Abstract
Milk fat globule‐epidermal growth factor factor 8 (MFG‐E8) is secreted from macrophages and is known to induce immunological tolerance mediated by regulatory T cells. However, the roles of the MFG‐E8 that is expressed by cancer cells have not yet been fully examined. Expression of MFG‐E8 was examined using immunohistochemistry in surgical samples from 134 patients with esophageal squamous cell carcinoma. The relationships between MFG‐E8 expression levels and clinicopathological factors, including tumor‐infiltrating lymphocytes, were evaluated. High MFG‐E8 expression was observed in 23.9% of the patients. The patients with tumors highly expressing MFG‐E8 had a significantly higher percentage of neoadjuvant chemotherapy (NAC) history (P < .0001) and shorter relapse‐free survival (P = 0.012) and overall survival (OS; P = .0047). On subgroup analysis, according to NAC history, patients with high MFG‐E8 expression had significantly shorter relapse‐free survival (P = .027) and OS (P = .0039) only when they had been treated with NAC. Furthermore, tumors with high MFG‐E8 expression had a significantly lower ratio of CD8+ T cells/regulatory T cells in tumor‐infiltrating lymphocytes (P = .042) only in the patients treated with NAC, and those with a lower ratio had a shorter OS (P = .026). High MFG‐E8 expression was also found to be an independent prognostic factor in multivariate analysis. The abundant MFG‐E8 expression in esophageal squamous cell carcinoma might have a negative influence on the long‐term survival of patients after chemotherapy by affecting T‐cell regulation in the tumor microenvironment.
Keywords: apoptosis, cancer microenvironment, immunological escape, neoadjuvant chemotherapy, regulatory T cell
1. INTRODUCTION
In recent years, the human immune system has been recognized as having important roles in tumor progression.1, 2 Because immune escape, which is caused by various factors such as regulatory cells, molecules, and cytokines, is associated with tumor development, the inhibition of these factors has been considered a promising treatment for cancer.3, 4, 5 This strategy has proven effective with the successful development of antibody therapies blocking immune checkpoints, including anti‐cytotoxic T‐lymphocyte associated protein‐4 or programmed cell death‐1 (PD‐1) therapy.4 Therefore, it might be worth investigating other factors involved in immune suppression that are related to tumor progression.
Milk fat globule‐epidermal growth factor factor 8 (MFG‐E8), also known as lactadherin in humans, was originally identified as a glycoprotein that exists on the membrane of milk fat globule in the mammary duct6, 7 and is known to be associated with adhesion to integrin‐expressing cells.8 This molecule is also secreted from various types of cells, including mammary epithelial cells, macrophages, and immature dendritic cells, and mediates the efficient engulfment of apoptotic cells by binding to αVβ3 integrin on phagocytes.9, 10, 11 Mice deficient in MFG‐E8 have defects in the removal of apoptotic cells and tend to develop autoimmune disease.12 These results suggest a critical role for MFG‐E8 in phagocytosis and the suppression of the immune response against apoptotic cells.
Furthermore, MFG‐E8 expression in the tumor microenvironment was suggested to regulate the function of granulocyte macrophage colony‐stimulating factor and to suppress antitumor immune responses through regulatory T cell (Treg) propagation.13 When an anti‐MFG‐E8 blocking antibody was administered accompanied with the treatments that induce tumor cell apoptosis in mouse tumors, systemic and potent antitumor effects were observed with efficient dendritic cell cross‐presentation, which leads to the activation of cytotoxic CD8+ T cells through the suppression of Treg induction.14
Milk fat globule‐epidermal growth factor factor 8 is reported to be expressed in several types of human cancer cells, as well as immune‐related cells. Such expression has been found to be associated with tumor proliferation, epithelial‐mesenchymal transition, cell migration, M2 macrophage polarization, and the induction of Tregs.15, 16, 17, 18, 19 However, there are limited reports that clarified its prognostic impact on cancer patients.16, 20 Furthermore, although MFG‐E8 is predicted to affect the balance of the expression of tumor‐infiltrating lymphocytes such as CD8+ T cells and Tregs,14 this relationship and its influence on survival are yet to be revealed.
In this study, we investigated MFG‐E8 expression in surgical samples of esophageal squamous cell carcinoma patients, including those who underwent preoperative chemotherapy, and evaluated the relationship with clinicopathological factors, including the immunological situation in the tumor microenvironment.
2. MATERIALS AND METHODS
2.1. Patients
Among the 403 esophageal cancer patients who underwent curative esophagectomy between May 2000 and January 2008 in Osaka University Hospital (Suita, Japan), the 134 patients with squamous cell cancer whose surgical specimens and clinical information were obtainable were enrolled in this study. Patients who underwent preoperative chemoradiotherapy were excluded.
Of the 134 patients, 19, 32, 60, and 23 patients were pathologically diagnosed as stage I, II, III, and IV, respectively, according to the UICC classification (7th edition). Sixty‐six patients (49.3%) underwent esophagectomy without preoperative therapy. Sixty‐eight patients (50.7%) received neoadjuvant chemotherapy (NAC) that consisted of cisplatin, doxorubicin, and 5‐fluorouracil (Table 1). The precise regimen is described elsewhere.21 In the study period, the NAC was given to patients with tumors in any T category (cT1‐4) accompanied with metastasis only to the regional or non‐regional lymph nodes. The NAC was also given to patients with tumors classified as cT2 or more in depth with or without lymph node metastasis. The patients with metastatic lesions in distant organs other than the lymph nodes at the preoperative evaluation were excluded from the study.22, 23 The characteristics of patients with and without NAC are described in Table 2. Surgical treatment was carried out 3‐8 weeks after 2 courses of NAC. The surgical procedure has been described previously.24 The median follow‐up period after the operation was 89.3 months.
Table 1.
Relationship between milk fat globule‐epidermal growth factor factor 8 (MFG‐E8) expression and clinicopathological factors in 134 patients with esophageal squamous cell carcinoma
| Patient characteristics | Total | MFG‐E8 low | MFG‐E8 high | P‐value | |
|---|---|---|---|---|---|
| n = 134 (%) | n = 102 (%) | n = 32 (%) | |||
| Age, years | ≤65 | 73 (54.5) | 52 (38.8) | 21 (15.7) | .15 |
| >65 | 61 (45.5) | 50 (37.3) | 11 (8.2) | ||
| Sex | Male | 117 (87.3) | 86 (64.2) | 31 (23.1) | .06 |
| Female | 17 (12.7) | 16 (11.9) | 1 (0.8) | ||
| Location[Link] | Ut | 18 (13.4) | 10 (7.5) | 8 (6.0) | .028 |
| Mt‐Lt | 116 (86.6) | 92 (68.7) | 24 (17.9) | ||
| Tumor differentiation[Link] | Well‐Mod | 102 (76.1) | 81 (60.5) | 21 (15.7) | .11 |
| Por | 32 (23.9) | 21 (15.7) | 11 (8.2) | ||
| Neoadjuvant chemotherapy | With | 68 (50.7) | 41 (30.6) | 27 (20.2) | <.0001 |
| Without | 66 (49.3) | 61 (45.5) | 5 (3.7) | ||
| Tumor depth[Link] | pT1‐2 | 47 (35.1) | 37 (27.6) | 10 (7.5) | .6000 |
| pT3‐4 | 87 (64.9) | 65 (48.5) | 22 (16.4) | ||
| Lymph node metastasis[Link] | pN0‐1 | 83 (61.9) | 69 (51.5) | 14 (10.5) | .0150 |
| pN2‐3 | 51 (38.1) | 33 (24.6) | 18 (13.4) | ||
| Non‐regional lymph node metastasis[Link] | pM0 | 111 (82.8) | 91 (67.9) | 20 (14.9) | .0005 |
| pM1 | 23 (17.2) | 11 (8.2) | 12 (9.0) | ||
| Tumor stage[Link] | I‐II | 51 (38.0) | 42 (31.3) | 9 (6.7) | .1800 |
| III‐IV | 83 (62.0) | 60 (44.8) | 23 (17.2) | ||
Upper (Ut), middle (Mt), and lower (Lt) third of thorax.
Well, moderately (Mod), and poorly (Por) differentiated squamous cell carcinoma.
Pathological classification according to UICC (7th edition).
Table 2.
Difference in clinical and pathological characteristics of patients with esophageal squamous cell carcinoma treated with or without neoadjuvant chemotherapy (NAC)
| Patient characteristics | Total | NAC− | NAC+ | P‐value | |
|---|---|---|---|---|---|
| n = 134 (%) | n = 66 (%) | n = 68 (%) | |||
| Age, years | ≤65 | 73 (54.5) | 30 (22.4) | 43 (32.1) | .0390 |
| >65 | 61 (45.5) | 36 (26.9) | 25 (18.7) | ||
| Sex | Male | 117 (87.3) | 57 (42.5) | 60 (44.8) | .7400 |
| Female | 17 (12.7) | 9 (6.7) | 8 (6.0) | ||
| Location[Link] | Ut | 18 (13.4) | 12 (9.0) | 6 (4.5) | .1100 |
| Mt‐Lt | 116 (86.6) | 54 (40.3) | 62 (46.3) | ||
| Tumor differentiation[Link] | Well‐Mod | 102 (76.1) | 52 (38.8) | 50 (37.3) | .4800 |
| Por | 32 (23.9) | 14 (10.5) | 18 (13.4) | ||
| cT[Link] | cT1‐2 | 57 (42.5) | 37 (27.6) | 20 (14.9) | .0018 |
| cT3‐4 | 77 (57.5) | 29 (21.6) | 48 (35.8) | ||
| cN[Link] | cN0 | 42 (31.3) | 36 (26.9) | 6 (4.5) | <.0001 |
| cN1 | 92 (68.7) | 30 (22.4) | 62 (46.3) | ||
| cM[Link] | cM0 | 107 (79.9) | 62 (46.3) | 45 (33.6) | <.0001 |
| cM1 | 27 (20.2) | 4 (3.0) | 23 (17.2) | ||
| cStage[Link] | cI‐II | 51 (38.1) | 39 (29.1) | 12 (9.0) | <.0001 |
| cIII‐IV | 83 (61.9) | 27 (20.2) | 56 (41.8) | ||
| pT[Link] | pT1‐2 | 47 (35.1) | 28 (20.9) | 19 (14.2) | .0800 |
| pT3‐4 | 87 (64.9) | 38 (28.4) | 49 (36.6) | ||
| pN[Link] | pN0‐1 | 42 (31.3) | 41 (30.6) | 83 (61.9) | .6900 |
| pN2‐3 | 24 (17.9) | 27 (20.2) | 51 (38.1) | ||
| pM[Link] | pM0 | 111 (82.8) | 61 (45.5) | 50 (37.3) | .0037 |
| pM1 | 23 (17.2) | 5 (3.7) | 18 (13.4) | ||
| pStage[Link] | pI‐II | 51 (38.1) | 30 (22.4) | 21 (15.7) | 0.0800 |
| pIII‐IV | 83 (61.9) | 36 (26.9) | 47 (35.1) | ||
Upper (Ut), middle (Mt), and lower (Lt) third of thorax.
Well, moderately (Mod), and poorly (Por) differentiated squamous cell carcinoma.
Clinical classification of tumor depth (cT), lymph node metastasis (cN), non‐regional lymph node metastasis (cM), and stage (cStage) in UICC (6th edition).
Pathological classification of tumor depth (pT), lymph node metastasis (pN), non‐regional lymph node metastasis (pM), and stage (pStage) in UICC (7th edition).
This study was approved by the appropriate institutional review boards of Osaka University Hospital (approved no. 08226‐6) and was carried out in accordance with the Declaration of Helsinki.
2.2. Clinical and pathological evaluation of response to chemotherapy
The clinical response was assessed according to the following criteria. Patients with complete regression or a partial response (with >50% reduction in the tumor on computed tomography) were defined as responders. Patients with progressive disease (PD; with >25% enlargement of the tumor or with appearance of new lesions) or with stable disease (with residual tumor and neither categorized as partial response nor PD) were defined as non‐responders.25 The pathological response was categorized into 5 groups according to the criteria of the Japanese Society for Esophageal Diseases.26 The grades were as follows: grade 0, no therapeutic effect; grade1a, viable cancer cells accounting for 2/3 or more tumor tissue; grade 1b, viable cancer cells accounting for 1/3 to 2/3 of tumor tissue; grade 2, viable cancer cells accounting for less than 1/3 of tumor tissue; and grade 3, no viable cancer cells. In this study, patients with grades 1b‐3 were defined as responders and those with grades 0‐1a were defined as non‐responders.
2.3. Immunohistochemistry
Surgical samples were fixed in 10% phosphate‐buffered formalin and embedded in paraffin. These formalin‐fixed paraffin‐embedded (FFPE) tissues were sliced into 3.5‐μm‐thick sections. Our immunohistochemistry (IHC) method is described elsewhere.27 Briefly, the FFPE sections were deparaffinized in xylene and rehydrated in a graded series of ethanol solutions. Heat‐induced epitope retrieval was carried out at 110°C for 15 minutes in citrate buffer (pH 6.0) using a decloaking chamber (Biocare Medical, Walnut Creek, CA, USA). Endogenous peroxidase was blocked with 0.3% H2O2 in methanol for 20 minutes. The specimens were blocked with normal horse serum (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA, USA) for 20 minutes at room temperature. Subsequently, the specimens were incubated overnight at 4°C with mouse mAbs specific to human MFG‐E8 (14A‐11B produced in The Institute of Medical Science, University of Tokyo, Tokyo, Japan) at a dilution of 1:100, Foxp3 (Clone 236A/E7; Abcam, Cambridge, UK) at a dilution of 1:100 or CD8 (Clone C8/144B; Dako, Glostrup, Denmark) without dilution. A secondary antibody reaction was carried out using biotinylated rabbit anti‐mouse secondary antibodies (Vectastain Elite ABC kit; Vector Laboratories) for 20 minutes at room temperature. The samples were then incubated for 30 minutes with avidin‐biotin complex reagent (Vectastain Elite ABC kit; Vector Laboratories) at room temperature. The reaction products were developed in liquid 3,3′‐diaminobenzidine (DAB Chromogen tablets; Dako) dissolved with 0.05 M Tris‐HCl buffer (pH 7.5). As positive controls, human tonsils were used for Foxp3 and CD8 staining.
2.4. Evaluation of MFG‐E8 expression and quantification of tumor‐infiltrating T cells
The MFG‐E8 expression of the tumor cells was examined. Other cells including macrophages and dendritic cells in tumor stroma showed sporadic MFG‐E8 expression at significantly lower level. The intensity of MFG‐E8 expression was examined and classified according to the highest intensity of the staining in each sample. The grades of intensity were determined by comparison with that of endothelial cells of muscular blood vessels found in the same specimen in high‐power (100×) microscopic fields28 (Figure 1A). Intensity scores 2, 1, or 0 were given to those with stronger, equal, or weaker staining intensity than vascular endothelial cells, respectively (Figure 1A‐C). The tumors scored as 2 were classified as high expression, and those with score 1 or 0 were defined as low expression. Foxp3 was used to detect Tregs. Five independent areas of the tumor with the most abundant lymphocytes were selected in each specimen to evaluate the number of tumor‐infiltrating Foxp3+ and CD8+ T cells. The positive cells were counted manually in each high‐power (200×) microscopic field (Figure 1D,E), and the mean values of the intratumoral tumor‐infiltrating T cells were calculated. Based on these results, CD8+/Foxp3+ T‐cell ratios (CD8/Foxp3 ratios) were evaluated. The samples were assessed by two investigators in a blinded manner.
Figure 1.
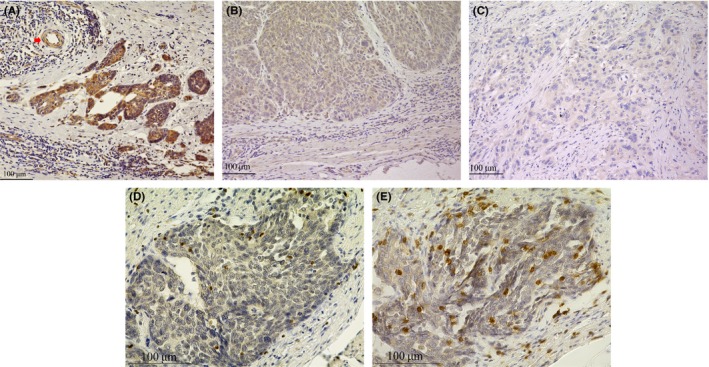
Representative staining patterns of immune‐reactive milk fat globule‐epidermal growth factor factor 8 (MFG‐E8), CD8, and Foxp3 in tumor samples of esophageal squamous cell carcinoma. A, Endothelial cells of muscular blood vessels were used as internal positive control of MFG‐E8 expression (red arrow). Squamous cell carcinoma with MFG‐E8 expression stronger than that of the positive control was defined to have intensity score 2 (100×). B, Tumor cells with MFG‐E8 expression equal to that of the positive control were defined to have intensity score 1 (100×). C, Tumor cells with MFG‐E8 expression weaker than that of the positive control were defined to have intensity score 0 (100×). D, Immunohistochemical staining of intratumoral Foxp3+ T cells (200×). E, Immunohistochemical staining of intratumoral CD8+ T cells (200×)
2.5. Statistical analyses
The associations between MFG‐E8 expression and clinicopathological factors were analyzed by the χ2 test. Relapse‐free survival (RFS) and overall survival (OS) were calculated using the Kaplan‐Meier method, and the log‐rank test was used to assess the association between the survival and MFG‐E8 expression or CD8/Foxp3 ratios. A subgroup analysis of RFS and OS according to NAC history was undertaken using the same method. Hazard ratios and 95% confidence intervals of the variables were calculated using the Cox proportional hazard regression model. A multivariate Cox regression analysis was undertaken for the potential variables that could influence the survival. The difference of the CD8/Foxp3 ratios between high and low MFG‐E8 expression groups was compared by the Wilcoxon rank sum test. Receiver operating characteristic (ROC) curve analysis was applied to determine the cut‐off value for decreased CD8/Foxp3 ratio that affects the survival by using the 3‐year OS as the end‐point.29 A two‐sided P value less than 0.05 was considered significant. All statistical analyses were carried out with JMP version 13 (SAS, Cary, NC, USA).
3. RESULTS
3.1. Relationship between MFG‐E8 expression and clinicopathological factors
Among the 134 patients, high MFG‐E8 expression levels in the tumors were observed in 32 patients (23.9%). The relationships between MFG‐E8 expression and clinicopathological factors are shown in Table 1. In the group with high MFG‐E8 expression, the frequency of patients with tumors located in the upper thoracic esophagus (P = .028), tumors with pathologically diagnosed advanced lymph nodes metastasis (pN2‐3) (P = 0.015), or non‐regional lymph node metastasis (pM1) (P = .0005) and a history of NAC (P < .0001) were significantly higher compared with the patients with low MFG‐E8 expression. There was no significant relationship between the intensity of MFG‐E8 and other pathological factors such as sex, poorly differentiated tumor, tumor depth (pT), or tumor stage (pStage).
3.2. Relationship between MFG‐E8 expression and patient survival
Among all 134 patients, the RFS and OS of the high MFG‐E8 expression group were significantly worse than those of the low‐expression group (P = .012 in RFS and P = .0047 in OS; Figure 2A). To minimize the differences in patient backgrounds (Table 2), further analysis was undertaken in the subgroups divided according to the NAC history. Within the patients without NAC, there was no significant difference in the RFS or OS between those with high MFG‐E8 expression and those with low expression (RFS, P = .65; OS, P = .68; Figure 2B]. However, among patients treated with NAC, those with high MFG‐E8 expression showed significantly shorter RFS and OS compared with those with low expression (RFS, P = .027; OS, P = .0039; Figure 2C).
Figure 2.
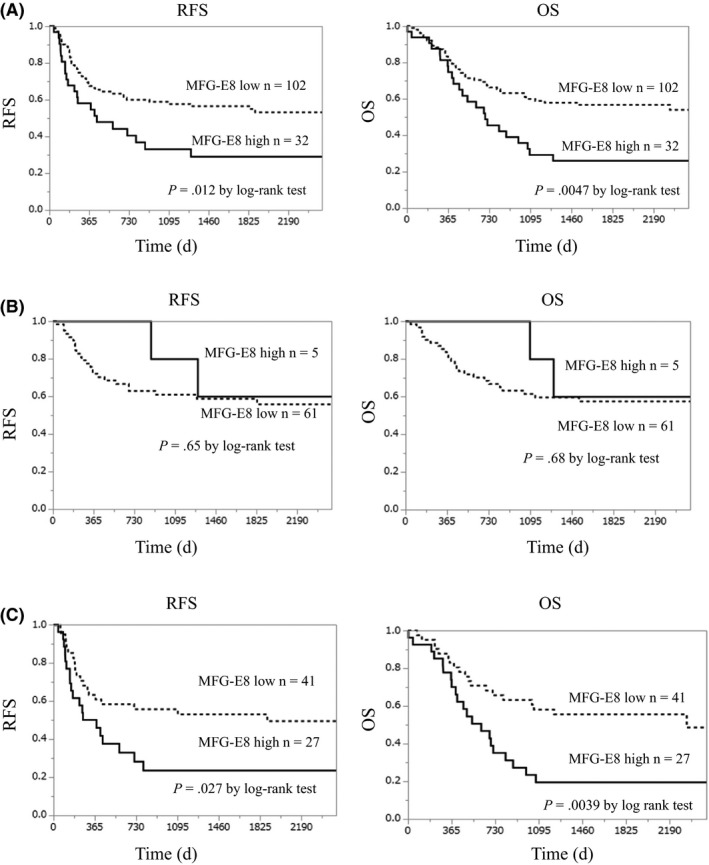
Comparison of relapse‐free survival (RFS) and overall survival (OS) between patients with high and low milk fat globule‐epidermal growth factor factor 8 (MFG‐E8) expression. A, Patients with high MFG‐E8 expression showed significantly worse relapse‐free survival (RFS) and overall survival (OS) compared with patients with low MFG‐E8 expression in total 134 patients examined. B, There was no significant difference in RFS and OS between the patients with high and low MFG‐E8 expression in subgroup without neoadjuvant chemotherapy. C, Patients with high MFG‐E8 expression showed significantly worse RFS and OS compared with the patients with low MFG‐E8 expression in subgroup with neoadjuvant chemotherapy
As shown in Table 3, the high MFG‐E8 expression group among patients who received NAC had a significantly larger percentage of N2‐3 lymph node metastasis and non‐regional lymph node metastasis. This observation was not made for the group without NAC (Table S1).
Table 3.
Relationship between milk fat globule‐epidermal growth factor factor 8 (MFG‐E8) expression and clinicopathological factors in 68 patients with esophageal squamous cell carcinoma treated with neoadjuvant chemotherapy (NAC)
| Patient characteristics | NAC+ | |||
|---|---|---|---|---|
| MFG‐E8 Low | MFG‐E8 High | P‐value | ||
| N = 41 (%) | N = 27 (%) | |||
| Age, years | ≤65 | 25 (36.8) | 18 (26.5) | .630 |
| >65 | 16 (23.5) | 9 (13.2) | ||
| Sex | Male | 34 (50.0) | 26 (38.2) | .090 |
| Female | 7 (10.3) | 1 (1.5) | ||
| Tumor location[Link] | Ut | 0 (0) | 6 (8.8) | .002 |
| Mt‐Lt | 41 (60.3) | 21 (30.9) | ||
| Tumor differentiation[Link] | Well‐Mod | 32 (47.1) | 18 (26.5) | .300 |
| Por | 9 (13.2) | 9 (13.2) | ||
| Tumor depth[Link] | pT1‐2 | 12 (17.7) | 7 (10.3) | .760 |
| pT3‐4 | 29 (42.7) | 20 (29.4) | ||
| Lymph node metastasis[Link] | pN0‐1 | 30 (44.1) | 11 (16.2) | .008 |
| pN2‐3 | 11 (16.2) | 16 (23.5) | ||
| Non‐regional lymph node metastasis[Link] | pM0 | 34 (50.0) | 16 (23.5) | .030 |
| pM1 | 7 (10.3) | 11 (16.2) | ||
| Tumor stage[Link] | pStageI‐II | 15 (22.0) | 6 (8.8) | .210 |
| pStageIII‐IV | 26 (38.2) | 21 (31.0) | ||
| Clinical response to NAC | Responder | 23 (33.8) | 15 (22.0) | .960 |
| Non responder | 18 (26.5) | 12 (17.7) | ||
| Pathological response to NAC | Responder | 10 (14.7) | 6 (8.8) | .840 |
| Non responder | 31 (45.6) | 21 (30.9) | ||
Upper (Ut), middle (Mt), and lower (Lt) third of thorax.
Well, moderately (Mod), and poorly (Por) differentiated squamous cell carcinoma.
Pathological classification in UICC (7th edition).
To analyze the causes that led to the worse survival rate of patients with high MFG‐E8 expression after receiving NAC, the relationships between MFG‐E8 expression and both the clinical and pathological response to therapy were examined. There were no relationships between these factors (Table 3).
3.3. Relationship between expression of MFG‐E8 and CD8+ T cell/Treg (Foxp3+ T cell) ratio
To investigate the possible influence of MFG‐E8 expression on immune suppression through Treg induction, the CD8+ T cell/Treg (Foxp3+ T cell) ratio was examined in the patients with NAC. Among 134 cases whose MFG‐E8 expression was evaluated, the same FFPE sections were available for CD8+ T cell and Foxp3+ T cell assessment in 127 cases (61 cases without NAC and 66 cases with NAC). Within the 61 patients without NAC, there were no significant differences in the CD8/Foxp3 ratio between those with high MFG‐E8 expression and those with low expression (median value, 1.0 vs 1.71; P = .34) (Figure 3A). Within the 66 patients who underwent NAC, the CD8/Foxp3 ratios were significantly lower in patients with high MFG‐E8 expression compared with those with low expression (median value, 1.53 vs 3.28; P = .042) (Figure 3B). These results indicated the effect of MFG‐E8 on the balance of infiltrating CD8+ T cells and Tregs in tumors within patients who received NAC.
Figure 3.
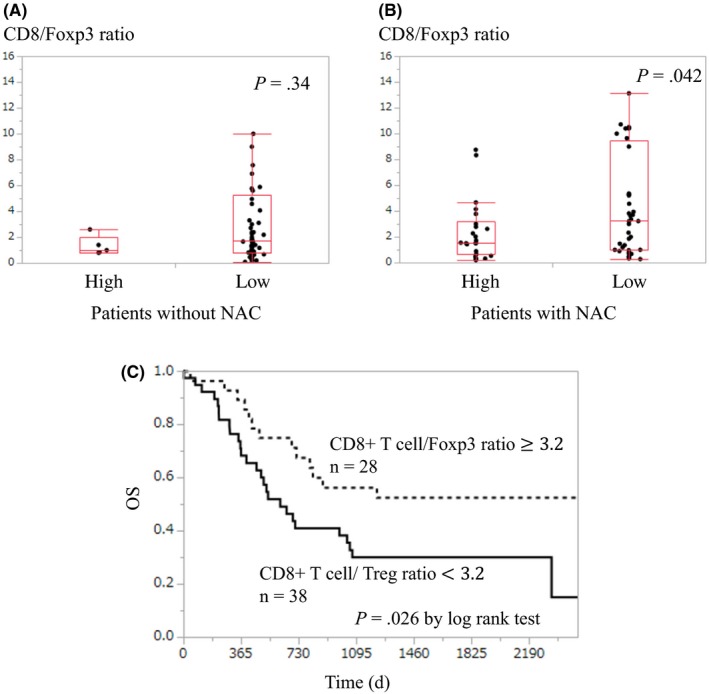
Examination of CD8+/Foxp3+ T‐cell ratio (CD8/Foxp3) in tumor samples of esophageal squamous cell carcinoma. A, There was no significant difference in the CD8/Foxp3 between the patients with high and low milk fat globule‐epidermal growth factor factor 8 (MFG‐E8) expression in the subgroup without neoadjuvant chemotherapy (NAC). B, Patients with high MFG‐E8 expression showed a significantly lower CD8/Foxp3 compared with that of the patients with low expression in the subgroup with NAC. C, Patients with a CD8/Foxp3 below 3.2 showed significantly worse overall survival compared with that of the patients with a CD8/Foxp3 equal to or higher than 3.2
3.4. Relationship between CD8+ T cell/Treg ratio and survival in patients with NAC
To assess the influence of immunological status on tumor progression, the relationship between the CD8+ T cell/Treg (Foxp3+ T cell) ratio and the survival of patients with NAC was evaluated. The patients were divided into 2 groups according to the cut‐off value determined with ROC curve analysis. The cut‐off value was 3.2 with an area under the curve of 0.61. Twenty‐eight patients had a CD8/Foxp3 ≥ 3.2 (high CD8/Foxp3 group), and 38 patients had a CD8/Foxp3 < 3.2 (low CD8/Foxp3 group). When the OS was compared between the two groups, the low CD8/Foxp3 group showed a significantly worse prognosis (P = .026; Figure 3C). This finding might suggest that CD8+ T‐cell suppression with Treg propagation leads to worse OS of patients treated with NAC.
3.5. Univariate and multivariate analyses for factors associated with OS in patients treated with NAC
In the univariate analysis among patients with NAC history, poor tumor differentiation, pathologically diagnosed N2‐3 lymph node metastasis, non‐regional lymph node metastasis, tumor stage III‐IV, and high MFG‐E8 expression were the factors correlated with worse OS (Table 4). In the multivariate analysis, poor tumor differentiation, tumor stage III‐IV, and high MFG‐E8 expression were found to be independent prognostic factors (Table 4).
Table 4.
Univariate and multivariate analyses of factors associated with overall survival in patients with esophageal squamous cell carcinoma treated with neoadjuvant chemotherapy
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age, years | ||||||
| >65 | 0.98 | 0.50‐1.84 | 0.95 | 0.71 | 0.35‐1.37 | 0.31 |
| Sex | ||||||
| Male | 1.29 | 0.51‐4.31 | 0.62 | |||
| Tumor location[Link] | ||||||
| Ut | 0.76 | 0.18‐2.11 | 0.64 | |||
| Tumor differentiation[Link] | ||||||
| Por | 3.74 | 1.93‐7.12 | 0.0002 | 3.04 | 1.54‐5.92 | 0.0016 |
| Tumor depth[Link] | ||||||
| pT3‐4 | 1.53 | 0.75‐3.41 | 0.24 | |||
| Lymph node metastasis[Link] | ||||||
| pN2‐3 | 6.06 | 3.08‐12.38 | <0.0001 | |||
| Non‐regional lymph node metastasis[Link] | ||||||
| pM1 | 3.93 | 2.02‐7.61 | <0.0001 | |||
| Tumor stage[Link] | ||||||
| III‐IV | 4.03 | 1.80‐10.71 | 0.0003 | 3.77 | 1.63‐10.24 | 0.0013 |
| MFG‐E8 expression | ||||||
| High | 2.46 | 1.30‐4.65 | 0.0054 | 2.07 | 1.08‐3.99 | 0.028 |
Upper (Ut), middle (Mt), and lower (Lt) third of thorax.
Well, moderately (Mod), and poorly (Por) differentiated squamous cell carcinoma.
Pathological classification in UICC (7th edition).
CI, confidence interval; HR, hazard ratio; MFG‐E8, milk fat globule‐epidermal growth factor factor 8.
4. DISCUSSION
We examined MFG‐E8 expression in human esophageal squamous cell carcinoma using IHC staining and found that some of the cells abundantly express MFG‐E8, as shown in other types of cancer.15, 16, 17, 18, 19, 20, 28, 30, 31 As the IHC staining of the samples showed that the expression of MFG‐E8 was more abundant in cancer cells than in macrophages or other stromal cells, MFG‐E8 from cancer cells appears to play an important role in the tumor microenvironment. Analysis of all patients examined showed that tumors with high MFG‐E8 expression had a significantly higher possibility of having advanced lymph node and non‐regional lymph node metastasis (M1), as previously suggested in malignant melanoma patients.16 Our study also showed that the RFS and OS of the high MFG‐E8 expression group were significantly worse than those of the low‐expression group. These findings are consistent with the previous reports.16, 20, 31
In the subgroup analysis according to the history of NAC showed unexpected characteristics associated with MFG‐E8 expression of the tumors. In the patient group with NAC history, high MFG‐E8 expression in the tumors correlated with worse RFS and OS. In both univariate and multivariate analyses, high MFG‐E8 expression was found to be a statistically significant negative prognostic factor.
Because the unfavorable influence of abundant MFG‐E8 expression on survival was only observed in patients who received NAC, we initially speculated that the effect might be associated with tumor resistance for the chemotherapy. However, our data showed no significant relationship between the MFG‐E8 expression and the extent of tumor shrinkage in response to the chemotherapy (Table 3). Therefore, the negative effect of MFG‐E8 on survival does not appear to be the result of enhanced resistance of the tumor cell to the treatment. Thus, we hypothesized that immunosuppression induced by abundant MFG‐E8 expression, which is shown in mouse tumor models14 and in other human cancers,18 might have long‐term immunosuppressive effects and cause the recurrence and poor survival. To investigate this hypothesis, we examined the influence of MFG‐E8 on the characteristics of TILs. Multiple reports elsewhere have shown that lower CD8/Foxp3, not the absolute number of Foxp3+ or CD8+ T cells, better indicates the extent of suppression of antitumor immunity and has a relationship with worse patient survival in multiple cancer types, including esophageal squamous cell carcinoma.32, 33, 34, 35, 36, 37 In the current study, among patients treated with NAC, tumors with high MFG‐E8 expression had a significantly lower CD8/Foxp3 ratio compared with those with low MFG‐E8 expression. Furthermore, the patients with lower CD8/Foxp3 ratio had worse survival compared with those with high ratio, consistent with previous reports.32, 37 These findings in patients treated with NAC suggest that high MFG‐E8 expression in the tumors treated with chemotherapy might induce Treg propagation to suppress antitumor immunity exerted by CD8+ T cells. It is of interest that the substantial influence of high MFG‐E8 expression is observed only in patients treated with NAC. Because MFG‐E8 mediates the phagocytosis of apoptotic cells and suppresses the immune responses, the immune‐regulatory effects of this molecule might become apparent when extensive apoptosis is induced with chemotherapy, as shown in mouse tumor models.14
Soki et al reported the impact of MFG‐E8 on tumor‐associated macrophage polarization in prostate cancer,17 therefore this interaction could be important in esophageal cancer as well. Thus, we examined the relationship between MFG‐E8 and tumor‐associated macrophages by IHC on our surgical samples using methods similar to the previous study from our group23 which examined the expression of total macrophages (M1 and M2) and independent M2 in esophageal cancer by CD68 and CD163 IHC staining, respectively. As a result, MFG‐E8 expression in esophageal cancer had no relationship with total macrophages (Figure S1) or M2 macrophages (Figure S2). The finding shown in prostate cancer was not seen in the surgical samples of esophageal cancer.
In the current study, there was a significantly higher rate of high MFG‐E8 expression in the tumors of patients treated with NAC compared with those not treated with NAC. Immune checkpoint molecules, such as PD‐1, cytotoxic T‐lymphocyte associated protein‐4 and PD‐1 ligand (PD‐L1), have been suggested to increase after chemotherapy.27, 38, 39 We hypothesized that MFG‐E8 also might be induced by preoperative chemotherapy in esophageal cancer cells. The promotion of MFG‐E8 expression could be clarified by comparing IHC before and after the therapy. However, as preoperative biopsy samples did not include vascular endothelial cells used as internal control, objective comparison was not possible. Instead, we found increased MFG‐E8 expression from esophageal cancer cell lines after treatment with chemotherapeutic agents (cisplatin, 5‐fluorouracil, and adriamycin) in RT‐quantitative PCR and ELISA assay (Figures S3,S4). This might suggest induced MFG‐E8 expression after chemotherapy, although further analysis of this matter would be beneficial.
The prognosis of esophageal cancer patients improved after NAC was introduced;40, 41 however, even when treated with NAC, the 5‐year survival rate remains as low as 59.2% among those who showed a good response to therapy.42 Our results suggested that immunosuppression, which is possibly occurring after chemotherapy, might influence the long‐term postoperative outcomes of patients. Our previous study also showed that strong PD‐L1 expression in esophageal squamous cancer cells after NAC was associated with reduced activity of CD8+ T cells and worse survival.27 These results might suggest that various types of immunological escape occur in the tumor microenvironment, particularly after chemotherapy, and serve as critical events that strongly influence the outcome of the treatment. Therefore, the inhibition of the responsible molecules, including MFG‐E8, could be a useful strategy to significantly improve the survival of esophageal cancer patients treated with chemotherapy.
In conclusion, abundant MFG‐E8 expression in esophageal squamous cell carcinomas might have a negative influence on the long‐term survival of patients after chemotherapy by affecting T‐cell regulation in the tumor microenvironment.
CONFLICT OF INTEREST
H.T. has received grants from the Japanese Ministry of Education, Culture, Sports Science and Technology, Sanofi KK, and Ono Pharmaceutical. The other authors have no conflict of interest related to this research.
Supporting information
Kanemura T, Miyata H, Makino T, et al. Immunoregulatory influence of abundant MFG‐E8 expression by esophageal cancer treated with chemotherapy. Cancer Sci. 2018;109:3393–3402. 10.1111/cas.13785
REFERENCES
- 1. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991‐998. [DOI] [PubMed] [Google Scholar]
- 2. Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807‐839. [DOI] [PubMed] [Google Scholar]
- 3. Stewart TJ, Smyth MJ. Improving cancer immunotherapy by targeting tumor‐induced immune suppression. Cancer Metastasis Rev. 2011;30:125‐140. [DOI] [PubMed] [Google Scholar]
- 4. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sakaguchi S. Naturally arising CD4 + regulatory t cells for immunologic self‐tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531‐562. [DOI] [PubMed] [Google Scholar]
- 6. Ceriani RL, Thompson K, Peterson JA, Abraham S. Surface differentiation antigens of human mammary epithelial cells carried on the human milk fat globule. Proc Natl Acad Sci USA. 1977;74:582‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stubbs JD, Lekutis C, Singer KL, et al. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor‐like domains linked to factor VIII‐like sequences. Proc Natl Acad Sci USA. 1990;87:8417‐8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taylor MR, Couto JR, Scallan CD, Ceriani RL, Peterson JA. Lactadherin (formerly BA46), a membrane‐associated glycoprotein expressed in human milk and breast carcinomas, promotes Arg‐Gly‐Asp (RGD)‐dependent cell adhesion. DNA Cell Biol. 1997;16:861‐869. [DOI] [PubMed] [Google Scholar]
- 9. Hanayama R, Tanaka M, Miwa K, Nagata S. Expression of developmental endothelial locus‐1 in a subset of macrophages for engulfment of apoptotic cells. J Immunol. 2004;172:3876‐3882. [DOI] [PubMed] [Google Scholar]
- 10. Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182‐187. [DOI] [PubMed] [Google Scholar]
- 11. Miyasaka K, Hanayama R, Tanaka M, Nagata S. Expression of milk fat globule epidermal growth factor 8 in immature dendritic cells for engulfment of apoptotic cells. Eur J Immunol. 2004;34:1414‐1422. [DOI] [PubMed] [Google Scholar]
- 12. Hanayama R, Tanaka M, Miyasaka K, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG‐E8‐deficient mice. Science. 2004;304:1147‐1150. [DOI] [PubMed] [Google Scholar]
- 13. Jinushi M, Nakazaki Y, Dougan M, Carrasco DR, Mihm M, Dranoff G. MFG‐E8‐mediated uptake of apoptotic cells by APCs links the pro‐ and antiinflammatory activities of GM‐CSF. J Clin Invest. 2007;117:1902‐1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jinushi M, Sato M, Kanamoto A, et al. Milk fat globule epidermal growth factor‐8 blockade triggers tumor destruction through coordinated cell‐autonomous and immune‐mediated mechanisms. J Exp Med. 2009;206:1317‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jinushi M, Nakazaki Y, Carrasco DR, et al. Milk fat globule EGF‐8 promotes melanoma progression through coordinated Akt and twist signaling in the tumor microenvironment. Cancer Res. 2008;68:8889‐8898. [DOI] [PubMed] [Google Scholar]
- 16. Oba J, Moroi Y, Nakahara T, Abe T, Hagihara A, Furue M. Expression of milk fat globule epidermal growth factor‐VIII may be an indicator of poor prognosis in malignant melanoma. Br J Dermatol. 2011;165:506‐512. [DOI] [PubMed] [Google Scholar]
- 17. Soki FN, Koh AJ, Jones JD, et al. Polarization of prostate cancer‐associated macrophages is induced by milk fat globule‐EGF factor 8 (MFG‐E8)‐mediated efferocytosis. J Biol Chem. 2014;289:24560‐24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sugano G, Bernard‐Pierrot I, Lae M, et al. Milk fat globule–epidermal growth factor–factor VIII (MFGE8)/lactadherin promotes bladder tumor development. Oncogene. 2011;30:642‐653. [DOI] [PubMed] [Google Scholar]
- 19. Tibaldi L, Leyman S, Nicolas A, et al. New blocking antibodies impede adhesion, migration and survival of ovarian cancer cells, highlighting MFGE8 as a potential therapeutic target of human ovarian carcinoma. PLoS ONE. 2013;8:e72708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao Q, Xu L, Sun X, et al. MFG‐E8 overexpression promotes colorectal cancer progression via AKT/MMPs signalling. Tumour Biol. 2017;39:1010428317707881. [DOI] [PubMed] [Google Scholar]
- 21. Yasuda T, Yano M, Miyata H, et al. Systemic control and evaluation of the response to neoadjuvant chemotherapy in resectable thoracic esophageal squamous cell carcinoma with 18F‐fluorodeoxyglucose positron emission tomography‐positive lymph nodes. Surg Today. 2015;45:335‐345. [DOI] [PubMed] [Google Scholar]
- 22. Miyata H, Yamasaki M, Kurokawa Y, et al. Survival factors in patients with recurrence after curative resection of esophageal squamous cell carcinomas. Ann Surg Oncol. 2011;18:3353‐3361. [DOI] [PubMed] [Google Scholar]
- 23. Sugimura K, Miyata H, Tanaka K, et al. High infiltration of tumor‐associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol. 2015;111:752‐759. [DOI] [PubMed] [Google Scholar]
- 24. Takata A, Takiguchi S, Murakami K, et al. Effects of ghrelin administration on the early postoperative inflammatory response after esophagectomy. Surg Today. 2015;45:1025‐1031. [DOI] [PubMed] [Google Scholar]
- 25. Sugimura K, Miyata H, Tanaka K, et al. Let‐7 expression is a significant determinant of response to chemotherapy through the regulation of IL‐6/STAT3 pathway in esophageal squamous cell carcinoma. Clin Cancer Res. 2012;18:5144‐5153. [DOI] [PubMed] [Google Scholar]
- 26. Japan Esophageal S . Japanese classification of esophageal cancer, 11th edition: part I. Esophagus. 2017;14:1‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanaka K, Miyata H, Sugimura K, et al. Negative influence of programmed death‐1‐ligands on the survival of esophageal cancer patients treated with chemotherapy. Cancer Sci. 2016;107:726‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamazaki M, Maruyama S, Abe T, et al. MFG‐E8 expression for progression of oral squamous cell carcinoma and for self‐clearance of apoptotic cells. Lab Invest. 2014;94:1260‐1272. [DOI] [PubMed] [Google Scholar]
- 29. Shimizu T, Ishizuka M, Kubota K. A lower neutrophil to lymphocyte ratio is closely associated with catarrhal appendicitis versus severe appendicitis. Surg Today. 2016;46:84‐89. [DOI] [PubMed] [Google Scholar]
- 30. Carrascosa C, Obula RG, Missiaglia E, et al. MFG‐E8/lactadherin regulates cyclins D1/D3 expression and enhances the tumorigenic potential of mammary epithelial cells. Oncogene. 2012;31:1521‐1532. [DOI] [PubMed] [Google Scholar]
- 31. Jia M, Yao H, Chen C, et al. Prognostic correlation between MFG‐E8 expression level and colorectal cancer. Arch Med Res. 2017;48:270‐275. [DOI] [PubMed] [Google Scholar]
- 32. Hatogai K, Kitano S, Fujii S, et al. Comprehensive immunohistochemical analysis of tumor microenvironment immune status in esophageal squamous cell carcinoma. Oncotarget. 2016;7:47252‐47264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ladoire S, Mignot G, Dabakuyo S, et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol. 2011;224:389‐400. [DOI] [PubMed] [Google Scholar]
- 34. Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8 + tumor‐infiltrating lymphocytes and a high CD8 + /regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538‐18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sayour EJ, McLendon P, McLendon R, et al. Increased proportion of FoxP3 + regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol Immunother. 2015;64:419‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sideras K, Biermann K, Yap K, et al. Tumor cell expression of immune inhibitory molecules and tumor‐infiltrating lymphocyte count predict cancer‐specific survival in pancreatic and ampullary cancer. Int J Cancer. 2017;141:572‐582. [DOI] [PubMed] [Google Scholar]
- 37. Zhu Y, Li M, Mu D, et al. CD8 + /FOXP3 + ratio and PD‐L1 expression associated with survival in pT3N0M0 stage esophageal squamous cell cancer. Oncotarget. 2016;7:71455‐71465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Der Kraak L, Goel G, Ramanan K, et al. 5‐Fluorouracil upregulates cell surface B7‐H1 (PD‐L1) expression in gastrointestinal cancers. J Immunother Cancer. 2016;4:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bohm S, Montfort A, Pearce OM, et al. Neoadjuvant chemotherapy modulates the immune microenvironment in metastases of tubo‐ovarian high‐grade serous carcinoma. Clin Cancer Res. 2016;22:3025‐3036. [DOI] [PubMed] [Google Scholar]
- 40. Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study–JCOG9204. J Clin Oncol. 2003;21:4592‐4596. [DOI] [PubMed] [Google Scholar]
- 41. Party MRCOCW . Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727‐1733. [DOI] [PubMed] [Google Scholar]
- 42. Miyata H, Yamasaki M, Takahashi T, et al. Determinants of response to neoadjuvant chemotherapy for esophageal cancer using 18F‐fluorodeoxiglucose positron emission tomography (18F‐FDG‐PET). Ann Surg Oncol. 2014;21:575‐582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


