Abstract
The present study is the first phase II clinical trial aimed to evaluate the efficacy and safety of S‐1 plus nanoparticle albumin‐bound paclitaxel (Nab‐PTX) as first‐line chemotherapy for advanced gastric cancer (AGC). Previously untreated patients with metastatic gastric adenocarcinoma received S‐1 in oral doses of 40 mg (BSA <1.25 m2), 50 mg (1.25 ≤ BSA < 1.50 m2) and 60 mg (BSA ≥1.50 m2) b.i.d. on days 1‐14 in combination with Nab‐PTX (120 mg/m2, on days 1 and 8) for each 21‐day cycle. Primary endpoint was progression‐free survival (PFS), and secondary endpoints were overall response rate (ORR), overall survival (OS), disease control rate (DCR), and toxicity. A total of 73 gastric cancer patients with metastatic and measurable lesions were enrolled in the first‐line setting. Median PFS and OS were 9.63 months and 14.60 months, respectively. Four (5.5%) patients had complete responses, 39 (53.4%) had partial responses (PRs), 21 (28.8%) had stable disease, four (5.5%) progressed and five (6.8%) were not evaluable. ORR and DCR were 58.9% and 87.7%, respectively. Most toxicities were mild, and no treatment‐related deaths occurred. Grade 3 to 4 toxicities occurred in 22 patients (30.1%) as follows: leukopenia (13.7%), neutropenia (12.3%), anemia (5.5%), thrombocytopenia (1.4%), diarrhea (6.8%), vomiting (2.7%), stomatitis (1.4%), peripheral neuropathy (1.4%), and hand‐foot syndrome (1.4%). Seven patients achieved good responses and underwent gastrectomy plus metastasectomy. Thirty (41.1%) patients had S‐1 maintenance with a median of four cycles. S‐1 plus Nab‐PTX is an efficient and safe regimen as first‐line treatment for patients with AGC.
Keywords: advanced gastric cancer, chemotherapy, first‐line, nanoparticle albumin‐bound paclitaxel, S‐1
1. INTRODUCTION
Gastric cancer remains the third leading cause of cancer‐related death worldwide and is especially frequent in East Asia.1 Despite many drug combinations and even targeting human epidermal growth factor receptor 2 (HER2) in a small subset of HER2‐positive patients, the outcomes of patients with advanced gastric cancer (AGC) are still not satisfactory.2
Fluoropyrimidines combined with platinums are the most common first‐line chemotherapy for AGC.3, 4 S‐1, an oral fluoropyrimidine, is prevalently used in Asian countries including China due to its simplicity and convenience of drug administration.5, 6 Addition of docetaxel to fluorouracil plus cisplatin (DCF), a standard triplet first‐line option used especially in Europe and USA, is superior to fluorouracil plus cisplatin (CF) in terms of efficacy. However, the benefit of overall survival (OS) is moderate, and high rates of severe toxicities limit its routine use.7 In the first‐line setting, taxanes are also important components of non‐platinum doublets that are accepted in some Asian areas with similar efficacy and similar or lower toxicity compared to platinum regimens. A meta‐analysis that we conducted found no statistically significant increase in response or overall survival when platinum therapies were used compared to new‐generation non‐platinum regimens (S‐1, taxanes and irinotecan) as first‐line treatment, and the toxicity of platinum therapies was significantly higher for hematological toxicity, nausea, vomiting, and neurotoxicity.8 The synergistic effect of paclitaxel and S‐1 was reported in the mouse model, and paclitaxel or docetaxel in combination with S‐1 was reported effective and well tolerated as first‐line chemotherapy for AGC by phase II or phase III clinical trials, respectively.9, 10, 11 Furthermore, in particular cases where patients cannot receive platinums as a result of renal dysfunction or other treatment‐related adverse effects such as ototoxicity, an efficient first‐line chemotherapy regimen without platinum is needed, and taxanes are valid options for platinum‐intolerant patients. It is significative to explore equivalent and low toxic taxane‐based non‐platinum doublets as first‐line treatment.
Nanoparticle albumin‐bound paclitaxel (Nab‐PTX [Abraxane]; Celgene, Summit, NJ, USA) has an advantage over solvent‐based paclitaxel as it can deliver a higher dose of paclitaxel into tumors and decrease the incidence of serious toxicities. To date, Nab‐PTX has been indicated for breast cancer, pancreatic cancer and non‐small‐cell lung cancer.12, 13, 14 Taxanes including paclitaxel and docetaxel are commonly used as the second‐line treatment for AGC after failure to first‐line chemotherapy of fluoropyrimidine plus platinum. However, very little is known about the role of Nab‐PTX in gastric cancer. A Japanese phase II trial reported that Nab‐PTX (260 mg/m2 on d1, q3w) has promising activity and well‐tolerated toxicities as second‐line chemotherapy against previously treated AGC. Thus, Nab‐PTX has been approved as a second‐line option for AGC in Japan.15 A randomized phase III ABSOLUTE trial showed that 3/4 weekly Nab‐PTX (100 mg/m2, on days 1, 8, and 15, q4w) was non‐inferior to weekly solvent‐based paclitaxel in terms of overall survival, with a similar safety profile and a lower incidence of hypersensitivity reactions, and the advantages of the Nab‐PTX formulation make it a potential regimen for second‐line treatment of gastric cancer.16 The safety of S‐1 plus Nab‐PTX was confirmed in a phase I study of metastatic breast cancer.17 The synergistic effect of capecitabine and Nab‐PTX was observed in a phase II study of metastatic breast cancer.18 Recently, a phase I study for the first time showed that the combination of S‐1 plus Nab‐PTX was well tolerated and had antitumor efficacy with an overall response rate (ORR) of 54.5% and median progression‐free survival (PFS) of 5.8 months as first‐line treatment for AGC patients.19
We previously conducted dose escalation of Nab‐PTX plus standard S‐1 in Chinese patients with AGC in which Nab‐PTX was set at three dose levels: 80 mg/m2, 100 mg/m2, or 120 mg/m2, on days 1 and 8, q3w. The maximum‐tolerated dose was not met. For patients receiving 120 mg/m2 on d1 and d8, q3w, which was the recommended dose, both the efficacy and the tolerance were good. We did not explore a dose above 120 mg/m2 on d1 and d8, q3w, for Nab‐PTX‐based combined chemotherapy, as the approved dose of Nab‐PTX monotherapy in China for breast cancer (the only Chinese indication) is 260 mg/m2, d1, q3w. As the first phase II study of fluoropyrimidines plus Nab‐PTX in gastric cancer patients, the present study aimed to evaluate the efficacy and safety of S‐1 plus Nab‐PTX with the recommended dose as first‐line treatment for patients with metastatic gastric cancer.
2. MATERIALS AND METHODS
As a one‐arm, single‐center, open phase II clinical trial, this study was approved by the independent Institute Research Ethics Committee at the Sun Yat‐sen University Cancer Center and conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. Patients provided informed consent to participate.
2.1. Patient selection
Eligibility criteria included the following: (i) age ≥ 18 years; (ii) ECOG performance status 0, 1, or 2; (iii) histologically confirmed metastatic gastric adenocarcinoma; (iv) no previous chemotherapy for advanced disease (prior adjuvant/neoadjuvant therapy was allowed if at least 6 months had elapsed between completion of adjuvant/neoadjuvant therapy and enrolment in the study); (v) at least one measurable lesion according to RECIST v1.1; (vi) adequate oral intake and bone marrow, heart, hepatic, and renal function; and (vii) signed informed consent.
Exclusion criteria included the following: (i) prior palliative chemotherapy; (ii) other malignancy within the last 5 years, except for carcinoma in situ of the cervix or basal cell carcinoma; (iii) history or clinical evidence of brain metastases; (iv) serious uncontrolled systemic concomitant illness; (v) pregnancy; (vi) subjects with reproductive potential who were unwilling to use an effective method of contraception; (vii) known hypersensitivity to any of the study drugs; and (viii) neurological toxicity ≥grade 2 according to the NCI Common Terminology Criteria for Adverse Events version 4.0 (NCI‐CTCAE v4.0).
2.2. Treatment schedule
All patients were orally treated with S‐1 in doses of 40 mg (BSA <1.25 m2), 50 mg (1.25 ≤ BSA < 1.50 m2) and 60 mg (BSA ≥1.50 m2) b.i.d. on days 1‐14 in combination with Nab‐PTX (120 mg/m2 on d1 and d8, i.v. for 30 minutes) of each 21‐day cycle. Treatment was planned for six cycles or until progression, unacceptable toxicity, or patient refusal.
2.3. Response and adverse event assessment
Clinical and laboratory examinations were carried out within 7 days before enrolment and each chemotherapy cycle afterward. Tumors were measured using computed tomographic scans, within 15 days before enrolment and every two cycles afterward, according to RECIST v1.1. Patients were considered response‐assessable if they had overt clinical or radiological evidence of early progression (PD) within the first two cycles. All treatment‐related toxicities were categorized according to NCI‐CTCAE v4.0.
2.4. Statistical analyses
Primary endpoint of this study was PFS, whereas secondary endpoints were ORR, OS, disease control rate (DCR), and toxicity.
Primary endpoint of this study was to test the hypothesis that S‐1 plus Nab‐PTX would improve median PFS from 5.6 months (on the basis of parent DCF regimen) to 8.0 months.7 With α = 0.05 and β = 0.2, a sample size of 65 patients was required. Considering a drop‐off of 10%, 72 patients were required in this study.
The Kaplan‐Meier method was used to estimate the distribution of time to events. PFS was determined from the date of treatment to PD or death. OS was calculated from the date of treatment to death from any cause or the last follow‐up date. Two‐sided log‐rank test was used for subgroup analyses. Cox proportional hazards model was used for multivariate analyses of survival. Statistical analyses were carried out using SPSS 19.0.
3. RESULTS
3.1. Patient characteristics
Between February 2012 and November 2015, 73 gastric cancer patients with metastatic and measurable lesions (M/F = 45/28, median age 53 years, ECOG 0‐2) were enrolled in the first‐line setting at Sun Yat‐sen University Cancer Center and, thus, comprised the full analysis population and were analysed for efficacy and safety. The last follow up was on August 28, 2017. Baseline patient characteristics are summarized in Table 1. In total, 298 cycles of S‐1 plus Nab‐PTX were given, with a median of four cycles (range 1‐6). Thirty (41.1%) patients received S‐1 maintenance treatment with a median of four cycles (range 1‐20).
Table 1.
Baseline characteristics of patients receiving S‐1 plus Nab‐PTX for advanced gastric cancer
| Characteristics | S‐1 plus Nab‐PTX[Link] (N = 73) | |
|---|---|---|
| No. | % | |
| Gender (male/female) | 45/28 | 61.6/38.4 |
| Age (years, median, range) | 53 (28‐83) | |
| ECOG performance status | ||
| 0 | 2 | 2.7% |
| 1 | 63 | 86.3% |
| 2 | 8 | 11.0% |
| Primary tumor location | ||
| Proximal | 17 | 23.3% |
| Body | 15 | 20.5% |
| Antrum | 26 | 35.6% |
| Multiple/diffuse | 12 | 16.4% |
| Cancer of gastric remnant | 3 | 4.1% |
| Histology | ||
| Well differentiated | 0 | 0 |
| Moderately differentiated | 15 | 20.5% |
| Poorly differentiated | 36 | 49.3% |
| Mucinous | 5 | 6.8% |
| Signet‐ring cell | 17 | 23.3% |
| Lauren classification | ||
| Diffuse type | 20 | 27.4% |
| Intestinal type | 17 | 23.3% |
| Mixed type | 5 | 6.8% |
| NA | 31 | 42.5% |
| Her‐2 gene type | ||
| Positive | 6 | 8.2% |
| Negative | 54 | 74.0% |
| NA | 13 | 17.8% |
| Site of metastases | ||
| Liver | 22 | 30.1% |
| Lung | 2 | 2.7% |
| Lymph nodes | 42 | 57.5% |
| Peritoneum | 36 | 49.3% |
| Others | 13 | 17.8% |
| No. of involved organs | ||
| 1 | 10 | 13.7% |
| 2 | 28 | 38.4% |
| 3 | 30 | 41.1% |
| 4 | 4 | 5.5% |
| 5 | 1 | 1.4% |
| Prior surgery | ||
| Curative gastrectomy | 17 | 23.3% |
| Palliative gastrectomy/metastasectomy | 10 | 13.7% |
| Exploration/bypass | 8 | 10.9% |
| No | 38 | 52.1% |
| Prior adjuvant chemotherapy | ||
| Yes | 15 | 20.5% |
| No | 58 | 79.5% |
NA, data not available; Nab‐PTX, nanoparticle albumin‐bound paclitaxel.
3.2. Efficacy
Of the 73 patients, four (5.5%) had complete response, 39 (53.4%) had partial response (PR), 21 (28.8%) had stable disease, four (5.5%) progressed, and five (6.8%) could not be evaluated. ORR and DCR were 58.9% (95% confidence interval [CI] 47.3%‐70.5%) and 87.7% (95% CI 79.9%‐95.4%), respectively.
At a median follow‐up time of 33.0 months, 62 (84.9%) of 73 patients had progressive cancer. Median PFS was 9.63 months (95% CI 7.67‐11.59) (Figure 1). The fraction of progression‐free patients at 1 year was 29.0%. Fifty‐three (72.6%) of 73 patients had died. Median OS was 14.60 months (95% CI 11.69‐17.51) (Figure 2). The fraction of patients alive at 1 year and at 2 years was 54.6% and 25.2%, respectively.
Figure 1.
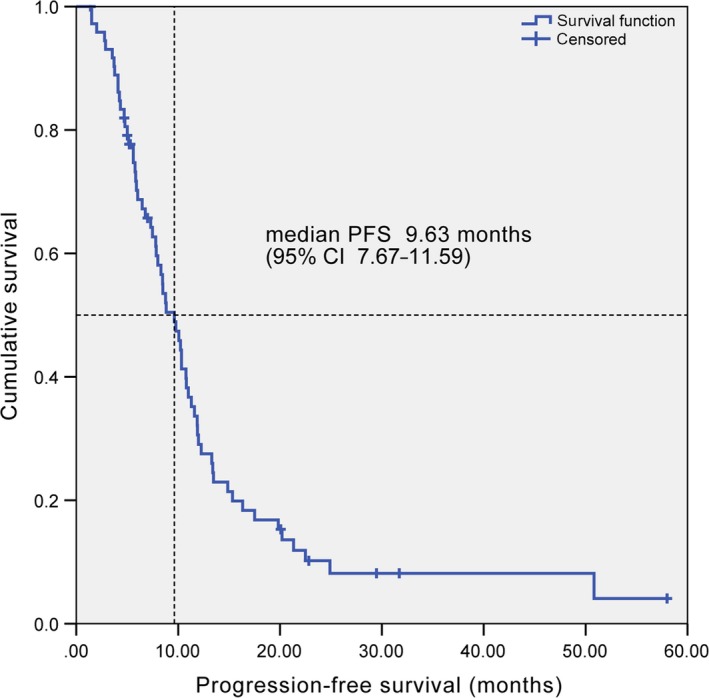
Kaplan‐Meier curve of progression‐free survival. CI, confidence interval; PFS, progression‐free survival
Figure 2.
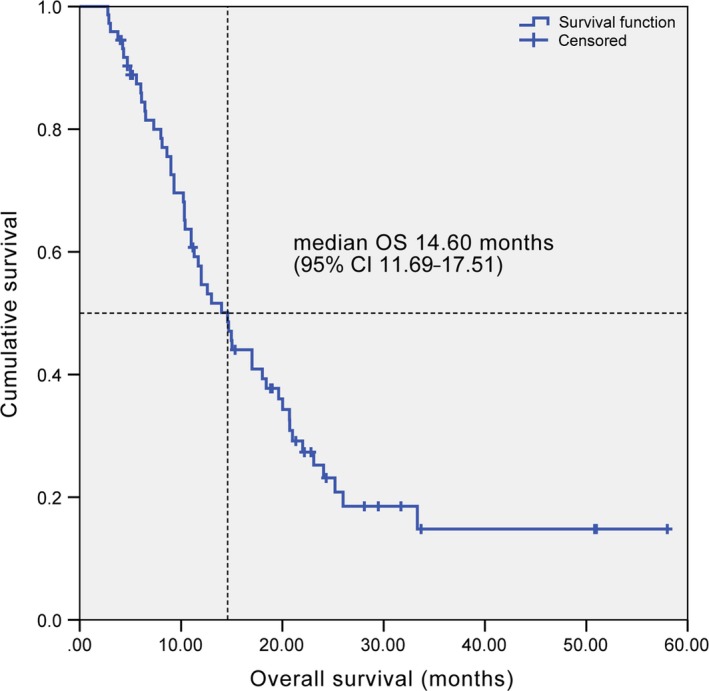
Kaplan‐Meier curve of overall survival. CI, confidence interval; OS, overall survival
Of note, thirty‐three (45.2%) of the 73 patients were provided with additional treatment, among whom seven patients (9.6%) (6 with lymph nodes and 1 with liver metastases) achieved good response and underwent gastrectomy plus metastasectomy after two to six cycles. For these patients, the multidisciplinary treatment group discussed the reduction in tumors so that gastrectomy plus metastasectomy would be feasible and beneficial. Both surgeons and postoperative pathology confirmed R0 resection. Meanwhile, 35.6% of patients received second‐line chemotherapy, including 20.5% platinums plus fluoropyrimidines, 9.6% irinotecan‐based chemotherapy for peripheral neuropathy or creatinine elevation with first‐line treatment, 1.4% S‐1 plus Nab‐PTX reintroduction after a relatively long first‐line PFS, and 4.1% pemetrexed in a free clinical trial as second‐line chemotherapy for AGC (Table S1).
Subgroup analysis showed significantly prolonged PFS (median 14.87 vs 8.50 months, P = 0.038) and non‐significantly prolonged OS (median 23.10 vs 12.60 months, P = 0.116) in patients with gastrectomy plus metastasectomy than without, with separated PFS and OS curves (Figure S1). Subgroup analysis according to peritoneal metastases or not showed no significant differences in terms of ORR (57.1% vs 60.5%, P = 0.944), PFS (median 8.00 vs 10.77 months, P = 0.769), or OS (median 11.70 vs 17.00 months, P = 0.367).
Multivariate analysis showed that ascites, gastrectomy plus metastasectomy, and baseline lactate dehydrogenase were significantly associated with PFS (Table S2). Multivariate analysis showed that ECOG performance status was significantly associated with OS (Table S3).
3.3. Toxicity, feasibility and treatment compliance
A total of 62 (84.9%) patients experienced toxicity. Most cases of toxicity were mild, and no treatment‐related deaths occurred. The most frequent hematological toxicities were leukopenia (58.9%) and neutropenia (37.0%), and febrile neutropenia was observed in one patient (1.4%). The most frequent non‐hematological toxicities were diarrhea (15.1%), transaminase elevation (13.7%), peripheral neuropathy (12.3%), and stomatitis (11.0%). Grade 3 to 4 toxicities occurred in 22 (30.1%) patients, and grade 4 toxicity (neutropenia) occurred in one (1.4%) patient. Grade 3 toxicities included leukopenia (13.7%), neutropenia (12.3%), anemia (5.5%), thrombocytopenia (1.4%), diarrhea (6.8%), vomiting (2.7%), stomatitis (1.4%), peripheral neuropathy (1.4%), and hand‐foot syndrome (1.4%) (Table 2). There were no new safety concerns related to Nab‐PTX.
Table 2.
Toxicity of S‐1 plus Nab‐PTX in patients with advanced gastric cancer
| Toxicity | S‐1 plus nanoparticle albumin‐bound paclitaxel (N = 73) | ||||
|---|---|---|---|---|---|
| Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Grade 4 (%) | All grades (%) | |
| Leukopenia | 14 (19.2) | 19 (26.0) | 10 (13.7) | 0 | 43 (58.9) |
| Neutropenia | 4 (5.5) | 13 (17.8) | 9 (12.3) | 1 (1.4) | 27 (37.0) |
| Anemia | 6 (8.2) | 0 | 4 (5.5) | 0 | 10 (13.7) |
| Thrombocytopenia | 2 (2.7) | 4 (5.5) | 1 (1.4) | 0 | 7 (9.6) |
| Asthenia | 2 (2.7) | 2 (2.7) | 0 | 0 | 4 (5.5) |
| Anorexia | 5 (6.8) | 0 | 0 | 0 | 5 (6.8) |
| Nausea | 4 (5.5) | 0 | 0 | 0 | 4 (5.5) |
| Vomiting | 2 (2.7) | 0 | 2 (2.7) | 0 | 2 (2.7) |
| Diarrhea | 4 (5.5) | 2 (2.7) | 5 (6.8) | 0 | 11 (15.1) |
| Constipation | 4 (5.5) | 0 | 0 | 0 | 4 (5.5) |
| Abdominal pain | 3 (4.1) | 0 | 0 | 0 | 3 (4.1) |
| Skin rash | 2 (2.7) | 2 (2.7) | 0 | 0 | 4 (5.5) |
| Hand‐foot syndrome | 1 (1.4) | 0 | 1 (1.4) | 0 | 2 (2.7) |
| Pigmentation | 1 (1.4) | 0 | 0 | 0 | 1 (1.4) |
| Stomatitis | 4 (5.5) | 3 (4.1) | 1 (1.4) | 0 | 8 (11.0) |
| Peripheral neuropathy | 6 (8.2) | 2 (2.7) | 1 (1.4) | 0 | 9 (12.3) |
| Alopecia | 2 (2.7) | 2 (2.7) | 0 | 0 | 4 (5.5) |
| Creatinine elevation | 3 (4.1) | 0 | 0 | 0 | 3 (4.1) |
| Transaminase elevation | 10 (13.7) | 0 | 0 | 0 | 10 (13.7) |
| Bilirubin elevation | 6 (8.2) | 0 | 0 | 0 | 6 (8.2) |
| Hypoalbuminemia | 3 (4.1) | 0 | 0 | 0 | 3 (4.1) |
| Total | 40 (54.8) | 36 (49.3) | 22 (30.1) | 1 (1.4) | 62 (84.9) |
Severity was graded according to NCI Common Terminology Criteria for Adverse Events, version 4.0.
Nine (12.3%) patients had a dose reduction of S‐1. Main reasons for S‐1 dose reduction were grade 3 leukopenia or neutropenia (8/73, 11.0%) and grade 3 diarrhea (3/73, 4.1%). One (1.4%) patient had a dose reduction of both S‐1 and Nab‐PTX as a result of grade 3 leukopenia and thrombocytopenia. The course of one (1.4%) patient was delayed by 21 days as a result of grade 2 leukopenia with herpes zoster. Three (4.1%) patients discontinued S‐1 plus Nab‐PTX as a result of grade 3 hand‐foot syndrome, febrile neutropenia, and grade 3 peripheral neuropathy, respectively, whereas three patients (4.1%) discontinued Nab‐PTX as a result of grade 2 peripheral neuropathy and grade 3 stomatitis (Table S4). Grade 3 peripheral neuropathy was reversible and improved to grade 1 within 22 days after discontinuation.
4. DISCUSSION
In the present study, the combination of 240 mg/m2 three‐weekly Nab‐PTX doses evenly divided on d1 and d8 and S‐1 d1‐14 every 3 weeks met the primary endpoint of PFS, and this treatment was well tolerated despite the higher cumulative paclitaxel dose and shorter infusion schedules (30 minutes vs 3 hours for paclitaxel) delivered without premedication for unselected patients with metastatic gastric cancer.
Globally, fluoropyrimidine‐based combination chemotherapy regimens, including fluorouracil or its oral derivatives S‐1 or capecitabine, platinum, taxane, and irinotecan compounds, have yielded an ORR of 26.9‐54%, PFS of 2‐7 months and OS of 10.5‐13 months in first‐line settings.3, 4, 5, 6, 7, 11, 20, 21, 22 Combination of S‐1 and Nab‐PTX in the present study compared favorably with those phase III trials, although no direct comparison data are available. ORR and PFS of the current phase II trial also compared favorably with the ORR of 54.5% and PFS of 5.8 months for the recent phase I study of S‐1 plus Nab‐PTX with 16 AGC patients enrolled.19 In particular, S‐1 plus Nab‐PTX yielded a complete response rate of 5.5%, which is higher than data from those phase III trials. Furthermore, the hypothesis that S‐1 plus Nab‐PTX would improve median PFS from 5.6 months (on the basis of parent DCF regimen) to 8.0 months was met.7 At the last follow up, 62 (84.9%) of 73 patients had progressive cancer. Among the 11 censored patients, five (6.8%) patients still had PR or stable disease, whereas six patients were lost to follow up after treatment with a drop‐out rate of 8.2%. Even though we excluded drop‐out patients, median PFS and OS were 8.83 months and 14.60 months, respectively and, thus, the hypothesis was still met. The majority included in the present study were HER2‐negative patients, whereas the minority were HER2‐positive patients (8.2%). According to the study protocol, this trial mainly included HER2‐negative patients, as well as HER2‐positive patients who refused to use trastuzumab as a result of low financial status. Trastuzumab is expensive and was not covered by Chinese medical insurance until 2017. Hence, we excluded the influence of trastuzumab in the observation of S‐1 plus Nab‐PTX. Nevertheless, the efficacy of S‐1 plus Nab‐PTX for both the entire population and the six HER2‐positive patients seemed to compare favorably with what is reported in the Trastuzumab for Gastric Cancer (ToGA) study, with no direct comparison data.2 Further phase III study of S‐1 plus Nab‐PTX combined with trastuzumab for HER2‐positive AGC patients is expected to provide more information on this subject.
Safety advantages were observed in the present study for the rates of grade 3 to 4 toxicities compared with fluoropyrimidines plus platinums reported in historical trials, although no direct comparison data are available.4, 5, 7 In particular, in the present study, creatinine elevation was mild, with no ototoxicity and infrequent grade 3 to 4 peripheral neuropathy. The most frequent overall and grade 3 to 4 hematological toxicities were leukopenia and neutropenia, which occurred at a lower rate than those reported for Nab‐PTX in breast, pancreatic, non‐small‐cell and gastric cancer.12, 13, 14, 15, 19 The most frequent grade 3 to 4 non‐hematological toxicities were diarrhea, vomiting, peripheral neuropathy, hand‐foot syndrome, and stomatitis. Historical trials reported that peripheral neuropathy and fatigue were the most common grade 3 to 4 non‐hematological toxicities of Nab‐PTX,12, 13, 14, 15 whereas diarrhea, vomiting, hand‐foot syndrome, and stomatitis were common toxicities related to S‐1. There seemed to be a correlation between the dose schedule and rate of grade 3 to 4 peripheral neuropathy. The three‐weekly Nab‐PTX (260 mg/m2) dose yielded 7%‐23.6% grade 3 to 4 peripheral neuropathy, significantly higher than paclitaxel.12, 15, 16 Weekly Nab‐PTX (100 mg/m2) yielded 3%, significantly lower than paclitaxel,14 3/4 weekly Nab‐PTX (125 mg/m2 on d1, d8, and d15, q4w) yielded 17%, significantly higher than paclitaxel,13 whereas 3/4 weekly Nab‐PTX (100 mg/m2 on d1, d8, and d15, q4w) yielded 2%, non‐inferior to paclitaxel.16 The 2/3 weekly Nab‐PTX (120 mg/m2 on d1 and d8, q3w) in the present study was confirmed to improve tolerance by observations of less frequent toxicities, especially grade 3 to 4 peripheral neuropathy, and infrequent toxicity‐related discontinuations, dose reductions, and dose delays.
In the present study, age was not associated with the response, PFS, OS, grade 3 to 4 toxicities, dose reduction, or discontinuation, indicating that age did not affect the efficacy or tolerance of S‐1 plus Nab‐PTX. A previous study found no differences for pharmacodynamic variables of Nab‐PTX in patients with metastatic breast cancer based on age and concluded that 3/4 weekly Nab‐PTX was well tolerated across all age groups.23 Considering its convenience, compliance, and feasibility, this 2/3 weekly Nab‐PTX (120 mg/m2 on d1 and d8, q3w) plus S‐1 was a favorable option across all age groups, including elderly patients.
Seven patients achieved good responses and underwent gastrectomy plus metastasectomy with R0 resection. These patients had a prolonged PFS and OS compared to the other patients, as indicated by separated PFS and OS curves. Nevertheless, a PFS of 8.50 months still met the primary endpoint even though patients with gastrectomy plus metastasectomy were excluded. Multivariate analysis showed gastrectomy plus metastasectomy was an independent prognostic factor for PFS; however, it was not prognostic for OS. Currently, there are debates on the role of palliative gastrectomy, and a phase III randomized‐controlled REGATTA trial reported that gastrectomy plus chemotherapy had no survival benefit over chemotherapy alone for AGC patients with a single non‐curable factor.24 However, little is known about the value of gastrectomy plus metastasectomy after first‐line chemotherapy. Our data showed gastrectomy plus metastasectomy may have a preliminary role in improving progression‐free survival, and we expect a phase III trial of conversion chemotherapy with or without gastrectomy plus metastasectomy for AGC patients with limited metastasis.
The proportion of Chinese AGC patients receiving second‐line chemotherapy is not high,25, 26 which is similar to that reported for European and USA patients,5, 7, 27 and lower than that for Japanese and Korean patients in the phase III trials.2, 11, 20 The same thing happened in our present study and 35.6% of patients received second‐line chemotherapy, which limits the contribution to overall survival. Apart from patients who died of progressive cancers during or shortly after first‐line chemotherapy, who still had PR or stable diseases until the last follow up, and patients lost to follow up, there were actually 16 (21.9%) patients who were reluctant to receive second‐line chemotherapy after failure with first‐line, even though platinums, irinotecan, and fluoropyrimidines are partially covered by Chinese medical insurance. These patients had weak treatment will for second‐line treatment, low financial status, or the proportions of their medical compensations were not high based on different residential areas.
Dose schedules of Nab‐PTX varied across phase I to III trials, and three‐weekly 260 mg/m2 doses were used in breast and gastric cancers.15, 17, 19 The ABSOLUTE trial that explored 3/4 weekly Nab‐PTX (100 mg/m2 on d1, d8, and d15, q4w) was non‐inferior to weekly solvent‐based paclitaxel as second‐line treatment of gastric cancer, which added evidence for divided doses of Nab‐PTX.16 Based on the dose‐escalation of Nab‐PTX with divided doses in combination with standard S‐1, in the present study, we use the recommended dose for Nab‐PTX 120 mg/m2 on d1 and d8, q3w, achieving an efficacy/toxicity balance. A previous study showed the peritoneal metastases are an independent prognostic factor of poor survival in AGC patients.28 In this one‐arm trial where all patients received the same regimen, we found patients with peritoneal metastases had no significantly different efficacy or prognosis than other patients, which may indicate S‐1 plus Nab‐PTX had similar efficacy in patients with peritoneal metastases or without. Because the sample sizes of the subgroups in our study were small, further study is required to confirm this good observation for patients with peritoneal metastases. The ABSOLUTE trial found patients with peritoneal metastases benefit more from Nab‐PTX than solvent‐based paclitaxel,16 the reasons for which are worth further study and may help define the benefiting group of Nab‐PTX.
The excellent efficacy of nab‐paclitaxel may, in part, be explained by the high and preferential intratumor paclitaxel concentration achieved by nab‐technology. Albumin receptor‐mediated transcytosis of PTX and binding of albumin‐bound Nab‐PTX to interstitial albumin‐binding proteins, such as secreted protein acidic and rich in cysteine (SPARC), play an important role. Whether there is mechanistic synergy between Nab‐PTX with S‐1 and whether SPARC predicts outcomes remain to be explored in a further study.
In conclusion, S‐1 plus Nab‐PTX (120 mg/m2 on d1 and d8, q3w) is an encouraging option as first‐line treatment for patients with metastatic gastric cancer, and it had promising efficacy, acceptable safety, and convenience, warranting phase III evaluation.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
Supporting information
ACKNOWLEDGMENT
This study was funded by National Natural Science Foundation of China (Nos 81372570, 81572392).
He M‐M, Wang F, Jin Y, et al. Phase II clinical trial of S‐1 plus nanoparticle albumin‐bound paclitaxel in untreated patients with metastatic gastric cancer. Cancer Sci. 2018;109:3575‐3582. 10.1111/cas.13813
Ming‐ming He, Feng Wang and Ying Jin contributed equally as first authors.
Dong‐sheng Zhang and Rui‐hua Xu contributed equally as senior authors.
Trial registration: ClinicalTrials.gov Identifier: NCT02229058.
References
- 1. Chen W, Zheng R, Zeng H, Zhang S. The updated incidences and mortalities of major cancers in China, 2011. Chin J Cancer. 2015;34:502‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet. 2010;376:687‐697. [DOI] [PubMed] [Google Scholar]
- 3. Ajani JA, Buyse M, Lichinitser M, et al. Combination of cisplatin/S‐1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: Results of noninferiority and safety analyses compared with cisplatin/5‐fluorouracil in the First‐Line Advanced Gastric Cancer Study. Eur J Can. 2013;49:3616‐3624. [DOI] [PubMed] [Google Scholar]
- 4. Al‐Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435‐1442. [DOI] [PubMed] [Google Scholar]
- 5. Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S‐1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547‐1553. [DOI] [PubMed] [Google Scholar]
- 6. Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S‐1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063‐1069. [DOI] [PubMed] [Google Scholar]
- 7. Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first‐line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991‐4997. [DOI] [PubMed] [Google Scholar]
- 8. Chen WW, Wang F, Xu RH. Platinum‐based versus non‐platinum‐based chemotherapy as first line treatment of inoperable, advanced gastric adenocarcinoma: a meta‐analysis. PLoS One. 2013;8:e68974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nukatsuka M, Fujioka A, Nakagawa F, et al. Antimetastatic and anticancer activity of S‐1, a new oral dihydropyrimidine‐dehydrogenase‐inhibiting fluoropyrimidine, alone and in combination with paclitaxel in an orthotopically implanted human breast cancer model. Int J Oncol. 2004;25:1531‐1536. [PubMed] [Google Scholar]
- 10. Ueda Y, Yamagishi H, Ichikawa D, et al. Multicenter phase II study of weekly paclitaxel plus S‐1 combination chemotherapy in patients with advanced gastric cancer. Gastric Cancer. 2010;13:149‐154. [DOI] [PubMed] [Google Scholar]
- 11. Koizumi W, Kim YH, Fujii M, et al. Addition of docetaxel to S‐1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol. 2014;140:319‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin‐bound paclitaxel compared with polyethylated castor oil‐based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794‐7803. [DOI] [PubMed] [Google Scholar]
- 13. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab‐paclitaxel in combination with carboplatin versus solvent‐based paclitaxel plus carboplatin as first‐line therapy in patients with advanced non‐small‐cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30:2055‐2062. [DOI] [PubMed] [Google Scholar]
- 15. Sasaki Y, Nishina T, Yasui H, et al. Phase II trial of nanoparticle albumin‐bound paclitaxel as second‐line chemotherapy for unresectable or recurrent gastric cancer. Cancer Sci. 2014;105:812‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shitara K, Takashima A, Fujitani K, et al. Nab‐paclitaxel versus solvent‐based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open‐label, randomised, non‐inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:277‐287. [DOI] [PubMed] [Google Scholar]
- 17. Tsurutani J, Kuroi K, Iwasa T, et al. Phase I study of weekly nab‐paclitaxel combined with S‐1 in patients with human epidermal growth factor receptor type 2‐negative metastatic breast cancer. Cancer Sci. 2015;106:734‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwartzberg LS, Arena FP, Mintzer DM, Epperson AL, Walker MS. Phase II multicenter trial of albumin‐bound paclitaxel and capecitabine in first‐line treatment of patients with metastatic breast cancer. Clin Breast Cancer. 2012;12:87‐93. [DOI] [PubMed] [Google Scholar]
- 19. Nakayama N, Ishido K, Chin K, et al. A phase I study of S‐1 in combination with nab‐paclitaxel in patients with unresectable or recurrent gastric cancer. Gastric Cancer. 2017;20:350‐357. [DOI] [PubMed] [Google Scholar]
- 20. Koizumi W, Narahara H, Hara T, et al. S‐1 plus cisplatin versus S‐1 alone for first‐line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215‐221. [DOI] [PubMed] [Google Scholar]
- 21. Narahara H, Iishi H, Imamura H, et al. Randomized phase III study comparing the efficacy and safety of irinotecan plus S‐1 with S‐1 alone as first‐line treatment for advanced gastric cancer (study GC0301/TOP‐002). Gastric Cancer. 2011;14:72‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luo HY, Xu RH, Wang F, et al. Phase II trial of XELOX as first‐line treatment for patients with advanced gastric cancer. Chemotherapy. 2010;56:94‐100. [DOI] [PubMed] [Google Scholar]
- 23. Yardley DA. nab‐Paclitaxel mechanisms of action and delivery. J Controlled Release. 2013;170:365‐372. [DOI] [PubMed] [Google Scholar]
- 24. Fujitani K, Yang HK, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non‐curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309‐318. [DOI] [PubMed] [Google Scholar]
- 25. Wang J, Xu R, Li J, et al. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first‐line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer. 2016;19:234‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen L, Li J, Xu J, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double‐blind, phase III study (AVATAR study). Gastric Cancer. 2015;18:168‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shah MA, Xu RH, Bang YJ, et al. HELOISE: Phase IIIb Randomized Multicenter Study Comparing Standard‐of‐Care and Higher‐Dose Trastuzumab Regimens Combined With Chemotherapy as First‐Line Therapy in Patients With Human Epidermal Growth Factor Receptor 2‐Positive Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma. J Clin Oncol. 2017;35:2558‐2567. [DOI] [PubMed] [Google Scholar]
- 28. Fuchs CS, Muro K, Tomasek J, et al. Prognostic factor analysis of overall survival in gastric cancer from two phase III studies of second‐line Ramucirumab (REGARD and RAINBOW) using pooled patient data. J Gastric Can. 2017;17:132‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


