Abstract
We previously reported the efficacy of anti‐cancer therapy with hyperthermia using an alternating magnetic field (AMF) and a magnetic compound. In the course of the study, unexpectedly, we found that an AMF enhances the cytotoxicity of Compound C, an activated protein kinase (AMPK) inhibitor, although this compound is not magnetic. Therefore, we examined the cellular mechanism of AMF‐induced cytotoxicity of Compound C in cultured human glioblastoma (GB) cells. An AMF (280 kHz, 250 Arms) for 30 minutes significantly enhanced the cytotoxicity of Compound C and promoted apoptosis towards several human GB cell lines in vitro. The AMF also increased Compound C‐induced cell‐cycle arrest of GB cells at the G2 phase and, thus, inhibited cell proliferation. The AMF increased Compound C‐induced reactive oxygen species production. Furthermore, the AMF decreased ERK phosphorylation in the presence of Compound C and suppressed the protective autophagy induced by this compound. The application of an AMF in cancer chemotherapy may be a simple and promising method, which might reduce the doses of drugs used in future cancer treatment and, therefore, the associated side effects.
Keywords: alternating magnetic field, compound C, cytotoxicity, glioblastoma, proliferation
1. INTRODUCTION
Glioblastoma (GB), known as a WHO Grade IV astrocytoma, is the most common primary brain tumor.1 It has a very poor prognosis because of its invasiveness, its resistance to treatment, and the difficulty of total resection. Currently, the standard of treatment for glioblastoma patients is adjuvant chemotherapy with temozolomide (TMZ) combined with extended focal radiotherapy.2 However, the addition of chemotherapy only prolongs the average survival period (median 14.6 months) compared to radiation alone (median 12.1 months). The treatment outcome has changed little in recent decades. Therefore, a novel and more effective medical treatment with few complications needs to be developed.
It is well known that AMP‐activated protein kinase (AMPK) is a serine/threonine kinase and a molecular hub for cellular metabolic control.3 AMPK is the downstream component of a kinase cascade as a sensor of the intracellular energy charge, which is activated by increasing AMP coupled with decreasing ATP.4 AMPK is activated under environmental conditions in various cells and plays a critical role in systemic energy balance. Rois et al demonstrate that AMPK activation is essential for the proliferation of astrocytic tumor cells by promoting cell‐cycle progression in both mice and humans.5
Compound C (6‐[4‐(2‐Piperidin‐1‐ylethoxy) phenyl]‐3‐pyridin‐4‐ylpyrazolo [1,5‐a]pyrimidine) is a cell permeable AMPK inhibitor, which is the only available agent. Compound C (BML‐275) induced apoptosis in myeloma, glioma, breast carcinoma and prostate cancer cells.6, 7, 8, 9 Compound C also induced the accumulation of G2‐M in the cell cycle in glioma.8 Lui et al report that Compound C is an extremely potent anti‐glioma agent; however, it has little effect on the human astrocyte.10
Recently, intratumoral hyperthermic therapy for GB using magnetic iron‐oxide nanoparticles (Fe3O4) directly injected into the tumor and exposed to an alternating magnetic field (AMF) to generate heat has been investigated.11, 12 Furthermore, Optune (formerly known as NovoTTF‐100A, Novocure, Jersey, Israel) was approved for use with TMZ for the treatment of adults with newly diagnosed supratentorial glioblastoma multiforme (GBM) after maximal surgical reduction and completion of radiation.13 The common feature of these novel treatments is the application of a physical phenomenon.
We have previously examined hyperthermia treatment for tumors using magnetic material and an AMF.14, 15, 16, 17 In the course of our study, we unexpectedly found that an AMF increased the cytotoxicity of an AMP‐activated protein kinase (AMPK) inhibitor, that is, Compound C, which is not a magnetic material. Several reports have explored the application of an AMF. In 1994, whole body pulsed magnetic field (PMF) exposure for 1 hour suppressed tumor growth when combined with cisplatin, carboplatin or doxorubicin treatment in vivo.18 Similarly, a PMF (1.5 mT peak, repeated at 1 and 25 Hz) increased the cytotoxicity of vincristine (VCR), mitomycin C (MMC) and cisplatin.19 However, the mechanism remains elusive. Our condition of the AMF (280 kHz, 250 Arms) is much different from the previous reports, especially the frequency.
This is the first report of an AMF being applied in the treatment of GB in combination with the anti‐tumor agent, Compound C. We propose that the application of an AMF for cancer therapy may be a simple and promising method, which might reduce the dose and, thus, the side effects of chemotherapy in future cancer treatments.
2. MATERIALS AND METHODS
2.1. Reagents and cell culture
Compound C (6‐[4‐[2‐(1‐Piperidinyl)ethoxy]phenyl]‐3‐(4‐pyridinyl)pyrazolo[1,5‐a]pyrimidine dihydrochloride: Dorsomorphin dihydrochlo ride) was purchased from Abcam. TMZ, carmustine (1,3‐bis(2‐chlorethyl)‐1‐nitrosourea; BCNU) and U0126 were purchased from Sigma. Chloroquine (CQ) was purchased from Thermo Fisher Scientific. Human GB cell lines, U251 (U251MG‐Luc, JCRB1386) and A172 (A‐172, JCRB0228), and the human metastatic mammary carcinoma cell line MCF7 (MCF7, JCRB0134) was purchased from the Japanese Collection of Research Bioresources (JCRB) Cell Bank. The U251 cell line was engineered to express the firefly luciferase gene. The human GB cell line T98 (T98‐G, CRL‐1690) was purchased from the ATCC. The human pancreatic cancer cell line PANC1 (PANC‐1, RCB2095) was purchased from RIKEN BioResource Research Center (RIKEN BRC). Normal human astrocytes (NHA) were purchased from the Lonza group. In all cases, early‐passage cultures were stored and used for the experiments. The GB cell lines were cultured in DMEM (Sigma‐Aldrich) containing 10% FBS and 1% penicillin‐streptomycin. MCF7 and PANC‐1 were cultured in RPMI‐1640 with L‐glutamine and phenol red medium containing 10% FBS and 1% penicillin‐streptomycin. The normal human astrocytes (NHA) were cultured in specialized medium (AGM BulletKit) purchased from the Lonza group. D‐Luciferin was purchased from Promega (Madison, WI, USA).
2.2. Electric devices
An alternating magnetic field was driven by a transistor inverter (Hot Shot, Ameritherm Inc., New York, NY, USA) and generated by a solenoid copper coil (resistivity: 1.673 × 10−8 Ωm) with an inner diameter of 4 cm and an outer diameter of 5 cm. The experiments were performed at a frequency of 280 kHz and a current of 250 Arms for 15‐90 minutes.15, 16, 17, 20
2.3. Thermometer and thermography
A thermometer (fibre optic thermometer FL‐2400, Anritsu Meter Co., Tokyo, Japan) or a thermograph (InfraRed camera, Nippon Avionics Co., Ltd., Tokyo, Japan) was used to determine the temperature in vitro.21
2.4. XTT assay
A cell proliferation assay was performed using a commercial kit, the XTT Cell Proliferation Assay Kit (Biological Industries, Beit Haemek, Israel), as previously described.21, 22 U251, T98, A172, MCF7, PANC1 and NHA cells were seeded on 96‐well plates at 5.0 × 103 cells (Figure 1F, Figure 2B, Figure 4D and Figure 6A) or 1.0 × 104 cells (Figure 1E, Figure 2A and Figure 5D) per well. The cells were incubated for 24 hours in an atmosphere of 5% CO2 in air at 37°C in the presence of Compound C, with or without 30 minutes of exposure to an AMF.
Figure 1.
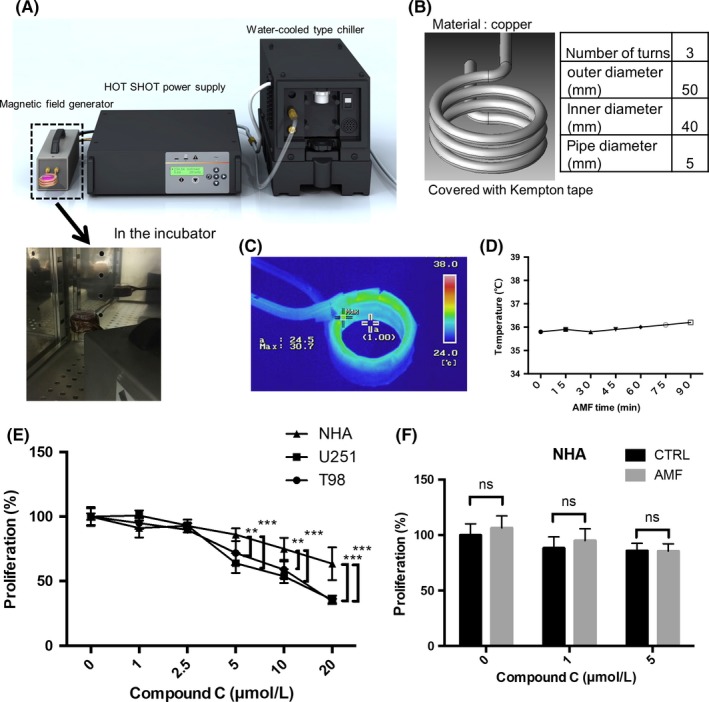
The alternating magnetic field (AMF) apparatus and coil. A, Illustration of the AMF generator and representative picture of the coil and dish in the incubator. B, Illustration of the solenoid coil. C, Representative thermography of the solenoid coil. D, The temperature of the coil for 90 min. E, The cytotoxic effect of Compound C on the proliferation of U251 and T98 glioblastoma (GB) cell lines and normal human astrocytes (n = 4, **P < 0.01, ***P < 0.001). F, The effect of Compound C (0, 1, 5 μmol/L) for 24 h with/without the AMF on NHA (n = 4; ns, not significant)
Figure 2.
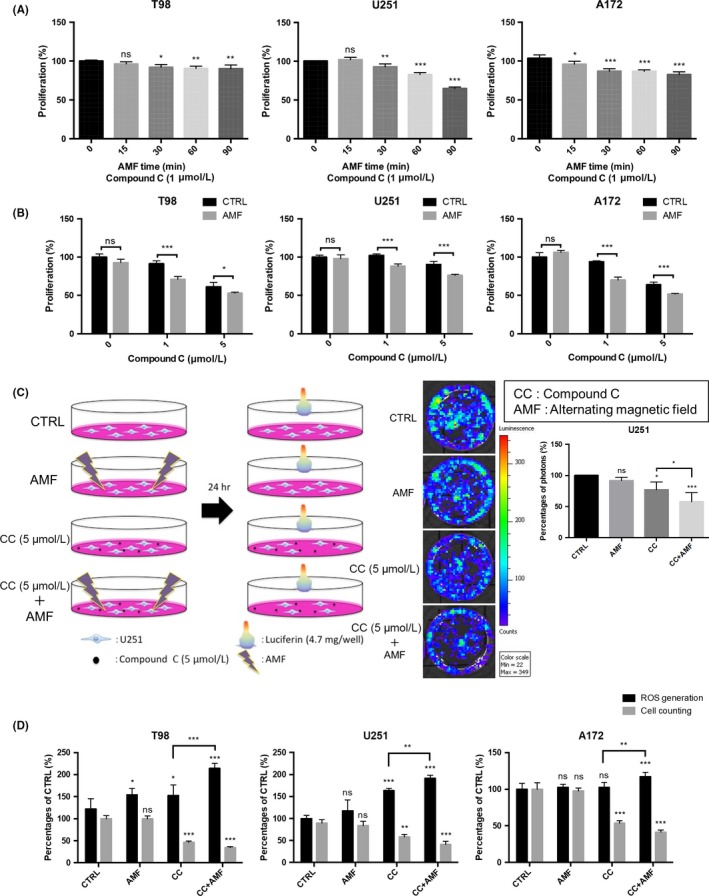
The alternating magnetic field (AMF) decreased the cell viability of glioblastoma (GB) cell lines in the presence of Compound C. A, Applications of the AMF for longer than 30 min increased the Compound C‐induced cytotoxicity in T98, U251, and A172 cells (n = 4; ns, not significant; *P < 0.05, ***P < 0.001). B, Compound C at 1 or 5 μmol/L exhibited cytotoxicity when combined with the AMF for 30 min in T98, U251 and A172 cells (n = 4; ns, not significant; *P < 0.05,**P < 0.01, ***P < 0.001). C, Viability analysis in the presence/absence of Compound C with/without the AMF for 30 min in U251 cells, which have been engineered to express the firefly luciferase gene. The viability of U251 cells was measured in terms of photon flux measured with an in vivo imaging system (IVIS). D, Quantification of cell viability with an IVIS imaging system. D, Effect of AMF for 30 min on ROS production in the absence/presence of Compound C (5 μmol/L) and the cell number counts for T98, U251 and A172 cells using the trypan blue assay (n = 4; ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001)
2.5. In vivo imaging system analysis
The in vivo imaging system (IVIS) analysis was performed as reported.21, 23 U251 cells expressing the luciferase gene were seeded on a 4‐cm dish (1.5 × 105 cells per dish) and incubated for 1 day.21 Then, 5 μmol/L Compound C was added, and the cells were stimulated with or without AMF for 30 minutes. At 24 hours after combined therapy with Compound C and an AMF, D‐luciferin (4.7 mg/well) was added. After 15 minutes, the bioluminescence signal (proportional to the number of surviving cells) was examined using an IVIS.
2.6. The measurement of reactive oxygen species
The measurement of reactive oxygen species (ROS) was performed as previously reported.15, 24 The GB cells were incubated overnight in 96‐well plates (2.0 × 104 cells per well) and then exposed to Compound C with or without 30 minutes of AMF exposure at 37°C for 24 hours.
2.7. Apoptosis assay
Apoptosis assays were performed as previously described.15 U251, A172, T98, MCF7 and PANC1 cells were seeded in 4‐cm dishes at 1.5 × 105 cells per dish and incubated for 24 hours. Compound C was added (5 μmol/L), and the cells were incubated at 37°C for 24 hours in an atmosphere of 5% CO2 in air.
2.8. Cell cycle analysis
Cell cycle analysis was performed using the Cycletest TM Plus DNA Reagent Kit (BD Biosciences) according to the manufacturer's protocol.23
2.9. Western blotting
Western blot analyses were performed as previously described.25, 26 Briefly, the cells were lysed and sonicated in RIPA buffer (Thermo Scientific, Rockford, IL, USA). Equal amounts of protein were subjected to SDS‐PAGE. After electrophoretic separation, the protein bands were transferred to a Millipore Immobilon‐P membrane followed by immunoblotting with antibodies against molecules of interest. The following primary antibodies were used for immunoblotting: Bcl2, BAX, cleaved caspase‐3, phospho‐ERK, Akt, phospho‐Akt, mTOR, phospho‐mTOR, raptor, phospho‐raptor, p70S6K, phospho‐p70S6K, 4EBP1, phospho‐4EBP1,GAPDH and LC3B, obtained from CST (Cell Signaling Technology, Beverly, MA, USA), as well as ERK, obtained from SCBT (Santa Cruz Biotechnology, Dallas, TX, USA). Detection was performed using anti‐rabbit or anti‐mouse secondary antibodies followed by Clarity Western ECL Substrate (Bio‐Rad, Hercules, CA, USA).
2.10. Detection of autophagic flux
The formation of autophagosomes and autophagolysomes was detected in T98 cells using the Premo Autophagy Tandem Sensor RFP‐GFP‐LC3B Kit (Thermo Fischer Scientific, Grand Island, NY, USA).27 The cells were grown on coverslips and incubated with component A containing the RFPGFP‐LC3B overnight. The cells were then treated with vehicle or 5 μmol/L Compound C for 24 hours with/without AMF for 30 minutes.
2.11. Data analysis and statistics
Values represent the means ± SEM. Statistical comparisons among groups were performed using Student's t‐test or 1‐factor ANOVA with the Bonferroni post‐hoc test. A P value of less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Compound C suppressed cell growth in glioblastoma cells and normal human astrocytes
We previously reported that hyperthermia generated with ferucarbotran (Risovist) in an AMF enhanced cisplatin‐induced apoptosis of oral cancer cells in culture.16 Similarly, we demonstrated that the use of hyperthermia and chemotherapy with μ‐oxo N,N′‐bis(salicylidene)ethylenediamine iron [Fe(Salen)], a magnetic organic compound, greatly enhanced its cytotoxicity in oral cancer and glioblastoma because Fe(Salen) generates heat when exposed to an AMF and exhibited a hyperthermic effect.15, 17 In the course of our study, we hypothesized that an AMF enhanced the cytotoxicity of an anti‐tumor agent that does not possess magnetism.
To examine the effect of an AMF in the presence of Compound C, we used a commercial AMF generator equipped with a solenoid coil in the same condition (280 kHz, 250 Arms) as previously reported (Figure 1A).15, 16, 17 The sample dish was placed above the center of the coil (Figure 1B). To exclude the effect of heat generation from the coil, we checked its temperature using thermography. The results showed that the temperature of the solenoid coil rose to less than 36°C for 90 minutes (Figure 1C,D). Similarly, the temperature of the coil according to a thermometer was not increased above 36°C for 30 minutes (280 kHz, 250 Arms) (Figure S1a). The AMF did not change the expression of heat shock protein (HSP) 70 in the presence/absence of Compound C 24 hours after exposure to the AMF for 30 minutes (Figure S1b,c).
We first examined the cytotoxicity of Compound C. As mentioned above, Compound C is a cell‐permeable AMPK inhibitor. Compound C exhibited cytotoxicity in gliomas.10 In the current study, Compound C suppressed the cell proliferation of GB cells (T98 and U251) and normal human astrocytes (NHA) in a dose‐dependent manner (Figure 1E). However, Compound C exhibited cytotoxicity in NHA, and its effect in normal human astrocytes was less than that in glioma cells, indicating that the difference in efficacy between cancer cells and normal cells may contribute to a reduction in its side effects.
We next examined whether an AMF exhibited cytotoxicity in normal cells, namely, NHA. We found that the AMF did not exhibit cytotoxicity in NHA in this condition. (Figure 1F). Furthermore, the AMF did not enhance the cytotoxicity in NHA in the presence of 1 or 5 μmol/L Compound C. Collectively, the AMF had no adverse effects on the NHA in the presence/absence of Compound C. In the treatment of brain tumors, it is extremely important to avoid injuring normal cells, such as NHA.
3.2. An alternating magnetic field increased cytotoxicity and promoted reactive oxygen species production in the presence of Compound C in glioblastoma cells
We examined whether an AMF enhanced the cytotoxicity of Compound C in GB cell lines. Application of an AMF for longer than 30 minutes showed time‐dependent cytotoxicity in the presence of 1 μmol/L Compound C in T98, U251 and A172 GB cell lines (Figure 2A). Furthermore, the AMF in the presence of 5 μmol/L Compound C showed greater cytotoxicity than that in the presence of 0 or 1 μmol/L Compound C in T98, U251 and A172 GB cell lines (Figure 2B).
The in vivo imaging system (IVIS) analysis also showed that the AMF resulted in greater Compound C‐induced cytotoxicity than 5 μmol/L Compound C alone or the AMF alone in U251 cells, which were engineered to express the firefly luciferase gene (Figure 2C). These results are in accord with the result in Figure 2A.
It is reported that Compound C‐induced ROS generation in skin cancer and pancreatic cancer cells.28, 29 Therefore, we next evaluated whether the AMF promotes Compound C‐induced ROS production. We found that Compound C increased ROS production, and the AMF further promoted Compound C‐induced ROS production in T98, U251 and A172 cell lines (Figure 2D). In contrast, AMF did not change ROS generation in normal cells (NHA) (Figure S2). These data suggested that an AMF in the presence of Compound C enhanced cytotoxicity by increasing ROS production in GB cell lines.
3.3. An alternating magnetic field promoted Compound C‐induced apoptosis and the accumulation of G2‐M in the cell cycle, resulting in the inhibition of cell proliferation
It was reported that Compound C showed cytotoxicity towards glioma cells through multiple mechanisms, including the activation of the calpain/cathepsin pathway, inhibition of AKT and mTORC1/C2, cell‐cycle block at G2‐M, and induction of apoptosis and autophagy.10, 29 Therefore, we evaluated whether an AMF promotes Compound C‐induced apoptosis and accumulation of G2‐M in the cell cycle. We found that Compound C increased apoptosis, and the AMF further promoted Compound C‐induced apoptosis of T98, U251 and A172 cells (Figure 3A,B), not NHA (Figure S2). We also found that Compound C created a block at G2‐M, and the AMF further promoted cell‐cycle blockade of T98, U251 and A172 cells at the G2 phase (Figure 3C,D).
Figure 3.
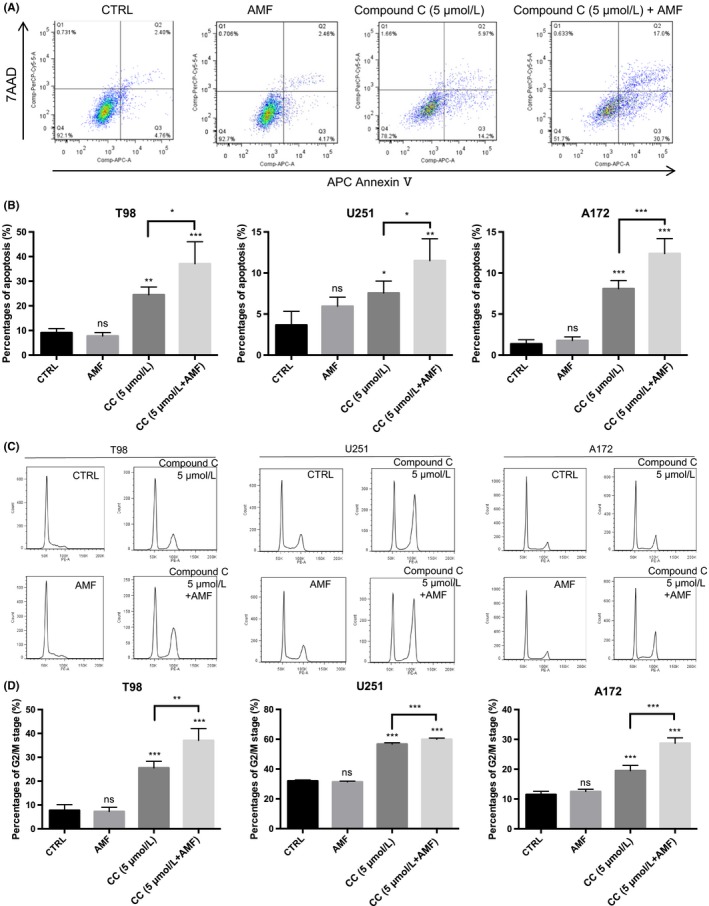
The alternating magnetic field (AMF) promoted Compound C‐induced apoptosis and the accumulation of G2‐M in the cell cycle, resulting in the inhibition of glioblastoma (GB) cell proliferation. A, Representative analysis of apoptosis of T98 cells in the presence of Compound C (5 μmol/L) with or without the AMF. Early apoptosis and late apoptosis are shown in Q3 and Q2, respectively. B, Percentages of apoptosis in the stimulation of 5 μmol/L Compound C for 24 h with/without the AMF for 30 min in human GB cells (n = 4; ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001). C, Representative histogram of flow cytometry‐based cell cycle analysis in the absence/presence of Compound C (5 μmol/L) with/without the AMF for 30 min. D, Percentages of G2/M arrest in the presence/absence of Compound C (5 μmol/L) with/without the AMF for 30 min in human GB cells (n = 4; ns, not significant; **P < 0.01, ***P < 0.001)
3.4. An alternating magnetic field decreased Compound C‐induced ERK phosphorylation and increased BAX/Bcl2 and caspase‐3 expression in the presence of Compound C
AMPK is a key regulator of cellular energy metabolism and is activated under metabolic stress for survival.10 To examine the effect of an AMF, we next investigated a mechanism whereby an AMF enhanced the cytotoxicity of Compound C in GB cells. Compound C blocked the Akt/mTOR pathway through AMPK inhibition as previously reported.30 The Raf/MEK/ERK and PI3K/AKT signaling pathways cooperatively link with each other to enhance the proliferation and apoptotic resistance capacity in cancer cells.31 Both signaling pathways are activated by growth factor receptors. These 2 pathways can exert complementary and redundant functions when only a single pathway is inhibited.32
Our results showed that Compound C or Compound C+ AMF, inhibited the phosphorylation of Akt in T98 and U251 cells (Figure S3). The AMF did not significantly enhance Akt/mTOR signaling inhibition.
In contrast, the ERK signaling pathway is known to play a major role in cell proliferation, and the effect of Compound C on the phosphorylation of ERK is not fully understood. In the current study, we found that Compound C promoted the phosphorylation of ERK in a dose‐dependent manner in T98 cells. To our knowledge, this is the first study to report that Compound C increased the phosphorylation of ERK (Figure 4A). Interestingly, Compound C did not increase but rather decreased ERK signaling in NHA (Figure S2).
Figure 4.
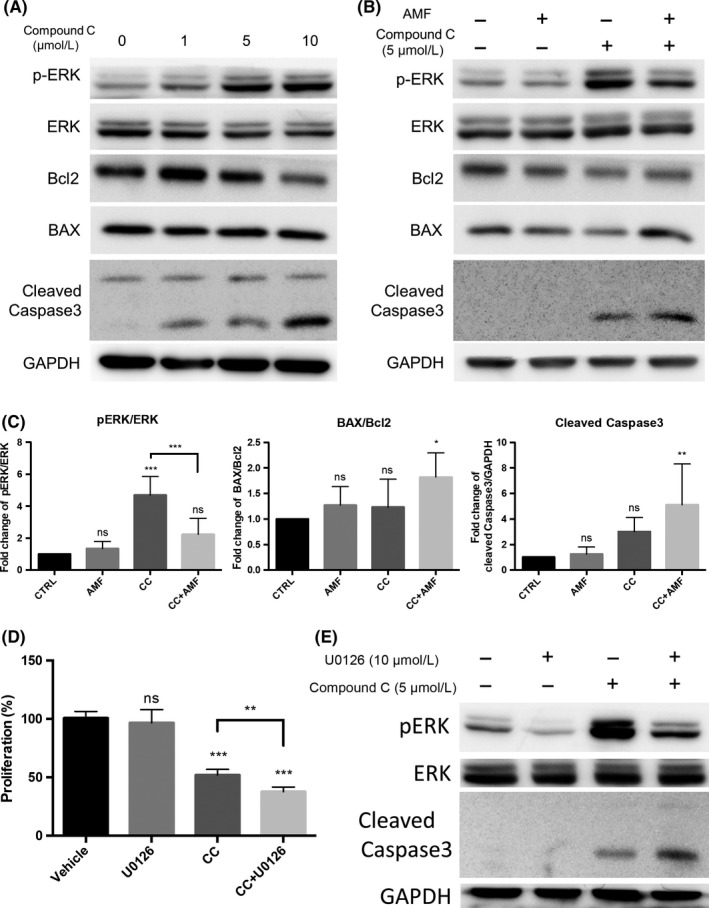
The alternating magnetic field (AMF) inhibited Compound C‐induced phosphorylation of ERK and, thus, increased apoptosis in T98 cells. A, Western blot analysis of the protein expression of ERK, p‐ERK, Bcl2, BAX and cleaved caspase‐3 in T98 cells stimulated by Compound C (0, 1, 5, 10 μmol/L) for 24 h. B, Western blot analysis of the protein expression of ERK, p‐ERK, Bcl2, BAX and cleaved caspase‐3 in T98 cells stimulated by Compound C (5 μmol/L) for 24 h with/without the AMF. C, Densitometric analysis (bar graph) of the western blot showed that the AMF decreased the Compound C‐induced phosphorylation of ERK and increased the BAX/Bcl2 ratio and cleaved caspase‐3 expression (n = 4; ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001). D, The cytotoxic effect of U0126 was analyzed using the XTT assay. T98 cells were pretreated with U0126 (10 μmol/L) for 1 h and then treated with 5 μmol/L Compound C for 24 h (n = 4; ns, not significant; **P < 0.01, ***P < 0.001). E, Representative images of the western blot analysis showed that U0126 dephosphorylated ERK and increased cleaved caspase‐3 expression in T98 cells in the presence/absence of 5 μmol/L Compound C for 24 h
Because the AMF enhanced the apoptotic effect in the presence of Compound C, we examined the protein expression of BCL‐2 and BAX. These proteins are known to be key regulators of apoptosis represented by anti‐apoptotic proteins.33, 34 They regulate the efflux of pro‐apoptotic molecules from mitochondria and other organelles. Compound C also increased the protein expression of BAX/Bcl2 and caspase‐3 in T98 cells in a dose‐dependent manner (Figure 4A).
Taken together, we assumed that this Compound C‐induced ERK phosphorylation is a response to the cytotoxicity of Compound C and is responsible for the drug resistance. Interestingly, the AMF negated the Compound C‐induced phosphorylation of ERK. The AMF further increased the protein expression of BAX/Bcl2 and caspase‐3 in the presence of Compound C in T98 cells (Figure 4B,C). U0126, MEK inhibitor, decreased phosphorylation of ERK, resulting in increasing ROS generation and cleaved caspase 3 in the presence of Compound C (Figure S4). These results suggested that the AMF promoted cytotoxicity via inhibition of Compound C‐induced ERK phosphorylation.
To confirm this hypothesis, we examined whether U0126, an MEK1 and MEK2 inhibitor, also negated Compound C‐induced ERK phosphorylation and suppressed cell proliferation. In fact, U0126 decreased the phosphorylation of ERK in the presence of Compound C and increased the expression of cleaved caspase‐3 (Figure 4D,E). This result is similar to the effect of an AMF in the presence of Compound C in T98 cells. Collectively, these results suggested that the AMF decreased the Compound C‐induced phosphorylation of ERK and enhanced the cytotoxicity of Compound C in T98 cells.
3.5. An alternating magnetic field inhibited compound C‐induced autophagy, resulting in increasing cytotoxicity of compound C in T98 cells
Autophagy is a catabolic process whereby cells self‐digest intracellular organelles.34 Autophagy is an evolutionarily conserved and genetically controlled process, resulting in the selection of cellular proteins and organelles for degradation by the lysosomes. Vucicevic et al report that Compound C‐induced protective autophagy, and the autophagy inhibitor bafilomycin A1 further increased the cytotoxicity of Compound C in U251 glioma cells.30 Therefore, we investigated whether the AMF could regulate autophagy in the presence of Compound C in GB cells and NHA. Compound C increased LC3BII expression in a dose‐dependent manner in T98 cells (Figure 5A). Although Compound C increased autophagy, Compound C did not increase the expression of BAX and cleaved Caspase 3, and did not decrease the expression of BCL‐2 in NHA (Figure S2). Interestingly, the AMF negated Compound C‐induced autophagy in T98 cells (Figure 5B). U0126 also decreased Compound C‐induced autophagy in T98 cells (Figure S4).
Figure 5.
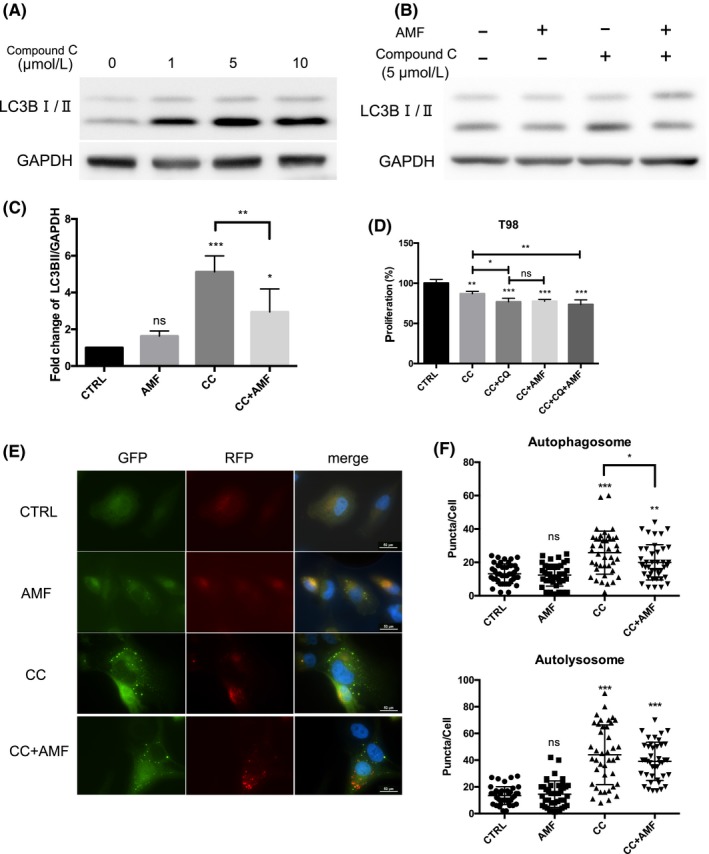
The alternating magnetic field (AMF) inhibited Compound C‐induced autophagy and enhanced Compound C‐induced cytotoxicity in T98 cells. A, The representative western blot analysis showed that stimulation by Compound C for 24 h decreased the ratio of LC3BI/IIin T98 cells in a dose‐dependent manner. B, The representative western blot analysis showed that the AMF decreased Compound C‐induced autophagy in T98 cells in the presence of 5 μmol/L Compound C. C, The densitometric analysis showed that the AMF inhibited Compound C‐induced autophagy in T98 cells stimulated by 5 μmol/L Compound C (n = 4; ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001). D, The effect of chloroquine (CQ) (10 μmol/L) in cells stimulated by Compound C (5 μmol/L) for 24 h with/without the AMF. Proliferation was analyzed using the XTT assay (n = 4; ns, not significant;*P < 0.05, **P < 0.01, ***P < 0.001). E, Representative images of puncta in T98 cells in the presence of 5 μmol/L Compound C with/without the AMF for 30 min. F, The number of puncta in each cell (n = 50 cells; ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001)
This result might indicate that the AMF increased the cytotoxicity of Compound C via the inhibition of autophagy. Similarly, Compound C increased the number of autophagosomes (Figure 5E,F). In contrast, the AMF decreased the number of autophagosomes.
A randomized, double‐blind, placebo‐controlled trial using chloroquine, an autophagy inhibitor, was performed for glioblastoma multiforme.35 This study concluded that adding chloroquine to conventional therapy improved the treatment outcomes. To investigate the effect of an AMF, we evaluated the effect of chloroquine in the presence of Compound C. Chloroquine increased the cytotoxicity of Compound C in T98 and U251 cells (Figure S5). These results are similar to that of the AMF. Thus, these results indicated that the AMF increased the cytotoxicity of Compound C via the inhibition of Compound C‐induced protective autophagy. Taken together, MAPK signaling pathway is important, and is located in the upstream of ROS generation and autophagy in this cascade.
3.6. An alternating magnetic field increases the cytotoxicity of Compound C in breast carcinoma and pancreatic cell lines MCF7 and PANC1
Compound C‐induced apoptosis in glioma and breast carcinoma.9 Compound C also inhibited cell proliferation in glioma and human pancreatic cancer cell lines.8, 29 We examined whether an AMF increases the cytotoxicity of Compound C in the breast carcinoma and pancreatic cell lines MCF7 and PANC1. The XTT assay showed that an AMF for 30 minutes enhanced Compound C‐induced cytotoxicity in a dose‐dependent manner in MCF7 and PANC1 cell lines (Figure 6A). The AMF also induced cell apoptosis in the presence of 5 μmol/L Compound C (Figure 6B,C) and promoted autophagy, resulting in increased levels of cleaved caspase‐3 (Figure 6D,E). Interestingly, the AMF also increased the cytotoxicity of TMZ and BCNU induced‐cytotoxicity in U251 glioma cells (Figure S6).
Figure 6.
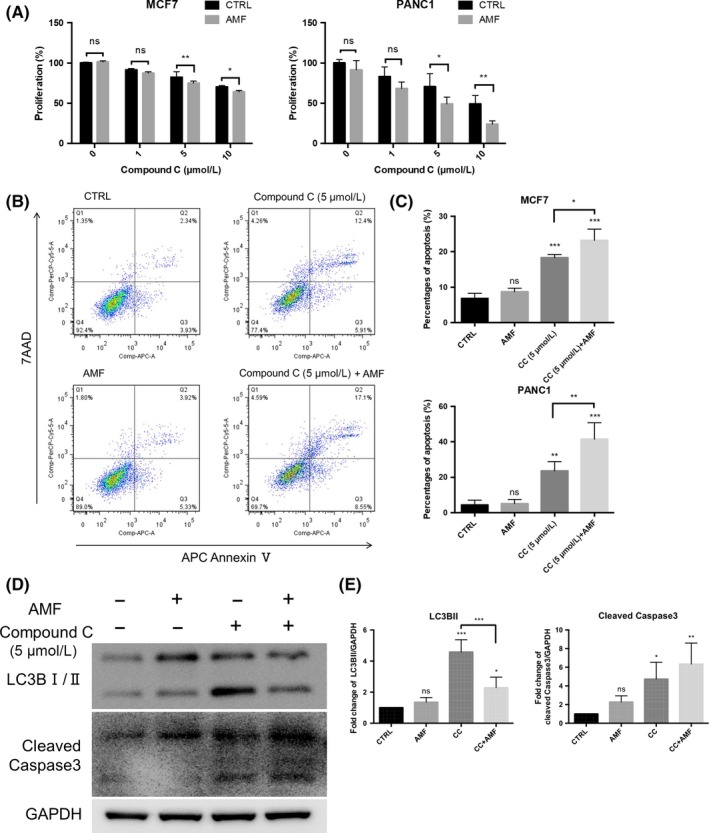
The alternating magnetic field (AMF) enhanced Compound C‐induced cytotoxicity in MCF7 and PANC‐1 cell lines. A, Application of the AMF for longer than 30 min enhanced Compound C‐induced cytotoxicity in MCF7 and PANC1 cells (n = 4; ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001). B, Representative analysis of apoptosis in the presence of 5 μmol/L Compound C with/without the AMF in MCF7 and PANC1 cells. Early apoptosis and late apoptosis are shown in Q3 and Q2, respectively. C, Percentages of apoptosis in the presence of 5 μmol/L Compound C with/without the AMF for 30 min (n = 4; ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.000). D, Representative images of western blot analysis. The AMF inhibited Compound C‐induced autophagy and induced apoptosis in MCF7 cells. E, The densitometric analysis showed that the AMF inhibited Compound C‐induced autophagy and increased Compound C‐induced cleaved caspase‐3 in MCF7 cells
4. DISCUSSION
In the present study, we demonstrated that an AMF increased the cytotoxicity of Compound C via the de‐phosphorylation of ERK and inhibition of autophagy in various cancer cells, including GB cells. In contrast, AMF did not affect the cytotoxicity of Compound C in normal cells (NHA). The AMF also enhanced the cytotoxicity of various anti‐cancer drugs (TMZ and BCNU), which are used clinically in GB cells. An AMF may help to decrease the dose of Compound C and other anti‐cancer drugs, resulting in the reduction of side effects in the future.
The present study demonstrated that an AMF decreased the phosphorylation of ERK in the presence of Compound C. The AMF did not increase the phosphorylation of ERK in the absence of Compound C. As mentioned above, it was not previously known that Compound C increases the phosphorylation of ERK. To our knowledge, this is the first report of this phenomenon. However, there is currently no evidence that supports how the AMF regulates the phosphorylation of ERK in the presence of Compound C. Further studies regarding the effect of the AMF are needed to investigate the mechanism of the anti‐tumor effect of the AMF.
It is known that autophagy is activated by chemotherapy or radiation.36, 37 Therefore, autophagy is attracting attention as a target of cancer treatment. Our study indicated that an AMF increased the cytotoxicity of Compound C through the suppression of autophagy.
An AMF has a potential therapeutic antitumor effect in the presence of Compound C, whereas the underlying mechanism remains unknown. It is well known that the mitochondria play an important role in energy transduction. Mitochondrial iron and the other transition metals that are magnetic bodies are used by cytochrome c oxidase (CcO), the final enzyme complex in the electron transport chain. Therefore, the AMF may affect the metal in the mitochondria and induce instability of the mitochondrial electron transport system. The mechanism connecting the effect of the AMF and anti‐cancer drugs, including Compound C, needs to be determined in future studies.
Other issues also remain before clinical application will be possible. First, the different conditions (kHz or Arms) of the electric device need to be further investigated. However, it is difficult to change the experimental conditions because our device is an industrial machine. There is the possibility that other conditions of the AMF further increase the cytotoxicity of Compound C on GB cells or other cancer cells. The adequate conditions may differ according to the types of cancer cells or anti‐cancer drugs. In our data, the AMF did not enhance the cytotoxicity of paclitaxel (data not shown). Therefore, the effect of the AMF may be dependent on the molecular size of the anti‐cancer drug because the molecular size of paclitaxel is much larger than that of other anticancer drugs. It may be desirable to develop an AMF generator that can be adjusted in terms of the detailed condition or coil to optimize the efficacy upon exposure to an AMF.
In conclusion, the AMF enhanced the cytotoxicity of Compound C‐induced cytotoxicity via the de‐phosphorylation of ERK and inhibition of autophagy in GB cells. The AMF enhanced the cytotoxicity of Compound C for not only GB cells but also breast cancer cells and pancreatic cancer cells. The application of an AMF may a promising method to reduce the dose and the side effects of chemotherapy in cancer treatment.
DISCLOSURE
The authors disclose no conflict of interests.
Supporting information
ACKNOWLEDGMENTS
The Japan Society for the Promotion of Science (JSPS) KAKENHI Grant 24390200, 25670131, Japan Agency for Medical Research and Development (AMED) 66890005, 66890011, 66890001, 66890023, The Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI Grant 22136009, The National Cerebral and Cardiovascular Center (NCVC) 22‐2‐3, the New Energy and Industrial Technology Development Organization 60890021.
Akimoto T, Umemura M, Nagasako A, et al. Alternating magnetic field enhances cytotoxicity of Compound C. Cancer Sci. 2018;109:3483–3493. 10.1111/cas.13781
Funding Information
The Japan Society for the Promotion of Science (JSPS) KAKENHI Grant 24390200, 25670131, Japan Agency for Medical Research and Development (AMED) 66890005, 66890011, 66890001, 66890023, The Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI Grant 22136009, The National Cerebral and Cardiovascular Center (NCVC) 22‐2‐3, the New Energy and Industrial Technology Development Organization 60890021.
Contributor Information
Masanari Umemura, Email: umemurma@yokohama-cu.ac.jp.
Yoshihiro Ishikawa, Email: yishikaw@med.yokohama-cu.ac.jp.
REFERENCES
- 1. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19:v1‐v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987‐996. [DOI] [PubMed] [Google Scholar]
- 3. Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP‐activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329‐3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kahn BB, Alquier T, Carling D, Hardie DG. AMP‐activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15‐25. [DOI] [PubMed] [Google Scholar]
- 5. Ríos M, Foretz M, Viollet B, et al. AMPK activation by oncogenesis is required to maintain cancer cell proliferation in astrocytic tumors. Cancer Res. 2013;73:2628‐2638. [DOI] [PubMed] [Google Scholar]
- 6. Baumann P, Mandl‐Weber S, Emmerich B, Straka C, Schmidmaier R. Inhibition of adenosine monophosphate‐activated protein kinase induces apoptosis in multiple myeloma cells. Anticancer Drugs. 2007;18:405‐410. [DOI] [PubMed] [Google Scholar]
- 7. Park HU, Suy S, Danner M, et al. AMP‐activated protein kinase promotes human prostate cancer cell growth and survival. Mol Cancer Ther. 2009;8:733‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vucicevic L, Misirkic M, Janjetovic K, et al. AMP‐activated protein kinase‐dependent and ‐independent mechanisms underlying in vitro antiglioma action of compound C. Biochem Pharmacol. 2009;77:1684‐1693. [DOI] [PubMed] [Google Scholar]
- 9. Jin J, Mullen TD, Hou Q, et al. AMPK inhibitor Compound C stimulates ceramide production and promotes Bax redistribution and apoptosis in MCF7 breast carcinoma cells. J Lipid Res. 2009;50:2389‐2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu X, Chhipa RR, Nakano I, Dasgupta B. The AMPK inhibitor compound C is a potent AMPK‐independent antiglioma agent. Mol Cancer Ther. 2014;13:596‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maier‐Hauff K, Rothe R, Scholz R, et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. J Neurooncol. 2007;81:53‐60. [DOI] [PubMed] [Google Scholar]
- 12. Maier‐Hauff K, Ulrich F, Nestler D, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron‐oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103:317‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mittal S, Klinger NV, Michelhaugh SK, Barger GR, Pannullo SC, Juhász C. Alternating electric tumor treating fields for treatment of glioblastoma: rationale, preclinical, and clinical studies. J Neurosurg. 2018;128(2):414‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim J‐H, Eguchi H, Umemura M, et al. Magnetic metal‐complex‐conducting copolymer core–shell nanoassemblies for a single‐drug anticancer platform. NPG Asia Mater. 2017;9:e367. [Google Scholar]
- 15. Sato I, Umemura M, Mitsudo K, et al. Simultaneous hyperthermia‐chemotherapy with controlled drug delivery using single‐drug nanoparticles. Sci Rep. 2016;6:24629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sato I, Umemura M, Mitsudo K, et al. Hyperthermia generated with ferucarbotran (Resovist®) in an alternating magnetic field enhances cisplatin‐induced apoptosis of cultured human oral cancer cells. J Physiol Sci. 2014;64:177‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohtake M, Umemura M, Sato I, et al. Hyperthermia and chemotherapy using Fe(Salen) nanoparticles might impact glioblastoma treatment. Sci Rep. 2017;7:42783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hannan CJ Jr, Liang Y, Allison JD, Pantazis CG, Searle JR. Chemotherapy of human carcinoma xenografts during pulsed magnetic field exposure. Anticancer Res. 1994;14:1521‐1524. [PubMed] [Google Scholar]
- 19. Ruiz‐Gómez MJ, de la Peña L, Prieto‐Barcia MI, Pastor JM, Gil L, Martínez‐Morillo M. Influence of 1 and 25 Hz, 1.5 mT magnetic fields on antitumor drug potency in a human adenocarcinoma cell line. Bioelectromagnetics. 2002;23:578‐585. [DOI] [PubMed] [Google Scholar]
- 20. Eguchi H, Umemura M, Kurotani R, et al. A magnetic anti‐cancer compound for magnet‐guided delivery and magnetic resonance imaging. Sci Rep. 2015;5:9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sato I. Simultaneous hyperthermia‐chemotherapy with controlled drug delivery using single‐drug nanoparticles. Sci Rep. 2016;6:24629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Umemura M, Baljinnyam E, Feske S, et al. Store‐operated Ca2+ entry (SOCE) regulates melanoma proliferation and cell migration. PLoS ONE. 2014;9:e89292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakakaji R, Umemura M, Mitsudo K, et al. Treatment of oral cancer using magnetized paclitaxel. Oncotarget. 2018;9:15591‐15605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Umemura M, Kim J‐H, Aoyama H, et al. The iron chelating agent, deferoxamine detoxifies Fe(Salen)‐induced cytotoxicity. J Pharmacol Sci. 2017;134:203‐210. [DOI] [PubMed] [Google Scholar]
- 25. Ryo T, Masanari U, Masatoshi N, et al. Hydrostatic pressure suppresses fibrotic changes via Akt/GSK‐3 signaling in human cardiac fibroblasts. Physiol Rep. 2018;6:e13687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Narikawa M, Umemura M, Tanaka R, et al. Acute hyperthermia inhibits TGF‐β1‐induced cardiac fibroblast activation via suppression of Akt signaling. Sci Rep. 2018;8:6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yong Z, Xinyu L, Hui C, et al. Ampelopsin‐induced autophagy protects breast cancer cells from apoptosis through Akt‐mTOR pathway via endoplasmic reticulum stress. Cancer Sci. 2014;105:1279‐1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang S‐W, Wu C‐Y, Wang Y‐T, et al. p53 modulates the AMPK inhibitor compound C induced apoptosis in human skin cancer cells. Toxicol Appl Pharmacol. 2013;267:113‐124. [DOI] [PubMed] [Google Scholar]
- 29. Duong H‐Q, Hwang JS, Kim HJ, Seong Y‐S, Bae I. BML‐275, an AMPK inhibitor, induces DNA damage, G2/M arrest and apoptosis in human pancreatic cancer cells. Int J Oncol. 2012;41:2227‐2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vucicevic L, Misirkic M, Janjetovic K, et al. Compound C induces protective autophagy in cancer cells through AMPK inhibition‐independent blockade of Akt/mTOR pathway. Autophagy. 2011;7:40‐50. [DOI] [PubMed] [Google Scholar]
- 31. Inman CK, Li N, Shore P. Oct‐1 counteracts autoinhibition of Runx2 DNA binding to form a novel Runx2/Oct‐1 complex on the promoter of the mammary gland‐specific gene beta‐casein. Mol Cell Biol. 2005;25:3182‐3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barnes GL, Javed A, Waller SM, et al. Osteoblast‐related transcription factors Runx2 (Cbfa1/AML3) and MSX2 mediate the expression of bone sialoprotein in human metastatic breast cancer cells. Cancer Res. 2003;63:2631‐2637. [PubMed] [Google Scholar]
- 33. Edinger AL, Thompson CB. Defective autophagy leads to cancer. Cancer Cell. 2003;4:422‐424. [DOI] [PubMed] [Google Scholar]
- 34. Degenhardt K, Mathew R, Beaudoin B, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sotelo J, Briceño E, López‐González M. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double‐blind, placebo‐controlled trial. Ann Intern Med. 2006;144:337‐343. [DOI] [PubMed] [Google Scholar]
- 36. Paglin S, Hollister T, Delohery T, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439‐444. [PubMed] [Google Scholar]
- 37. Sui X, Chen R, Wang Z, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


