Abstract
Objective
The effect of combined oral contraceptives (COCs) on female sexuality has long been a matter of discussion, but placebo-controlled studies are lacking. Thus, the aim of the present study was to investigate if an oestradiol-containing COC influences sexual function.
Design
Investigator-initiated, randomised, double-blinded, placebo-controlled clinical trial where 202 healthy women were randomised to a combined oral contraceptive (1.5 mg oestradiol and 2.5 mg nomegestrol acetate) or placebo for three treatment cycles.
Methods
Sexual function at baseline and during the last week of the final treatment cycle was evaluated by the McCoy Female Sexuality Questionnaire. Serum and hair testosterone levels were assessed at the same time points.
Results
Compared to placebo, COC use was associated with a small decrease in sexual interest (COC median change score: −2.0; interquartile range (IQR): −5.0 to 0.5 vs placebo: −1.0; IQR: −3.0 to 2.0, P = 0.019), which remained following adjustment for change in self-rated depressive symptoms (B = −0.80 ± 0.30, Wald = 7.08, P = 0.008). However, the proportion of women who reported a clinically relevant deterioration in sexual interest did not differ between COC or placebo users (COC 18 (22.2%) vs placebo 16 (17.8%), P = 0.47). Change in other measured aspects of sexual function as well as total score of sexual function did not differ between the two treatments.
Conclusions
This study suggests that use of oestradiol-based COCs is associated with reduced sexual interest. However, the changes are minute, and probably not of clinical relevance.
Keywords: combined oral contraceptive, McCoy Female Sexuality Questionnaire, placebo, randomised clinical trial, sexual function, sexual interest, testosterone
Introduction
The effect of combined oral contraceptives (COCs) on female sexuality has long been a matter of discussion (1). According to a systematic review in the field, however, mainly based on observational studies, approximately 15% of COC users report decreased sexual desire (2). At present, despite the bulk of literature, only two randomised placebo-controlled trials have investigated the causal influence of COCs on sexual function (3, 4). Both of these studies evaluated a pill introduced in the 1970s, containing ethinyl oestradiol (EE) and levonorgestrel, and the findings pointed to a COC-induced reduction in sexual desire (4, 5). However, since the 1970s, attempts have been made to reduce side effects from combined oral contraceptives by introducing less androgenic progestogens and by also substituting the synthetic oestrogen ethinyl with oestradiol. At present, placebo-controlled studies on sexual function in women using oestradiol-containing COCs are lacking.
Receptors for sex steroid hormones have widespread expression in genital tissues and the brain, and sex hormones are thought to play a role in female sexuality (6). While no clear-cut relationship between androgen levels and sexual desire has been established in COC users (2, 4), many still believe that the COC-induced decrease in bioavailable testosterone may negatively affect sexual function (2, 7, 8). Hormone measurement in hair is a new method that allows non-invasive sampling of the unbound testosterone fraction (9, 10). Further, the method enables retrospective evaluation of hormone levels across time, making it suitable for longitudinal assessment in COC users (11, 12).
Because of the lack of knowledge regarding the influence of oestradiol-based COCs on female sexual function, this randomised controlled trial (RCT) aimed to compare changes in sexual function between women treated with an oestradiol-based COC or placebo. A secondary aim was to investigate associations between sexual function and testosterone in serum and hair.
Subjects and methods
Participants
This investigator-initiated RCT was conducted at the Departments of Obstetrics and Gynaecology at Uppsala University Hospital, the Karolinska University Hospital, Södersjukhuset Stockholm, Linköping University Hospital, Örebro University Hospital, Umeå University Hospital and Närhälsan Maternity Health Care Centre, Gothenburg. The study investigates several aspects of COC use, including mood side effects (13).
Enrolment took place between September 2013 and May 2015. Healthy, non-obese (BMI <30 kg/m2) women aged 18–35 years were recruited by advertisements in local newspapers, on local notice boards and students’ websites. The women had to confirm they would use barrier contraceptives or copper intrauterine device (IUD) while participating. No study-specific exclusion criteria were used. As in clinical routine, women with prior history of venous thromboembolism, known predisposition for venous thromboembolism, systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg, known dyslipidaemia, migraine with focal symptoms, inflammatory bowel disease, first-degree relatives with cardiovascular disease at a young age, previous cancer, liver disease and previous pancreatitis were excluded.
Notably, current psychiatric disorders or use of psychotropic drugs were not reasons for exclusion. Ongoing primary depressive or anxiety disorders were assessed by use of the Mini International Neuropsychiatric Interview 6.0.0 (14). Women were categorised as suffering from any mood disorder if they fulfilled criteria for major or minor depressive disorder or dysthymia. Similarly, women who fulfilled diagnostic criteria for panic disorder, generalised anxiety disorder, social phobia or obsessive-compulsive disorder were classified as having an anxiety disorder.
All women gave written informed consent prior to inclusion. The study was approved by the regional Ethical Review Board, EPN 2013/161 Uppsala, and the Medical Products Agency in Sweden and was pre-registered: EUDRA-CT 2013-000925-30.
Study design
The study was a double-blind, randomised, parallel-group clinical trial during which the participants were treated with a COC (1.5 mg oestradiol and 2.5 mg nomegestrol acetate) or placebo during three 24/4 treatment cycles. During the screening visit blood and hair samples were collected and women filled out the McCoy Female Sexuality Questionnaire (MFSQ) (15). Following the screening visit, at least one menstrual cycle (baseline cycle) passed before randomisation and start of treatment. During the baseline cycle, no hormonal contraceptive use was allowed. Women switching from another hormonal contraceptive (HC) filled out the MFSQ and donated blood and hair samples at the randomisation visit, that is, following at least 1-month washout.
The National Corporation of Swedish Pharmacies in Stockholm prepared identical capsules containing either COC or placebo, and performed the packaging and randomisation (computerised random-number generator in blocks of four). At randomisation, the container with the lowest available randomization number was distributed to the participant. During the study, the participants and study personnel were not informed about which treatment the patient received, and randomisation codes were kept at the Uppsala University Hospital Pharmacy until completion of the study.
Following randomisation, women started taking the COC or placebo capsules once daily on the first day of the next-coming menstruation and continued treatment for 24 days, followed by four pill-free days. The study lasted three treatment cycles. After two treatment cycles, the participants met with the study coordinator to receive treatment for the third and final cycle. The fourth and final visit was made during the last week of treatment. Any adverse events or changes in concomitant medication were actively asked for and registered at each visit. Compliance was checked by counting remaining capsules at study completion. Pregnancy tests were taken once at baseline and before the start of each treatment cycle.
The primary outcome was change in the MFSQ scores (15). The MFSQ was chosen as it originally was designed to measure aspects of female sexuality likely to be affected by changing hormone levels (14). The questionnaire assesses sexual function during the past 4 weeks and consists of 19 questions, 18 answered using a 7-point Likert scale, where 1 represents negative answers such as ‘not at all enjoyable’ or ‘never’, whereas 7 represents positive answers such as ‘very enjoyable’ or ‘every time’, Supplementary Table 1 (see section on supplementary data given at the end of this article). One question assessed intercourse frequency, reported as number of intercourses per day, week or month.
The MFSQ questions are categorised into six domains; Sexual Interest, Satisfaction with Frequency of Sexual Activity, Vaginal Lubrication, Orgasm, Partner and Attractiveness, Supplementary Table 1. According to McCoy, the domain Satisfaction with Frequency of Sexual Activity is a summary score of the items Satisfaction with Frequency of Sexual Activity, Decreased Satisfaction due to Partner’s Interest and Frequency of Intercourse. As many women reported that they did not have a steady partner at baseline, the question on frequency was analysed separately. The items for Vaginal Lubrication and Orgasm were only answered by women who had engaged in sexual intercourse during the past 4 weeks. A clinically relevant deterioration in sexual desire and vaginal lubrication was defined as at least 30% decrease in the score from baseline to the last visit.
Depressive mood was captured at the screening visit and the final treatment visit, by use of the self-rated version of the Montgomery-Åsberg Depression Rating Scale (MADRS-S) (16). The MADRS-S scores reflect depressive symptoms during the past 3 days on a scale ranging from 0 to 54.
Hormonal analyses
Blood and hair samples from participants were taken at the screening or randomisation visit and at the final visit. A lock of hair was cut as close as possible to the scalp, with the proximal end marked. The most proximal 3 cm hair segment was finely cut with scissors and samples of about 10 mg of hair were prepared.
Testosterone in hair was measured using a competitive radioimmunoassay in speed-vaced methanol extracts of homogenised hair (10). The radio ligand was 125I-labelled testosterone-3-CMO-histamine and rabbit antiserum (T4276, Sigma Aldrich) was used which cross-reacts 23.0, 1.5, 0.2 and 1.7% with 5α-dihydrotestosterone, 17α-epitestosterone, dehydroepiandrosterone and androstenedione, respectively. The calibrator was testosterone (Sigma Aldrich T5411) verified with a European pharmacopoeia reference standard (EDQM, Strasbourg, France). All hair samples were analysed in the same assay, the intra-assay coefficient of variation (CV)% for testosterone in hair was 9.6% at 10 pg/mL, 3.0% at 45 pg/mL and 2.9% at 90 pg/mL.
Testosterone and sex hormone binding globulin (SHBG) in serum were measured with chemiluminescence (10). The analyses were performed with a Cobas EE (Roche Diagnostics). Total CV for testosterone was 5% at 2.2 nmol/L and 3% at 23 nmol/L. No samples were below detection level. Total CV for SHBG was 3% at 38 nmol/L and 3% at 109 nmol/L. All blood analyses were performed at the Department of Clinical Chemistry, Uppsala University hospital, which is an accredited laboratory. Free androgen index was calculated as (testosterone/SHBG) ×100.
Statistics
The power analysis was based on data from Graham and colleagues who used a five-point scale for assessment of sexual desire (7). Assuming that the SD was twice the size (0.6 scale steps) of the reported difference between the COC and placebo (0.3 scale steps), this study would reach 80% power to detect a significant difference (P < 0.05) between treatments, with 80 subjects in each treatment arm completing the trial.
Demographic data were compared between groups by Student’s t-test or chi-square tests. Delta (Δ)-scores were calculated for the MFSQ domains as difference between the final treatment cycle and baseline, meaning that negative Δ-scores indicate worsening and positive Δ-scores indicate improvement. Baseline and delta scores were compared between treatment groups by Mann–Whitney U test. Ordinal regression analyses on the MFSQ items sexual interest and lubrication were performed, adjusted for change in self-rated depression scores (ΔMADRS-s). Correlations between serum testosterone, hair testosterone, free androgen index and MFSQ domains obtained during the final treatment cycle were performed by use of Spearman’s rank correlation. In addition, correlation analyses were performed between Δ-scores in the sexual function variables and Δ-scores in testosterone variables. The SPSS 22.0 statistical package was used (IBM), and P values less than 0.05 were considered statistically significant.
Results
Study participants
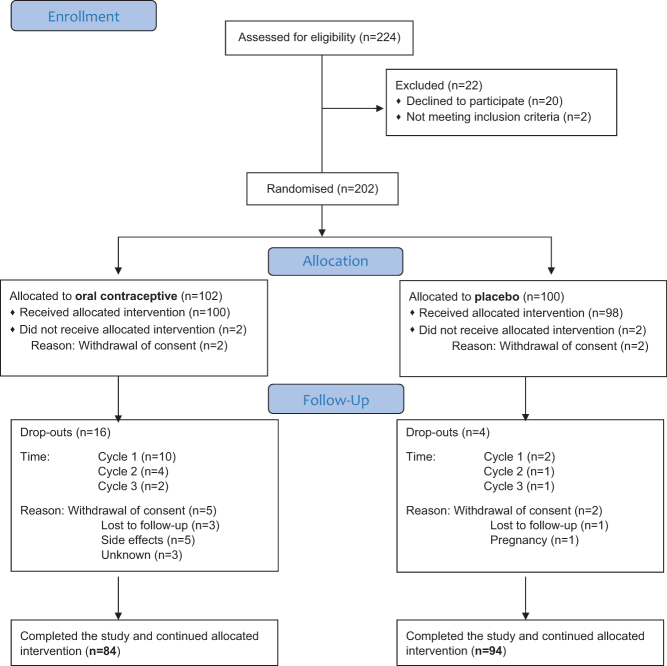
Two-hundred twenty-four women were screened for the study. Of these, 202 women were randomised; 102 to the COC and 100 women to placebo (Fig. 1). Eighteen women allocated to COC and six women allocated to placebo dropped out at various stages of the study, with an overall drop-out rate of 11.9%. Withdrawal of consent was the most common reason for drop-out, however, five women in the COC group discontinued due to side effects, and one woman in the placebo group became pregnant. Among the women who discontinued due to side effects, one did so because of a combination of sexual side effects and mood side effects, whereas two complained of mood side effects, one developed bleeding problems and one reported on worsening of migraine. In the end, 84 women in the COC group and 94 women in the placebo group continued with the allocated intervention and completed the trial. Compliance was very good; none of the women had more than 1 or 2 capsules remaining at the end of the study.
Figure 1.
Flowchart of the study population.
Eighty-one women randomised to COC and 90 women randomised to placebo answered the MFSQ both at the baseline and at the final assessment. Fifty-seven (67.9%) women randomised to COC and 59 (63.4%) women randomised to placebo correctly guessed their treatment upon completion of the study. Adverse events are presented in Supplementary Table 2. Thirteen COC users (12.7%) spontaneously reported sexual side effects, whereas the corresponding number among placebo users was three (3.0%), P = 0.01 (Supplementary Table 2).
No baseline differences in demographic and clinical variables or baseline MFSQ scores were noted between the two treatment groups (Tables 1 and 2).
Table 1.
Demographic and clinical variables in the study population (n = 202).
| Clinical variables | Combined oral contraceptive (n = 102) | Placebo (n = 100) | P |
|---|---|---|---|
| Age (years) | 23.8 ± 4.2 | 24.8 ± 4.2 | 0.097 |
| Married/partner, n (%) | 61 (59.8) | 49 (49.5) | 0.158 |
| University education, n (%) | 78 (76.5) | 75 (76.5) | 0.992 |
| BMI (kg/m2) | 22.3 ± 2.6 | 22.5 ± 2.5 | 0.532 |
| Smokers, n (%) | 7 (6.9) | 8 (8.2) | 0.727 |
| Previous hormonal contraceptive use, n (%) | 84 (82.4) | 82 (82.0) | 0.947 |
| Duration of previous hormonal contraceptive use (years) | 4.8 ± 3.5 | 4.8 ± 3.3 | 0.945 |
| Intercourse last four weeks | 75 (74.3) | 74 (77.9) | 0.551 |
| Dysmenorrhoea, n (%) | 3 (2.9) | 8 (8.0) | 0.113 |
| Endometriosis, n (%) | 1 (1.0) | 0 (0.0) | 1.00 |
| PCOS, n (%) | 2 (2.0) | 0 (0.0) | 0.498 |
| Any mood disorder, n (%) | 9 (8.8) | 5 (5.0) | 0.285 |
| Any anxiety disorder, n (%) | 7 (6.9) | 8 (8.0) | 0.758 |
| Current use of psychotropic drugs | 9 (8.8) | 7 (7.0) | 0.631 |
| Serotonin reuptake inhibitors | 8 (7.8) | 5 (5.0) | 0.410 |
| Other | 2 (2.0) | 2 (2.0) | 1.000 |
Results are presented as mean ± s.d. or n (%). Frequencies are reported in relation to available responses, missing cases evident in 2–4 cases depending on variable. P value according to chi-square test.
BMI, body mass index; PCOS, polycystic ovary syndrome.
Table 2.
Sexual function scores on the McCoy Female Sexuality Questionnaire at baseline.
| Maximum score | N | Combined oral contraceptive | N | Placebo | P | |
|---|---|---|---|---|---|---|
| Sexual interest | 28 | 101 | 19.0 (16.0–21.0) | 96 | 19.0 (16.0–23.0) | 0.704 |
| Satisfaction sexual activity | 14 | 100 | 10.0 (7.3–11.0) | 95 | 10.0 (7.0–11.0) | 0.989 |
| Vaginal lubricationa | 21 | 75 | 16.0 (13.0–18.0) | 74 | 16.0 (13.0–18.3) | 0.368 |
| Orgasma | 28 | 75 | 19.0 (14.0–22.0) | 74 | 19.0 (13.8–22.5) | 0.932 |
| Partner | 21 | 85 | 19.0 (16.0–21.0) | 76 | 19.0 (16.0–20.0) | 0.437 |
| Attractiveness | 14 | 101 | 9.0 (6.5–10.5) | 95 | 9.0 (7.0–11.0) | 0.297 |
| Frequency, intercourse/week | 96 | 1 (0.0–2.0) | 95 | 1 (0.25–2.50) | 0.435 | |
| Total score | 101 | 88.0 (54.5–100.5) | 96 | 90.5 (62.7–100.0) | 0.720 |
Data presented as median (IQR). Available responses in each item are presented. Statistical analyses by Mann–Whitney U test.
aItems ‘Vaginal lubrication’ and ‘Orgasm’ presented only in women who reported intercourse in the last 4 weeks.
Sexual function during treatment
Sexual function delta scores are displayed in Table 3. Women randomised to the COC reported a significant decrease in sexual interest at the completion of the trial compared with women randomised to placebo (P = 0.019). This finding remained following adjustment for self-rated change in depressive symptoms; B = −0.80 ± 0.30, Wald = 7.08, P = 0.008. However, the proportion of women with clinically relevant reduction in sexual desire scores did not differ between treatments (COC 18 (22.2%) vs placebo 16 (17.8%), P = 0.47).
Table 3.
Delta sexual function scores on the McCoy Female Sexuality Questionnaire during the final treatment cycle.
| n | Combined oral contraceptive | N | Placebo | P | |
|---|---|---|---|---|---|
| Sexual interest | 81 | −2.0 (−5.0 to 0.5) | 90 | −1.0 (−3.0 to 2.0) | 0.019 |
| Satisfaction sexual activity | 80 | 0.0 (−2.0 to 0.0) | 89 | 0.0 (−1.0 to 1.0) | 0.060 |
| Vaginal lubricationa | 57 | −2.0 (−3.0 to 1.0) | 58 | 0.0 (−1.0 to 2.0) | 0.005 |
| Orgasma | 57 | −1.0 (−4.0 to 1.0) | 58 | 0.0 (−2.3 to 2.0) | 0.147 |
| Partner | 67 | 0.0 (−2.0 to 0.0) | 66 | 0.0 (−2.0 to 1.3) | 0.398 |
| Attractiveness | 80 | 0.0 (−2.0 to 1.0) | 90 | 0.0 (−2.0 to 1.0) | 0.488 |
| Frequency, intercourse/week | 71 | 0.0 (−1.0 to 0.25) | 86 | 0.0 (−0.75 to 0.25) | 0.800 |
| Total score | 81 | −5 (−17.0 to 2.1) | 90 | −2.0 (−13.0 to 10.0) | 0.086 |
Data presented as median (IQR). Statistical analyses by Mann–Whitney U test.
aItems ‘Vaginal lubrication’ and ‘Orgasm’ presented only in women who reported intercourse during baseline and treatment.
Among the women who had engaged in sexual intercourse both at baseline and during treatment, COC users reported a decrease in vaginal lubrication compared with women in the placebo group (P = 0.005) (Table 3). This finding did not remain following adjustment for change in self-rated depressive symptoms, B = −0.22 ± 0.32, Wald = 0.48, P = 0.487. Further, the proportion of women with clinically relevant reduction in lubrication scores did not differ between treatments (COC 11 (14.7%) vs placebo 12 (15.6%), P = 0.87).
No differences in total score, satisfaction with sexual activity, frequency of sexual activity, satisfaction with partner, orgasm or attractiveness were noted between the treatment groups (Table 3).
Testosterone
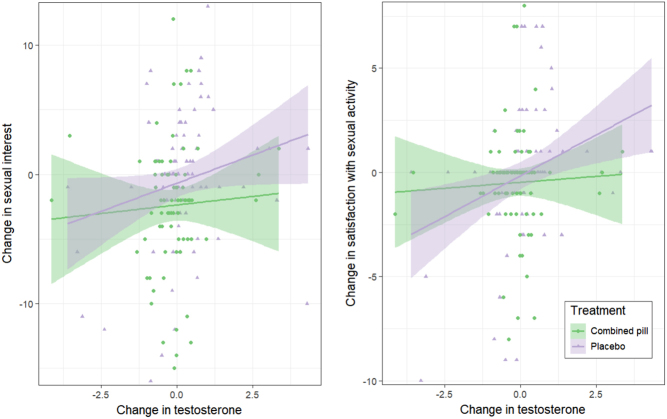
On average, serum testosterone levels decreased, SHBG levels increased and free androgen index decreased during treatment with the COC (Table 4). However, for most women testosterone levels remained stable throughout the trial (Fig. 2). In addition, levels of testosterone in hair were unaltered after three cycles of COC or placebo (Table 4). No correlation between testosterone variables and sexual function was noted at the end of the study, data not shown. However, when including both women on active treatment and placebo, we found weak positive correlations between change in testosterone levels and free androgen levels and change in sexual desire and satisfaction with sexual activities (Table 5). These associations were driven by a sub-group of women with greater change in testosterone levels, predominantly in the placebo group (Fig. 2) (COC users; testosterone and sexual interest rho = 0.07, P = 0.564, satisfaction with sexual activities rho = 0.01, P = 0.915, placebo users; testosterone and sexual interest rho = 0.27, P = 0.012, satisfaction with sexual activities rho = 0.36, P = 0.001).
Table 4.
Testosterone, sex hormone-binding globulin, free androgen index and hair testosterone at baseline and during treatment with a combined oral contraceptive or placebo.
| Baseline | P | Treatment | Pa | |||
|---|---|---|---|---|---|---|
| COC (n = 97) | Placebo (n = 99) | COC (n = 76) | Placebo (n = 89) | |||
| Testosterone (nmol/L) | 1.54 (1.22–2.10) | 1.53 (1.25–2.00) | NS | 1.37 (1.04–1.77) | 1.67 (1.27–2.15) | 0.002 |
| SHBG | 72 (47–104) | 75 (54–99) | NS | 103 (74–129) | 62 (44–81) | 0.000 |
| Free androgen index | 2.40 (1.39–4.04) | 2.11 (1.39– 3.28) | NS | 1.44 (0.95–2.08) | 2.87 (1.80–4.57) | 0.000 |
| (n = 90) | (n = 87) | (n = 75) | (n = 78) | |||
| Hair testosterone (pg/mg) | 1.33 (1.02–1.92) | 1.28 (0.95–1.69) | NS | 1.26 (1.02–1.74) | 1.41 (1.00–1.81) | NS |
Data displayed as median (IQR).
aSignificant P values have been calculated on delta values treatment – baseline, Mann–Whitney U test.
COC, combined oral contraceptive; NS, not significant; SHBG, sex hormone-binding globulin.
Figure 2.
Spearman rank correlations between change in total testosterone and change in sexual function scores in each treatment group. The associations were mainly driven by the placebo users (testosterone and sexual interest rho = 0.27, P = 0.012, satisfaction with sexual activities rho = 0.36, P = 0.001), whereas no association between testosterone and sexual function scores were noted in the COC users (testosterone and sexual interest rho = 0.07, P = 0.564, satisfaction with sexual activities rho = 0.01, P = 0.915).
Table 5.
Spearman rank correlation coefficients between serum testosterone, sex hormone-binding globulin, free androgen index, and hair testosterone and sexual function scores at the end of the study.
| Testosterone (rho) | SHBG (rho) | Free androgen index (rho) | Hair testosterone (rho) | |
|---|---|---|---|---|
| Sexual interest | 0.229b | −0.148 | 0.231b | −0.129 |
| Satisfaction sexual activity | 0.252b | −0.119 | 0.272b | 0.023 |
| Vaginal lubrication | 0.154 | −0.83 | 0.146 | 0.147 |
| Orgasm | 0.173 | −0.33 | 0.026 | 0.198 |
| Partner | 0.161 | 0.092 | 0.037 | 0.135 |
| Attractiveness | 0.145 | −0.106 | 0.203a | −0.032 |
| Frequency, intercourse/week | 0.192a | −0.007 | 0.128 | 0.060 |
aP < 0.05, Spearman rank correlation; bP < 0.01, Spearman rank correlation.
Discussion
Main findings
Our study demonstrates that use of an oral contraceptive containing 1.5 mg of oestradiol and 2.5 mg of nomegestrolacetate is associated with a small decrease in sexual interest, even following adjustment for change in depressive symptoms throughout the study course. However, no difference in the proportion of women who reported clinically relevant deterioration in sexual interest was noted between treatments. In a sub-group of women, the change in testosterone levels may also influence sexual desire.
Strengths and limitations
Our study is the first randomised placebo-controlled trial on sexual function using an oestradiol-containing COC. Oestradiol-based COCs have less impact on SHBG levels than EE-containing pills, consequently less impact on bioavailable testosterone levels and have been proven beneficial in women with COC-induced sexual dysfunction (17, 18). This is also the first study evaluating the effects of COCs on long-term bioavailable testosterone levels by measurement of hair testosterone. Strengths of the study include the fact that we used no study-specific exclusion criteria, which should increase the generalizability of the results. For instance, studies that have only included women not using HCs for at least 6 months prior to inclusion, are at increased risk of selectively recruiting women with previous negative experiences of hormonal contraception (3). The study design, with six geographically dispersed centres including patients, should also increase generalizability. Further, the drop-out rate was low and compliance with treatment was high.
However, the study is not without limitations. First, blinding might have been jeopardised since bleeding irregularities are common during COC use. Indeed, about two thirds of the women in both the COC and in the placebo groups correctly guessed which treatment they had received. Secondly, the duration of study treatment was only three treatment cycles, and long-term effects on sexual function were not assessed. However, more prolonged studies may lead to selection bias and a ‘healthy survivor effect’, which would limit the interpretations that can be drawn. In line with this, it may be speculated that the higher discontinuation rate among the women allocated to active treatment have diminished the differences regarding sexual function found between the groups. In support of this, spontaneously reported sexual side effects were more common in the active treatment group, and at least one of the women who dropped out did so because of sexual side effects. Finally, our study population was young with a median age of 24 years, and 54% reported being in a stable relationship upon inclusion. Given the fairly young cohort, it is possible that relationship status fluctuated during the study, in turn affecting responses to the MFSQ. Hopefully, however, randomisation made the proportion of changed relationship similar in the two groups.
Interpretation
The relation between oral contraceptive use and sexual function has been extensively discussed, but causality has been difficult to prove. Results from the present study are in line with two recently published studies, one placebo-controlled (4) and one randomised without placebo (5). Together, these studies add to the evidence that COC may negatively affect sexual interest in women. However, the differences in median sexual interest change scores between treatments was small, and the proportion of women with clinically relevant impairment did not differ between treatments. Overall, these findings suggest that an oestradiol-based COC may impair sexual desire at the statistical level, but with unclear clinical relevance.
It has been suggested that COC-induced effects on sexual desire may be mediated by COC-induced depressed mood (3, 19) or change in quality of life (20, 21). A previous publication from the present study (13) reported that the COC was associated with increased reporting of anxiety, irritability and mood swings and suggested that the progestogen component could contribute to these effects. We found no evidence of a mediation effect by COC-induced depressed mood on sexual desire. Instead, the relationship between HC use and sexual desire was strengthened when change in self-rated depressive symptoms was adjusted for in the regression analysis.
Weak positive correlations between change in serum testosterone and free androgen index and sexual desire and satisfaction with sexual activity were noted, in line with some previous findings in the field (2). As most women had relatively stable levels of total and free testosterone throughout the trial, these associations seem driven by a sub-group of women with greater change in total and free testosterone, predominantly in the placebo group. Testosterone levels in hair remained unchanged throughout the trial. However, the incorporation and stability of steroid hormones in scalp hair may be affected by yet unknown variables, which warrant further methodological research on the clinical utility of hair testosterone analysis.
Conclusion
Our study suggests that a combined oral contraceptive with 1.5 mg oestradiol and 2.5 mg nomegestrolacetate is associated with a small, but not clinically relevant, decrease in sexual desire. Further studies are needed to explore which HCs would be most beneficial for women in terms of sexual function, preferably head-to-head comparisons between different preparations.
Supplementary Material
Declaration of interest
I Sundstrom-Poromaa serves occasionally on advisory boards or act as invited speaker at scientific meetings for MSD, Bayer Health Care, and Lundbeck A/S. K Gemzell-Danielsson serves on an ad hoc basis as invited speaker and on advisory boards for MSD/Merck, Bayer AG, Gedeon Richter, HRA-Pharma, Exelgyn, Natural Cycles, Mithra, Exeltis and Actavis. None of the other authors have any conflicts of interest.
Funding
The Swedish Research Council project K2013-99X-22269-01-3 and the Family Planning Foundation supported this study. The funder sources had no involvement in study design, collection, analysis and interpretation of data or writing and submitting the article. No support was obtained from any pharmaceutical company.
Author contribution statement
Authors I S P, K G D, M B, M H and E T were involved in the conception and design of the study. Authors C L, A M, J S, L M, H B, L M and I L participated in the acquisition of data. A M and C L did the data analyses, and authors K G D, M B, I S P, M H and E T contributed to the interpretation. A M and C L drafted the article, and all authors revised the manuscript critically for important intellectual content. All authors have approved the final version of the manuscript.
References
- 1.Bakker CB, Dightman CR. Side effects of oral contraceptives. Obstetrics and Gynecology 1966. 28 373–379. [PubMed] [Google Scholar]
- 2.Pastor Z, Holla K, Chmel R. The influence of combined oral contraceptives on female sexual desire: a systematic review. European Journal of Contraception and Reproductive Health Care 2013. 18 27–43. ( 10.3109/13625187.2012.728643) [DOI] [PubMed] [Google Scholar]
- 3.Graham CA, Sherwin BB. The relationship between mood and sexuality in women using an oral contraceptive as a treatment for premenstrual symptoms. Psychoneuroendocrinology 1993. 18 273–281. ( 10.1016/0306-4530(93)90024-F) [DOI] [PubMed] [Google Scholar]
- 4.Zethraeus N, Dreber A, Ranehill E, Blomberg L, Labrie F, von Schoultz B, Johannesson M, Hirschberg AL. Combined oral contraceptives and sexual function in women-a double-blind, randomized, placebo-controlled trial. Journal of Clinical Endocrinology and Metabolism 2016. 101 4046–4053. ( 10.1210/jc.2016-2032) [DOI] [PubMed] [Google Scholar]
- 5.Ciaplinskiene L, Zilaitiene B, Verkauskiene R, Zalinkevicius R, Bumbuliene Z, Vanagiene V, Bitzer J. The effect of a drospirenone-containing combined oral contraceptive on female sexual function: a prospective randomised study. European Journal of Contraception and Reproductive Health Care 2016. 21 395–400. ( 10.1080/13625187.2016.1217324) [DOI] [PubMed] [Google Scholar]
- 6.Goldstein I, Traish A, Kim N, Munarriz R. The role of sex steroid hormones in female sexual function and dysfunction. Clinics in Obstetrics and Gynaecology 2004. 47 471–484. ( 10.1097/00003081-200406000-00022) [DOI] [PubMed] [Google Scholar]
- 7.Graham CA, Ramos R, Bancroft J, Maglaya C, Farley TM. The effects of steroidal contraceptives on the well-being and sexuality of women: a double-blind, placebo-controlled, two-centre study of combined and progestogen-only methods. Contraception 1995. 52 363–369. ( 10.1016/0010-7824(95)00226-X) [DOI] [PubMed] [Google Scholar]
- 8.Johannesson U, Blomgren B, Hilliges M, Rylander E, Bohm-Starke N. The vulval vestibular mucosa-morphological effects of oral contraceptives and menstrual cycle. British Journal of Dermatology 2007. 157 487–493. ( 10.1111/j.1365-2133.2007.08066.x) [DOI] [PubMed] [Google Scholar]
- 9.Chan J, Sauve B, Tokmakejian S, Koren G, Van Uum S. Measurement of cortisol and testosterone in hair of obese and non-obese human subjects. Experimental and Clinical Endocrinology and Diabetes 2014. 122 356–362. ( 10.1055/s-0034-1374609) [DOI] [PubMed] [Google Scholar]
- 10.Slezak JK, Strom JO, Theodorsson E. Testosterone-like immunoreactivity in hair measured in minute sample amounts – a competitive radioimmunoassay with an adequate limit of detection. Scientific Reports 2017. 7 17636 ( 10.1038/s41598-017-17930-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao W, Stalder T, Foley P, Rauh M, Deng H, Kirschbaum C. Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. Journal of Chromatography: Part B, Analytical Technologies in the Biomedical and Life Sciences 2013. 928 1–8. ( 10.1016/j.jchromb.2013.03.008) [DOI] [PubMed] [Google Scholar]
- 12.Wester VL, van Rossum EF. Clinical applications of cortisol measurements in hair. European Journal of Endocrinology 2015. 173 M1–M10. ( 10.1530/EJE-15-0313) [DOI] [PubMed] [Google Scholar]
- 13.Lundin C, Danielsson KG, Bixo M, Moby L, Bengtsdotter H, Jawad I, Marions L, Brynhildsen J, Malmborg A, Lindh I, et al. Combined oral contraceptive use is associated with both improvement and worsening of mood in the different phases of the treatment cycle-A double-blind, placebo-controlled randomized trial. Psychoneuroendocrinology 2017. 76 135–143. ( 10.1016/j.psyneuen.2016.11.033) [DOI] [PubMed] [Google Scholar]
- 14.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 1998. 59 (Supplement 20) 22–33; quiz 34–57. [PubMed] [Google Scholar]
- 15.McCoy NL. The McCoy female sexuality questionnaire. Quality of Life Research 2000. 9 739–745. ( 10.1023/A:1008925906947) [DOI] [Google Scholar]
- 16.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979. 134 382–389. ( 10.1192/bjp.134.4.382) [DOI] [PubMed] [Google Scholar]
- 17.Caruso S, Cianci S, Cariola M, Fava V, Di Pasqua S, Cianci A. Improvement of low sexual desire due to antiandrogenic combined oral contraceptives after switching to an oral contraceptive containing 17beta-estradiol. Journal of Women’s Health 2017. 26 728–734. ( 10.1089/jwh.2016.5801) [DOI] [PubMed] [Google Scholar]
- 18.Davis SR, Bitzer J, Giraldi A, Palacios S, Parke S, Serrani M, Mellinger U, Nappi RE. Change to either a nonandrogenic or androgenic progestin-containing oral contraceptive preparation is associated with improved sexual function in women with oral contraceptive-associated sexual dysfunction. Journal of Sexual Medicine 2013. 10 3069–3079. ( 10.1111/jsm.12310) [DOI] [PubMed] [Google Scholar]
- 19.Warner P, Bancroft J. Mood, sexuality, oral contraceptives and the menstrual cycle. Journal of Psychosomatic Research 1988. 32 417–427. ( 10.1016/0022-3999(88)90025-6) [DOI] [PubMed] [Google Scholar]
- 20.Caruso S, Cianci S, Vitale SG, Fava V, Cutello S, Cianci A. Sexual function and quality of life of women adopting the levonorgestrel-releasing intrauterine system (LNG-IUS 13.5 mg) after abortion for unintended pregnancy. European Journal of Contraception and Reproductive Health Care 2018. 23 24–31. ( 10.1080/13625187.2018.1433824) [DOI] [PubMed] [Google Scholar]
- 21.Caruso S, Cianci S, Malandrino C, Cicero C, Lo Presti L, Cianci A. Quality of sexual life of women using the contraceptive vaginal ring in extended cycles: preliminary report. European Journal of Contraception and Reproductive Health Care 2014. 19 307–314. ( 10.3109/13625187.2014.914488) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a