Abstract
The innate immune receptors, such as toll‐like receptor 3 (TLR3), melanoma differentiation‐associated 5 (MDA5) and retinoic acid‐inducible gene‐I (RIG‐I), have been shown to be differentially expressed in neuroblastoma (NB) and promote dsRNA poly (I:C)‐induced NB suppression in vitro and in vivo. However, the role of another important innate immune cytosolic sensor, laboratory of genetics and physiology 2 (LGP2), in the cancer behavior of NB remains unclear. Here, we demonstrated that the expression levels of LGP2 were either low or undetectable in all NB cell lines tested with or without MYCN amplification. LGP2 expression levels were significantly increased only in NB cells without MYCN amplification, including SK‐N‐AS and SK‐N‐FI after poly (I:C) treatment in vitro and in mouse xenograft models. Ectopic expression of LGP2 in NB cells significantly enhanced poly (I:C)‐induced NB cell death associated with downregulation of MDA5, RIG‐I, MAVS and Bcl‐2, as well as upregulation of Noxa and tBid. By immunofluorescence analyses, LGP2 localized mainly in the cytoplasm of NB cells after poly (I:C) treatment. In human NB tissue samples, cytoplasmic LGP2 expression was positively correlated with histological differentiation and inversely correlated with MYCN amplification. Positive cytoplasmic LGP2 expression in tumor tissues could predict a favorable outcome in NB patients independent of other prognostic factors. In short, LGP2 was effective in promoting poly (I:C)‐induced NB suppression and cytoplasmic LGP2 can serve as an independent favorable prognostic factor in NB patients.
Keywords: LGP2, MDA5, neuroblastoma, polyinosinic‐polycytidylic acid, RIG‐I
1. INTRODUCTION
Neuroblastoma (NB) is the most common extracranial solid cancer in children under 10 years of age.1 Patients with NB can be classified into 4 risk groups according to several clinic‐biological factors, including age, tumor stage, grade of tumor differentiation, and the status of the MYCN oncogene as defined by the International Neuroblastoma Risk Group (INRG).1 Moreover, a high level of MYC/MYCN protein expression has been reported to be associated with aggressive clinical behavior and poor prognosis.2, 3 Children with low‐risk NB have a 5‐year survival rate >90%, whereas those with high‐risk NB have a rate <40%.4 Recent studies revealed that innate immune responses could be used as an indicator to classify patients with high‐risk and low‐risk NB. Compared to high‐risk NB patients, low‐risk NB patients tend to exhibit higher levels of innate immune response,5 suggesting the potential involvement of the innate immune reaction in NB progression.
Cytosolic retinoic acid‐inducible gene (RIG)‐I‐like receptors (RLR) recognize viral double‐stranded (ds) RNA to initiate innate immune responses against pathogens.6 Laboratory of genetics and physiology 2 (LGP2), an RLR family member with the highest RNA‐binding affinity, shares homologous DECH‐box helicase regions with melanoma differentiation‐associated 5 (MDA5) and RIG‐I. Nevertheless, LGP2 is ineffective in triggering downstream signaling by itself due to a lack of tandem N‐terminal interacting caspase activation and recruitment domains (CARD) to interact with mitochondrial antiviral‐signaling protein (MAVS).7 Among these innate immune receptors, MDA5 senses virus‐derived long dsRNA (>1 kbp), whereas RIG‐I is responsible for the recognition of short dsRNA (<1 kbp).8, 9 Intriguingly, LGP2 has both positive and negative effects on the regulation of MDA5 and RIG‐I signaling. While LGP2 binding with RNA can promote MDA5 activation, LGP2 may also function as an inhibitor of MDA5 signaling during Sendai virus infection.10, 11 Moreover, LGP2 may display a biphasic switch to activate MDA5 and RIG‐I in a concentration‐dependent manner.12
Toll‐like receptor 3 (TLR3) and MDA5 have been implicated in the tumor behavior and therapy of NB.13, 14, 15, 16, 17 Stimulation with either high molecular weight (HMW) or low molecular weight (LMW) polyinosinic‐polycytidylic acid [poly (I:C)] can upregulate MDA5 and RIG‐I expression in NB cell lines.13 However, poly (I:C)‐induced NB suppression effect is still limited by induction of MDA5 and TLR3 alone.16
In human melanoma cells and in NOD/SCID mice inoculated with human lung cancer cells, both MDA5 and RIG‐I are involved in pro‐apoptotic signaling.18 Endogenous MDA5 and ATP hydrolysis activity are required for poly (I:C)‐stimulated LGP2 signaling, which is independent of RIG‐I.19, 20
In this study, we aimed to clarify the significance of LGP2 expression in poly (I:C)‐induced NB cell death as well as in clinical tumor behavior.
2. MATERIALS AND METHODS
2.1. Cells and mice
Human NB cell lines SK‐N‐AS, SK‐N‐DZ, IMR‐32, SK‐N‐FI, BE(2)‐M17 and SH‐SY5Y were purchased from the ATCC and maintained according to accepted guidelines. SK‐N‐AS, SK‐N‐DZ and SK‐N‐FI cells were cultured in DMEM supplemented with L‐glutamine; IMR‐32 cells were cultured in Eagle's Minimum Essential Medium supplemented with sodium pyruvate; BE(2)‐M17 cells were cultured in a 1:1 mixture of MEM and F‐12 medium supplemented with sodium pyruvate; SH‐SY‐5Y cells were cultured in a 1:1 mixture of DMEM and F‐12 medium. All the above culture mediums contain 10% (v/v) heat‐inactivated FBS, 10 mM nonessential amino acids and antibiotic‐antimycotic. The cells were cultured at 37°C humidified atmosphere containing 5% CO2.
Male nonobese diabetic‐severe combined immunodeficient (NOD‐SCID) mice, 4 weeks of age, were purchased from BioLASCO Taiwan (Ilan, Taiwan); 10 mg/kg of polyinosinic‐polycytidylic acid high molecular weight [poly (I:C)HMW; Invitrogen, San Diego, CA, USA] administration and xenograft sample processing were performed as described previously.15 Tissues from 3 mice in each group were used for immunohistochemical staining on day 17 or day 27 post–injection.
2.2. Patients and tumor samples
From January 2000 to December 2014, this study enrolled 94 patients with NB who had complete follow‐up data and sufficient tumor tissues for analysis. This study was approved by the Institutional Ethics Committee. The age at diagnosis, sex and primary tumor site for all patients were recorded and analyzed. The histological grades of differentiation were categorized according to the criteria of the International Neuroblastoma Pathology Classification into 4 groups: undifferentiated NB, poorly differentiated NB, differentiating NB and ganglioneuroblastoma.21, 22 Tumor staging was classified according to the International NB Staging System.23 MYCN status was evaluated using chromogenic in situ hybridization analysis of formalin‐fixed paraffin‐embedded tissues.24 Risk‐directed therapies were applied to all patients.25
2.3. Immunohistochemistry
Laboratory of genetics and physiology 2 protein expression in human tumor specimens and in the SK‐N‐AS xenograft tissues was assessed by immunohistochemical analysis. Immunohistochemical staining was performed as previously described.15 Paraffin sections (2 μm) cut from formalin‐fixed paraffin‐embedded block of the xenograft tissue (day 17, n = 3/each group; day 27, n = 5/each group) were incubated overnight at 55°C, and then deparaffinized in xylene and hydrated. The sections were incubated with the anti‐LGP2 antibody (1:200; Proteintech Group) for 1 hour at room temperature. Tissue sections were visualized with ImmPACT diaminobenzidine (DAB) peroxidase substrate (Vector Laboratories, Burlingame, CA, USA). Finally, sections were counterstained with Mayer's hematoxylin (ScyTek Laboratories, Logan, UT, USA) and mounted. Staining scores were defined as 2+ (strong cytoplasmic LGP2 staining, the proportion of stained cells > 60%); 1+ (faint or weak cytoplasmic LGP2 staining, 60% > the proportion of stained cells > 30%); or 0 (no staining and only nuclear LGP2 staining). All the sections were initially scored by a clinical pathologist. Because there was a significant difference in the survival possibility between these 2 groups of patients, both scores 1 and 2 as positive staining and score 0 as negative staining for the further statistical analysis.
2.4. Immunoblotting
Lysates for immunoblotting were prepared from isolated NB cells after treatment with 50 μg/mL of polyinosinic‐polycytidylic acid high molecular weight [poly (I:C)HMW; Invitrogen] or polyinosinic‐polycytidylic acid low molecular weight [poly (I:C)LMW; Invitrogen]. Total protein was extracted by PRO‐PREPTM Protein Extraction Solution (iNtRON Biotechnology; Seoul, Korea). Equal amounts of protein (30 μg) were separated on a 10%‐15% SDS–PAGE and transferred to nitrocellulose membranes. Immunoblotting was performed using antibodies to LGP2 (Proteintech Group), MDA5 (D74E4; Cell Signaling Technology, Danvers, MA, USA), RIG‐I (D14G6; Cell Signaling Technology), TLR3 (Abcam, Cambridge, MA, USA), MAVS (Santa Cruz Biotechnology, Dallas, TX, USA), Bcl‐2 (50E3; Cell Signaling Technology), t‐Bid (Cell Signaling Technology), Noxa (Abcam), β‐actin (Millipole, Billerica, MA, USA) and GAPDH (GeneTex, Irvine, CA, USA). After washing 3 times, membranes were incubated with HRP‐conjugated anti‐mouse (Jackson ImmunoResearch Europe, Newmarket, Suffolk, UK) and anti‐rabbit (Jackson ImmunoResearch) secondary antibody for 60 minutes at room temperature. Immunoblotting analysis was performed as described previously.13
2.5. Immunofluorescence staining
SK‐N‐AS cells stimulated by poly (I:C) HMW for 24 hours were incubated in the chamber slide. Cells were fixed in 3.7% formaldehyde, washed, and then permeabilized with .1% Triton X‐100 in PBS for 10 minutes. Cells were labeled with mouse monoclonal anti‐human LGP2 (1:100; Santa Cruz Biotechnology) antibody, rabbit monoclonal anti‐human MDA5 (1:100; D74E4; Cell Signaling Technology) antibody or rabbit monoclonal anti‐human RIG‐I (1:100; D14G6; Cell Signaling Technology) antibody. Secondary antibodies included Alexa 594‐conjugated goat anti‐rabbit and Alexa 488‐conjugated donkey anti‐mouse (Abcam). The incubated slides of antibodies were washed and then mounted by DAPI fluoromount‐G (SouthernBiotech, Birmingham, AL, USA). Images were acquired by using a fluorescence microscope (Leica, Wetzlar, Germany).
2.6. Immunoprecipitation
For each sample, 200 μg of total protein was performed and incubated with 5 μg of anti‐LGP2 antibody (Proteintech Group) or normal mouse IgG (Millipore, Billerica, MA, USA) as negative control at 4°C overnight. PureProteome Protein A Magnetic Beads (50 μL; Millipore) were added to all samples and control as per the manufacturer's instructions. Pre‐formed antibody‐antigen complex was combined with the beads and incubated for 10 minutes at room temperature, and then washed multiple times with PBS. Immunoprecipitated proteins isolated from magnetic beads were subjected to electrophoresis and immunoblotting analysis.
2.7. Flow cytometric analysis of apoptosis, cell viability and cell cycle
Apoptosis and cell viability cells were measured by flow cytometric analysis following propidium iodide (PI; BD Biosciences, San Jose, CA, USA) staining and trypan blue exclusion assays (BioWhittaker, MD, USA), respectively. After siRNA or plasmid transfection, cells were harvested and incubated in PBS containing PI (2.5 μg/mL) for 15 minutes or .04% trypan blue for 10 minutes at room temperature. The staining procedure was performed as described previously13 and analyzed using a FACS (BD Biosciences).
The percentage of cells in G0/G1, S and G2/M phases was determined by flow cytometry analysis by propidium iodide staining (PI; BD Biosciences). SK‐N‐AS cells were transfected with 0, 2, 4 and 6 μg of LGP2 plasmid. Cells were fixed with ice‐cold 70% PBS‐ethanol overnight at −20°C. Then, the fixed cells were washed with PBS and incubated in PBS containing 10% Triton X‐100, PI (20 mg/mL) and RNase A (Boehringer Mannheim, Indianapolis, IN, USA) for 30 minutes at room temperature. Samples were analyzed using a FACSCaliber (BD Biosciences).
2.8. Quantitative Real‐time PCR
Total RNA was extracted by using TRIzol Reagent (Invitrogen) from cells. After quantification, total RNA (3 μg) was used to produce cDNA using M‐MLV Reverse Transcriptase following the protocol for transcription (Promega, Madison, WI, USA). Quantitative PCR analysis was performed using LightCycler 480 SYBR Green I Master (Roche diagnostics, Mannheim, Germany). β‐actin was used as an internal control gene to analyze the mRNA expression. The quantification was achieved by means of the Roche LightCycler Sequence Detection System (Roche). Primers were used as follows:
DHX58 (LGP2) forward primer 5′‐ CCATTTCTGATTTCTGCTCTCTGC‐3′, reverse primer 5′‐ AGATGGAAACTGAAACTGCGCC‐3′; IFIH1(MDA5) forward primer 5′‐ GACTCGGGAATTCGTGGAGG‐3′, reverse primer 5′‐ CTCAAACGATGGAGAGGGCA‐3′; DDX58 (RIG‐I) forward primer 5′‐ CTCCCGGCACAGAAGTGTAT‐3′, reverse primer 5′‐ CTTCCTCTGCCTCTGGTTTG‐3′, MAVS forward primer 5′‐ GCAATGCCGTTTGCTGAAGA‐3′, reverse primer 5′‐ GTCCGAGGTCCCTGGTCTCT‐3′; NOXA forward primer 5′‐ TCACCGTGTGTAGTTGG CAT‐3′, reverse primer 5′‐ AGCTGAACACGAACAGTCCT‐3′; BCL2 forward primer 5′‐ CTGGGATGCCTTTGTGGAAC‐3′, reverse primer 5′‐ GCAGGCATGTTGACTTCACT‐3′; ACTB (β‐actin) forward primer 5′‐ TCACCCACACTGTGCCCATCTACGA‐3′, reverse primer 5′‐ CAGCGGAACCGCTCATTGCCAATGG‐3′.
2.9. Ethics statement
The study was approved by the Ethics Committee of Chang Gung Medical Foundation (103‐6573B issued by IRB of Chang Gung Medical Foundation on 31 December 2014), and all patients or their guardians provided written informed consent to participate in the study in accordance with the Declaration of Helsinki. Animal procedures were approved and performed by the Institutional Animal Care and Use Committee (IACUC) of the Kaohsiung Chang Gung Memorial Hospital. All experiments were performed in accordance with relevant guidelines and regulations.
2.10. Statistical analysis
The data represent at least triplicate analyses for each experiment and are expressed as mean ± standard deviation (SD). Statistical comparisons between groups were conducted using the 2‐tailed Student's t test for continuous data and the χ2‐test for categorical data. Survival analysis was performed using the Kaplan‐Meier method with the Wilcoxon log‐rank test for comparing differences between groups. P‐values < .05 were considered to indicate statistical significance.
3. RESULTS
3.1. Differential expression of laboratory of genetics and physiology 2 in neuroblastoma cell lines and mouse xenograft models after poly (I:C) administration
To characterize the responses of LGP2 expression in NB cells after poly (I:C) stimulation, the expression levels of LGP2 in 6 human NB cell lines, including 3 without MYCN amplification (SK‐N‐AS, SK‐N‐FI, and SH‐SY5Y) and 3 with MYCN amplification (SK‐N‐DZ, IMR‐32 and BE(2)‐M17), were evaluated before and after poly (I:C) treatment. In the absence of poly (I:C), the expression levels of LGP2 were either low (IMR‐32 and BE(2)‐M17) or barely detected (SK‐N‐AS, SK‐N‐DZ, SK‐N‐FI, and SH‐SY5Y) in all cell lines. Treatment with either HMW or LMW poly (I:C) only enhanced LGP2 expression in 2 NB cell lines without MYCN amplification (SK‐N‐AS and SK‐N‐FI, Figure 1A). This finding was similar to that of MDA5 as reported in our previous study.13
Figure 1.
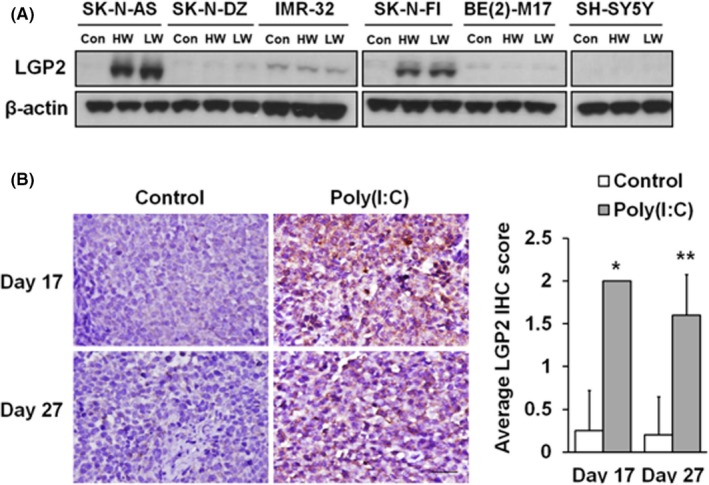
Laboratory of genetics and physiology 2 (LGP2) expression is associated with poly (I:C) stimulation in neuroblastoma (NB) cells and mouse xenografts. A, Immunoblotting analysis of relative levels of LGP2 expression is shown in 6 human NB cell lines in the presence/absence of poly (I:C) stimulation. Upregulation of LGP2 was observed in SK‐N‐AS and SK‐N‐FI but not in the other cell lines after treatment. Con, control. HW, high molecular weight poly (I:C). LW, low molecular weight poly (I:C). B, Representative images (left) and quantitative analysis (right) of immunohistochemical staining for LGP2 performed on tissue sections from SK‐N‐AS xenografts on day 17 (n = 3 in each group) and day 27 (n = 5 in each group). *P < .05, **P < .01 vs control group. Original magnification, ×400. Scale bar, 30 μm
We have shown the therapeutic results of poly (I:C) treatment that can inhibit tumor growth in mice bearing subcutaneous SK‐N‐AS xenografts.15 In this study, poly (I:C) administration strongly enhanced LGP2 expression in tumor tissues of SK‐N‐AS xenograft, and SK‐N‐DZ xenograft was used as a negative control (Figures 1A,S1).
3.2. Ectopic laboratory of genetics and physiology 2 overexpression may sensitize neuroblastoma cells to poly (I:C)‐induced cell death through activation of t‐Bid and Noxa with simultaneous suppression of MDA5, RIG‐I, MAVS and Bcl‐2
To further explore the functions of LGP2 expression in NB, full‐length LGP2 cDNA was transfected into SK‐N‐AS and SK‐N‐FI cells, and the levels of LGP2, MDA5, RIG‐I, MAVS, Bcl‐2, t‐Bid and Noxa expression, as well as cell survival were evaluated. The results showed that ectopic expression of LGP2 could upregulate MDA5 and RIG‐I mRNA and protein expression in a dose‐dependent manner (Figures 2A,S2A,B,S3,S4). In addition, a gradual increase to a maximal 20% of apoptotic cell death following LGP2 overexpression was also observed (Figures 2B,S5).
Figure 2.
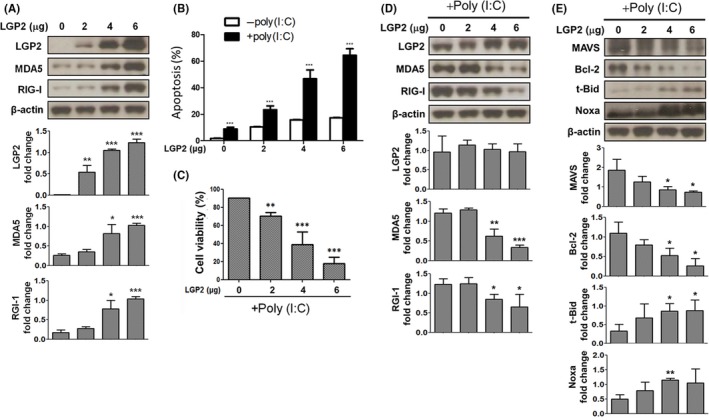
Overexpression of laboratory of genetics and physiology 2 (LGP2) enhanced poly (I:C)‐induced neuroblastoma (NB) suppression and downstream signals. SK‐N‐AS cells were transfected with increasing doses of LGP2 expressing constructs (0, 2, 4, 6 μg) in the presence/absence of poly (I:C) stimulation. A, Protein expression levels of MDA5 and RIG‐I induced by increasing of LGP2 were examined by immunoblotting. B, Apoptosis of NB cells was measured by PI staining in the absence of poly (I:C). Effects of LGP2 overexpression on (B) apoptosis and (C) cell survival in NB cells followed by poly (I:C) stimulation. D, Cell lysates were collected at the 48 h transfection times followed by poly (I:C) treatment for 24 h. Expression levels of poly (I:C)‐induced signals were evaluated by immunoblotting. Data represent mean ± SD of 3 independent experiments. *P < .05
Upon poly (I:C) stimulation, LGP2‐overexpressed NB cells further displayed a dramatic dose‐dependent increase in apoptotic cell death, with the highest level of 70% (Figures 2B,C,S6). The protein expression of MDA5 and RIG‐I decreased gradually in NB cells following LGP2 overexpression and poly (I:C) stimulation (Figure 2D). The adaptor protein MAVS and anti‐apoptotic Bcl‐2 protein downregulation, and BH3‐only proteins, t‐Bid and Noxa, upregulation were also demonstrated in NB cells transfected with a high dose of LGP2 cDNA and poly (I:C) (Figure 2E). However, except for MAVS, the protein level changes in Bcl‐2/Noxa/t‐Bid after poly (I:C) treatment were less significant in mRNA levels (Figures 2E,S2C). In the cell cycle, we also found an increase in the rate of cells in Sub‐G0 phase when cells were LGP2‐overexpressed or treated by poly (I:C) (Figure S7).16
3.3. Predominant cytosolic localization of laboratory of genetics and physiology 2 in neuroblastoma cells, in association with MDA5 and RIG‐I after poly (I:C) stimulation
To clarify the interaction of LGP2 with MDA5 or RIG‐I, we assessed the subcellular distribution of LGP2 triggered by poly (I:C). As shown in Figures 3A,B and S8, 24 hours of poly (I:C) treatment triggered predominant cytosolic localization of LGP2, MDA5 and RIG‐I in SK‐N‐AS cells. LGP2 was further demonstrated to be colocalized with MDA5 and RIG‐I in the cytosol of SK‐N‐AS cells by using an immunofluorescence assay (Figure 3B) and had a direct physical association with both MDA5 and RIG‐I upon stimulation with poly (I:C), which was determined by immunoprecipitation and immunoblotting (Figure 3C).
Figure 3.
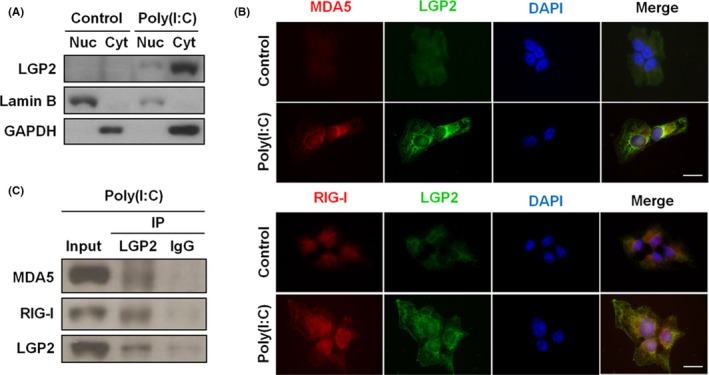
Laboratory of genetics and physiology 2 (LGP2) colocalizes with MDA5 or RIG‐I in the cytosol of SK‐N‐AS cells after poly (I:C) stimulation. A, Immunoblot analysis for nuclear (Nuc) and cytosolic (Cyt) extracts from cells in the presence or absence of poly (I:C) using anti‐LGP2, anti‐lamin B and anti‐GAPDH antibodies. Lamin B and GAPDH were used as nuclear and cytosolic markers, respectively. B, Representative immunofluorescence images of cells showed colocalization of nuclear DNA (DAPI) (blue), LGP2 (green) with MDA5 (red, upper panel) or RIG‐I (red, lower panel) following poly (I:C) stimulation. (C) Cell lysate was extracted from cells in the presence of poly (I:C), and then, immunoprecipitation (IP) was performed using anti‐LGP2 or anti‐IgG antibody. The expressions of MDA5, RIG‐I and LGP2 were detected by immunoblotting. IgG mouse was used as negative control
3.4. Cytoplasmic laboratory of genetics and physiology 2 expression in human neuroblastoma tissues correlates with histological grade of differentiation and favorable outcome of the neuroblastoma patients
To further explore the clinical significance of LGP2 expression, LGP2 immunostaining in human NB tumor tissues was performed to correlate with prognostic factors and the outcome of NB patients. Of the 94 NB tumors, 41 demonstrated LGP2‐positive immunostaining (1+ and 2+) in the cytoplasm of NB cells. No LGP2 immunostaining was detected in the stromal cells. Nine cases showing only nuclear staining were designated as negative immunostaining in the clinical analysis because the in vitro assays indicated that LGP2 carried out its cellular functions primarily in the cytoplasm. Further analysis revealed that LGP2‐positive cytoplasmic immunostaining was strongly correlated with the histological grade of differentiation (P = .006, Figure 4A‐D and Table 1), but inversely correlated with MYCN amplification (P = .032, Table 1).
Figure 4.
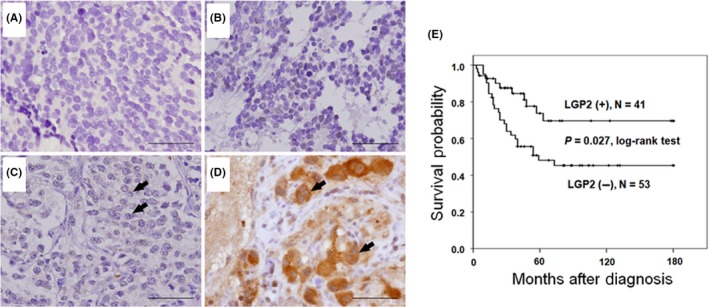
Immunostaining for laboratory of genetics and physiology 2 (LGP2) in human neuroblastoma (NB) tissues and patient survival. Representative pictures of immunostaining in NB tumors with various degrees of histological differentiation, including (A) undifferentiated neuroblastoma, (B) poorly differentiated neuroblastoma, (C) differentiated neuroblastoma and (D) ganglioneuroblastoma. Stronger cytoplasmic staining of LGP2 was found in ganglioneuroblastoma than in the other 3 histological types. Arrows indicate positive LGP2 staining. Original magnification, ×400. Scale bar, 50 μm. E, Kaplan‐Meier survival analysis in patients with NB according to LGP2 immunostaining. P‐value was calculated using a log‐rank test
Table 1.
Laboratory of genetics and physiology 2 (LGP2) expression and clinicopathologic and biologic characteristics of neuroblastoma
| Cases | Positive cytoplasmic LGP2 expression (%) | P value | |
|---|---|---|---|
| Sex | |||
| Male | 58 | 23 (39.7) | .221 |
| Female | 36 | 18 (50.0) | |
| Primary tumor site | |||
| Adrenal | 66 | 29 (43.9) | .553 |
| Extra‐adrenal | 28 | 12 (42.9) | |
| Age at diagnosis | |||
| ≤1.5 year | 37 | 15 (40.5) | .394 |
| >1.5 year | 57 | 26 (45.6) | |
| Clinical stage | |||
| 1, 2, 4S | 28 | 13 (46.4) | .447 |
| 3, 4 | 66 | 28 (42.4) | |
| Tumor histology | |||
| Undifferentiated NB | 26 | 6 (23.1) | .006 |
| Poorly differentiated NB | 38 | 21 (55.3) | |
| Differentiating NB | 11 | 3 (27.3) | |
| GNB | 19 | 12 (63.2) | |
| MYCN | |||
| Amplified | 21 | 5 (23.8) | .032 |
| Non‐amplified | 73 | 36 (49.3) | |
GNB, ganglioneuroblastoma; NB, neuroblastoma.
Kaplan‐Meier analysis revealed that NB patients with positive LGP2 cytoplasmic immunostaining tumors had a higher likelihood of predictive 5‐year survival than those with negative LGP2 immunostaining tumors (P = .027, log‐rank test; Figure 4E). In advanced stage, positive cytoplasmic LGP2 predicted a better survival probability for NB patients (P = .044, log‐rank test; Figure S9). In addition to clinical stage, MYCN status, and histological grade of differentiation, multivariate analysis revealed that cytoplasmic LGP2 immunostaining could also serve as an independent prognostic factor of NB patients (Table 2).
Table 2.
Clinicopathologic and biologic factors affecting survival rate
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P‐value | RR | 95% CI | P‐value | |
| Age at diagnosis: >1 year vs ≤1 year | 4.382 | 2.242‐8.567 | <.001 | 2.147 | .815‐5.654 | .122 |
| Clinical stage: advanced (3, 4) vs early (1, 2, 4S) | 12.484 | 4.536‐34.361 | <.001 | 5.354 | 1.094‐26.208 | .038 |
| MYCN: amplified vs non‐amplified | 3.840 | 2.379‐6.197 | <.001 | 2.261 | 1.118‐4.576 | .023 |
| LGP2 expression: negative vs positive | 2.282 | 1.100‐4.735 | .027 | 2.300 | 1.098‐4.818 | .027 |
| Histology: undifferentiated vs differentiated | 2.312 | 1.308‐4.087 | .004 | 3.354 | 1.370‐8.209 | .008 |
4. DISCUSSION
This study demonstrated for the first time that poly (I:C) treatment could significantly increase LGP2 expression in the NB cell lines SK‐N‐AS and SK‐N‐FI, and in SK‐N‐AS xenograft tumor tissues. This character of LGP2 reaction to poly (I:C) treatment in NB cells is similar to those of MDA5 and TLR3 as described in our previous studies.13, 15 Colocalization of LGP2 with MDA5 and RIG‐I in response to poly (I:C) treatment implicates the requirement of MDA5 and RIG‐I for LGP2 to carry out its cellular functions as LGP2 lacks a CARD domain. Exogenous overexpression of LGP2 can sensitize NB cells to poly (I:C)‐triggered cell death through increasing sub‐G1 phase and activation of t‐Bid and Noxa with the suppression of MAVS and Bcl‐2. The unique characteristics of LGP2 expression in NB cells were further confirmed in human tumors by the finding that high cytoplasmic expression of LGP2 in NB tumor tissues was associated with differentiated tumor histology and predicted a favorable patient outcome. The above evidence suggests that LGP2 may potentially be a tumor suppressor in NB.
In mammalian cells, low abundance of LGP2 is exhibited during steady‐state conditions, and antiviral stimulation induces increased LGP2 expression.26 LGP2 deficiency leads to susceptibility to infection by dsRNA viruses.27, 28, 29 During infection with RNA virus, LGP2 mediates the activation of MDA5 and RIG‐I, and alters RNA‐dependent protein‐protein interactions.10 In the lymphoid organs, LGP2 is not essential for induction of innate immune responses, even if it could suppress CD95‐mediated apoptosis signaling by promoting CD8 (+) T cell survival and fitness.30 These studies reveal that LGP2 has a multifaceted function in the regulation of innate immune response.
Upregulation of LGP2 expression is observed in many types of cancer cells, including colorectal adenocarcinoma cells, glioblastoma cells, and head and neck squamous cell carcinoma cells, presumably to protect tumor cells from ionizing radiation‐induced killing.31 Our data showed that overexpression of exogenous LGP2 could sensitize NB cell death, particularly upon poly (I:C) stimulation. Using nuclear and cytoplasmic fractionation, immunoblotting and immunofluorescence staining, we found that most LGP2, MDA5 and RIG‐I were localized in the cytosol after poly (I:C) administration. In cytosol, LGP2 has been shown to modulate RLR signaling incorporation with MDA5 or RIG‐I as LGP2 lacks CARD to activate MAVS.7, 32 Moreover, we also observed a significant decrease in MDA5 and RIG‐I in LGP2‐overexpressed NB cells upon poly (I:C) stimulation that may be due to negative interaction between LGP2 and MDA5 or RIG‐I. The results of immunoprecipitation and immunoblotting (Figure 3B,C) demonstrated the direct association of LGP2 with MDA5 and RIG‐I. However, the mechanism underlying their relationships upon poly (I:C) stimulation is still unclear.
Our previous studies demonstrated that poly (I:C)‐induced NB cell death involves the intrinsic mitochondrial apoptotic pathway.16 Noxa and t‐Bid can induce apoptosis by causing mitochondrial dysfunction.33 The present study showed that LGP2 overexpression could increase the expression of Noxa and t‐Bid and decrease the expression of Bcl‐2. Noxa has been shown to be essential for MDA‐5‐ and RIG‐I‐initiated apoptosis.18 Furthermore, Noxa‐mediated apoptosis induced by modified vaccinia virus Ankara was found to be through MDA5/RIG‐I/MAVS signaling.34 In human melanoma cells, MDA5 and RIG‐I activate Noxa to induce the IFN‐independent and p53‐independent pro‐apoptotic signaling pathway.18 It has also been shown that LGP2 facilitates the MDA5‐RNA interaction. However, LGP2 accumulation could interfere with MAVS to block MDA5 signaling.20, 26 Except for MAVS, the downregulation of the mRNA levels of Bcl‐2/Noxa/t‐Bid by overexpressed LGP2 and poly (I:C) treatment was less evident than for protein levels, suggesting that LGP2 might induce cell death through Bcl‐2/Noxa/t‐Bid pathway via translational rather than transcriptional regulation.
Laboratory of genetics and physiology 2 has been defined as a cytosolic RNA sensor that preferentially binds double‐stranded RNA.6 After poly (I:C) stimulation, we found that LGP2 is localized mainly in the cytoplasm of NB cells. Our previous study has shown that poly (I:C) stimulation may promote the expression of neuronal differentiation markers.14 Consistent with this finding, positive cytoplasmic LGP2 staining in human NB tissues is correlated with the histological grade of differentiation and predicted favorable clinical tumor behavior.
In summary, we demonstrated a potential role of LGP2 in the suppression of NB. Overexpression of LGP2 may play a positive role on poly (I:C)‐induced cell death through the activation of Noxa and t‐Bid with the suppression of Bcl‐2, and MDA5, RIG‐I and MAVS signaling. Cytoplasmic LGP2 may be involved in the poly (I:C)‐mediated innate immune mechanism and, thus, provides new insight into the pathogenesis and therapy of NB.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Supporting information
Lin L‐L, Huang C‐C, Wu M‐T, Hsu W‐M, Chuang J‐H. Innate immune sensor laboratory of genetics and physiology 2 suppresses tumor cell growth and functions as a prognostic marker in neuroblastoma. Cancer Sci. 2018;109:3494–3502. 10.1111/cas.13790
Contributor Information
Wen‐Ming Hsu, Email: billwmhsu@gmail.com.
Jiin‐Haur Chuang, Email: jhchuang@cgmh.org.tw.
REFERENCES
- 1. Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27:289‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang LL, Suganuma R, Ikegaki N, et al. Neuroblastoma of undifferentiated subtype, prognostic significance of prominent nucleolar formation, and MYC/MYCN protein expression: a report from the Children's Oncology Group. Cancer. 2013;119:3718‐3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Westermann F, Muth D, Benner A, et al. Distinct transcriptional MYCN/c‐MYC activities are associated with spontaneous regression or malignant progression in neuroblastomas. Genome Biol. 2008;9:R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maris JM, Hogarty MD, Bagatell R, et al. Neuroblastoma. Lancet. 2007;369:2106‐2120. [DOI] [PubMed] [Google Scholar]
- 5. Gowda M, Godder K, Kmieciak M, et al. Distinct signatures of the immune responses in low risk versus high risk neuroblastoma. J Transl Med. 2011;9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783‐801. [DOI] [PubMed] [Google Scholar]
- 7. Yoneyama M, Kikuchi M, Matsumoto K, et al. Shared and unique functions of the DExD/H‐box helicases RIG‐I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851‐2858. [DOI] [PubMed] [Google Scholar]
- 8. Hornung V, Ellegast J, Kim S, et al. 5′‐Triphosphate RNA is the ligand for RIG‐I. Science. 2006;314:994‐997. [DOI] [PubMed] [Google Scholar]
- 9. Kato H, Takeuchi O, Mikamo‐Satoh E, et al. Length‐dependent recognition of double‐stranded ribonucleic acids by retinoic acid‐inducible gene‐I and melanoma differentiation‐associated gene 5. J Exp Med. 2008;205:1601‐1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uchikawa E, Lethier M, Malet H, et al. Structural analysis of dsRNA binding to anti‐viral pattern recognition receptors LGP2 and MDA5. Mol Cell. 2016;62:586‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Komuro A, Horvath CM. RNA‐ and virus‐independent inhibition of antiviral signaling by RNA helicase LGP2. J Virol. 2006;80:12332‐12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodriguez KR, Bruns AM, Horvath CM. MDA5 and LGP2: accomplices and antagonists of antiviral signal transduction. J Virol. 2014;88:8194‐8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsu WM, Huang CC, Lee HY, et al. MDA5 complements TLR3 in suppression of neuroblastoma. Oncotarget. 2015;6:24935‐24946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsu WM, Huang CC, Wu PY, et al. Toll‐like receptor 3 expression inhibits cell invasion and migration and predicts a favorable prognosis in neuroblastoma. Cancer Lett. 2013;336:338‐346. [DOI] [PubMed] [Google Scholar]
- 15. Lin LL, Huang CC, Wu CL, et al. Downregulation of c‐Myc is involved in TLR3‐mediated tumor death of neuroblastoma xenografts. Lab Invest. 2016;96:719‐730. [DOI] [PubMed] [Google Scholar]
- 16. Chuang JH, Lin TK, Tai MH, et al. Preferential involvement of mitochondria in Toll‐like receptor 3 agonist‐induced neuroblastoma cell apoptosis, but not in inhibition of cell growth. Apoptosis. 2012;17:335‐348. [DOI] [PubMed] [Google Scholar]
- 17. Chuang JH, Chuang HC, Huang CC, et al. Differential toll‐like receptor 3 (TLR3) expression and apoptotic response to TLR3 agonist in human neuroblastoma cells. J Biomed Sci. 2011;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Besch R, Poeck H, Hohenauer T, et al. Proapoptotic signaling induced by RIG‐I and MDA‐5 results in type I interferon‐independent apoptosis in human melanoma cells. J Clin Invest. 2009;119:2399‐2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Childs KS, Randall RE, Goodbourn S. LGP2 plays a critical role in sensitizing mda‐5 to activation by double‐stranded RNA. PLoS ONE. 2013;8:e64202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bruns AM, Pollpeter D, Hadizadeh N, et al. ATP hydrolysis enhances RNA recognition and antiviral signal transduction by the innate immune sensor, laboratory of genetics and physiology 2 (LGP2). J Biol Chem. 2013;288:938‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimada H, Ambros IM, Dehner LP, et al. Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer. 1999;86:349‐363. [PubMed] [Google Scholar]
- 22. Shimada H, Ambros IM, Dehner LP, et al. The international neuroblastoma pathology classification (the Shimada system). Cancer. 1999;86:364‐372. [PubMed] [Google Scholar]
- 23. Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466‐1477. [DOI] [PubMed] [Google Scholar]
- 24. Tsai HY, Hsi BL, Hung IJ, et al. Correlation of MYCN amplification with MCM7 protein expression in neuroblastomas: a chromogenic in situ hybridization study in paraffin sections. Hum Pathol. 2004;35:1397‐1403. [DOI] [PubMed] [Google Scholar]
- 25. Castleberry RP. Neuroblastoma. Eur J Cancer. 1997;33:1430‐1437. [DOI] [PubMed] [Google Scholar]
- 26. Bruns AM, Horvath CM. Activation of RIG‐I‐like receptor signal transduction. Crit Rev Biochem Mol Biol. 2012;47:194‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kato H, Takeuchi O, Sato S, et al. Differential roles of MDA5 and RIG‐I helicases in the recognition of RNA viruses. Nature. 2006;441:101‐105. [DOI] [PubMed] [Google Scholar]
- 28. Satoh T, Kato H, Kumagai Y, et al. LGP2 is a positive regulator of RIG‐I‐ and MDA5‐mediated antiviral responses. Proc Natl Acad Sci USA. 2010;107:1512‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deddouche S, Goubau D, Rehwinkel J, et al. Identification of an LGP2‐associated MDA5 agonist in picornavirus‐infected cells. Elife. 2014;3:e01535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suthar MS, Ramos HJ, Brassil MM, et al. The RIG‐I‐like receptor LGP2 controls CD8(+) T cell survival and fitness. Immunity. 2012;37:235‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Widau RC, Parekh AD, Ranck MC, et al. RIG‐I‐like receptor LGP2 protects tumor cells from ionizing radiation. Proc Natl Acad Sci USA. 2014;111:E484‐E491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reikine S, Nguyen JB, Modis Y. Pattern recognition and signaling mechanisms of RIG‐I and MDA5. Front Immunol. 2014;5:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Strasser A. The role of BH3‐only proteins in the immune system. Nat Rev Immunol. 2005;5:189‐200. [DOI] [PubMed] [Google Scholar]
- 34. Eitz Ferrer P, Potthoff S, Kirschnek S, et al. Induction of Noxa‐mediated apoptosis by modified vaccinia virus Ankara depends on viral recognition by cytosolic helicases, leading to IRF‐3/IFN‐beta‐dependent induction of pro‐apoptotic Noxa. PLoS Pathog. 2011;7:e1002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


