Abstract
Purpose
The authors performed this study to investigate the risk factors for predicting stent failure and to evaluate its impact on prognosis.
Materials and Methods
Between January 2002 and March 2017, we retrospectively reviewed 117 consecutive patients who underwent retrograde ureteral stenting and exchanging at least once every 3 months for malignant ureteral obstruction. The patients were classified according to their pre-stenting chronic kidney disease (CKD) stage. The factors affecting stent failure were analyzed using a logistic regression model. Overall survival (OS) was estimated, and the prognostic significance of each variable was estimated using Cox proportional-hazards regression modeling.
Results
Before stenting, 91 patients were CKD stages 1–3 and 26 patients were CKD stages 4–5. These two groups differed significantly only in pre-stenting estimated glomerular filtration rate (eGFR), bilateral obstruction, and pre-stenting pyuria. Among the 117 patients, stent failure occurred in 30 patients (25.6%), and there were no differences between the groups. Pre-stenting pyuria and post-stenting complications were significant predictors of stent failure. There were 79 deaths in total, including 56 in the CKD stages 1–3 group and 23 in the CKD stages 4–5 group. In the multivariate analysis predicting patient OS, pre-stenting eGFR and post-stenting disease progression were significant factors.
Conclusions
Internal ureteral stenting was effective for maintaining renal function in malignant ureteral obstruction. However, it did not restore renal function, which is related to the prognosis of the patients. Therefore, to improve patients' renal function and prognosis, patients who require stenting must be quickly recognized and treated.
Keywords: Kidney, Prognosis, Stent, Ureteral obstruction
INTRODUCTION
Extrinsic malignant ureteral obstruction can damage ureteral patency and cause to renal dysfunction [1,2]. Malignant ureteral strictures occurs secondary to extrinsic compression caused by primary tumor, direct tumor invasion and metastatic disease [2,3]. Another possible cause is retroperitoneal lymphadenopathy due to advanced malignancy [2,4]. Malignant ureteral obstruction usually occurs slowly and is often ambiguous. However, if untreated, progressive obstruction can cause electrolyte imbalance, uremia, and urinary tract infections (UTIs) [5]. Therefore, upon the detection of malignant ureteral obstruction, immediate decompression of the ureter is required to restore renal function [2,6]. Ideally, surgical reconstruction is the most appropriate treatment for correcting these occlusions. However, most of these patients are often poor surgical candidates and are accompanied by a various risk of morbidity; therefore, it is not easy to determine whether to perform the operation. Furthermore, it is difficult to manage urological diseases because clinical problems and ethical dilemmas related to quality of life, disease prognosis, and rapid symptom relief should be solved with minimal complications [7].
An internal ureteral stent has been used for some time to relieve upper urinary tract obstruction in patients [2,3,8]. Although these treatments are performed frequently, there are many reports of high failure rates of stenting [3,9]. Other studies have found that internal stents are more susceptible to urosepsis and less effective than percutaneous nephrostomy (PCN) when improving azotemia [5,10]. Therefore, another form of upper urinary diversion should be considered in patients who are predicted to have stent failure. Thus far, stent failure has been extensively studied [1,3,5,9]. However, few studies have addressed the outcomes of stent failure and its impact on prognosis [1]. Therefore, the authors performed this study to investigate the risk factors for predicting stent failure and to evaluate its effect on prognosis.
MATERIALS AND METHODS
1. Study participants
Between January 2002 and March 2017, we retrospectively reviewed 117 consecutive patients who received retrograde ureteral stenting and exchanging at least once every 3 months for malignant ureteral obstruction. Ureteral obstruction was defined as the presence of hydronephrosis confirmed by computerized tomography or ultrasound, with/without the presence of flank pain and increased serum creatinine. Patients who underwent antegrade ureteral stent insertion after initial management with PCN placement were excluded from this study. The clinical characteristics of the patients, type of malignancy, laboratory and image data, and survival were reviewed. The number of stent exchanges was determined and whether the stents were placed unilaterally or bilaterally was confirmed. Renal function was assessed before and after the procedure via serum creatinine levels at each stent change.
2. Outcome measure
Stent failure was defined as persistence of hydronephrosis accompanied by flank pain or sustained elevation of serum creatinine after 2 weeks of stenting [9]. The estimated glomerular filtration rate (eGFR) was calculated by the Modification of Diet in Renal Disease formula, and patients were classified according to their pre-stenting chronic kidney disease (CKD) stage defined by the National Kidney Foundation [11]. UTI was defined as irritative symptoms and a positive urine culture (>100,000 CFU/mL). Urosepsis was defined as the recovery of a common organism from both blood and urine cultures. Complications included infection, renal failure, refractory hematuria and pain, and hospitalization for any of the above issues.
3. Statistical analysis
Clinicopathological features according to pre-stenting CKD stage were compared using Pearson's chi-square test for categorical variables and the Student's t-test for continuous variables. Quantitative data are expressed as the mean±standard deviation. Data were assessed to verify the difference in mean eGFR during the follow-up period according to CKD stage (pre-stenting, 3, 6, 9, and 12 months post-stenting). Overall survival (OS) was defined as the time from initial ureteral stenting to death from any cause. Survivors were censored at the date of last contact. Kaplan–Meier survival curves were generated to estimate OS and were compared by the log-rank test. A Cox proportional hazards model was used to estimate the prognostic significance of each variable. The correlations between outcomes and the assessed variables are expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). All statistical tests were two sided, with p<0.05 considered significant. The data were analyzed using IBM SPSS Statistics ver. 21.0 software (IBM Co., Armonk, NY, USA).
4. Ethics statement
This study was conducted after receiving approval from the Ulsan University Hospital Institutional Review Board (approval number: UUH-2018-07-007). Clinical data, including patient information and lab data, were obtained by retrospective review, so informed consent was omitted.
RESULTS
All patients had malignancies, and retrograde ureteral stenting was used to manage ureteral obstruction. The most common causes of malignancy were gynecologic tumors (53.0% in the uterus and 8.5% in the ovary), followed by colon cancer (21.4%) (Table 1).
Table 1. Primary sites of malignant disease causing malignant ureteral obstruction (n=117).
| Primary cancer site | Value |
|---|---|
| Uterus | 62 (53.0) |
| Ovary | 10 (8.5) |
| Colon | 25 (21.4) |
| Upper gastrointestinal | 7 (6.0) |
| Cholangiocarcinoma | 6 (5.1) |
| Bladder cancer | 5 (4.3) |
| Breast cancer | 2 (1.7) |
Values are presented as number (%).
The characteristics of the 117 patients are described in Table 2. The mean age of the patients was 56.8 years, and they had been followed-up for a mean duration of 23.3 months. Before stenting, 91 patients had CKD stages 1–3 and 26 patients had CKD stages 4–5. The two groups were significantly different only in pre-stenting eGFR, bilateral obstruction, and pre-stenting pyuria. Thus, although the mean pre-stenting eGFR was 53.4 mL/min/1.73 m2, the mean pre-stenting eGFR of the CKD stage 1–3 and stage 4–5 groups were 63.7 and 17.4, respectively (p=0.001). Bilateral obstruction and pyuria were higher in the CKD stage 4–5 group (p<0.05). However, there was no difference in the other variables between the two groups. Complications that could affect renal function and outcomes occurred in 14 (12.0%) patients.
Table 2. Clinical/pathological characteristics of the patient cohort.
| Variable | Overall | CKD 1–3 | CKD 4–5 | p-value |
|---|---|---|---|---|
| No. of patient | 117 | 91 | 26 | |
| Mean age (y) | 56.8±14.1 | 55.8±14.0 | 60.7±14.1 | 0.985 |
| Gender | 0.604 | |||
| Male | 27 (23.1) | 20 (22.0) | 7 (26.9) | |
| Female | 90 (76.9) | 71 (78.0) | 19 (73.1) | |
| Diabetes | 14 (12.0) | 9 (9.9) | 5 (19.2) | 0.300 |
| Hypertension | 28 (23.9) | 20 (22.0) | 8 (30.8) | 0.435 |
| Mean body mass index (kg/m2) | 22.4±4.0 | 22.7±4.2 | 21.7±3.4 | 0.749 |
| Pre-stent eGFR (mL/min/1.73 m2) | 53.4±27.3 | 63.7±21.3 | 17.4±9.2 | 0.001 |
| Ureteral obstruction site | 0.817 | |||
| Proximal | 29 (24.8) | 23 (25.3) | 6 (23.1) | |
| Mid | 25 (21.4) | 21 (23.1) | 4 (15.4) | |
| Distal | 58 (49.6) | 44 (48.3) | 14 (53.8) | |
| Multiple or diffuse | 5 (4.3) | 3 (3.3) | 2 (7.7) | |
| Bilateral obstruction | 47 (40.2) | 27 (29.7) | 20 (76.9) | 0.001 |
| Symptom (pain) | 69 (59.0) | 49 (53.8) | 20 (76.9) | 0.043 |
| Pre-stent pyuria | 34 (29.1) | 21 (23.1) | 13 (50.0) | 0.014 |
| Post-stent systemic therapy | 75 (64.1) | 59 (64.8) | 16 (61.5) | 0.818 |
| Post-stent disease progression | 86 (73.5) | 64 (70.3) | 22 (84.6) | 0.208 |
| Mean number of stent changes | 4.9±5.7 | 4.9±5.9 | 4.4±5.4 | 0.754 |
| Stent failure | 30 (25.6) | 20 (22.0) | 10 (38.5) | 0.125 |
| Complications | 14 (12.0) | 11 (12.1) | 3 (11.5) | 0.516 |
| UTI or Sepsis | 8 | 6 | 2 | |
| Hematuria | 6 | 5 | 1 |
Values are presented as number only, mean±standard deviation, or number (%).
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; UTI, urinary tract infection.
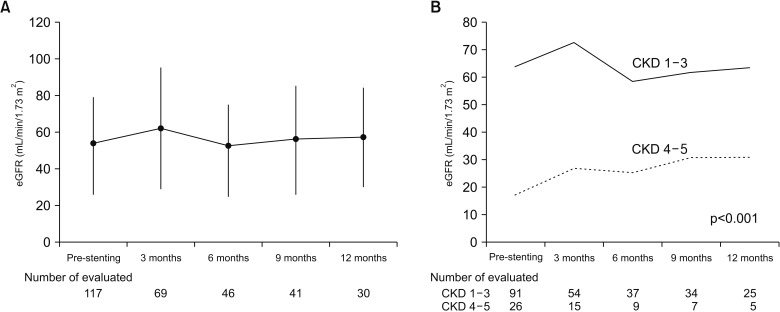
After stenting, eGFR was significantly elevated during the first 3 months but remained between 53.4 and 57.8 mL/min/1.73 m2 for the following year (Fig. 1A). The eGFR remained between 63.7 and 63.2 mL/min/1.73 m2 in the CKD stage 1–3 group. Although the eGFR increased from 17.3 to 30.8 mL/min/1.73 m2 in the CKD stage 4–5 group for the year following stenting, the eGFR of the CKD 4–5 group remained significantly lower (p<0.001) (Fig. 1B).
Fig. 1. Estimated glomerular filtration rate (eGFR) changes after stenting. (A) In all patients. (B) According to pre-stenting renal function. CKD, chronic kidney disease.
Among the 117 patients, stent failure occurred in 30 patients (25.6%), and there were no differences between the groups. Pre-stenting pyuria (odds ratio [OR], 2.68; 95% CI, 1.10–7.19; p=0.045) and post-stenting complications (OR, 8.02; 95% CI, 2.05–31.37; p=0.003) were significant predictors of stent failure (Table 3).
Table 3. Factors predictive of stent failure.
| Variable | Univariate | Multivariatea | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (continuous) | 1.00 (0.98–1.04) | 0.560 | ||
| Sex (female) | 0.48 (0.19–1.22) | 0.486 | ||
| Diabetes (yes) | 1.18 (0.34–4.10) | 0.789 | ||
| Hypertension (yes) | 0.55 (0.19–1.62) | 0.284 | ||
| Body mass index (continuous) | 0.98 (0.85–1.12) | 0.765 | ||
| Pre-stent eGFR (continuous) | 0.99 (0.98–1.01) | 0.568 | ||
| Ureteral obstruction site | ||||
| Proximal | Reference | |||
| Mid | 1.22 (0.36–4.13) | 0.747 | ||
| Distal | 0.91 (0.31–2.59) | 0.857 | ||
| Multiple or diffuse | 1.57 (0.12–9.06) | 0.728 | ||
| Bilateral obstruction (yes) | 2.06 (0.89–4.78) | 0.091 | ||
| Symptom (yes) | 0.73 (0.31–1.69) | 0.467 | ||
| Pre-stent pyuria (yes) | 2.84 (1.18–6.82) | 0.019 | 2.68 (1.10–7.19) | 0.045 |
| Post-stent systemic therapy (yes) | 1.76 (0.71–4.41) | 0.225 | ||
| Post-stent disease progression (yes) | 4.27 (1.19–15.28) | 0.026 | ||
| Number of stent change (continuous) | 0.96 (0.88–1.04) | 0.354 | ||
| Complications (yes) | 7.03 (2.13–23.18) | <0.001 | 8.02 (2.05–31.37) | 0.003 |
OR, odds ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate.
a:Logistic regression model (stepwise selection).
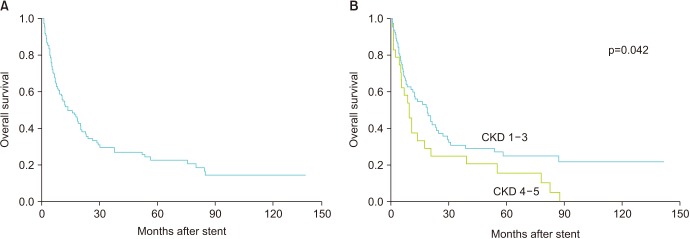
There were 79 deaths in total, including 56 in the CKD 1–3 group and 23 in the CKD stage 4–5 group. The overall 5 and 10 year OS rates were 22.9% and 14.7%, respectively; however, the 5 year OS rate of the CKD 4–5 group was significantly lower than that of the CKD 1–3 group (15.6% vs. 25.0%; p=0.042) (Fig. 2). In the multivariate analysis of predicting patient OS, pre-stenting eGFR (HR, 0.97) and post-stenting disease progression were significant factors (Table 4).
Fig. 2. Kaplan–Meier analysis of overall survival after stenting. (A) All cohorts. (B) According to pre-stenting renal function. CKD, chronic kidney disease.
Table 4. Univariate and multivariate analysis of factor influencing overall survival.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (continuous) | 1.02 (1.01–1.04) | 0.042 | 0.98 (0.96–1.02) | 0.580 |
| Sex (female) | 0.56 (0.33–0.92) | 0.023 | 0.55 (0.27–1.13) | 0.067 |
| Diabetes (yes) | 0.53 (0.24–1.15) | 0.108 | 0.49 (0.14–1.64) | 0.499 |
| Hypertension (yes) | 0.79 (0.45–1.40) | 0.432 | 1.53 (0.26–3.80) | 0.484 |
| Body mass index (continuous) | 0.98 (0.92–1.05) | 0.580 | 0.95 (0.88–1.03) | 0.963 |
| Pre-stent eGFR (continuous) | 0.98 (0.98–0.99) | 0.006 | 0.97 (0.96–0.99) | 0.001 |
| Ureteral obstruction site | ||||
| Proximal | Reference | Reference | ||
| Mid | 0.81 (0.04–1.64) | 0.564 | 1.09 (0.04–3.11) | 0.992 |
| Distal | 0.79 (0.46–1.36) | 0.405 | 1.08 (0.49–2.37) | 0.798 |
| Multiple or diffuse | 0.62 (0.14–2.69) | 0.530 | 0.30 (0.03–2.48) | 0.471 |
| Bilateral obstruction (yes) | 1.25 (0.80–1.95) | 0.327 | 0.46 (0.20–1.07) | 0.182 |
| Symptom (yes) | 0.71 (0.45–1.12) | 0.142 | 0.79 (0.39–1.62) | 0.350 |
| Pre-stent pyuria (yes) | 0.93 (0.58–1.51) | 0.787 | 0.61 (0.27–1.35) | 0.174 |
| Post-stent systemic therapy (yes) | 2.33 (1.35–4.01) | 0.002 | 0.98 (0.38–2.55) | 0.675 |
| Post-stent disease progression (yes) | 15.83 (4.95–50.60) | <0.001 | 28.99 (5.12–164.36) | <0.001 |
| Stent failure (yes) | 1.84 (1.14–2.95) | 0.012 | 1.44 (0.56–3.73) | 0.217 |
| Complications (yes) | 1.34 (0.70–2.55) | 0.373 | 1.65 (0.58–4.66) | 0.229 |
HR, hazard ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate.
DISCUSSION
Urologists frequently encounter patients with ureteral obstruction that is confirmed by imaging during a follow-up of the malignancy. In these cases, it is necessary to decide whether to divert the urinary tract, which should consider various factors, such as the opinion of the primary physician, oncologist, and radiologist, the patient's quality of life, predicted survival and cost [1]. In addition to these factors, most of these patients are accompanied by a significant risk of morbidity and poor surgical conditions; therefore, a treatment method with minimal complications must be addressed [7]. Currently, the most commonly used methods are PCN, internal stenting, and balloon dilatation with/without internal stenting [7,12]. Among these methods, stent change through cystoscopy is less invasive procedure that accompany with a low probability of complications and therefore is recommended [1,9]. This form of diversion provides a better quality of life than nephrostomy tubes, which is in the body and easy to manage [1,7]. Although we did not investigate the tolerability of the stents for this study, many patients well accepted to having multiple procedures, sometimes for years (average number of exchanges=4.78, maximum of 30 in our study).
Nevertheless, many of these patients experience frequent UTI, discomfort from the stent/tube, and decreased renal function [9,12,13]. Over the years, the success rate of internal stenting has been reported to vary [1,5,9,14]. We report a 25.6% stent failure rate in 117 patients after 23.3 months of follow-up. After conducting statistical analysis, we confirmed that two predictors of stent failure were accompaniment with pre-stenting pyuria and complications. Pyuria and several complications including UTIs are associated with stent failure because they induce decreased renal function and flank pain. Therefore, it may be possible to reduce stent failure by treating pyuria with antibiotics before the procedure. However, although stent failure has been defined as persistent hydronephrosis or persistently increased serum creatinine, there are several factors involved [9]. Many other factors are involved in the deterioration of renal function, so further research is necessary.
The OS of malignant cancer patients who require treatment for ureteral obstruction is poor [1,9,15]. Therefore, until recently, it was argued that this urinary diversion should be performed only if therapeutic methods for their malignancy remained [16]. In addition, because of the rapid progression of tumors in breast cancer, urinary diversion is not recommended for asymptomatic ureteral obstruction without further antitumor therapy or life-threatening medical problems [17]. However, in recent years, cancer treatment has developed dramatically, and the survival rate of cancer patients has been steadily increasing because of the development of various medical technologies [18]. Our median survival rate after stenting was 15 months, which is longer than that reported in prior quoted series. If stenting was not performed in these patients, the uremia would persist, and the survival rate of the patients would remain poor. In our study, eGFR was well maintained after stenting. As well, eGFR was elevated in CKD stage 4–5 patients. However, renal function did not improve in all cohorts, and eGFR levels in CKD stage 4–5 patients with improved renal function after stenting was still lower than that in CKD 1–3 patients (p<0.001, Fig. 1B). If we recognize the need for internal stenting and perform this stenting before renal function deteriorates, renal function can be maintained. Moreover, in the multivariate analysis of OS, eGFR before stenting was the only significant predictor, except for progression of the underlying malignancy. Therefore, if patients who require stenting are quickly recognized and treated, renal function and prognosis can be improved. However, more research is necessary because survival and renal function are affected by various factors.
This study has some limitations in addition to the inherent limitations associated with retrospective studies in small populations. First, there were clinical differences between patients, particularly regarding different types of malignancy causing ureteral obstruction that have different prognoses. In the multivariate analysis, the progression of these malignant tumors was also a stronger predictor than pre-stenting eGFR. Second, patient loss to follow-up and the exclusion of some patients who lacked long-term creatinine data may have introduced selection bias. In addition, the effect of various treatment modalities on prognosis was not considered in stenting failure patients. Further research involving more patients is necessary to address these problems.
CONCLUSIONS
Internal ureteral stenting was effective for maintaining renal function in malignant ureteral obstruction. However, it did not restore renal function, which is related to the prognosis of the patients. Therefore, to improve patients' renal function and prognosis, patients who require stenting must be quickly recognized and treated.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Rosenberg BH, Bianco FJ, Jr, Wood DP, Jr, Triest JA. Stent-change therapy in advanced malignancies with ureteral obstruction. J Endourol. 2005;19:63–67. doi: 10.1089/end.2005.19.63. [DOI] [PubMed] [Google Scholar]
- 2.Yachia D. Recent advances in ureteral stents. Curr Opin Urol. 2008;18:241–246. doi: 10.1097/MOU.0b013e3282f46d81. [DOI] [PubMed] [Google Scholar]
- 3.Liatsikos EN, Karnabatidis D, Katsanos K, Kallidonis P, Katsakiori P, Kagadis GC, et al. Ureteral metal stents: 10-year experience with malignant ureteral obstruction treatment. J Urol. 2009;182:2613–2617. doi: 10.1016/j.juro.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 4.Hafron J, Ost MC, Tan BJ, Fogarty JD, Hoenig DM, Lee BR, et al. Novel dual-lumen ureteral stents provide better ureteral flow than single ureteral stent in ex vivo porcine kidney model of extrinsic ureteral obstruction. Urology. 2006;68:911–915. doi: 10.1016/j.urology.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Song SH, Pak S, Jeong IG, Kim KS, Park HK, Kim CS, et al. Outcomes of stent-change therapy for bilateral malignancy-related ureteral obstruction. Int Urol Nephrol. 2015;47:19–24. doi: 10.1007/s11255-014-0858-z. [DOI] [PubMed] [Google Scholar]
- 6.Sountoulides P. Are metal ureteral stents indicated in cases of benign upper urinary tract obstruction? Indian J Urol. 2011;27:305–306. doi: 10.4103/0970-1591.85419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aravantinos E, Anagnostou T, Karatzas AD, Papakonstantinou W, Samarinas M, Melekos MD. Percutaneous nephrostomy in patients with tumors of advanced stage: treatment dilemmas and impact on clinical course and quality of life. J Endourol. 2007;21:1297–1302. doi: 10.1089/end.2006.0104. [DOI] [PubMed] [Google Scholar]
- 8.Andriole GL, Bettmann MA, Garnick MB, Richie JP. Indwelling double-J ureteral stents for temporary and permanent urinary drainage: experience with 87 patients. J Urol. 1984;131:239–241. doi: 10.1016/s0022-5347(17)50324-9. [DOI] [PubMed] [Google Scholar]
- 9.Chung SY, Stein RJ, Landsittel D, Davies BJ, Cuellar DC, Hrebinko RL, et al. 15-year experience with the management of extrinsic ureteral obstruction with indwelling ureteral stents. J Urol. 2004;172:592–595. doi: 10.1097/01.ju.0000130510.28768.f5. [DOI] [PubMed] [Google Scholar]
- 10.Hyppolite JC, Daniels ID, Friedman EA. Obstructive uropathy in gynecologic malignancy. Detrimental effect of intraureteral stent placement and value of percutaneous nephrostomy. ASAIO J. 1995;41:M318–M323. [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Russo P. Ureteral obstruction and stents: still a difficult problem for patients and urologists alike. J Urol. 2005;174:2088. doi: 10.1097/01.ju.0000187105.30751.77. [DOI] [PubMed] [Google Scholar]
- 13.Ganatra AM, Loughlin KR. The management of malignant ureteral obstruction treated with ureteral stents. J Urol. 2005;174:2125–2128. doi: 10.1097/01.ju.0000181807.56114.b7. [DOI] [PubMed] [Google Scholar]
- 14.Yu SH, Ryu JG, Jeong SH, Hwang EC, Jang WS, Hwang IS, et al. Predicting factors for stent failure-free survival in patients with a malignant ureteral obstruction managed with ureteral stents. Korean J Urol. 2013;54:316–321. doi: 10.4111/kju.2013.54.5.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf JS., Jr 15-year experience with the management of extrinsic ureteral obstruction with indwelling ureteral stents. Int Braz J Urol. 2004;30:340–341. [PubMed] [Google Scholar]
- 16.Harrington KJ, Pandha HS, Kelly SA, Lambert HE, Jackson JE, Waxman J. Palliation of obstructive nephropathy due to malignancy. Br J Urol. 1995;76:101–107. doi: 10.1111/j.1464-410x.1995.tb07841.x. [DOI] [PubMed] [Google Scholar]
- 17.Zadra JA, Jewett MA, Keresteci AG, Rankin JT, St Louis E, Grey RR, et al. Nonoperative urinary diversion for malignant ureteral obstruction. Cancer. 1987;60:1353–1357. doi: 10.1002/1097-0142(19870915)60:6<1353::aid-cncr2820600632>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]