Abstract
Cisplatin plus 5‐fluorouracil is regarded as standard neoadjuvant chemotherapy for esophageal squamous cell carcinoma (ESCC) in Japan, but the prognosis remains poor. We have previously described how definitive chemoradiotherapy with docetaxel, nedaplatin, and 5‐fluorouracil (DNF) led to a very high response rate and promising survival times. We therefore undertook a phase II trial to evaluate the feasibility and efficacy of neoadjuvant DNF. The study included patients with clinical stage Ib‐III ESCC. Chemotherapy consisted of i.v. docetaxel (30 mg/m2) and nedaplatin (50 mg/m2) on days 1 and 8, and a continuous infusion of 5‐fluorouracil (400 mg/m2/day) on days 1‐5 and 8‐12, every 3 weeks. After three courses of chemotherapy, esophagectomy was carried out. The primary end‐point was the completion rate of the protocol treatment. Twenty‐eight patients were enrolled (cStage Ib/II/III, 2/3/23) and all received at least two cycles of chemotherapy. Twenty‐five patients underwent surgery, all of whom achieved an R0 resection, leading to a completion rate of 89.3%. The overall response rate was 87.0%. A pathological complete response was confirmed in eight (32.0%) cases. Grade 3/4 adverse events included leukopenia (32.1%), neutropenia (39.3%), febrile neutropenia (10.7%), thrombocytopenia (10.7%), and diarrhea (14.3%), but were manageable. Treatment‐related deaths and major surgical complications did not occur. Estimated 2‐year progression‐free and overall survival rates were 70.4% and 77.2%, respectively. Thus, DNF therapy was well tolerated and deemed feasible, with a strong tumor response in a neoadjuvant setting for ESCC. This trial is registered with the University Hospital Medical Information Network (UMIN ID: 000014305).
Keywords: docetaxel, esophageal cancer, esophageal squamous cell carcinoma, nedaplatin, neoadjuvant chemotherapy
Abbreviations
- 5‐FU
5‐fluorouracil
- CDDP
cisplatin
- CDGP
nedaplatin (cis‐diamine‐glycolate platinum)
- CF
cisplatin plus 5‐fluorouracil
- CI
confidence interval
- CR
complete response
- CRT
chemoradiotherapy
- CT
computed tomography
- DCF
docetaxel, cisplatin, and 5‐fluorouracil
- DNF
docetaxel, nedaplatin, and 5‐fluorouracil
- DOC
docetaxel
- ESCC
esophageal squamous cell carcinoma
- G‐CSF
granulocyte colony‐stimulating factor
- JCEC
Japanese Classification of Esophageal Cancer
- JCOG
Japan Clinical Oncology Group
- NAC
neoadjuvant chemotherapy
- NACRT
neoadjuvant chemoradiotherapy
- OS
overall survival
- pCR
pathological complete response
- PFS
progression‐free survival
- PS
performance status
- RR
response rate
1. INTRODUCTION
Esophageal cancer is a highly malignant disease with a poor prognosis. Worldwide, in 2012, 456 000 cases of esophageal cancer were diagnosed, and 400 000 people died from this life‐threatening disease. With an overall mortality to incidence ratio as high as 0.88, esophageal cancer is the sixth leading cause of death due to a malignant tumor.1
Continual improvements have been made in the treatment of advanced esophageal cancer with surgery but these seem to have plateaued, with a 5‐year OS rate of 50%‐60%.2 This has led to urgently implementing a multimodality treatment approach, combining chemotherapy, radiotherapy, and surgery. In Europe and North America, NACRT is regarded as standard treatment in response to several studies describing significant improvements in OS by NACRT followed by surgery for esophageal cancer, compared with surgery alone.2, 3 However, in Japan, such evidence has largely been disregarded, and the use of perioperative chemotherapy has instead been investigated for the treatment of esophageal cancer.4, 5 This is partly because of differences in the histological type of tumor found, with squamous cell carcinoma more likely to be identified in East Asian countries, whereas adenocarcinoma accounts for about half of esophageal cancer cases in Europe and North America. A disparity in operative procedures, such as the extent of lymphadenectomy, has also led to largely longer PFS in Japan compared to that in other developed nations. Additionally, it is thought that NACRT might increase the likelihood of postoperative complications.6, 7
From the results of the JCOG9907 study, in which NAC was deemed superior in terms of OS in comparison to adjuvant chemotherapy, esophagectomy following two courses of CDDP plus 5‐FU (CF) is regarded as a standard strategy for resectable stage Ib‐III ESCC.5 The rationales for the use of NAC included increasing curability by tumor reduction, and eradicating minimal residual disease that could cause recurrence, among others; failure to achieve a tumor response might result in an inability to undertake curative surgery, or early recurrence after an operation. Therefore, a regimen with strong antitumor activity is needed for NAC. The response rate (RR) for neoadjuvant CF in the JCOG9907 study was limited to 38%; 2.5% of patients were considered to be unresectable because of disease progression. Also, in a subgroup analysis of the study, NAC therapy failed to benefit cohorts with advanced disease, such as stage III or T3, compared with those with stage II or T1/T2. These results suggest that a more effective regimen than CF is desirable, especially for patients with an advanced tumor.
To improve the current poor rate of survival for ESCC, triple‐combination regimens have been evaluated in recent years. Docetaxel (DOC), a taxane‐derived agent that exerts antitumor efficacy by the stabilization of microtubules, has shown effectiveness against ESCC.8 Combination chemotherapy with DCF, in which DOC is added to CF, is regarded as one of the standard regimens in gastric cancer, as well as in head and neck cancers, as judged from the results of phase III studies in which DCF was superior to CF in terms of RR and OS.9, 10, 11 Good results with RR of 34.5%‐83.3% have also been reported in several phase II trials of DCF for metastatic ESCC;12, 13, 14, 15, 16 this regimen was thought to be a good candidate for NAC, leading to clinical studies in a neoadjuvant setting. Two studies undertaken in Japan, mainly for ESCC, revealed the promising efficacy of NAC‐DCF with RR of 53.7%‐64.3%, an R0 resection rate of 92.0%‐98.0%, and a 2‐year survival rate of 78.0%‐88.0%. However, severe hematological toxicities were noted, with grade 3/4 and febrile neutropenia in 53.7%‐64.3% and 2.4%‐14.5% of patients, respectively. In addition, more than 40% of patients experienced a postoperative complication, suggesting the need for safer regimens.17, 18
We previously undertook a phase I/II study of definitive CRT using DOC and CDGP, which has almost the same antitumor activity and less gastrointestinal and renal toxicity compared to CDDP,19, 20 plus 5‐FU (DNF). We subsequently showed strong anticancer efficacy with a RR of 100% including a CR rate of 88.2%, and with a 2‐year PFS of 60.0% in patients with stage Ib‐III disease. In addition, grade 3/4 and febrile neutropenia were limited to 42.8% and 7.1%, respectively; cases with grade 3/4 nephrotoxicity were not observed with a treatment completion rate of 94.7%, indicating a good tolerability of DNF.21
Based on these results, we concluded DNF was suitable for NAC, and therefore undertook a phase II trial to evaluate the feasibility and efficacy of NAC with DNF in patients with clinical stage Ib‐III resectable ESCC.
2. MATERIALS AND METHODS
2.1. Patient eligibility
Eligibility criteria were as follows: histologically confirmed ESCC; clinical stage IB‐III disease according to the UICC TNM classification (7th edition);22 no prior chemotherapy or CRT; Eastern Cooperative Oncology Group performance status (ECOG PS) of 0‐1; aged 20‐80 years; adequate bone marrow function (leukocyte count >3000/μL, neutrophil count >1500/μL, hemoglobin >8.0 g/dL, and platelet count >100 000/μL); adequate liver function (serum bilirubin level <1.5 mg/dL, and serum aspartate aminotransferase and alanine aminotransferase levels less than twice the upper limit of normal); adequate renal function (creatinine clearance ≥50 mL/min); life expectancy of at least 3 months; no other serious medical conditions; medically fit for surgery; no pregnancy or breast‐feeding; and written informed consent.
2.2. Treatment and dose modifications
Treatment consisted of three cycles of DOC (30 mg/m2) and CDGP (50 mg/m2) on days 1 and 8, and a continuous i.v. infusion of 5‐FU, 400 mg/m2/day, on days 1‐5 and 8‐12, repeated every 3 weeks. Docetaxel was diluted in 250 mL normal saline and infused i.v. over a period of 2 hours. Nedaplatin diluted in 500 mL saline was then infused over 2 hours. After preparation in saline (250 mg/500 mL saline), 5‐FU was drip‐infused continuously over a period of 120 hours. Concomitant medications routinely given i.v. before DOC included 3 mg granisetron plus 6.6 mg dexamethasone. The prophylactic use of antibiotics was not allowed. Granulocyte colony‐stimulating factor was given when febrile or grade 4 neutropenia were observed until symptoms recovered to grade 2. If G‐CSF was given, prophylactic G‐CSF was allowed in subsequent cycles. If tolerability was found in the first course, which was undertaken in hospital, then chemotherapy in an outpatient setting was allowed for in subsequent cycles.
Chemotherapy was interrupted in the event of grade 3/4 hematological toxicity, or grade 3/4 non‐hematological toxicity (except for fatigue, nausea, and alopecia), and was resumed when an adverse event resolved to grade 1 or less with a 10% reduction in doses of all drugs. After the repeated occurrence of grade 3/4 toxicity in a subsequent cycle, a 20% reduction of all drugs from baseline doses was required. Any patient who required more than 4 weeks for recovery from adverse reactions was taken off the study. Treatment was also terminated when disease progression was observed, or patients refused to continue.
Esophagectomy with three‐field lymphadenectomy (cervical, thoracic, and abdominal) was carried out 4‐8 weeks after the completion of the last cycle of DNF. Surgery consisted of right thoracotomy followed by laparotomy and a neck incision with cervical anastomosis. Thoracoscopic surgery was allowed. Reconstruction was undertaken using a gastric tube or the jejunum through a posterior mediastinal route.
2.3. Assessment and follow‐up
Pretreatment assessment included a physical examination, laboratory tests, a chest X‐ray, an esophagogastroduodenoscopy, and a CT scan. Magnetic resonance imaging (MRI), 18F‐fluorodeoxyglucose positron emission tomography/CT and/or radionuclide bone imaging were also undertaken, if necessary. Bronchoscopy was carried out if bronchial invasion by the tumor was suspected. During treatment, a complete blood count, serum chemistry, and urinalysis were undertaken at least twice a week. Toxicity was assessed according to US NCI's Common Terminology Criteria for Adverse Events (version 4.0).23
An assessment of responses to chemotherapy for measurable lesions was undertaken with CT and/or MRI after each course of chemotherapy according to RECIST guidelines version 1.1.24 The response of the primary lesion was examined by endoscopy according to the guidelines of the JCEC, 10th edition.25 An independent review committee confirmed observed responses by radiological and endoscopic examinations. Each patient was assessed every 3 months after surgery for 1 year, then every 6 months for 4 years, and then annually until death.
After surgery, the pathological response of the primary lesion was also evaluated by JCEC according to the proportion of viable tumor cells after chemotherapy as follows: grade 0, no part of tumor affected; grade 1a, less than one‐third affected; grade 1b, between one‐third and two‐thirds affected; grade 2, between two‐thirds and entire tumor affected; and grade 3, no viable cancer cells (pCR).
Evaluations of residual tumor (R) were classified by the definition listed in the 7th UICC TNM staging system.22 Surgical complications were evaluated from the day of surgery until the time of discharge according to the Clavien‐Dindo classification and its Japanese extended version.26, 27
2.4. Study design and statistical analysis
The primary end‐point was to estimate the completion rate of the protocol treatment. Patients were deemed to have completed the protocol treatment when they received at least two courses of NAC and achieved a pathologically complete resection (R0). Secondary objectives included adverse events, RR including pathological response, PFS, OS, R0 resection rate, and operative morbidity. Given that the expected completion rate was 90% and the threshold incidence was 70%, based on previously reported data in this cohort,5, 17 with an alpha value of 0.1 (one‐sided) and a beta value of 0.1, the required minimum number of patients was 25. The projected sample size was 28 patients in total.
Progression‐free survival was defined as the time from the date of registration to that of disease progression or death resulting from any cause, and OS was measured from the date of registration to that of death resulting from any cause. Statistical analyses were undertaken using GraphPad Prism version 7.0d for Mac (GraphPad Software, La Jolla, CA, USA; http://www.graphpad.com). Both PFS and OS were analyzed according to the Kaplan‐Meier method and were updated to February 1, 2018.
This study was registered at the University Hospital Medical Information Network (UMIN ID: 000014305) and carried out in accordance with the principles of the Declaration of Helsinki and Ethical Guidelines for Medical and Health Research Involving Human Subjects.28 The protocol was approved by the ethics committee of each participating institution.
3. RESULTS
3.1. Patient characteristics
Patients’ characteristics are summarized in Table 1. Twenty‐eight patients were enrolled from June 2014 to October 2017. The median age was 68.5 years (range, 52‐77 years), and most patients were men (85.7%). All patients had an ECOG PS of 0 or 1. Approximately 70% of the patients had a T3/T4a tumor, and more than 80% were clinical stage III. All were assessed for both the presence of any adverse events and their response to treatment.
Table 1.
Characteristics of Japanese patients with resectable esophageal squamous cell carcinoma treated with docetaxel, nedaplatin, and 5‐fluorouracil
| Characteristics | No. of patients (n = 28) | % |
|---|---|---|
| Age, years | ||
| Median (range) | 68.5 (52‐77) | |
| Sex | ||
| Male | 24 | 85.7 |
| Female | 4 | 14.3 |
| ECOG performance status | ||
| 0 | 14 | 50.0 |
| 1 | 14 | 50.0 |
| Histology | ||
| Squamous cell carcinoma | 28 | 100.0 |
| Others | 0 | 0.0 |
| Tumor location | ||
| Ut | 6 | 21.4 |
| Mt | 13 | 46.4 |
| Lt | 9 | 32.1 |
| Clinical T stagea | ||
| T1 | 0 | 0.0 |
| T2 | 8 | 28.6 |
| T3 | 17 | 60.7 |
| T4a | 3 | 10.7 |
| Clinical N stagea | ||
| N0 | 5 | 17.9 |
| N1 | 7 | 25.0 |
| N2 | 9 | 32.1 |
| N3 | 7 | 25.0 |
| Clinical stagea | ||
| IB | 2 | 7.1 |
| IIA | 3 | 10.7 |
| IIB | 0 | 0.0 |
| IIIA | 11 | 39.3 |
| IIIB | 3 | 10.7 |
| IIIC | 9 | 32.1 |
According to the UICC TNM system (7th edition).
Lt, lower thoracic esophagus; Mt, middle thoracic esophagus; Ut, upper thoracic esophagus.
3.2. Treatment and compliance
The trial profile is shown in Figure 1. All patients received at least two courses of NAC. Three patients discontinued the third cycle of DNF and proceeded to surgery: two due to patient refusal and one owing to disease progression. A dose reduction in NAC occurred in seven patients in the second cycle and five in the third, mainly because of neutropenia. Seven patients experienced a delay in treatment of 7 days or more, due to protracted neutropenia in five cases and thrombocytopenia in two. Of all 81 treatment cycles in the study, 23 courses were undertaken by patients in an outpatient setting.
Figure 1.
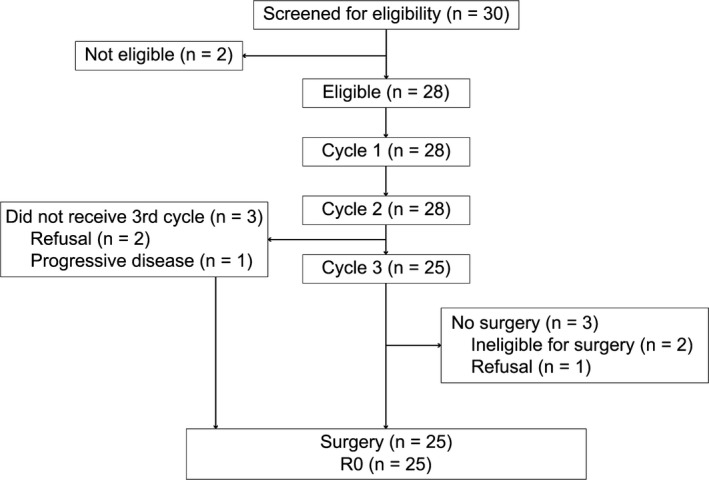
Profile of phase II study of docetaxel, nedaplatin, and fluorouracil for resectable esophageal cancer
In all, 25 patients received subsequent definitive surgery after NAC. Two patients failed to undergo surgery because they were deemed ineligible due to a low forced expiratory volume because of pulmonary emphysema. One refused surgery. These three cases received definitive CRT.
3.3. Toxicity
Adverse events during chemotherapy are listed in Table 2. Leukocytopenia and neutropenia were major toxicities in 32.1% and 39.3% of grade 3/4 cases, respectively. Grade 3 febrile neutropenia was observed in 10.7% of patients. Although 10.7% of patients experienced grade 3 thrombocytopenia, grade 4 cases were not observed. In terms of non‐hematological toxicities, grade 4 events were not observed, and grade 3 anorexia, diarrhea, stomatitis, and fatigue were detected at rates of 32.1%, 14.3%, 7.1%, and 3.6%, respectively. Grade 3/4 renal impairment was not observed in any patient. All events resolved with appropriate care, and treatment‐related deaths were not observed.
Table 2.
Toxicity of treatment with docetaxel, nedaplatin, and 5‐fluorouracil in Japanese patients with resectable esophageal squamous cell carcinoma
| Toxicity grade (NCI‐CTC version 4.0) | Patients (n = 28) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | All (%) | 3/4 (%) | |
| White blood cell decreased | 2 | 7 | 9 | 0 | 64.3 | 32.1 |
| Neutrophil count decreased | 2 | 6 | 5 | 6 | 67.9 | 39.3 |
| Febrile neutropenia | – | – | 3 | 0 | 10.7 | 10.7 |
| Anemia | 5 | 3 | 5 | 0 | 46.4 | 17.9 |
| Platelet count decreased | 5 | 1 | 3 | 0 | 28.6 | 10.7 |
| Anorexia | 4 | 7 | 9 | 0 | 71.4 | 32.1 |
| Fatigue | 7 | 6 | 1 | 0 | 50.0 | 3.6 |
| Fever | 1 | 0 | 0 | 0 | 3.6 | 0.0 |
| Nausea | 3 | 10 | 2 | 0 | 53.6 | 7.1 |
| Vomiting | 2 | 1 | 0 | 0 | 10.7 | 0.0 |
| Mucositis oral | 5 | 8 | 2 | 0 | 53.6 | 7.1 |
| Constipation | 4 | 1 | 0 | 0 | 17.9 | 0.0 |
| Diarrhea | 5 | 6 | 4 | 0 | 53.6 | 14.3 |
| Alopecia | 3 | 3 | 0 | 0 | 21.4 | 0.0 |
| Edema | 2 | 0 | 0 | 0 | 7.1 | 0.0 |
| Sensory neuropathy | 1 | 0 | 0 | 0 | 3.6 | 0.0 |
| AST increased | 6 | 0 | 0 | 0 | 21.4 | 0.0 |
| ALT increased | 9 | 0 | 0 | 0 | 32.1 | 0.0 |
| ALP increased | 0 | 1 | 0 | 0 | 3.6 | 0.0 |
| Hyponatremia | 3 | 0 | 1 | 0 | 14.3 | 3.6 |
| Creatinine increased | 2 | 1 | 0 | 0 | 10.7 | 0.0 |
| Skin hyperpigmentation | 2 | 0 | 0 | 0 | 7.1 | 0.0 |
–, a grade is not available. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTC, Common Toxicity Criteria.
3.4. Response
Table 3 shows the best overall response recorded during NAC according to RECIST version 1.1. Of the 23 patients with measurable lesions, 20 patients achieved a response (87.0%; 95% CI, 73.3%‐100%), with four (17.4%) showing a CR. Of the five patients without measurable lesions, a CR was observed in two cases (40.0%). A patient who had confirmed stable disease after the first cycle of chemotherapy showed progressive disease after the second course.
Table 3.
Response to treatment with docetaxel, nedaplatin, and 5‐fluorouracil in Japanese patients with resectable esophageal squamous cell carcinoma
| n | % | |
|---|---|---|
| Patient with target disease | 23 | |
| CR | 4 | 17.4 |
| PR | 16 | 69.6 |
| SD | 3 | 13.0 |
| PD | 0 | 0.0 |
| Overall response rate | 87.0a | |
| Patient without target disease | 5 | |
| CR | 2 | 40.0 |
| Non‐CR/Non‐PD | 3 | 60.0 |
| PD | 0 | 0.0 |
95% confidence interval, 73.3%‐100%.
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
3.5. Surgery and complications
Twenty‐five patients received surgery after NAC as listed in Figure 1. Surgical outcomes are shown in Table 4. Twenty patients underwent a thoracoscopic subtotal esophagectomy with three‐field lymphadenectomy, and a right thoracotomy was carried out in five patients. Of the patients who underwent an esophagectomy, a radical R0 resection was achieved in all 25 cases, leading to a completion rate for protocol treatment of 89.3% (25/28; 95% CI, 77.9%‐100%). Although postoperative complications were observed in seven patients (28.0%), most of these were minor (Clavien‐Dindo grade 1‐2). All major (Clavien‐Dindo grade 3‐5) complications were grade IIIa; anastomotic stenosis in four cases, and one case each showed a wound infection or chylothorax. These events resolved with endoscopic dilation, antibiotics, and thoracic drainage, respectively. Postoperative mortality was not observed.
Table 4.
Details of surgery in Japanese patients with resectable esophageal squamous cell carcinoma treated with docetaxel, nedaplatin, and 5‐fluorouracil
| n | % | |
|---|---|---|
| Surgical approach | ||
| Thoracoscopic surgery | 20 | 80.0 |
| Right thoracotomy | 5 | 20.0 |
| Residual tumora | ||
| R0 | 25 | 100 |
| R1/R2 | 0 | 0.0 |
| Postoperative 30‐d mortality | 0 | 0.0 |
| Pathological tumor responseb | ||
| 0 | 2 | 8.0 |
| 1a | 5 | 20.0 |
| 1b | 2 | 8.0 |
| 2 | 8 | 32.0 |
| 3 | 8 | 32.0 |
| Post‐therapy T stagea | ||
| ypT0 | 8 | 32.0 |
| ypT1 | 8 | 32.0 |
| ypT2 | 2 | 8.0 |
| ypT3 | 6 | 24.0 |
| ypT4 | 1 | 4.0 |
| Post‐therapy N stagea | ||
| ypN0 | 15 | 60.0 |
| ypN1 | 7 | 28.0 |
| ypN2 | 2 | 8.0 |
| ypN3 | 1 | 4.0 |
| Post‐therapy ypStagea | ||
| ypStage 0 | 6 | 24.0 |
| ypStage IA | 5 | 20.0 |
| ypStage IB | 0 | 0.0 |
| ypStage IIA | 4 | 16.0 |
| ypStage IIB | 6 | 24.0 |
| ypStage IIIA | 0 | 0.0 |
| ypStage IIIB | 2 | 8.0 |
| ypStage IIIC | 2 | 8.0 |
| Surgical complication | ||
| Recurrent laryngeal nerve palsy | 4 | 16.0 |
| Anastomotic leakage | 1 | 4.0 |
| Chylothorax | 1 | 4.0 |
| Gastrointestinal anastomotic stenosis | 4 | 16.0 |
| Wound infection | 2 | 8.0 |
| Pneumonia | 1 | 4.0 |
| Subcutaneous emphysema | 1 | 4.0 |
According to the UICC TNM system (7th edition).
According to the Japanese Classification of Esophageal Cancer (10th edition).
A histopathologically complete response (grade 3) was achieved in 8/25 patients, leading to a pCR rate of 32.0%. Grades 2, 1, and 0 were observed in 32.0%, 28.0%, and 8.0% of cases, respectively. Pathological tumor and nodal status generally improved compared with these prior to NAC, with eight cases showing no histological evidence of a primary tumor (ypT0). Downstaging according to a comparison between preoperative clinical and post‐surgery pathological stages was confirmed in 21 (84.0%) patients.
3.6. Survival analysis
With a median follow‐up time of 27.2 months, the median PFS was 28.5 months, and 1‐/2‐year PFS rates were 76.8%/70.4% (Figure 2A); the median survival time was not reached, and 1‐/2‐year OS rates were 91.6%/77.2% (Figure 2B).
Figure 2.
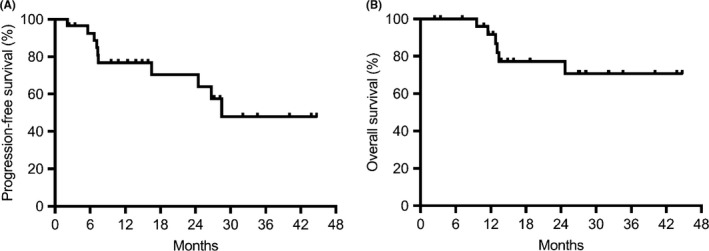
Kaplan‐Meier curves of progression‐free survival (A) and overall survival (B) in Japanese patients with resectable esophageal squamous cell carcinoma treated with docetaxel, nedaplatin, and 5‐fluorouracil. The estimated 1‐year progression‐free and overall survival rates were 76.8% and 91.6%, respectively
During follow‐up, seven patients presented with locoregional lymph node metastasis, two with distant organ metastasis, and one with both locoregional and distant metastases. Of eight cases who developed lymph node metastases, six involved cervical and superior mediastinal lymph nodes. All seven patients with only locoregional recurrence received definitive CRT, four of whom achieved a CR. Three cases with distant metastases underwent chemotherapy.
4. DISCUSSION
The current standard NAC used in Japan for ESCC is CF. However, the prognosis of patients with this cancer continues to remain poor. We previously found a high response rate and extended survival times in such patients in response to definitive CRT with DNF. We therefore undertook a phase II trial to evaluate the feasibility and efficacy of neoadjuvant DNF in patients with resectable stage Ib‐III ESCC. The treatment completion rate in the current study was 89.3%, with a 95% CI of 77.9%‐100%; this study met its primary end‐point. To our knowledge, this is the first study to prospectively examine DNF in a neoadjuvant setting.
Docetaxel, a microtubule inhibitor, CDGP, a DNA intrastrand cross‐linking agent, and 5‐FU, an antimetabolite, have different modes of action. This has led to the expectation of synergic effects and a lack of cross‐resistance when these three drugs are used in combination. Additionally, as these three agents show different major adverse effects that can lead to dose‐limiting toxicities (DOC, neutropenia; CDGP, thrombocytopenia; 5‐FU, diarrhea and mucositis), DNF is considered to be an ideal combination in terms of safety and feasibility.
With regard to the dosing method used for DOC, several reports have reported a lower incidence of adverse effects with a weekly dose of DOC compared to a conventional schedule of every 3 weeks.29, 30, 31 Zimatore et al31 also observed a higher dose intensity with a weekly schedule of DOC compared with a 3‐week administration in a comparative review. In addition, in our previous trial of definitive CRT using DNF, in which a similar schedule was used, few serious toxicities were observed.21 In contrast, in a phase II study undertaken in Japan examining the 3‐week administration of DOC, severe hematological toxicities were observed at an extremely high rate of 88% for grade 3/4 neutropenia and 18% for febrile neutropenia.8 Because of these observations, we adopted a weekly dosing schedule for DOC in the present study.
Although CDDP is widely used for ESCC, it frequently induces severe gastrointestinal toxicity and nephrotoxicity. Such adverse effects as well as the requirement for a high volume of hydration, recommended for the prevention of renal toxicity, limit the clinical use of CDDP, especially in elderly patients who are at greater risk of developing ESCC. Nedaplatin, a second‐generation platinum derivative, dissolves in water approximately 10 times more easily than CDDP, leading to less nephrotoxicity, and a lack of need for increased hydration.32 Zhang et al33 undertook a systematic review comparing CDDP‐ and CDGP‐based chemotherapies for metastatic or recurrent ESCC. They revealed that CDGP was comparable to CDDP in terms of RR and OS, but showed less toxicity in terms of a lower risk of nausea, vomiting, peripheral neuropathy, and nephrotoxicity. The weekly dosing schedule of 50 mg/m2 CDGP was determined from our previous phase I study of CRT using 5‐FU and CDGP.34 In the same phase I study, this 5‐FU was administered in the same way as the current DNF regimen, and its tolerability was shown.34 In addition, the dose intensity of 5‐FU in our trial is equal to that in the JCOG9907 study (800 mg/m2/3 weeks), and therefore its safety and efficacy are considered to be verified.5 Also, we postulate that 5‐FU, which induces time‐dependent cell growth inhibition, should be administered for a long‐time so as to maximize its efficacy. The schedule we chose for our DNF regimen was based on these observations.
Regarding the dosing frequency of NAC, the JCOG9907 study stipulated two courses of preoperative CF.5 In comparison, a trial assessing DCF in a neoadjuvant setting reported a high completion rate of 83% for three cycles of DCF.17 In addition to the above, all patients in our trial of CRT using DNF completed two courses of additional planned chemotherapy after CRT.21 Meredith et al35 reported that response and R0 resection rates after NAC correlated with disease‐free survival and OS. Based on these results, we specified two to three courses of DNF as the protocol therapy for NAC, after balancing the attributes of strong antitumor activity and feasibility.
Concerning the safety of NAC, the tolerability shown in the study was generally acceptable. The incidence of grade 3/4 neutropenia was found to be 39.3%, which is lower than that observed in studies using a DCF regimen (78.2%‐83.3%).17, 18 Cases with grade 4 thrombocytopenia, a dose‐limiting toxicity of CDGP, were not noted. Febrile neutropenia was observed in only 10.7% of patients without grade 4 events. In the current study, prophylactic antibiotics were not allowed, whereas the use of antibiotics for preventive purposes is generally permitted outside of a clinical trial.36 Therefore, on this basis, we can infer that the incidence of febrile neutropenia will be lower in actual clinical practice. Cases with grade 4 non‐hematological toxicity were not noted; patients also did not experience grade 3/4 nephrotoxicity, which is thought to be due to the use of CDGP instead of CDDP. As such adverse events were transient and manageable, it is thought that this favorable safety profile might have contributed to the high completion rate of NAC (two courses, 100%; three courses, 89.3%).
The completion rate for the protocol treatment was high at 89.3%. This was thought to be partly because a high response rate of 87.0% was achieved by DNF, which was higher than not only that reported in JCOG9907 (38%) but also that in trials using DCF (53.7%‐64.3%).5, 7, 18 In addition to using three potent agents, the high completion rate was supported by the low proportion of patients who needed a dose reduction or cessation of treatment; it might also be associated with a high dose intensity, and therefore, efficacy.
Despite a relatively short follow‐up period of 27.2 months, the 2‐year OS was 77.2%, which is comparable to that observed in trials of DCF.17, 18 In estimating long‐term survival, the high R0 resection rate of 100% for patients who underwent surgery in the current study is particularly meaningful. For example, Gertler et al37 analyzed the prognoses of 2920 resected cases with esophageal cancer and revealed that an R0 resection was an independent prognostic factor. Others reached the same conclusion in a reproducible manner, so the significance of an R0 resection as a prognostic factor is thought to be well established.38, 39 As with an R0 resection, it is noteworthy that the pCR rate was 32.0%, which is markedly superior to that obtained by CF (5%) and DCF (12%‐17%).5, 17, 18 The importance of a pCR as a prognostic factor has been pointed out in previous reports.40, 41 Furthermore, Tomasello et al42 reported a meta‐analysis of 17 studies with 3145 cases who received neoadjuvant therapy, and found that a pathologic response was strongly associated with a significant improvement in OS.
The rate of postoperative complications was found to be low, with an incidence of 28%, with most events not considered severe. In addition, mortality related to surgery was not noted. In particular, pneumonia, a major complication in surgery for esophageal cancer, was infrequent and mild, with only one case of grade 1 observed. One possible explanation for this favorable complication profile is the high rate (80.0%) of patients who underwent thoracoscopic esophagectomy. It is thought that a large incision of the chest by open thoracotomy prevents patients from taking deep breaths after surgery and thus raises the risk of pneumonitis because of atelectasis.43 In contrast, thoracoscopic esophagectomy has been evaluated as a less invasive method; two prospective trials have shown the safety of thoracoscopic esophagectomy compared to conventional open surgery in terms of a reduced risk of pneumonia.44, 45 Although few studies have reported the incidence of surgical morbidity, in two trials using DCF for NAC this was shown to be 32%‐39% and included pneumonia. In these studies, the proportion of patients who received thoracoscopic surgery was less than 5%.16, 46
Progression‐free survival in our study was almost identical to that observed in a trial using DCF.17 However, of the 10 patients who presented with recurrence in the current study, eight cases developed locoregional lymph node metastasis. The prevention and control of locoregional relapse is a crucial issue to be resolved before further improvement in long‐term outcomes can be achieved. Specifically, of the eight cases showing locoregional relapse, cervical or superior mediastinal lymph node metastases were observed in six patients. This might have been due to the difficulty of undertaking cervical and superior mediastinal lymphadenectomies because of anatomical constraints compared with those of lower mediastinal or abdominal lesions. Countries in Asia have not been very proactive in adopting non‐Asian evidence of NACRT thus far, as stated above. However, in Japan, following promising results of good tolerability and strong antitumor activity from a phase II study that investigated NACRT with CF,47 a three‐arm phase III trial (JCOG1109) is now ongoing to confirm DCF or CRT with CF as superior to CF as neoadjuvant therapy for ESCC.48 In the near future, NACRT could become one of the standard treatment options for resectable ESCC, depending on the result of JCOG1109, and, therefore, NACRT with DNF might also need to be evaluated, as well as DNF, for NAC. Our previous trial of definitive CRT with DNF achieved an excellent tumor response with a CR rate of 88.2% in stage Ib‐III cases;21 therefore, further improvements in local control could be achieved by NACRT with DNF. Nonetheless, this CRT regimen cannot be used in a neoadjuvant setting in its original form, partly because of the difference in radiation dose: approximately 40 Gy of radiation is generally accepted as a preoperative dosage, whereas definitive CRT with DNF consists of 59.4 Gy. The establishment of an optimal treatment schedule and a comparison with DNF for NAC in terms of efficacy and safety are possible problems that should be resolved in the future.
In conclusion, we have shown that combination chemotherapy with DNF is a promising preoperative regimen for resectable ESCC, showing an acceptable feasibility with a completion rate for protocol treatment of 89.3%, and strong antitumor efficacy. These results were quite promising, but the existence of several limitations of our investigation must be acknowledged, including the single‐armed nature of the study, and the relatively small number of patients recruited. Additional trials with larger cohorts and longer follow‐up periods are needed to confirm the findings of the current study.
CONFLICT OF INTEREST
Junji Kato received research funding from SymBio Pharmaceuticals, Alexion Pharmaceuticals, Ono Pharmaceutical, Asahi Kasei Pharma, Astellas Pharma, Eisai, Otsuka Pharmaceutical, Kyowa Hakko Kirin, Shionogi, Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharmaceutical, Toyama Chemical, and Pfizer Japan, outside the submitted work. The other authors have no conflict of interest.
Ohnuma H, Sato Y, Hayasaka N, et al. Neoadjuvant chemotherapy with docetaxel, nedaplatin, and fluorouracil for resectable esophageal cancer: A phase II study. Cancer Sci. 2018;109:3554–3563. 10.1111/cas.13772
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 2. Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta‐analysis. Lancet Oncol. 2007;8:226‐234. [DOI] [PubMed] [Google Scholar]
- 3. van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074‐2084. [DOI] [PubMed] [Google Scholar]
- 4. Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study ‐ JCOG9204. J Clin Oncol. 2003;21:4592‐4596. [DOI] [PubMed] [Google Scholar]
- 5. Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5‐fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68‐74. [DOI] [PubMed] [Google Scholar]
- 6. Kumagai K, Rouvelas I, Tsai JA, et al. Meta‐analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro‐oesophageal junctional cancers. Br J Surg. 2014;101:321‐338. [DOI] [PubMed] [Google Scholar]
- 7. Klevebro F, Johnsen G, Johnson E, et al. Morbidity and mortality after surgery for cancer of the oesophagus and gastro‐oesophageal junction: a randomized clinical trial of neoadjuvant chemotherapy vs. neoadjuvant chemoradiation. Eur J Surg Oncol. 2015;41:920‐926. [DOI] [PubMed] [Google Scholar]
- 8. Muro K, Hamaguchi T, Ohtsu A, et al. A phase II study of single‐agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol. 2004;15:955‐959. [DOI] [PubMed] [Google Scholar]
- 9. Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first‐line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991‐4997. [DOI] [PubMed] [Google Scholar]
- 10. Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695‐1704. [DOI] [PubMed] [Google Scholar]
- 11. Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705‐1715. [DOI] [PubMed] [Google Scholar]
- 12. Takahashi H, Arimura Y, Yamashita K, et al. Phase I/II study of docetaxel/cisplatin/fluorouracil combination chemotherapy against metastatic esophageal squamous cell carcinoma. J Thorac Oncol. 2010;5:122‐128. [DOI] [PubMed] [Google Scholar]
- 13. Osaka Y, Shinohara M, Hoshino S, et al. Phase II study of combined chemotherapy with docetaxel, CDDP and 5‐FU for highly advanced esophageal cancer. Anticancer Res. 2011;31:633‐688. [PubMed] [Google Scholar]
- 14. Yamasaki M, Miyata H, Tanaka K, et al. Multicenter phase I/II study of docetaxel, cisplatin and fluorouracil combination chemotherapy in patients with advanced or recurrent squamous cell carcinoma of the esophagus. Oncology. 2011;80:307‐313. [DOI] [PubMed] [Google Scholar]
- 15. Tamura S, Imano M, Takiuchi H, et al. Phase II study of docetaxel, cisplatin and 5‐fluorouracil (DCF) for metastatic esophageal cancer (OGSG 0403). Anticancer Res. 2012;32:1403‐1408. [PubMed] [Google Scholar]
- 16. Hironaka S, Tsubosa Y, Mizusawa J, et al. Phase I/II trial of 2‐weekly docetaxel combined with cisplatin plus fluorouracil in metastatic esophageal cancer (JCOG0807). Cancer Sci. 2014;105:1189‐1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hara H, Tahara M, Daiko H, et al. Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1455‐1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watanabe M, Baba Y, Yoshida N, et al. Outcomes of preoperative chemotherapy with docetaxel, cisplatin, and 5‐fluorouracil followed by esophagectomy in patients with resectable node‐positive esophageal cancer. Ann Surg Oncol. 2014;21:2838‐2844. [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi H, Takemura Y, Miyachi H, et al. Antitumor activities of new platinum compounds, DWA2114R, NK121 and 254‐S, against human leukemia cells sensitive or resistant to cisplatin. Invest New Drugs. 1991;9:313‐319. [DOI] [PubMed] [Google Scholar]
- 20. Sasaki Y, Amano T, Morita M, et al. Phase I study and pharmaco‐ logical analysis of cis‐diammine(glycolato)platinum (254‐S; NSC 375101D) administered by 5‐day continuous intravenous infusion. Cancer Res. 1991;51:1472‐1477. [PubMed] [Google Scholar]
- 21. Ohnuma H, Sato Y, Hirakawa M, et al. A Phase 1/2 study of definitive chemoradiation therapy using docetaxel, nedaplatin, and 5‐fluorouracil (DNF‐R) for esophageal cancer. Int J Radiat Oncol Biol Phys. 2015;93:382‐390. [DOI] [PubMed] [Google Scholar]
- 22. Sobin LH, Gospodarowicz MK, Wittekind CH. TNM Classification of Malignant Tumors, 7th edn Hoboken, NJ: Wiley‐Blackwell; 2010. [Google Scholar]
- 23. National Cancer Institute . National Cancer Institute Common Terminology Criteria for Adverse Events v 4.0. Bethesda, MD: National Cancer Institute; 2009. [Google Scholar]
- 24. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 25. Japan Esophageal Society . Japanese Classification of Esophageal Cancer, 10th edn Tokyo, Japan: Kanehara; 2008. [Google Scholar]
- 26. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katayama H, Kurokawa Y, Nakamura K, et al. Extended Clavien‐Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46:668‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ministry of Education Culture Sports Science and Technology, Ministry of Health Labour and Welfare, Ethical Guidelines for Medical and Health Research Involving Human Subjects. 2015, Ministry of Education Culture Sports Science and Technology and Ministry of Health Labour and Welfare: Tokyo. [Google Scholar]
- 29. Hainsworth JD, Burris HA, Erland JB, et al. Phase I trial of docetaxel administered by weekly infusion in patients with advanced refractory cancer. J Clin Oncol. 1998;16:2164‐2168. [DOI] [PubMed] [Google Scholar]
- 30. Kouroussis C, Agelaki S, Mavroudis D, et al. A dose escalation study of weekly docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2000;46:488‐492. [DOI] [PubMed] [Google Scholar]
- 31. Zimatore M, Danova M, Vassallo E, et al. Weekly taxanes in metastatic breast cancer (review). Oncol Rep. 2002;9:1047‐1052. [PubMed] [Google Scholar]
- 32. Shimada M, Itamochi H, Kigawa J. Nedaplatin: a cisplatin derivative in cancer chemotherapy. Cancer Manag Res. 2013;5:67‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang F, Wang Y, Wang ZQ, et al. Efficacy and safety of cisplatin‐based versus nedaplatin‐based regimens for the treatment of metastatic/recurrent and advanced esophageal squamous cell carcinoma: a systematic review and meta‐analysis. Dis Esophagus. 2017;30:1‐8. [DOI] [PubMed] [Google Scholar]
- 34. Sato Y, Takayama T, Sagawa T, et al. A phase I/II study of nedaplatin and 5‐fluorouracil with concurrent radiotherapy in patients with esophageal cancer. Cancer Chemother Pharmacol. 2006;58:570‐576. [DOI] [PubMed] [Google Scholar]
- 35. Meredith KL, Weber JM, Turaga KK, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol. 2010;17:1159‐1167. [DOI] [PubMed] [Google Scholar]
- 36. Flowers CR, Seidenfeld J, Bow EJ, et al. Antimicrobial prophylaxis and out‐ patient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31:794‐810. [DOI] [PubMed] [Google Scholar]
- 37. Gertler R, Stein HJ, Langer R, et al. Long‐term outcome of 2920 patients with cancers of the esophagus and esophagogastric junction. Ann Surg. 2011;253:689‐698. [DOI] [PubMed] [Google Scholar]
- 38. Wijnhoven BPL, Tran KTC, Esterman A, et al. An evaluation of prognostic factors and tumor staging of resected carcinoma of the esophagus. Ann Surg. 2007;245:717‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen SB, Weng HR, Wang G, et al. Prognostic factors and outcome for patients with esophageal squamous cell carcinoma underwent surgical resection alone: evaluation of the seventh edition of the American Joint Committee on Cancer staging system for esophageal squamous cell carcinoma. J Thorac Oncol. 2013;8:495‐501. [DOI] [PubMed] [Google Scholar]
- 40. Dittrick GW, Weber JM, Shridhar R, et al. Pathologic nonresponders after neoadjuvant chemoradiation for esophageal cancer demonstrate no survival benefit compared with patients treated with primary esophagectomy. Ann Surg Oncol. 2012;19:1678‐1684. [DOI] [PubMed] [Google Scholar]
- 41. Tiesi G, Park W, Gunder M, et al. Long‐term survival based on pathologic response to neoadjuvant therapy in esophageal cancer. J Surg Res. 2017;216:65‐72. [DOI] [PubMed] [Google Scholar]
- 42. Tomasello G, Petrelli F, Ghidini M, et al. Tumor regression grade and survival after neoadjuvant treatment in gastro‐esophageal cancer: a meta‐analysis of 17 published studies. Eur J Surg Oncol. 2017;43:1607‐1616. [DOI] [PubMed] [Google Scholar]
- 43. Grogan EL, Jones DR. VATS lobectomy is better than open thoracotomy: what is the evidence for short‐term outcomes? Thorac Surg Clin. 2008;18:249‐258. [DOI] [PubMed] [Google Scholar]
- 44. Luketich J, Pennathur A, Catalano P, et al. Results of a phase II multicenter study of minimally invasive esophagectomy (Eastern Cooperative Oncology Group Study E2202). J Clin Oncol. 2009;27(suppl 15): Abstract 4516. [Google Scholar]
- 45. Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open‐label, randomised controlled trial. Lancet. 2012;379:1887‐1892. [DOI] [PubMed] [Google Scholar]
- 46. Ferri LE, Ades S, Alcindor T, et al. Perioperative docetaxel, cisplatin, and 5‐fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: a multicenter phase II trial. Ann Oncol. 2012;23:1512‐1517. [DOI] [PubMed] [Google Scholar]
- 47. Kojima T, Hashimoto J, Kato K, et al. Feasibility study of neoadjuvant chemoradiotherapy with cisplatin plus 5‐fluorouracil and elective nodal irradiation for stage II/III esophageal squamous cell carcinoma. J Clin Oncol. 2012;37(suppl. 4): Abstract 130. [DOI] [PubMed] [Google Scholar]
- 48. Nakamura K, Kato K, Igaki H, et al. Three‐arm phase III trial comparing cisplatin plus 5‐FU (CF) versus docetaxel, cisplatin plus 5‐FU (DCF) versus radiotherapy with CF (CF‐RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT Study). Jpn J Clin Oncol. 2013;43:752‐755. [DOI] [PubMed] [Google Scholar]


