Abstract
Wnt proteins, a group of secreted glycoproteins, mainly combine with receptors Frizzled (FZD) and/or low‐density‐lipoprotein receptor‐related proteins 5/6 (LRP5/6), initiating β‐catenin‐dependent and ‐independent signaling pathways. These pathways, which can be regulated by some secreted antagonists such as secreted Frizzled‐related proteins (SFRP) and dickkopf‐related protein (DKK), play a critical role in embryo development and adult homeostasis. Overactivation of Wnt signaling has been implicated in some human diseases including cancer. Wnt transgenic mice provide convincing evidence that Wnt signaling is involved in breast cancer initiation and progression, which is further strengthened by observations on human clinical breast cancer patients and studies on in vitro cultured human breast cancer cells. This review focuses on the roles of Wnt ligands, receptors and antagonists in breast cancer development instead of molecules or signaling transactivating β‐catenin independent on Wnt upstream components.
Keywords: β‐catenin, DKK, FZD, SFRP, Wnt
Abbreviations
- CAF
cancer‐associated fibroblasts
- CRD
cysteine‐rich domain
- CSC
cancer stem cells
- DKK
dickkopf‐related protein
- EMT
epithelial‐mesenchymal transition
- ER
estrogen receptor
- FZD
Frizzled
- GSK3β
glycogen synthase kinase 3 beta
- HER2
human epidermal growth factor receptor 2
- LRP5/6
low‐density lipoprotein receptor‐related proteins 5/6
- MaSC
mammary stem cells
- MIC
metastasis‐initiating cells
- MMTV
mouse mammary tumor virus
- PCP
planar cell polarity
- PR
progesterone receptor
- Procr
protein C receptor
- Rspo
R‐spondin
- SFRP
secreted Frizzled‐related proteins
- α‐SMA
α‐smooth muscle actin
- TCF/LEF
T‐cell factor/lymphoid enhancer factor
- TGF‐β
transforming growth factor beta
- TNBC
triple‐negative breast cancer
- WIF‐1
Wnt inhibitory factor 1
1. INTRODUCTION
Since the discovery of the first Wnt gene, Wnt1 (initially named int‐1),1 19 human Wnt genes have been identified. These genes encode a group of highly conserved secreted glycoproteins, which are critical to embryo development and adult tissue homeostasis. FZD proteins are seven transmembrane receptors for Wnt ligands. There are 10 members in the FZD family, sharing a conserved extracellular CRD to which Wnt ligands bind. LRP5 and LRP6 are coreceptors for Wnt ligands. Both FZD and LRP5/6 are required for the Wnt/β‐catenin pathway.2
Conventionally, Wnt pathways are defined as canonical and noncanonical according to whether β‐catenin signaling is affected. Some members in the Wnt family such as Wnt1 and Wnt3a bind to FZD and LRP5/6, leading to the dissociation of the β‐catenin degrading complex in which β‐catenin is phosphorylated by GSK3β. Consequently, β‐catenin in the cytoplasm escapes from phosphorylation and subsequent degradation and translocates to the nucleus, where it combines with TCF/LEF, thereby promoting transcription of some target genes. Noncanonical Wnt pathways are independent of β‐catenin, and are usually initiated by Wnt5a and Wnt11. Until now, some secreted proteins have been found to antagonize Wnt signaling, including SFRP, DKK and WIF‐1.
2. TRANSGENIC MICE PROVIDE EVIDENCE FOR WNT‐DRIVEN BREAST CARCINOGENESIS
Mouse mammary tumor virus‐Wnt1 transgenic mice have provided solid evidence that Wnt signaling can initiate breast cancer. These mice were established by the insertion of MMTV‐LTR upstream of the gene Wnt1 in the opposite transcriptional orientation.3 MMTV‐Wnt1 mice show apparent ductal hyperplasia, and some of them can develop breast cancer as early as 6 months of age; histological, MMTV‐Wnt1 tumors show heterogeneous containing myoepithelial (basal‐like) cells and luminal epithelial cells.4 MMTV‐Wnt10b mice and MMTV‐LRP6 mice have a similar phenotype to MMTV‐Wnt1 mice.5, 6 Tumors from these mice show activation of the β‐catenin pathway (Figure 1). Consistently, active β‐catenin transgenic (MMTV‐βcatΔN) mice and MMTV‐c‐Myc mice also develop mammary hyperplasia and adenocarcinoma.4, 7
Figure 1.
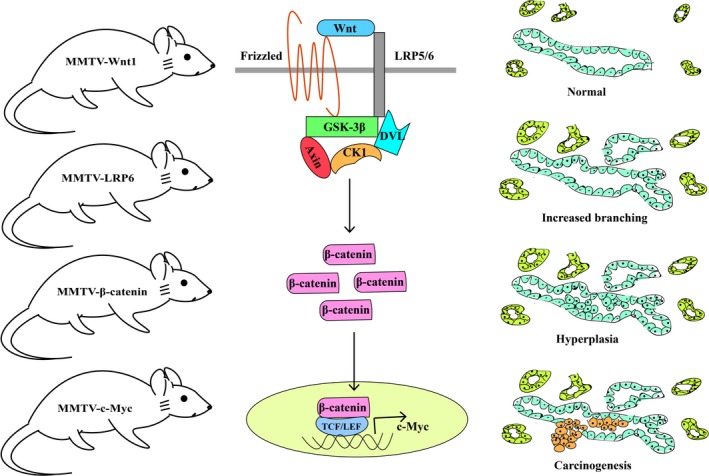
Mouse breast carcinogenesis induced by the activation of the canonical Wnt pathway. CK1, casein kinase 1; DVL, Dishevelled; GSK3β, glycogen synthase kinase 3 beta; LRP5/6, low‐density lipoprotein receptor‐related proteins 5/6; MMTV, mouse mammary tumor virus; TCF/LEF, T‐cell factor/lymphoid enhancer factor
3. WNT LIGANDS, RECEPTORS AND ANTAGONISTS ARE ABERRANTLY EXPRESSED IN HUMAN BREAST CANCERS
Although Wnt1 transgenic mice prove the capacity of the Wnt/β‐catenin pathway to initiate breast cancer, Wnt1 protein was hardly found overexpressed in human breast cancers.8 In contrast, Wnt10b is highly expressed in TNBC; Wnt10b activates the canonical β‐catenin pathway and contributes to increased cell proliferation and renewal.9 Wnt7b is expressed in several breast cancer cells and is overexpressed in approximately 10% of breast cancer patients.10 Moreover, Wnt receptors LRP6 and FZD7 are also overexpressed. LRP6 knockdown suppresses breast cancer cell growth, accompanied by a reduction in β‐catenin signaling activity.11 Similarly, FZD7 downregulation suppresses tumor formation in vivo as a result of inhibition of β‐catenin signaling.12 SFRP1 is expressed in normal breast epithelial cells but is frequently lost in invasive breast cancer tissues.13 Gene promoter methylation is responsible for SFRP1 expression loss and is correlated with unfavorable prognosis.14 Other SFRP such as SFRP2 and SFRP5 as well as DKK and WIF‐1 are also downregulated in breast cancer as a result of gene methylation.15, 16
4. WNT SIGNALING ENRICHES PROGENITOR CELLS/CANCER STEM CELLS IN BREAST CANCERS
Tumors from MMTV‐Wnt1 mice were shown containing mammary progenitor cells and CSC: also called tumor‐initiating cells, TIC), which can further differentiate into myoepithelial cells and luminal epithelial cells.4, 17, 18, 19 FZD7 knockdown in Wnt1 tumor cells reduces CSC subpopulation and tumor‐initiating capacity.20 Moreover, LRP5 deficiency delays Wnt1‐induced tumorigenesis accompanied by reduced progenitor cell accumulation.21 In contrast, a reduction in DKK1 or DKK3 promotes self‐renewal of progenitor cells/CSC by activating the β‐catenin pathway.22, 23
In human breast cancer, CD44+/highCD24−/low cells show stem‐like and high tumorigenicity.24 CD44+/highCD24−/low cells are enriched in basal‐like tumors25, 26 and show the EMT phenotype.27 Both canonical and noncanonical Wnt signalings are required for maintaining CD44+/highCD24−/low‐like cell EMT and stem phenotype.28 Moreover, DKK1 overexpression in breast cancer cells reduces CD44+/highCD24−/low subpopulation and inhibits tumorigenicity.29
Lgr5 has been identified as a stem cell marker in a series of organs.30 Subsequent studies further showed that Lgr5 is involved in the maintenance of stem cells. Binding of Lgr5 to Rspo sequesters E3 ubiquitin ligases RNF43 and ZNRF3 which ubiquitinate Wnt receptors FZD for degradation.31 Therefore, in the presence of Rspo and Wnt ligands, Lgr5 can potentiate Wnt signaling.32, 33 Actually, both Rspo and Wnt ligands are required for Lgr5+ stem cell renewal and to prevent them from differentiating.34 In this process, the Wnt/β‐catenin pathway is responsible for maintaining Lgr5 expression; Rspo/Lgr5 interaction is involved in stem cell expansion.34, 35, 36
In mice mammary gland, Lgr5+ cells are chiefly within the Lin−CD24+CD49fhigh subpopulation; they can differentiate into both basal and luminal mammary epithelial cells, and regenerate functional mammary glands.37, 38 In human breast cancers, both Lgr5 and Rspo are overexpressed with activation of the Wnt/β‐catenin pathway, which contributes to increased tumor growth, metastasis and stemness.39, 40 Tenascin C (TNC) is an extracellular matrix protein abundantly expressed by mammary stem cells.41 Produced by breast cancer cells in which ~90% is CD44+CD24−, TNC maintains the expression of Lgr5 and the response of Lgr5 to Wnt ligands, and is associated with aggressive lung metastasis.42 These data demonstrate a critical role of the Rspo/Lgr5/Wnt feedback loop in maintaining CD44+CD24−Lgr5+ cells and promoting breast cancer metastasis (Figure 2).
Figure 2.
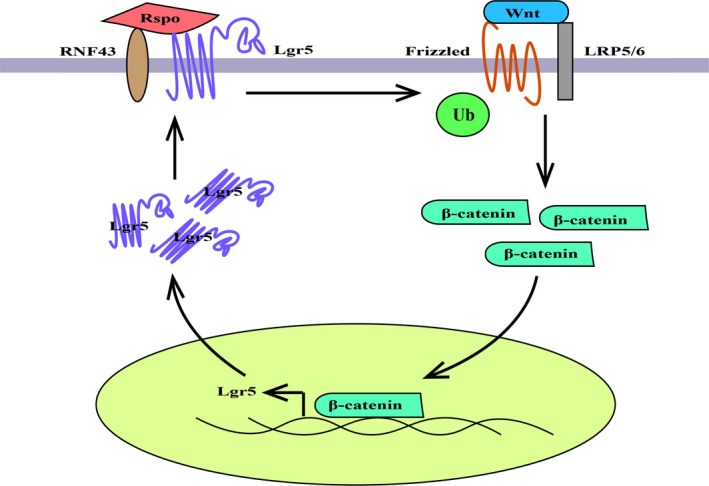
Positive feedback loop between the Rspo/Lgr5 pathway and the Wnt/β‐catenin pathway. LRP5/6, low‐density lipoprotein receptor‐related proteins 5/6; Rspo, R‐spondin
Protein C receptor is a single‐pass transmembrane protein and is expressed in hematopoietic, neuronal and epithelial progenitor populations.43 As a Wnt3a‐target protein, it is also expressed in mouse Lin−CD24+CD29hi mammary stem cells. Procr+CD24+CD29hi cells are multipotent, contribute to both basal and luminal cell lineages, and are important for mammary gland development.44 In human breast cancer, Procr is a CD44+ cell‐specific gene; Procr+ cells are enriched for genes involved in cell motility, chemotaxis, and angiogenesis.43, 45 Neuropilin‐1 (Nrp1), induced by Wnt/β‐catenin signaling, functions critically for the activity of Procr+ MaSC, and Nrp1 knockdown suppresses MMTV‐Wnt1 tumor growth.46 These studies indicate that Procr is a marker of mammary stem cells and cancer stem cells. However, unlike Lgr5, functional roles of Procr in these cells remain unknown.
5. WNT SIGNALING IS OVERACTIVATED IN BASAL‐LIKE BREAST CANCER/TNBC
Basal‐like breast cancers and TNBC are similar, although not identical, in molecular, clinical and pathological profiles, and they overlap by a high percentage. Although MMTV‐Wnt1 tumors express ER, they share a similar gene expression signature to human basal‐like tumors.47, 48 Crossing MMTV‐Wnt1 mice with ERα knockout mice showed that ERα absence delays, but cannot prevent, the appearance of tumors.49 MMTV‐Wnt10b tumors lack ERα, PR and HER2, and express the basal‐epithelial markers CK5 and CK6, therefore resembling human TNBC.9
Wnt10b expression is absent or low in ER+, PR+ and HER2+ human tumors, but high in TNBC, and is correlated with an unfavorable prognosis.9 Moreover, LRP6 and FZD7 are also predominantly overexpressed in basal‐like breast cancer or TNBC.11, 12, 50 The above observations suggest that the canonical Wnt10b/FZD7/LRP6 pathway triggers basal‐like breast cancer/TNBC initiation.
Some other Wnt ligands and receptors including Wnt7a, Wnt5a/5b, FZD6 and ROR1 were also found overexpressed in human basal‐like breast cancer/TNBC and correlated with poor clinical outcome. Wnt7a recruits and activates fibroblasts to facilitate tumor invasion and metastasis dependent on TGF‐β instead of on the β‐catenin pathway.51 Wnt5a/5b and ROR1 are involved in breast cancer invasiveness and metastasis independent of β‐catenin signaling.52, 53, 54 FZD6 promotes TNBC cell motility and distant metastasis through the fibronectin‐actin axis.55 These studies indicate that noncanonical Wnt signaling is involved in basal‐like breast cancer/TNBC progression.
6. WNT SIGNALING INDUCES BREAST CANCER CELL EPITHELIAL‐MESENCHYMAL TRANSITION
Epithelial‐mesenchymal transition plays critical roles in embryogenesis. This process is also involved in tumor metastasis, providing cancer cells increased motility. Additionally, EMT confers metastatic cancer cell stemness. Ectopic expression of EMT transcription factor such as twist or snail or exposure to TGF‐β1 induced EMT in non‐tumorigenic, immortalized human mammary epithelial cells, and these cells subsequently showed CD44highCD24low phenotype.56 In contrast, mouse Procr+ stem cells and CD49fhigh/CD24med stem cells, as well as human CD44highCD24low stem cells, all showed features of EMT.44, 56
Both canonical and noncanonical Wnt pathways are involved in EMT of breast stem cells or cancer cells. Wnt ligands (Wnt10b/3) or receptors (FZD7/LRP6) mediate activation of the β‐catenin pathway responsible for EMT induction.29, 57, 58, 59, 60 In contrast, Wnt5a/5b‐induced EMT is not dependent on β‐catenin, but is involved in FZD2/STAT3 signaling.61 Ectopic expression of DKK1/SFRP1 or recombinant DKK1/SFRP1 suppresses breast epithelial cell EMT, further proving the role of Wnt ligands and receptors in this process.28, 29 Rspo3 is expressed in mouse MaSC‐enriched basal (Lin−CD24+CD29high) epithelial populations, human basal ER−PR− cell lines, and human basal‐like tumors. Treatment of non‐tumor cells with recombinant Rspo3 induces EMT, whereas knockdown of Rspo3 in breast cancer cells induces mesenchymal‐epithelial transition (MET) with a reduction in cell migration capacity.62 Mechanically, Rspo3 controls EMT through modulating FZD ubiquitination by RNF43 and ZNRF3, thereby affecting the β‐catenin pathway triggered by Wnt ligands.
Transforming growth factor‐β pathways have crosstalk with the Wnt pathway in EMT induction (Figure 3). TGF‐β induces Wnt7a/7b through Smad2/3, which inversely enhances TGF‐β‐induced EMT of mammary epithelial cells.63 Moreover, TGF‐β can activate the β‐catenin pathway to induce EMT through other molecules such as P38 in a Wnt ligand‐nondependent method.64 Both TGF‐β and β‐catenin induce EMT involving a series of EMT transcription factors including Snail, Slug and Twist.65 A combination of SFRP1 with TGF‐β inhibitor exerts greater inhibitory action on mammary epithelial cell migration and mammosphere formation than either of them, further indicating that TGF‐β signaling synergizes with Wnt signaling in maintaining cell mesenchymal phenotype.28 Actually, TGF‐β is involved in MMTV‐Wnt1 tumors. These tumors show extensive nuclear phospho‐Smad2/3 staining, and TGF‐β abrogation significantly delays MMTV‐Wnt1 tumor development.66
Figure 3.
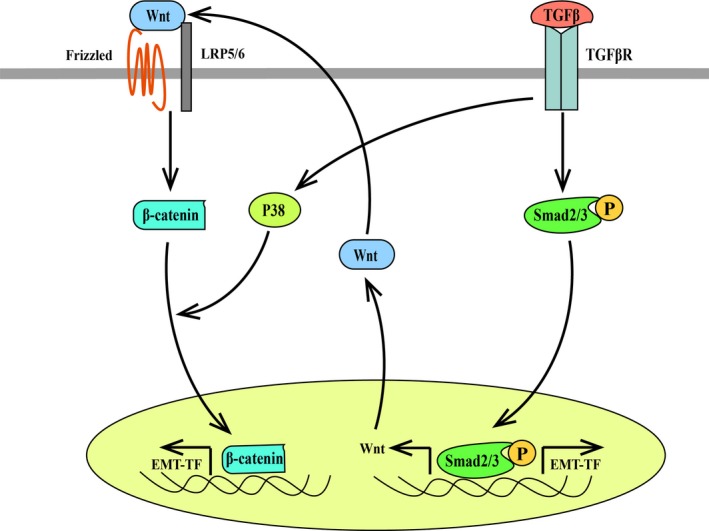
Crosstalk between the transforming growth factor‐beta (TGF‐β) pathway and the Wnt/β‐catenin pathway in the induction of breast cancer epithelial‐mesenchymal transition (EMT). LRP5/6, low‐density lipoprotein receptor‐related proteins 5/6. EMT‐TF, EMT‐transcription factor
7. WNT SIGNALING IS INVOLVED IN EPITHELIAL‐STROMAL CROSSTALK OF BREAST CANCER
It has been established that epithelial‐stromal crosstalk in the tumor microenvironment plays a crucial role in tumor development. In breast cancer stroma, fibroblasts are the major component. Fibroblasts in breast tumors of Wnt‐met mice show a myofibroblast/CAF phenotype characterized with expression of α‐SMA.67 Furthermore, Wnt ligand from breast cancer cells can activate fibroblasts into CAF in a TGF‐β signaling‐dependent way.51 CAF inversely secrete many molecules including Wnt ligands such as Wnt10b in exosomes to induce cancer cell EMT, metastasis and stemness.51, 57, 67 Interestingly, exosomes from fibroblasts can stimulate Wnt11 secretion by breast cancer cells, and then load Wnt11 to activate the Wnt/PCP pathway and drive cancer cell motility and metastasis.68 Together, these data indicate that Wnt signals in breast cancer environment are involved in epithelial‐stromal crosstalk and promote breast cancer progression (Figure 4).
Figure 4.
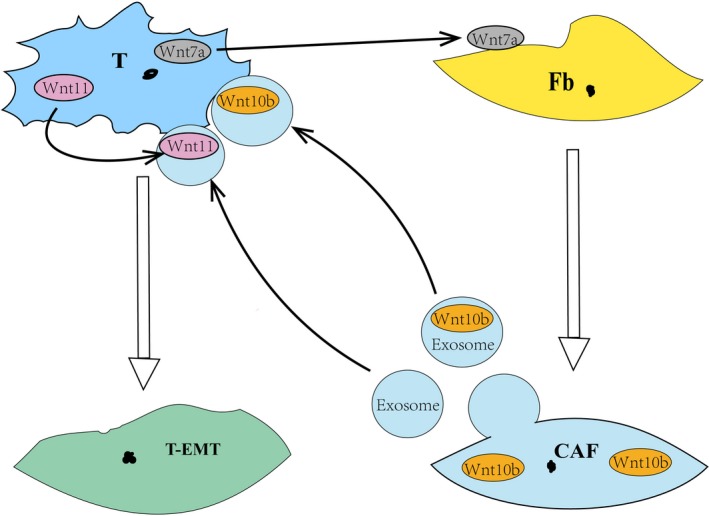
Crosstalk between breast cancer cells and mesenchymal fibroblasts in the breast cancer microenvironment. CAF, cancer‐associated fibroblasts; Fb, fibroblasts; T, tumor cells; T‐EMT, tumor cells with epithelial‐mesenchymal transition
Similarly, Rspo3 is not only secreted by mammary epithelial cells, but also by α‐SMA‐positive cells in breast cancer stroma.62 It is reasonable to think that, similar to autocrine Rspo3, paracrine Rspo3 may also be involved in the activation of the Wnt/β‐catenin pathway and in the induction of cancer cell EMT and stemness. It has been shown that myofibroblast‐derived Rspo3 is required for the expression of Axin2 and Lgr5 in gastric epithelial stem cells.69 Moreover, both Wnt ligands and Rspo3 from platelet‐derived growth factor receptor alpha (PdgfRα)‐positive myofibroblasts are required to support the intestinal stem‐cell niche.70
8. WNT SIGNALING PLAYS A ROLE IN BREAST CANCER METASTASIS‐INITIATING CELLS
It has now been accepted now that metastasis is an early event in cancer progression. Using HER2 transgenic mice, studies have shown that breast cancer cells can disseminate in tumor premalignant stage,71, 72 and EMT induced by both canonical and noncanonical Wnt signaling contributes to early dissemination.73 These early disseminated cells show stem‐like characteristics and have tumor‐initiating capability. They are quiescent, but they can release from dormancy leading to distant metastasis. Therefore, they are also defined as MIC which may be responsible for tumor metastasis even years after removal of the primary tumors. Notably, one recent study showed that during dormancy, DKK1 suppresses Wnt signaling in MIC which was defined as latent competent cancer (LCC); downregulation of Wnt signaling induces LCC into quiescence to avoid immune surveillance.74 Together, these findings raise a possibility that after early MIC dissemination, Wnt signaling activity may decline for subsequent MIC dormancy.
9. WNT LIGANDS/RECEPTORS MAY BE TARGETS FOR BREAST CANCER
Porcupine (PORCN), a membrane bound O‐acyltransferase, is required for Wnt secretion and activity. PORCN inhibitor C59 suppresses the growth of MMTV‐Wnt1 tumors, and these C59‐treated tumors show a decrease in β‐catenin, CyclinD1 and c‐Myc.75 Moreover, another PORCN inhibitor LGK974 similarly induces regression of MMTV‐Wnt1 tumors, accompanied by a reduction in LRP6 phosphorylation and AXIN2 expression.76 LRP6 antagonist Mesd/Mesd peptide or LRP6 antagonistic antibodies also inactivate the Wnt/β‐catenin pathway and suppress MMTV‐Wnt1 tumor growth.11 Similar effects were observed in MMTV‐Wnt1 tumors treated with a soluble Wnt Receptor Frizzled8 CRD‐hFc.77 Moreover, FZD7 antibody was shown to block the canonical Wnt pathway and suppress breast cancer cell growth in human tumor xenograft.78 These observations further confirm the roles of Wnt ligands and receptors in breast cancer development.
10. CONCLUSIONS
Studies from Wnt transgenic mice clearly indicate that Wnt signaling, especially the β‐catenin pathway, is capable of initiating breast carcinogenesis. Observations on clinical human patients show overactivated Wnt signaling in a section of breast cancers in part as a result of an upregulation of Wnt ligands and/or receptors, as well as a downregulation of Wnt antagonist. CSC are enriched in both mouse Wnt transgenic breast cancers and human Wnt overactivated breast cancers. Wnt‐driven breast cancers show basal‐like/triple‐negative and EMT phenotype, and canonical and noncanonical Wnt pathways both contribute to MIC generation and cancer early dissemination. Although not sufficient, evidence still suggests that Wnt signaling inactivation may help MIC into a dormant state to escape immune attack before overt metastasis occurs.
Targeting Wnt ligands/receptors has been shown to be effective on breast tumor growth in animal models and human xenografts, but its effect on breast cancer metastasis remains unclear. Wnt signaling block may suppress tumor cell EMT and early dissemination. However, a reduction in Wnt signaling activity may induce MIC dormancy. Moreover, recent studies have shown that DKK1 level is increased in patients with bone metastasis,79 and Wnt signaling inhibition by DKK1 promotes breast cancer bone metastasis.80 These findings cause us to question the application of Wnt inhibitors in breast cancer.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
ACKNOWLEDGMENT
This study was supported by a Grant from Science and Technology Agency of Liaoning Province (Number: 2017225028).
Yin P, Wang W, Zhang Z, Bai Y, Gao J, Zhao C. Wnt signaling in human and mouse breast cancer: Focusing on Wnt ligands, receptors and antagonists. Cancer Sci. 2018;109:3368–3375. 10.1111/cas.13771
REFERENCES
- 1. Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99‐109. [DOI] [PubMed] [Google Scholar]
- 2. He X, Semenov M, Tamai K, Zeng X. LDL receptor‐related proteins 5 and 6 in Wnt/beta‐catenin signaling: arrows point the way. Development. 2004;131:1663‐1677. [DOI] [PubMed] [Google Scholar]
- 3. Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int‐1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619‐625. [DOI] [PubMed] [Google Scholar]
- 4. Li Y, Welm B, Podsypanina K, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci USA. 2003;100:15853‐15858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lane TF, Leder P. Wnt‐10b directs hypermorphic development and transformation in mammary glands of male and female mice. Oncogene. 1997;15:2133‐2144. [DOI] [PubMed] [Google Scholar]
- 6. Zhang J, Li Y, Liu Q, Lu W, Bu G. Wnt signaling activation and mammary gland hyperplasia in MMTV‐LRP6 transgenic mice: implication for breast cancer tumorigenesis. Oncogene. 2010;29:539‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michaelson JS, Leder P. beta‐catenin is a downstream effector of Wnt‐mediated tumorigenesis in the mammary gland. Oncogene. 2001;20:5093‐5099. [DOI] [PubMed] [Google Scholar]
- 8. Meyers SL, O'Brien MT, Smith T, Dudley JP. Analysis of the int‐1, int‐2, c‐myc, and neu oncogenes in human breast carcinomas. Can Res. 1990;50:5911‐5918. [PubMed] [Google Scholar]
- 9. Wend P, Runke S, Wend K, et al. WNT10B/beta‐catenin signalling induces HMGA2 and proliferation in metastatic triple‐negative breast cancer. EMBO Mol Med. 2013;5:264‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huguet EL, McMahon JA, McMahon AP, Bicknell R, Harris AL. Differential expression of human Wnt genes 2, 3, 4, and 7B in human breast cell lines and normal and disease states of human breast tissue. Can Res. 1994;54:2615‐2621. [PubMed] [Google Scholar]
- 11. Liu CC, Prior J, Piwnica‐Worms D, Bu G. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc Natl Acad Sci USA. 2010;107:5136‐5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang L, Wu X, Wang Y, et al. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene. 2011;30:4437‐4446. [DOI] [PubMed] [Google Scholar]
- 13. Ugolini F, Charafe‐Jauffret E, Bardou VJ, et al. WNT pathway and mammary carcinogenesis: loss of expression of candidate tumor suppressor gene SFRP1 in most invasive carcinomas except of the medullary type. Oncogene. 2001;20:5810‐5817. [DOI] [PubMed] [Google Scholar]
- 14. Veeck J, Niederacher D, An H, et al. Aberrant methylation of the Wnt antagonist SFRP1 in breast cancer is associated with unfavourable prognosis. Oncogene. 2006;25:3479‐3488. [DOI] [PubMed] [Google Scholar]
- 15. Suzuki H, Toyota M, Carraway H, et al. Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br J Cancer. 2008;98:1147‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ai L, Tao Q, Zhong S, et al. Inactivation of Wnt inhibitory factor‐1 (WIF1) expression by epigenetic silencing is a common event in breast cancer. Carcinogenesis. 2006;27:1341‐1348. [DOI] [PubMed] [Google Scholar]
- 17. Vaillant F, Asselin‐Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Can Res. 2008;68:7711‐7717. [DOI] [PubMed] [Google Scholar]
- 18. Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84‐88. [DOI] [PubMed] [Google Scholar]
- 19. Cho RW, Wang X, Diehn M, et al. Isolation and molecular characterization of cancer stem cells in MMTV‐Wnt‐1 murine breast tumors. Stem Cells. 2008;26:364‐371. [DOI] [PubMed] [Google Scholar]
- 20. Chakrabarti R, Wei Y, Hwang J, et al. DeltaNp63 promotes stem cell activity in mammary gland development and basal‐like breast cancer by enhancing Fzd7 expression and Wnt signalling. Nat Cell Biol. 2014;16(1004–15):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindvall C, Evans NC, Zylstra CR, Li Y, Alexander CM, Williams BO. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1‐induced tumorigenesis. J Biol Chem. 2006;281:35081‐35087. [DOI] [PubMed] [Google Scholar]
- 22. Lv C, Li F, Li X, et al. MiR‐31 promotes mammary stem cell expansion and breast tumorigenesis by suppressing Wnt signaling antagonists. Nat Commun. 2017;8:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang XY, Yin Y, Yuan H, Sakamaki T, Okano H, Glazer RI. Musashi1 modulates mammary progenitor cell expansion through proliferin‐mediated activation of the Wnt and Notch pathways. Mol Cell Biol. 2008;28:3589‐3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Al‐Hajj M, Wicha MS, Benito‐Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983‐3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ricardo S, Vieira AF, Gerhard R, et al. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J Clin Pathol. 2011;64:937‐946. [DOI] [PubMed] [Google Scholar]
- 26. Honeth G, Bendahl PO, Ringner M, et al. The CD44+/CD24‐ phenotype is enriched in basal‐like breast tumors. Breast Cancer Res. 2008;10:R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu S, Cong Y, Wang D, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports. 2014;2:78‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scheel C, Eaton EN, Li SH, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DiMeo TA, Anderson K, Phadke P, et al. A novel lung metastasis signature links Wnt signaling with cancer cell self‐renewal and epithelial‐mesenchymal transition in basal‐like breast cancer. Can Res. 2009;69:5364‐5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leung C, Tan SH, Barker N. Recent Advances in Lgr5(+) Stem Cell Research. Trends Cell Biol. 2018;28:380‐391. [DOI] [PubMed] [Google Scholar]
- 31. Koo BK, Spit M, Jordens I, et al. Tumour suppressor RNF43 is a stem‐cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665‐669. [DOI] [PubMed] [Google Scholar]
- 32. Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R‐spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta‐catenin signaling. Proc Natl Acad Sci USA. 2011;108:11452‐11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Glinka A, Dolde C, Kirsch N, et al. LGR4 and LGR5 are R‐spondin receptors mediating Wnt/beta‐catenin and Wnt/PCP signalling. EMBO Rep. 2011;12:1055‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan KS, Janda CY, Chang J, et al. Non‐equivalence of Wnt and R‐spondin ligands during Lgr5(+) intestinal stem‐cell self‐renewal. Nature. 2017;545:238‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamamoto Y, Sakamoto M, Fujii G, et al. Overexpression of orphan G‐protein‐coupled receptor, Gpr49, in human hepatocellular carcinomas with beta‐catenin mutations. Hepatology. 2003;37:528‐533. [DOI] [PubMed] [Google Scholar]
- 36. Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003‐1007. [DOI] [PubMed] [Google Scholar]
- 37. Plaks V, Brenot A, Lawson DA, et al. Lgr5‐expressing cells are sufficient and necessary for postnatal mammary gland organogenesis. Cell Rep. 2013;3:70‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Visser KE, Ciampricotti M, Michalak EM, et al. Developmental stage‐specific contribution of LGR5(+) cells to basal and luminal epithelial lineages in the postnatal mammary gland. J Pathol. 2012;228:300‐309. [DOI] [PubMed] [Google Scholar]
- 39. Yang L, Tang H, Kong Y, et al. LGR5 promotes breast cancer progression and maintains stem‐like cells through activation of Wnt/beta‐catenin signaling. Stem Cells. 2015;33:2913‐2924. [DOI] [PubMed] [Google Scholar]
- 40. Coussy F, Lallemand F, Vacher S, et al. Clinical value of R‐spondins in triple‐negative and metaplastic breast cancers. Br J Cancer. 2017;116:1595‐1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253‐1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oskarsson T, Acharyya S, Zhang XH, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17:867‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ruf W, Schaffner F. Role of the protein C receptor in cancer progression. Thromb Res. 2014;133(suppl 2):S85‐S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang D, Cai C, Dong X, et al. Identification of multipotent mammary stem cells by protein C receptor expression. Nature. 2015;517:81‐84. [DOI] [PubMed] [Google Scholar]
- 45. Shipitsin M, Campbell LL, Argani P, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259‐273. [DOI] [PubMed] [Google Scholar]
- 46. Liu W, Wu T, Dong X, Zeng YA. Neuropilin‐1 is upregulated by Wnt/beta‐catenin signaling and is important for mammary stem cells. Sci Rep. 2017;7:10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Herschkowitz JI, Simin K, Weigman VJ, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lim E, Wu D, Pal B, et al. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res. 2010;12:R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bocchinfuso WP, Hively WP, Couse JF, Varmus HE, Korach KS. A mouse mammary tumor virus‐Wnt‐1 transgene induces mammary gland hyperplasia and tumorigenesis in mice lacking estrogen receptor‐alpha. Can Res. 1999;59:1869‐1876. [PubMed] [Google Scholar]
- 50. Lindvall C, Zylstra CR, Evans N, et al. The Wnt co‐receptor Lrp6 is required for normal mouse mammary gland development. PLoS ONE. 2009;4:e5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Avgustinova A, Iravani M, Robertson D, et al. Tumour cell‐derived Wnt7a recruits and activates fibroblasts to promote tumour aggressiveness. Nat Commun. 2016;7:10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Han B, Zhou B, Qu Y, et al. FOXC1‐induced non‐canonical WNT5A‐MMP7 signaling regulates invasiveness in triple‐negative breast cancer. Oncogene. 2018;37:1399‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Klemm F, Bleckmann A, Siam L, et al. Beta‐catenin‐independent WNT signaling in basal‐like breast cancer and brain metastasis. Carcinogenesis. 2011;32:434‐442. [DOI] [PubMed] [Google Scholar]
- 54. Cui B, Zhang S, Chen L, et al. Targeting ROR1 inhibits epithelial‐mesenchymal transition and metastasis. Can Res. 2013;73:3649‐3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Corda G, Sala G, Lattanzio R, et al. Functional and prognostic significance of the genomic amplification of frizzled 6 (FZD6) in breast cancer. J Pathol. 2017;241:350‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mani SA, Guo W, Liao MJ, et al. The epithelial‐mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen Y, Zeng C, Zhan Y, Wang H, Jiang X, Li W. Aberrant low expression of p85alpha in stromal fibroblasts promotes breast cancer cell metastasis through exosome‐mediated paracrine Wnt10b. Oncogene. 2017;36:4692‐4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gong C, Qu S, Lv XB, et al. BRMS1L suppresses breast cancer metastasis by inducing epigenetic silence of FZD10. Nat Commun. 2014;5:5406. [DOI] [PubMed] [Google Scholar]
- 59. Ahmed RA, Alawin OA, Sylvester PW. gamma‐Tocotrienol reversal of epithelial‐to‐mesenchymal transition in human breast cancer cells is associated with inhibition of canonical Wnt signalling. Cell Prolif. 2016;49:460‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ma J, Lu W, Chen D, Xu B, Li Y. Role of Wnt co‐receptor LRP6 in triple negative breast cancer cell migration and invasion. J Cell Biochem. 2017;118:2968‐2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gujral TS, Chan M, Peshkin L, Sorger PK, Kirschner MW, MacBeath G. A noncanonical Frizzled2 pathway regulates epithelial‐mesenchymal transition and metastasis. Cell. 2014;159:844‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tocci JM, Felcher CM, Garcia Sola ME, et al. R‐spondin3 is associated with basal‐progenitor behavior in normal and tumor mammary cells. Can Res. 2018;78(16):4497‐4511. [DOI] [PubMed] [Google Scholar]
- 63. Sundqvist A, Morikawa M, Ren J, et al. JUNB governs a feed‐forward network of TGFbeta signaling that aggravates breast cancer invasion. Nucleic Acids Res. 2018;46:1180‐1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hedrick E, Safe S. Transforming growth factor beta/NR4A1‐inducible breast cancer cell migration and epithelial‐to‐mesenchymal transition is p38alpha (Mitogen‐Activated Protein Kinase 14) dependent. Mol Cell Biol. 2017;37(18): pii: e00306‐17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Micalizzi DS, Farabaugh SM, Ford HL. Epithelial‐mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Labbe E, Lock L, Letamendia A, et al. Transcriptional cooperation between the transforming growth factor‐beta and Wnt pathways in mammary and intestinal tumorigenesis. Can Res. 2007;67:75‐84. [DOI] [PubMed] [Google Scholar]
- 67. Valenti G, Quinn HM, Heynen G, et al. Cancer stem cells regulate cancer‐associated fibroblasts via activation of Hedgehog signaling in mammary gland tumors. Can Res. 2017;77:2134‐2147. [DOI] [PubMed] [Google Scholar]
- 68. Luga V, Zhang L, Viloria‐Petit AM, et al. Exosomes mediate stromal mobilization of autocrine Wnt‐PCP signaling in breast cancer cell migration. Cell. 2012;151:1542‐1556. [DOI] [PubMed] [Google Scholar]
- 69. Sigal M, Logan CY, Kapalczynska M, et al. Stromal R‐spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature. 2017;548:451‐455. [DOI] [PubMed] [Google Scholar]
- 70. Greicius G, Kabiri Z, Sigmundsson K, et al. PDGFRalpha(+) pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc Natl Acad Sci USA. 2018;115:E3173‐E3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Husemann Y, Geigl JB, Schubert F, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58‐68. [DOI] [PubMed] [Google Scholar]
- 72. Hosseini H, Obradovic MM, Hoffmann M, et al. Early dissemination seeds metastasis in breast cancer. Nature. 2016; [Epub ahead of print] 10.1038/nature20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Harper KL, Sosa MS, Entenberg D, et al. Mechanism of early dissemination and metastasis in Her2(+) mammary cancer. Nature. 2016; [Epub ahead of print] 10.1038/nature20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Malladi S, Macalinao DG, Jin X, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016;165:45‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Proffitt KD, Madan B, Ke Z, et al. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT‐driven mammary cancer. Can Res. 2013;73:502‐507. [DOI] [PubMed] [Google Scholar]
- 76. Liu J, Pan S, Hsieh MH, et al. Targeting Wnt‐driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci USA. 2013;110:20224‐20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu BY, Soloviev I, Huang X, et al. Mammary tumor regression elicited by Wnt signaling inhibitor requires IGFBP5. Can Res. 2012;72:1568‐1578. [DOI] [PubMed] [Google Scholar]
- 78. Gurney A, Axelrod F, Bond CJ, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA. 2012;109:11717‐11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Galliera E, Marazzi MG, Drago L, Banfi G, Luzzati A, Corsi Romanelli MM. Wnt signaling pathway inhibitors as promising diagnostic serum markers of osteolytic bone metastasis. J Biol Regul Homeost Agents. 2016;30:399‐408. [PubMed] [Google Scholar]
- 80. Zhuang X, Zhang H, Li X, et al. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat Cell Biol. 2017;19:1274‐1285. [DOI] [PubMed] [Google Scholar]


