A., Warner S., Harvey H., Akin-Akinyosoye K., Richardson H. Knee pain and related health in the community study (KPIC): a cohort study protocol. BMC Muscoskel Disord. 2017;18:404. doi: 10.1186/s12891-017-1761-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Reilly S.C., Muir K.R., Doherty M. Screening for pain in knee osteoarthritis: which question? Ann Rheum Dis. 1996;55:931–933. doi: 10.1136/ard.55.12.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hochman J.R., Gagliese L., Davis A.M., Hawker G.A. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage. 2011;19:647–654. doi: 10.1016/j.joca.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Hawker G.A., Davis A.M., French M.R., Cibere J., Jordan J.M., March L. Development and preliminary psychometric testing of a new OA pain measure--an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16:409–414. doi: 10.1016/j.joca.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfe F., Smythe H.A., Yunus M.B., Bennett R.M., Bombardier C., Goldenberg D.L. The american college of rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 28.Mykletun A., Stordal E., Dahl A.A. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry. 2001;179:540–544. doi: 10.1192/bjp.179.6.540. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan M.J., Bishop S.R., Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524. [Google Scholar]
- 30.Ingham S.L., Moody A., Abhishek A., Doherty S.A., Zhang W., Doherty M. Development and validation of self-reported line drawings for assessment of knee malalignment and foot rotation: a cross-sectional comparative study. BMC Med Res Meth. 2010;10:57. doi: 10.1186/1471-2288-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer K.T. Occupational activities and osteoarthritis of the knee. Br Med Bull. 2012;102:147–170. doi: 10.1093/bmb/lds012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W., Robertson J., Doherty S., Liu J.J., Maciewicz R.A., Muir K.R. Index to ring finger length ratio and the risk of osteoarthritis. Arthritis Rheum. 2008;58:137–144. doi: 10.1002/art.23237. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W., Doherty M., Leeb B.F., Alekseeva L., Arden N.K., Bijlsma J.W. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann Rheum Dis. 2009;68:8–17. doi: 10.1136/ard.2007.084772. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh F.Y. Sample size tables for logistic regression. Stat Med. 1989;8:795–802. doi: 10.1002/sim.4780080704. [DOI] [PubMed] [Google Scholar]
- 35.Tosteson T.D., Buzas J.S., Demidenko E., Karagas M. Power and sample size calculations for generalized regression models with covariate measurement error. Stat Med. 2003;22:1069–1082. doi: 10.1002/sim.1388. [DOI] [PubMed] [Google Scholar]
- 36.Ingham S.L., Zhang W., Doherty S.A., Muir K.R., Doherty M. 2011. Natural History of Knee Pain in the Nottingham Community: Health States and Transition Probabilities in a 10 Year Retrospective Cohort Study. Unpublished manuscript. [DOI] [PubMed] [Google Scholar]
- 37.Westreich D., Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177:292–298. doi: 10.1093/aje/kws412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen T.V., Eisman J.A. Assessment of significant change in BMD: a new approach. J Bone Miner Res. 2000;15:369–370. doi: 10.1359/jbmr.2000.15.2.369. [DOI] [PubMed] [Google Scholar]
- 39.Akin-Akinyosoye K., Frowd N., Marshall L., Stocks J., Fernandes G.S., Valdes A. Traits associated with central pain augmentation in the Knee Pain in the Community (KPIC) cohort. Pain. 2018;159:1035–1044. doi: 10.1097/j.pain.0000000000001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlesso L.C., Segal N., Curtis J.R., Wise B.L., Law L.F., Nevitt M. Knee pain severity rather than structural damage is a risk factor for incident widespread pain: the multicenter osteoarthritis (MOST) study. Arthritis Care Res. 2017 Jun;69(6):826–832. doi: 10.1002/acr.23086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felson D.T., Niu J., Quinn E.K., Neogi T., Lewis C., Lewis C.E. Multiple nonspecific sites of joint pain outside the knees develop in persons with knee pain. Arthritis Rheumatol. 2017;69:335–342. doi: 10.1002/art.39848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staud R. Peripheral pain mechanisms in chronic widespread pain. Best Pract Res Clin Rheumatol. 2011;25:155–164. doi: 10.1016/j.berh.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riddle D.L., Kong X., Fitzgerald G.K. Psychological health impact on 2-year changes in pain and function in persons with knee pain: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2011;19:1095–1101. doi: 10.1016/j.joca.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins J.E., Katz J.N., Dervan E.E., Losina E. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2014;22:622–630. doi: 10.1016/j.joca.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mallen C.D., Peat G., Thomas E., Lacey R., Croft P. Predicting poor functional outcome in community-dwelling older adults with knee pain: prognostic value of generic indicators. Ann Rheum Dis. 2007;66:1456–1461. doi: 10.1136/ard.2006.067975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer K.T., Reading I., Calnan M., Linaker C., Coggon D. Does knee pain in the community behave like a regional pain syndrome? Prospective cohort study of incidence and persistence. Ann Rheum Dis. 2007;66:1190–1194. doi: 10.1136/ard.2006.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arendt-Nielsen L., Skou S.T., Nielsen T.A., Petersen K.K. Altered central sensitization and pain modulation in the CNS in chronic joint pain. Curr Osteoporos Rep. 2015;13:225–234. doi: 10.1007/s11914-015-0276-x. [DOI] [PubMed] [Google Scholar]
- 48.Ingham S.L., Zhang W., Doherty S.A., McWilliams D.F., Muir K.R., Doherty M. Incident knee pain in the Nottingham community: a 12-year retrospective cohort study. Osteoarthritis Cartilage. 2011;19:847–852. doi: 10.1016/j.joca.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 49.Sharma L., Song J., Felson D.T., Cahue S., Shamiyeh E., Dunlop D.D. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. J Am Med Assoc. 2001;286:188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 50.Walsh D.A. Editorial: arthritis pain: moving between early- and late-stage disease. Arthritis Rheumatol. 2017;69:1343–1345. doi: 10.1002/art.40126. [DOI] [PubMed] [Google Scholar]
- 51.Phillips K., Clauw D.J. Central pain mechanisms in the rheumatic diseases: future directions. Arthritis Rheum. 2013;65:291–302. doi: 10.1002/art.37739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pavlou M., Ambler G., Seaman S.R., Guttmann O., Elliott P., King M. How to develop a more accurate risk prediction model when there are few events. BMJ Br Med J (Clin Res Ed) 2015;351 doi: 10.1136/bmj.h3868. [DOI] [PMC free article] [PubMed] [Google Scholar]