Abstract
Although first‐line chemotherapy has a high rate of complete responses in ovarian cancer patients, the vast majority of patients present with recurrent disease that has become refractory to conventional chemotherapy. Peritoneal dissemination and malignant ascites are the hallmarks of recurrent or advanced ovarian cancer and severely reduce quality of life. Development of therapeutic measures to treat such patients is eagerly anticipated. Macrophage infiltration is observed in various types of cancer including epithelial ovarian cancer. In addition, macrophages are involved in the formation of spheroids in the malignant ascites of ovarian cancer and promote cancer growth. iPS‐ML, macrophage‐like myelomonocytic cells generated from human induced pluripotent stem (iPS) cells, made close contacts with ovarian cancer cells in vitro. We hypothesized that, if we inoculate iPS‐ML‐producing IFN‐β (iPS‐ML/IFN‐β) into the peritoneal cavity of patients with ovarian cancer, IFN‐β produced by the iPS‐ML/IFN‐β would efficiently act on the cancer cells to suppress cancer growth. To evaluate this hypothesis, we injected iPS‐ML/IFN‐β into SCID mice bearing peritoneally disseminated human ovarian cancer cells, SKOV3. Immunohistochemical analysis of the intraperitoneal tumors detected iPS‐ML/IFN‐β infiltrating into the cancer tissues. Therapy with iPS‐ML/IFN‐β significantly suppressed tumor progression. In addition, dramatic reduction of cancer‐related ascites was observed. Collectively, it is suggested that iPS‐ML/IFN‐β therapy offers a new approach for the treatment of patients with advanced ovarian cancer.
Keywords: interferon‐β, iPS cell, macrophage, ovarian cancer, peritoneal dissemination
1. INTRODUCTION
Ovarian cancer is the seventh most common cancer in women worldwide.1 The majority of ovarian cancers are diagnosed at advanced stages.2 The standard first‐line therapy for ovarian cancer is a combination of surgery and carboplatin/paclitaxel‐based chemotherapy. However, about 80% of advanced ovarian cancers relapse with more aggressive and drug‐resistant disease.3 The five‐year survival rate for patients with advanced ovarian cancer remains at less than 30%.4 There is also an unmet need for therapy which could improve the quality of life in patients with advanced or recurrent ovarian cancer.
The main symptom of advanced ovarian cancer in patients is accumulation of ascites. Recent molecular therapy research has primarily focused on the tumor microenvironment. The tumor microenvironment changes over the course of cancer progression and influences metastasis as a dynamic process. The detailed mechanisms underlying malignant ascites and tumor progression in the ovarian cancer tumor microenvironment have been elucidated. Our previous study showed that in epithelial ovarian cancer ascites, cell‐to‐cell interactions between tumor cells and macrophages led to Stat3 activation of both cell types.5 Stat3 activation in the cancer cells enhanced cell proliferation and induced production of interleukin (IL)‐6 and IL‐10 by macrophages. Another study showed that M2‐like macrophages promote spheroid formation and tumor metastases in ovarian cancer.6 It is well known that macrophages are derived from monocytes. M1‐like macrophages act as antitumor effector cells, whereas M2‐like macrophages act as suppressors of antitumor immune responses by producing immunosuppressive cytokines, including IL‐6, IL‐10, and transforming growth factor β (TGF‐β).7
Over the past decades, interferon (IFN)‐β has received considerable attention for the treatment of various cancers. It has been reported that IFN‐β either inhibits tumor cell proliferation or induces apoptosis of tumor cells through the Jak‐Stat1 intracellular signaling pathway. Activation of the host antitumor immune system and suppression of tumor angiogenesis by IFN‐β has also been described.8, 9 However, the successful use of IFN‐β as an anticancer agent is limited to therapy for melanoma, hairy cell leukemia, chronic myelogenous leukemia, renal cell cancer and brain tumors. This restricted efficacy of IFN‐β may be as a result of its short half‐life and rapid clearance from the circulation after i.v. injection. In addition, systemic toxicity precludes high‐dose administration. To reduce the undesirable side‐effects and enable IFN‐β to act on cancer more selectively, other approaches have been used. To treat ovarian cancer and other malignancies, researchers have developed gene‐based therapies or tried to stabilize IFN‐β by pegylation.10, 11, 12 Previously, we established a method to generate induced pluripotent stem (iPS)‐cell‐derived myelomonocytic cells (iPS‐ML) using lentivirus‐mediated transduction of cell proliferation and antisenescence factors into human iPS‐cell‐derived myeloid cells.13 iPS‐ML genetically modified to produce IFN‐β have shown therapeutic effects against gastric, pancreatic, and hepatocellular carcinomas in xenograft models.14, 15
In the present study, we developed an orthotopic xenograft model of dissemination of intraperitoneal ovarian cancer. Using this system, we evaluated the effect of iPS‐ML/IFN‐β on cancer progression and retention of ascites.
2. MATERIALS AND METHODS
2.1. Cell lines and culture conditions
Human ovarian cancer cell lines SKOV3, ES2, A2780 and SW626 were provided by ATCC (Manassas, VA, USA). SKOV3 and ES2 cells were transduced with a lentivirus vector encoding firefly luciferase. These cells were maintained in RPMI‐1640 medium (Wako Pure Chemical Industries, Osaka, Japan) supplemented with 10% FBS, 100 IU/mL penicillin G and 100 μg/mL streptomycin at 37°C in a fully humidified 5% CO2 incubator. The methods used for the generation of iPS‐ML producing a large amount of human IFN‐β will be described elsewhere manuscript in preparation. iPS‐ML and iPS‐ML producing IFN‐β (iPS‐ML/IFN‐β) were maintained in α‐MEM containing 20% FBS.
2.2. Clinical samples
After informed consent was obtained, samples of ascites were collected from patients with ovarian cancer that were undergoing surgery at Kumamoto University Hospital. Each sample was fixed with 10% neutral buffered formalin in line with the protocols approved by the Human Research and Ethics Committee of Kumamoto University.
2.3. MTS assay
Sensitivities of the human ovarian cancer cell lines, SKOV3, ES2, A2780, and SW626 to IFN‐β, and other cytokines including IFN‐γ, tumor necrosis factor (TNF)‐α, and TNF‐related apoptosis‐inducing ligand (TRAIL) were examined in vitro. The cells were cultured in 96‐well culture plates (3 × 103 cells/well in 100 μL) in the presence or absence of 30 ng/mL recombinant human IFNs, TNF‐α, or TRAIL. After 3 days, MTS tetrazonium solution (3‐[4,5‐dimethylthiazol‐2‐yl]‐5‐[3‐carboxymethoxyphenyl]‐2‐[4‐sulfophenyl]‐2H‐tetrazolium, inner salt) reagent (CellTiter 96; Promega, Madison, WI, USA) was added to the wells and incubated for 24 hours. The number of living cells in culture was measured using the optical absorbance on a plate reader at 490 nm (SpectraMax190; Molecular Devices, Sunnyvale, CA, USA).
2.4. Luciferase assay
Luciferase‐expressing SKOV3 and ES2 cells were either cultured alone in 96‐well culture plates (1 × 103 cells/well) or were cocultured with iPS‐ML/IFN‐β (5 × 102 to 5 × 103 cells/well). SKOV3 (1 × 103 cells/well) in 96‐well culture plates were cultured alone or were cocultured with ordinary type iPS‐ML (without production of IFN‐β, 1 × 103 cells/well) in the presence or absence of 30 ng/mL recombinant human IFN‐β. Three days later, luciferase substrate (SteadyLite Plus; Perkin‐Elmer, Waltham, MA, USA) was added to the wells, and luciferase activity was measured using a microplate reader (FilterMAX F5; Molecular Devices).
2.5. Flow cytometric analysis
We analyzed the expression of cell‐surface molecules on non‐IFN‐producing iPS‐ML and iPS‐ML/IFN‐β by a flow cytometric analysis. The following mAbs conjugated with phycoerythrin were purchased from BioLegend (San Diego, CA, USA) or Miltenyi Biotec (Bergisch, Gladbach, Germany): antihuman CD163 (GHI/61, mouse IgG1), antihuman CD169 (7‐239, mouseIgG1κ), antihuman CD206 (DCN228, mouse IgG1κ). Isotype‐matched mouse IgG1 (MOPC21) was used as control. Cell samples were treated with a human FcR‐blocking reagent (Miltenyi Biotec) for 10 minutes and washed three times with PBS/FCS (2%). Stained cell samples were analyzed using a BD FACSCalibur (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) flow cytometer.
2.6. Immunohistochemical analysis
A 200‐μL sample of non‐centrifuged ascites from a patient with high‐grade serous ovarian cancer was mixed with 1% sodium alginate. The cells were then separated as a precipitate and solidified by addition of 1 M calcium chloride. Finally, gelatinous specimens containing cancer cells and macrophages were embedded in paraffin using standard techniques. The tissue was cut into 3‐μm thick sections. Deparaffinized sections were pretreated for antigen retrieval and were then sequentially incubated with 5% goat serum and with mouse anti‐CD68 or mouse anti‐CD163 (Leica, Wetzlar, Germany). After rinsing, samples were incubated with HRP‐labeled goat antirabbit or antimouse immunoglobulin (Dako, Glostrup, Denmark). The immunoreaction was visualized using a diaminobenzidine (DAB) substrate kit (Vector, Burlingame, CA, USA).
SKOV3 cells (1 × 105 cells per chamber slide) were cocultured with iPS‐ML/IFN‐β in a 1:1 ratio for 24 hours. The cultured cells were fixed in 4% paraformaldehyde phosphate buffer solution (PFA) for 20 min and then washed twice in PBS. The samples were incubated with mouse anti‐CD68 antibody and then incubated with HRP‐labeled polymer and visualized using the DAB substrate system. Established intraperitoneal tumor tissues were fixed in vivo in 10% neutral buffered formalin. Next, the tumors were embedded in paraffin in the routine method. The tissue was cut into 3‐μm thick sections. Deparaffinized sections were stained with H&E for histological examination. For immunostaining, formalin‐fixed and paraffin‐embedded tissue sections were pretreated for antigen retrieval and were then sequentially incubated with 5% goat serum and stained with rabbit anti‐Iba‐1 (Wako Pure Chemical Industries), rabbit anti‐CD11b (ab52478, Abcam; Cambridge, UK), or rat anti‐CD204 (Bio‐Rad/DAKO, Hercules, CA, USA). The methods used for immunohistochemical analysis of rat anti‐CD204 have been described previously.16 After rinsing, the samples were incubated with HRP‐labeled polymer and the immunoreaction was visualized using a DAB substrate system.
2.7. Xenograft ovarian cancer model
Mouse experiments were approved by the Animal Research Committee of Kumamoto University. Five‐ to 6‐week‐old SCID C.B‐17/Icr‐scid/scid Jcl female mice were purchased from CLEA Japan. Mice were intraperitoneally (i.p.) injected with SKOV3 cells (2 × 107 cells/mouse) expressing luciferase. After 3 or 4 days, the mice were anesthetized by i.p. injection of medetomidine, midazolam, and butorphanol. The mice underwent bioluminescence imaging to examine the extent of ovarian cancer metastasis (NightOWL II; Berthold Technologies, Bad Wildbad, Germany). After confirmation of the engraftment of the cancer, mice were divided into control and treatment groups. The mice in the treatment group were injected twice each week with iPS‐ML (without production of IFN‐β, 1 × 107 cells/mouse) or iPS‐ML/IFN‐β (1 × 107 cells/mouse). All mice underwent luminescence image analysis to evaluate the effects of the treatment. The experimental data were analyzed using Indigo analysis software.
2.8. Statistical analysis
Statistical analyses were carried out using Student's t test (SPSS version 24, IBM; Armonk, NY, USA). A P‐value of <0.05 was considered statistically significant.
3. RESULTS
3.1. Sensitivity of ovarian cancer cell lines to IFN
We examined the sensitivity of four human ovarian cancer cell lines (SKOV3, ES2, A2780, SW626) to IFNs, TNF‐α and TRAIL. As shown in Figure 1, TRAIL (P < 0.05), and IFN‐α, IFN‐β, IFN‐γ, TNF‐α (P < 0.01) reduced the number of live SKOV3 cells. IFN‐α, IFN‐β, IFN‐γ, and TNF‐α (P < 0.01) also appeared to reduce the number of ES2 cells. A2780 cells showed sensitivity to IFN‐γ (P < 0.05), IFN‐α, TNF‐α, and TRAIL (P < 0.01). In contrast, only IFN‐β and TRAIL (P < 0.05) reduced the number of live SW626 cells.
Figure 1.
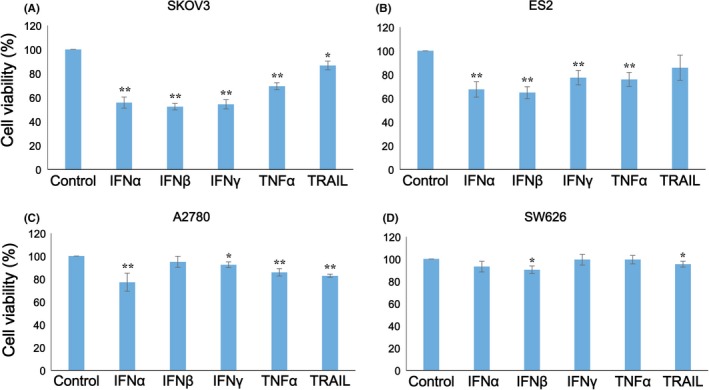
Sensitivity of ovarian cancer cell lines to cytokines in vitro. Four human ovarian cancer cell lines were examined for their sensitivity to interferons (IFNs), tumor necrosis factor (TNF)‐α, and TNF‐related apoptosis‐inducing ligand (TRAIL) in vitro. A, SKOV3, B, ES2, C, A2780, and D, SW626 cells were cultured in 96‐well culture plates (3 × 103 cells/well in 100 μL) with or without 30 ng/mL recombinant IFNs and TNFs. After 3 days, the number of living cells was measured by an MTS assay. The experiments were conducted three times to confirm the reproducibility of the assay, and representative results are presented. Data shown are the mean ± SD of triplicate assays. The difference from no cytokine condition was statistically significant (*P < 0.05; **P < 0.01 by Student's t test)
The effect of coculture with iPS‐ML/IFN‐β on the number of live SKOV3 and ES2 cells was also examined. We cocultured iPS‐ML/IFN‐β and the cancer cells expressing firefly luciferase. The number of live SKOV3 and ES2 cells was measured based on the luciferase activity after a 3‐day culture. iPS‐ML/IFN‐β reduced the number of live SKOV3 and ES2 cells in a dose‐dependent way (Figure 2). Coculture of ordinary‐type iPS‐ML (without production of IFN‐β) did not affect the number of SKOV3 cells in the absence or presence of recombinant IFN‐β (Figure S1). According to these findings, it is confirmed that iPS‐ML had no direct anticancer effect.
Figure 2.
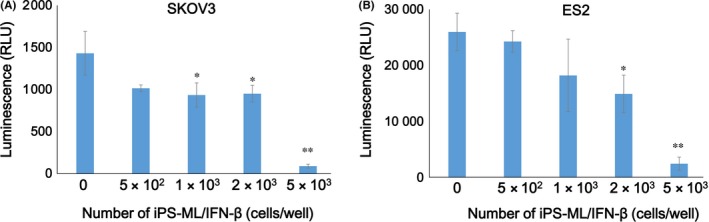
Sensitivity of ovarian cancer cell lines to induced pluripotent stem‐cell‐derived myelomonocytic cells (iPS‐ML)/interferon (IFN)‐β in vitro. Luciferase‐expressing A, SKOV3 or B, ES2 cells were cultured in a 96‐well culture plate (1 × 103 cells/well) with or without iPS‐ML/IFN‐β. Number of live cancer cells was measured by luciferase activity after 3 days. The difference from the control was statistically significant (*P < 0.05; **P < 0.01 by Student's t test. RLU, relative luminescent units)
3.2. Cognate interaction of tumor cells and macrophages
Direct interaction between macrophages and cancer cells plays a pivotal role in tumor progression. We previously reported the existence of abundant numbers of macrophages (106 cells/mL on average) in the ascites of patients with advanced stages of ovarian cancer, and the promotion of ovarian cancer cell growth by the interaction between macrophages and cancer cells.5 A similar phenomenon was observed in this study, and most of the cells formed aggregates in the ascites of patients with ovarian cancer (Figure 3A). In addition to cancer cells, sediments of the ascites included a large number of CD68+ CD163+ macrophages (Figure 3B). We cocultured SKOV3 cancer cells with iPS‐ML/IFN‐β, and the cells were fixed and stained with anti‐CD68 antibody to distinguish iPS‐ML/IFN‐β from the cancer cells. As shown in Figure 3C, iPS‐ML/IFN‐β were in close contact with SKOV3 cancer cells.
Figure 3.
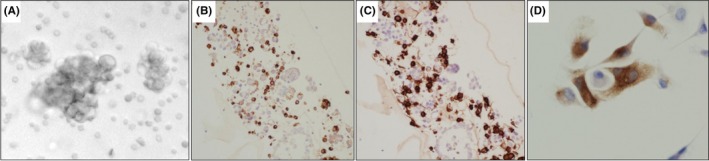
Interaction of macrophages with ovarian cancer cells. A, Spheres present in the ascites of serous carcinoma of the ovary (×400). B,C, Presence of CD68‐ and CD163‐positive cells in precipitates of ascites of ovarian carcinoma was examined (×200). D, Induced pluripotent stem‐cell‐derived myelomonocytic cells (iPS‐ML)/interferon (IFN)‐β were evaluated immunohistochemically using an anti‐CD68 antibody (×400). Distinct staining for CD68 showed that iPS‐ML/IFN‐β associated with SKOV3 cells
From these data, we hypothesized that inoculation of iPS‐ML into the peritoneal cavity of patients with ovarian cancer may result in intense interaction of the iPS‐ML with cancer cells in the ascites. Thereby, if the injected iPS‐ML produce a large amount of IFN‐β, the cytokine may efficiently act on the cancer cells. Based on this consideration, we decided to evaluate the therapeutic effect of iPS‐ML/IFN‐β on ovarian cancer in a xenograft model of peritoneal dissemination.
3.3. Therapeutic effect of iPS‐ML/IFN‐β in xenograft models
We injected SKOV3 cells expressing luciferase into the peritoneal cavity of SCID mice to establish a peritoneal dissemination model that we could use to quantitatively monitor in vivo cancer progression by imaging. After confirmation of the engraftment of the tumor, we divided the mice into treatment and control groups. We injected iPS‐ML/IFN‐β i.p. into the mice of the treatment group twice each week.
Luminescence image analysis showed a continuous progression of tumors from day 3 to day 24 in the mice of the control group. In contrast, tumor progression was inhibited in the mice of the treatment group (Figure 4A,B). The difference in tumor progression at day 24 between the treatment and control groups was statistically significant (P < 0.01).
Figure 4.
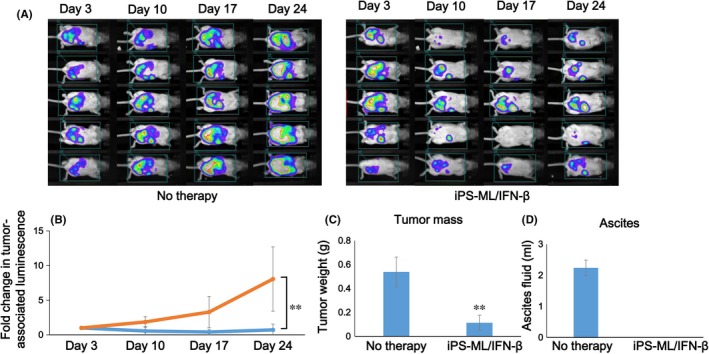
Therapeutic effect of induced pluripotent stem‐cell‐derived myelomonocytic cells (iPS‐ML)/interferon (IFN)‐β against SKOV3 cells in a peritoneal xenograft model. Aliquots of luciferase‐expressing SKOV3 cells (2 × 107 cells in 300 μL) were i.p. injected into mice. On day 3, the mice underwent a luminescence imaging analysis to evaluate the development of ovarian cancer metastases. After confirmation of the engraftment of the cancer, the mice were divided into a treatment (n = 5) and a control (n = 5) group. The mice in the treatment group were injected with iPS‐ML/IFN‐β (1 × 107 cells/mouse) twice each week, whereas those in the control group were left untreated. A, Tumor progression was monitored every week, using in vivo luminescence imaging analysis. B, The change in the luminescence signal in mice was calculated as a relative value, with the luminescence activity on day 3 defined as 1. Orange line and blue line in the graph indicate the control (no therapy) and iPS‐ML/IFN‐β‐treated mice, respectively. The imaging analysis was conducted twice. C,D, Three days after the last therapy and luminescence analysis (on day 31), the mice were killed and subjected to laparotomy to collect ascites and tumors, and the weight of tumors (C) and the volume of ascites (D) of each mouse was measured. Data shown are the mean ± SD of each mouse group. The difference from the control group was statistically significant (*P < 0.05; **P < 0.01 by Student's t test)
We killed the mice on day 31 and isolated the tumors. Tumor weights from the mice in the control and treatment groups were 0.54 ± 0.12 g and 0.11 ± 0.06 g, respectively (P < 0.01; Figure 4C). Tumor progression in the mice was accompanied by abdominal distension as a result of the retention of ascites. This distension is similar to that seen clinically in patients with advanced ovarian cancer. We measured the volume of ascites on day 31. The volume of ascites was more than 2 mL/mouse on average in the control group, whereas ascites were not detected in the mice of the treatment group (Figure 4D). Giving non‐IFN‐producing iPS‐ML did not affect the disseminated tumors of SKOV3 cells in the model mice (Figure S2).
Collectively, therapy with iPS‐ML/IFN‐β significantly suppressed tumor progression in SCID mice intraperitoneally engrafted with human ovarian cancer cells. In addition, the retention of ascites caused by the cancer was dramatically inhibited by the treatment.
3.4. Infiltration of iPS‐ML/IFN‐β into tumor tissues
Infiltration of macrophages is observed in various types of cancer tissues. We had expected that iPS‐ML/IFN‐β given to cancer‐bearing mice for therapeutic purposes would also infiltrate into the cancer. To examine this expectation, we histologically analyzed the tumor tissues of mice treated with iPS‐ML/IFN‐β. Three days after the final injection of iPS‐ML/IFN‐β (on day 31), the mice were killed and the tumors were isolated. Infiltration of macrophages was examined by immunohistochemical analysis of tumor tissue sections.
H&E staining of the sections identified inflammatory cells at the periphery of and within the tumors (Figure 5A,B). Staining with anti‐Iba‐1 antibody detected both intrinsic mouse macrophages and macrophages derived from injected human iPS‐ML/IFN‐β. As shown in Figure 5C,D, many cells positive for Iba‐1 were located at the periphery of and within the tumors. Antihuman CD11b antibody detected only iPS‐ML/IFN‐β (Figure 5E,F). Cells positive for human CD11b were detected in both the periphery of and within the tumor, although these cells were fewer than those positive for Iba‐1. The results indicate that both intrinsic mouse macrophages and administered human iPS‐ML/IFN‐β infiltrated into the cancer tissues. Furthermore, both iPS‐ML and iPS‐ML/IFN‐β cells cultured in vitro strongly expressed CD163 and CD206, and a fraction of cells expressed CD169 (Figure S3). Thus, iPS‐ML and iPS‐ML/IFN‐β showed mostly M2‐like phenotype. As shown in the Supporting Figure S4, the infiltrating mouse macrophages were positive for CD204 corresponding to M2‐like phenotype, with (Figure S4A‐C) or without (Figure S4D‐F) treatment with iPS‐ML/IFN‐β.
Figure 5.
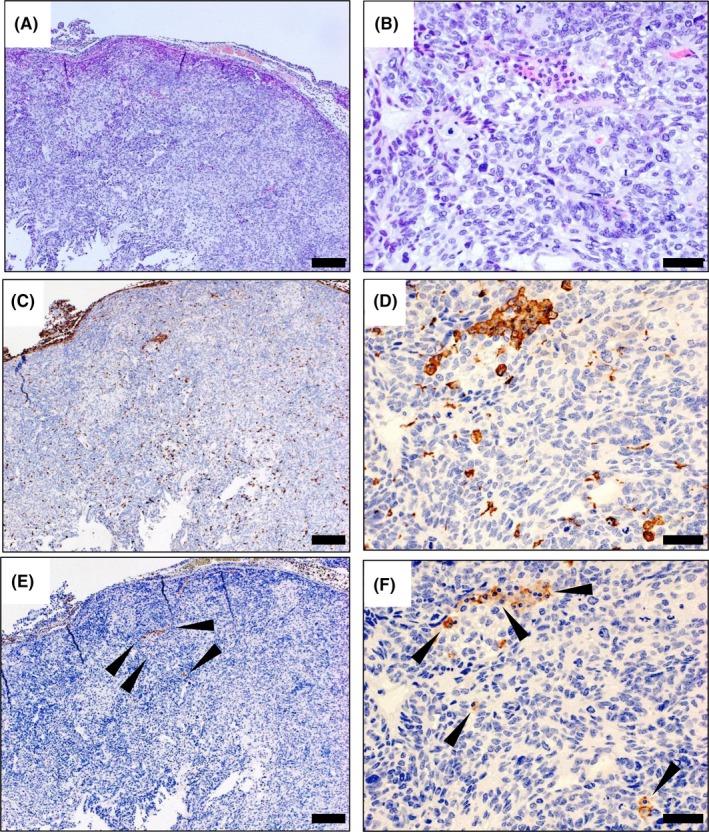
Immunohistochemical analysis of macrophages and induced pluripotent stem‐cell‐derived myelomonocytic cells (iPS‐ML)/interferon (IFN)‐β infiltrated into intraperitoneal tumor tissues of xenograft mice. Tumors were isolated from the mice on day 3 (31 days after the injection of SKOV3 cells), following the last injection of iPS‐ML/INF‐β in the experiments presented in Figure 4. Tumor tissue sections were prepared and stained with H&E (A, B) and immunohistochemistry was used to detect Iba‐1 (a marker for human and mouse myeloid cells. C,D) and CD11b (a marker for human myeloid cells. E,F) in the tumor tissues. The sections were analyzed on a microscope, human CD11b‐positive cells infiltrated into the tumor, although these cells were fewer than those positive for Iba‐1. Arrowheads indicate CD11b‐positive cells. Scale bar: A,C,E, 200 μm; B,D,F, 50 μm
We previously noted that the number of live iPS‐ML/IFN‐β rapidly decreased after their injection into SCID mice.15 The number of iPS‐ML/IFN‐β might have been higher at an earlier time point, and the small number of iPS‐ML/IFN‐β detected in the tumor tissues (Figure 5E,F) might reflect their subsequent decay in vivo. In any case, we observed that iPS‐ML/IFN‐β injected into the tumor‐bearing mice in our xenograft model migrated into the ovarian cancer tissues.
4. DISCUSSION
Macrophages found in clinical tumor samples are alternatively activated and polarized to the so‐called M2 phenotype.17 Accumulated studies have shown that M2‐like macrophages promote tumor progression by several mechanisms.5, 6, 18, 19, 20 M2‐like macrophages are the major producer of vascular endothelial growth factor (VEGF), which induces the angiogenesis that is essential for tumor growth. M2‐like macrophages, as well as myeloid derived suppressor cells (MDSC), suppress the anticancer activity of tumor‐infiltrating T cells and play a key role in the formation of the immunocompromised tumor microenvironment. The tissue‐remodeling activity of M2‐like macrophages that is mediated by macrophage‐derived matrix‐proteases aids in the local invasion and metastasis of the cancer. Moreover, in the peritoneal dissemination of colon, gastric and ovarian cancers, macrophages present in malignant ascites produce factors including IL‐6 and IL‐1β that induce cancer cell proliferation. As we previously reported, cognate cell‐to‐cell interactions between ovarian cancer cells and macrophages in malignant ascites results in activation of Stat3 in both cell types.5 Interactions between macrophages and cancer cells are pivotal in the pathogenesis of peritoneal metastases.5
The tumor‐tissue infiltration of macrophages depends on their expression of receptors for many kinds of leukocyte‐attracting factors, including chemokines, activated complements, and damage‐associated molecular pattern molecules (DAMP).21 A cascade of these factors is considered to be involved in the efficient tumor tissue infiltration of macrophages.
Induced pluripotent stem ML are myelomonocytic cells that are artificially generated from human iPS cells and possess proliferative capacity. They express myelomonocytic cell markers such as CD11b and CD68. In addition, iPS‐ML express various molecules involved in the tissue infiltration of macrophages, including chemokine receptors, complement receptors, Toll‐like receptors (TLR) and MMP. In our previous studies, we used fluorescence microscopy to observe efficient infiltration of iPS‐ML into NUGC4 gastric cancer tumors in the greater omentum and into MKN45 gastric cancer lesions formed in the liver.14, 15 In the current study, immunohistochemical analysis showed an infiltration of iPS‐ML/IFN‐β into SKOV3 ovarian cancer tumor lesions. Moreover, a clustering of iPS‐ML/IFN‐β and SKOV3 cells was observed in vitro.
Interferon‐beta exerts a direct anticancer effect by inducing growth arrest or apoptosis of various cancer cells. Induction of anticancer immunity and inhibition of tumor angiogenesis by IFN‐β have previously been described. In the current study, we evaluated a strategy to use iPS‐ML as a cell‐based drug‐delivery system to make IFN‐β specifically act on cancer cells. To this end, we established a xenograft model of peritoneal metastasis using ovarian cancer SKOV3 cells expressing firefly luciferase. This system enabled us to monitor the progression of the cancer and to assess the effect of the therapeutic intervention. As expected, i.p. injection of iPS‐ML/IFN‐β into tumor‐bearing mice resulted in significant suppression of the progression of i.p. cancer. Regarding the reason for the relatively short life span of iPS‐ML after administration into SCID mice, iPS‐ML may be killed by mouse endogenous natural killer (NK) cells or macrophages, the immune cells active even in immune‐compromised SCID mice. Another possibility may be that, although iPS‐ML proliferate for several months in the appropriate culture media, they cannot survive for long in in vivo environments.
More than half of the patients with advanced or recurrent ovarian cancer present with malignant ascites.22 They present with pain, abdominal swelling, nausea, dyspnea and their quality of life is severely affected. As indicated by the current study, therapy with iPS‐ML/IFN‐β dramatically reduced the volume of ascites as well as the tumor mass. Previous studies by other groups have also found that IFN‐β reduces the retention of ascites and that this effect is mediated by a decreased production of VEGF and maintenance of vascular integrity.8, 23 Our results suggest that i.p. injection of iPS‐ML/IFN‐β may be very useful not only to control cancer but also to control ascites in patients with ovarian cancer. iPS‐ML/IFN‐β given to SCID mice disappeared within 4 days.15 Thus, giving iPS‐ML/IFN‐β twice a week may be necessary to attain a therapeutic effect in clinical application. We are planning to use HLA‐A/B/DR haplotype‐homozygous iPS cells provided by iPS cell stock (Center for iPS Cell Research and Application [CiRA], Kyoto University) in a future clinical application. According to the announcement from CiRA, the iPS cell stock will cover more than 80% of the Japanese population. In addition, we have established a method to disrupt HLA‐A/B/DR genes in iPS‐ML. HLA‐A/B/DR‐deficient iPS‐ML/IFN‐β may be used for the treatment of any patient, irrespective of their HLA type.
In summary, the present study suggests that iPS‐ML/IFN‐β offers a new therapeutic approach for the treatment of patients with advanced or recurrent ovarian cancer. The therapy may be especially effective for controlling malignant ascites that are severely affecting quality of life.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
Supporting information
ACKNOWLEDGMENTS
This work was supported in part by a grant from JSPS KAKENHI (grant no. 17H04272) to SS, and (grant no. 16H05473) to HK. We thank Shelley Robison, PhD, from Edanz Group (http://www.edanzediting.com/ac) for editing a draft of this manuscript.
Imamura Y, Tashiro H, Tsend‐Ayush G, et al. Novel therapeutic strategies for advanced ovarian cancer by using induced pluripotent stem cell‐derived myelomonocytic cells producing interferon beta. Cancer Sci. 2018;109:3403–3410. 10.1111/cas.13775
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S161‐S192. [DOI] [PubMed] [Google Scholar]
- 3. Luvero D, Milani A, Ledermann JA. Treatment options in recurrent ovarian cancer: latest evidence and clinical potential. Ther Adv Med Oncol. 2014;6:229‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7‐30. [DOI] [PubMed] [Google Scholar]
- 5. Takaishi K, Komohara Y, Tashiro H, et al. Involvement of M2‐polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation. Cancer Sci. 2010;101:2128‐2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yin M, Li X, Tan S, et al. Tumor‐associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Invest. 2016;126:4157‐4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yuan X, Zhang J, Li D, Mao F, Du W, Ma X. Prognostic significance of tumor‐associated macrophages in ovarian cancer: a meta‐analysis. Gynecol Oncol. 2017;147:181‐187. [DOI] [PubMed] [Google Scholar]
- 8. Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15:405‐414. [DOI] [PubMed] [Google Scholar]
- 9. Parker BS, Rautela J, Hertzog PJ. Antitumor actions of interferons: implications for cancer therapy. Nat Rev Cancer. 2016;16:131‐144. [DOI] [PubMed] [Google Scholar]
- 10. Sterman DH, Gillespie CT, Carroll RG, et al. Interferon beta adenoviral gene therapy in a patient with ovarian cancer. Nat Clin Pract Oncol. 2006;3:633‐639. [DOI] [PubMed] [Google Scholar]
- 11. Iwamura T, Narumi H, Suzuki T, et al. Novel pegylated interferon‐β as strong suppressor of the malignant ascites in a peritoneal metastasis model of human cancer. Cancer Sci. 2017;108:581‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yi BR, Hwang KA, Aboody KS, Jeung EB, Kim SU, Choi KC. Selective antitumor effect of neural stem cells expressing cytosine deaminase and interferon‐beta against ductal breast cancer cells in cellular and xenograft models. Stem Cell Res. 2014;12:36‐48. [DOI] [PubMed] [Google Scholar]
- 13. Haruta M, Tomita Y, Yuno A, et al. TAP‐deficient human iPS cell‐derived myeloid cell lines as unlimited cell source for dendritic cell‐like antigen‐presenting cells. Gene Ther. 2013;20:504‐513. [DOI] [PubMed] [Google Scholar]
- 14. Koba C, Haruta M, Matsunaga Y, et al. Therapeutic effect of human iPS‐cell derived myeloid cells expressing IFN‐β against peritoneally disseminated cancer in xenograft models. PLoS One. 2013;8:e67567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sakisaka M, Haruta M, Komohara Y, et al. Therapy of primary and metastatic liver cancer by human iPS cell‐derived myeloid cells producing interferon‐β. J Hepatobiliary Pancreat Sci. 2017;24:109‐119. [DOI] [PubMed] [Google Scholar]
- 16. Nakagawa T, Ohnishi K, Kosaki Y, et al. Optimum immunohistochemical procedures for analysis of macrophages in human and mouse formalin fixed paraffin‐embedded tissue samples. J Clin Exp Hematop. 2017;57:31‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23‐35. [DOI] [PubMed] [Google Scholar]
- 19. Crowther M, Brown NJ, Bishop ET, Lewis CE. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol. 2001;70:478‐490. [PubMed] [Google Scholar]
- 20. Noy R, Pollard JW. Tumor‐associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laoui D, Van Overmeire E, De Baetselier P, Van Ginderachter JA, Raes G. Functional relationship between tumor‐associated macrophages and macrophage colony‐stimulating factor as contributors to cancer progression. Front Immunol. 2014;5:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fujimura M. Palliative medicine in the management of ovarian cancer In: Katabuchi H, ed. Frontiers in Ovarian Cancer Science. Singapore: Springer; 2017:305‐314. [Google Scholar]
- 23. Takano S, Ishikawa E, Matsuda M, Yamamoto T, Matsumura A. Interferon‐β inhibits glioma angiogenesis through downregulation of vascular endothelial growth factor and upregulation of vascular endothelial growth factor and upregulation of interferon inducible protein 10. Int J Oncol. 2014;45:1837‐1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


