Abstract
Aryl hydrocarbon receptor (AhR) regulates both innate and adaptive immune responses by sensing a variety of small synthetic and natural chemicals, which act as its ligands. AhR, which is expressed in dendritic cells (DCs), regulates the differentiation of DCs. However, effects of AhR on the differentiation of DCs are variable due to the heterogeneity of DCs in cell surface marker expression, anatomical location, and functional responses. The plasmacytoid DCs (pDCs), one of DC subsets, not only induce innate as well as adaptive immune responses by secreting type I interferons and pro-inflammatory cytokines, but also induce IL-10 producing regulatory T cell or anergy or deletion of antigen-specific T cells. We showed here that AhR ligands indoxyl 3-sulfate (I3S) and indole-3-carbinol (I3C) inhibited the development of pDCs derived from bone marrow (BM) precursors induced by FMS-like tyrosine kinase 3 ligand (Flt3L). I3S and I3C downregulated the expression of signal transducer and activator of transcription 3 (STAT3) and E2-2 (Tcf4). In mice orally treated with I3S and I3C, oral tolerance to dinitrofluorobenzene was impaired and the proportion of CD11c+B220+ cells in mesenteric lymph nodes was reduced. These data demonstrate that AhR negatively regulates the development of pDCs from BM precursors induced by Flt3L, probably via repressing the expression of STAT3.
Keywords: Plasmacytoid dendritic cells, Aryl hydrocarbon receptor, Cell differentiation, Signal transducer and activator of transcription 3, Immune tolerance, Tcf4
INTRODUCTION
Dendritic cells (DCs), of which morphology is characterized by dendrite-like extensions, play a central role in antigen recognition, activation of immediate and long-term immunity, and maintaining tolerance to self-antigens (1,2). DCs, which are heterogenous in terms of cell-surface markers, genetic program, anatomic location, and abilities to regulate T-cell fate, can be divided into 3 major populations in mice: 1) nonlymphoid tissue migratory DCs (also termed tissue DCs), 2) lymphoid tissue-resident DCs (also termed conventional DCs, and 3) plasmacytoid dendritic cells (pDCs) (3).
The pDCs was first identified as a cell type similar to plasma cells but lacking B cell and plasama cell markers (4). The pDCs secrete large amounts of type I interferons (IFNs) in response to viral antigens through Toll-like receptor (TLR) 7 and TLR9 (5), and pDCs can also secrete other pro-inflammatory cytokines and chemokines, including IL-12, CXC-chemokine ligand 8 and 10, promoting the polarization of CD4+ T cells into T helper (Th) 1 cells (6). In addition, pDCs secrete transforming growth factor (TGF)-β and express indoleamine 2,3-dioxygenase, promoting regulatory T cell (Treg) commitment. Unlike migratory DCs and tissue-resident DCs which are inherently efficient antigen-presenting cells (APCs) capable of eliciting lymphocyte responses, pDCs are considered to be poor APCs based on their low surface expression of major histocompatibility complex and costimulatory molecules (7). However, pDCs can become potent APCs capable of activating T cells upon stimulation with CD154 and TLRs (8). Thus, in addition to contributing innate immunity, pDCs can be immunogenic and tolerogenic.
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that belongs to the basic region-helix-loop-helix sub-family of DNA binding proteins with Per-Arnt-Sim domains (9). AhR is a cytosolic sensor of small synthetic and natural chemicals, which act as its ligands. Upon binding of a ligand to AhR, it undergoes a conformational change for translocation to the nucleus where it forms a heterodimeric complex with the Ah receptor nuclear translocator (10). This complex activates transcription of target genes such as Cyp1A1 and glutathione S-transferase by binding to the aromatic hydrocarbon-responsive elements in the 5′ flanking region of these genes (11). Environmental AhR ligands are exposed to the barrier organs such as the skin, lung and gut, with the gut accounting for most of human dioxin exposure and for other AhR ligands, including many dietary components such as quercetin in apples, resveratrol in red wine and indole-3-carbinol (I3C) in many cruciferous plants (12,13).
AhR, which is constitutively expressed in DCs (14), regulates the activation, maturation and differentiation of DCs. AhR activated bone marrow (BM)-derived DCs and was involved in Langerhans cell maturation (15,16). Effects of AhR on the differentiation of DCs are different depending on cell types used. In 1 study, AhR activation promoted human monocyte-derived DC differentiation (17), whereas in other studies, AhR inhibited in vitro differentiation of Langerhans DCs and myeloid DCs from CD34+ hematopoietic progenitor cells (18,19). Of interest, accumulating evidence indicates that AhR activation induces immune tolerance via a DC-mediated mechanism (20,21,22). AhR agonist VAF347 promoted in vivo allograft tolerance via DC-mediated effects on Tregs (20). The same compound exerted anti-inflammatory effects by inhibiting the production of inflammatory cytokines and the upregulation of costimulatory molecules on human monocyte-derived DCs (21). Activation of AhR by 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester, an endogenous ligand, induced not only FoxP3+ Treg that suppressed experimental autoimmune encephalomyelitis, but also tolerogenic DCs (22).
Although it is still not clear whether the regulatory functions of DCs is determined by their activation status or inherent of different lineages, in vitro and in vivo experiments have identified several subsets of DCs of tolerogenic characteristics (23): 1) CD11c+ DCs expressing perforin that enforces peripheral tolerance by deleting T cells, 2) CD103+ DCs present in the intestinal mucosa, where they play a key role in oral tolerance, and 3) pDCs.
We previously reported that AhR ligand 3,3′-diindolylmethane, an acid-stimulated conversion product of I3C, inhibited FMS-like tyrosine kinase 3 ligand (Flt3L)/granulocyte-macrophage colony-stimulating factor-induced BM-derived CD103+ DC differentiation (24). AhR antagonist StemRegenin 1 promoted human pDC development from CD34+ hematopoietic progenitor cells (19). In mice, lack of AhR promoted pDC development in vivo and AhR activation by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) inhibited pDC differentiation from BM in vitro (25). However, the underlying inhibitory mechanisms of AhR in pDC development and the physiological significance of AhR activation have not been addressed.
Since the first report that AhR is the cytosolic receptor for TCDD (26), a number of low-molecular weight, structurally diverse chemicals, including metabolites of tryptophan and arachidonic acid, indoles, and tetrapyroles, have been identified as naturally occurring exogenous and endogenous AhR ligands (27). Interestingly, although all AhR ligands bind AhR activating AhR, AhR functionality often depends on ligands used, showing ligand-selective effects on cell proliferation, death, and differentiation, due to differential affinity of AhR for structurally diverse ligands, stability of ligands, and differential binding of AhR ligands within the AhR ligand binding domain (28). TCDD, which is metabolically stable, induced Treg which suppressed experimental autoimmune encephalomyelitis, whereas 6-formylindolo[3,2-b]carbazole (FICZ), a photoproduct generated from tryptophan by ultraviolet B irradiation and an endogenous AhR ligand which is metabolically unstable (29), interfered with Treg development, increasing the severity of experimental autoimmune encephalomyelitis (30). In a mouse model of influenza virus infection, TCDD modulated inflammatory responses characterized by neutrophilia and virus-specific CD8 T cell response, whereas FICZ had no effect to infection (31). In various species, TCDD shows a spectrum of AhR-dependent toxic effects (i.e., wasting, dermal toxicity, thymic involution, and teratogenicity), which are not observed with nonhalogenated polycyclic aromatic hydrocarbons (28). Thus, interpretation of results studied with TCDD needs caution.
In the present study, using in vitro pDC differentiation model in which BM cells are cultured with FLt3L for 9 days, we examined effects of 2 AhR ligands, indoxyl 3-sulfate (I3S), a uremic toxin that originates from the metabolism of tryptophan (32), and I3C, a phytochemical that is abundant in cruciferous plants, on pDC differentiation and investigated underlying molecular mechanisms. Finally, using a mouse model of dinitrofluorobenzene (DNFB)-specific skin delayed type hypersensitivity (DTH), whether oral tolerance to DNFB is modulated by AhR activation was addressed.
MATERIALS AND METHODS
Mice
C57BL/6 mice, which were purchased from the Central Lab. Animal Inc. (Seoul, Korea), were used at the age of 6–12 weeks. The animals were housed 5 mice per cage in a laminar air flow room maintained at 22°C±2°C with relative humidity of 55%±5%. Mice were cared and treated in accordance with the guidelines established by the Changwon National University public health service policy on the use of laboratory animals.
Chemicals and reagents
I3S, I3C, and DNFB were purchased from Sigma-Aldrich (St. Louis, MO, USA); murine Flt3L from eBioscience (San Diego, CA, USA). Antibodies used in the present study are: anti-signal transducer and activator of transcription 3 (STAT3) from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-phospho-c-Src (Tyr416) from Cell Signaling Technology (Danvers, MA, USA); anti-phospho-STAT3 (Tyr705) from BD Biosciences (Franklin Lakes, NJ, USA); FITC anti-mouse CD11c, Alexa Fluor 647 anti-mouse B220, anti-ERK, and anti-phospho-ERK (Thr202/Tyr204) from Biolegend (San Diego, CA, USA). CpG oligodeoxyribonucleotides (CpG 2216: sequences are shown 5′-3′: small letters, phosphoorothioate linkage; capital letters, phosphodiester linkage 3′ of the base; bold, CpG-dinucleotides; ggGGGACGATCGTCgggggG) were manufactured by and purchased from Bioneer (Daejeon, Korea).
Development of CD11c+B220+ DC from BM cells
Development of CD11c+B220+ DC from BM cells was previously described (33). Briefly, BM cells were isolated by flushing femurs with RPMI 1640 (Roswell Park Memorial Institute, Buffalo, NY, USA) supplemented with 2.5 mM/L HEPES buffer (pH 7.4) and depleted of red blood cell by being treated in Tris-ammonium chloride at room temperature for 2 min. BM cells were cultured in a 24-well plate at a density of 1×106 cells/ml in RPMI 1640 with 10 mM HEPES, 2 mM L-glutamine, 10% fetal bovine serum, 50 μM β-mercaptoethanol, and Flt3L (200 ng/ml) for 9 days.
AhR ligand treatment
For in vitro experiments, AhR ligands dissolved in DMSO were added to the culture at the final concentration of 1 μM 1h before the addition of Flt3L. DMSO (0.1% v/v) was used as vector control. For gastric administration, 52 μg of I3S, 2 mg of I3C, or 10 μl of DMSO was mixed with 140 μl of corn oil and was given to mice at days 1, 3, 5, 7, and 9. At day 10, oral tolerance to DNFB was induced.
Flow cytometry and cell sorting
Cells were kept on ice or at 4°C at all time. Immunofluorescence staining was carried out in 96-well, round-bottomed cell culture plates. The cells were centrifuged and resuspended in 10 μl of primary antibody diluted with PBS at a previously determined optimal concentration. After 15 min of incubation, the cells were washed with washing buffer (PBS/0.5% bovine serum albumin (BSA)/0.09% sodium azide) 3 times and fixed with 0.9% buffered formalin. Cells were analyzed within 1 week on a FACSCalibur with CellQuest software (BD Biosciences). For cell sorting, BM cells cultured for 9 days in the presence of Flt3L were stained with FITC anti-mouse CD11c and Alexa Fluor 647 anti-mouse B220, and sorted with FACS Aria III. The cut-off point was the co-expression of CD11c and B220 at high levels.
IFN-α production assay
BM cells were incubated with FLt3L for 8 days and then treated with CpG oligodeoxyribonucleotides (CpG 2216) (3 μg/ml) for 24 h. Supernants were then collected and assayed by ELISA for murine IFN-α (MyBiosource, San Diego, CA, USA), according to the manufacturer's instructions. The limit of detection for IFN-α was 9 pg/ml.
RNA preparation and RT-PCR
Total cellular RNA was extracted from cells using the RNAzol method (Tel-Test Inc., Friendswood, TX, USA). For PCR analysis, RNA was used after contaminating DNA was completely removed by DNase I treatment. RT-PCR analysis was performed using pairs of oligonucleotide primers. The PCR products were confirmed to correspond to their original sequence by DNA sequencing. Gene specific primers, number of cycles of amplification, annealing temperature, and expected size of PCR product are listed in Table 1.
Table 1. Primers used in RT-PCR.
| Name | Nucleotide sequence | Annealing temperature (°C) | Cycles* | Size† (bp) |
|---|---|---|---|---|
| mAhRfw | 5′-ggccaagagcttctttgatg-3′ | 59 | 30 | 305 |
| mAhRrv | 5′-ctgggtttagagcccagtga-3′ | |||
| mCyp1A1fw | 5′-caccatcccccacagcac-3 | 60 | 30 | 125 |
| mCyp1A1rv | 5′-tcgtttgggtcaccccacag-3′ | |||
| mHGPRTfw | 5′-gctggtgaaaaggacctctc-3′ | 60 | 30 | 248 |
| mHGPRTrv | 5′-caggactagaacacctgc-3′ | |||
| mPu1fw | 5′-tgactactactccttcgtgg-3′ | 58 | 30 | 520 |
| mPu1rv | 5′-cttggacgagaactggaagg-3′ | |||
| mE2-2fw | 5′-agtgcgatgttttcgcctcc-3′ | 58 | 30 | 710 |
| mE2-2rv | 5′-tgtggatgcaggctacagta-3′ | |||
| mIRF8fw | 5′-atcaggaggtggatgcttcc-3′ | 58 | 30 | 440 |
| mIRF8rv | 5′-ataggcggcatatccggtca-3′ | |||
| mIDO-1fw | 5′-caccatggcgtatgtgtgga-3′ | 58 | 35 | 321 |
| mIDO-1rv | 5′-aggtcttgacgctctactgc-3′ | |||
| mIfnafw | 5′-atgagctactggccaacctgc-3′ | 58 | 30 | 489 |
| mIfnarv | 5′-aagacagggctctccagact-3′ | |||
| mTGF-βfw | 5′-tgacgtcactggagttgtacgg-3′ | 58 | 30 | 170 |
| mTGF-βrv | 5′-ggttcatgtcatggatggtgc-3′ |
*The number of cycles of amplification; †The expected size of PCR products.
Western blotting
Cells or tissues were homogenized in lysis buffer containing 20 mM Tris-HCl (pH 7.4), 1 mM EDTA (pH 8.0), 50 μM sodium vanadate, 20 mM p-nitrophenylphosphate, 50 mM sodium fluoride, leupeptin (0.5 μg/ml), aprotinin (10 μg/ml), and soybean trypsin inhibitor (10 μg/ml). Proteins size-fractionated on SDS/polyacrylamide gel electrophoresis were transferred to polyvinylidene difluoride membranes, and the blots were blocked with 3% BSA in tris-buffered saline (TBS) buffer (20 mM Tris-HCl pH 7.5/137 mM NaCl). The blots were sequentially treated with primary and secondary antibodies in TBS with Tween 20 (20 mM Tris-HCl pH 7.5/137 mM NaCl/0.1% Tween 20) with intermittent washing with TBS with Tween 20. Immunodetection was performed with the ECL-plus kit (Amersham Biosciences, Piscataway, NJ, USA).
Oral tolerance induction to DNFB
Oral tolerance to DNFB was induced by a single intragastric administration of 0.3 ml of 0.1% DNFB in acetone/olive oil (1/10, v/v) 7 days before skin sensitization with DNFB, as described (34).
Contact hypersensitivity to DNFB
Contact hypersensitivity to DNFB was determined by the mouse ear swelling test as described (34). Briefly, mice were sensitized epicutaneously onto 2 cm2 of shaved abdominal skin with 25 μl of 0.5% DNFB in acetone-olive oil (4:1, v/v) and challenged on day 5 with 4 μl of 0.2% DNFB, applied onto each side of the right ear. The left ear was painted with the vehicle alone. Ear thickness was measured using a digital caliper (Mitutoyo 500-197-30; Mitutoyo America Corporation, Aurora, IL, USA) before and after challenge. The ear swelling was calculated as (T-To of the right ear) – (T-To of the left ear), in which T and To represent the values of ear thickness after and before challenge, respectively.
DC isolation from mesenteric lymph nodes
Mesenteric lymph nodes were digested for 15 min at 37°C in RPMI 1640 medium with 2% fetal calf serum containing 1 mg/ml collagenase and 0.02 mg/ml DNase and were mixed with EDTA (5 mM final concentration). Then, cells were flow-cytometrically analyzed.
Statistical analysis
All experiments were performed 3 to 5 times, and a representative experiment is shown. Data are presented as the mean±standard deviation and analyzed by the paired Student's t-test. A value of p<0.05 was considered statistically significant.
RESULTS
Inhibition of pDC differentiation from BM cells by I3S and I3C
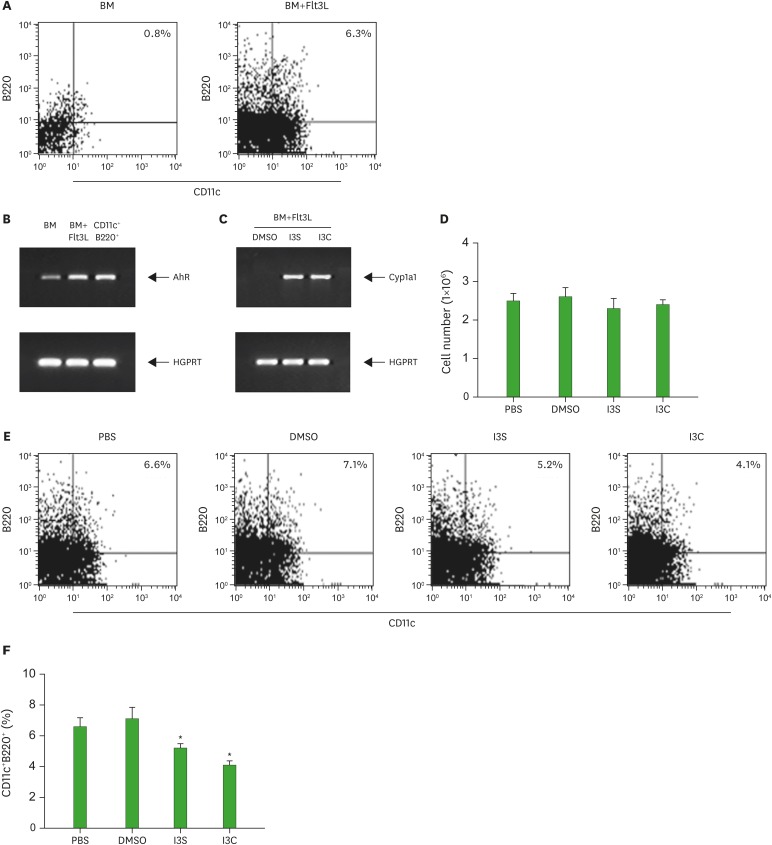
A large number of murine pDCs can be generated from BM precursors with Flt3L (35). Thus, BM cells were cultured for 9 days in the presence of Flt3L and then assayed for the expression of CD11c/B220, surface markers for murine pDCs (Fig. 1A). We performed this experiment 3 times. On average, about 6% of the harvested cells were CD11c+B220+. It is well known that hematopoietic stem cells respond to xenobiotics such as TCDD (36). To examine whether BM cells and CD11c+B220+ pDCs functionally express AhR, CD11c+B220+ cells were sorted using FACS Aria III, treated with I3S or I3C, and assayed for the expression of AhR and Cyp1A1, a target gene of AhR. AhR, which was expressed in BM was increased in Flt3L-treated BM cells and CD11c+B220+ cells (Fig. 1B). For Cyp1A1 expression, 3 days after BM cells were cultured in the presence of Flt3L with or without I3S and I3C, cells were harvested and analyzed for Cyp1A1 expression. Cyp1A1 was induced in Flt3L-treated BM cells by I3S or I3C (Fig. 1C), suggesting that AhR is functional in BM-derived DCs. To investigate the role of AhR activation in the differentiation of pDCs from BM in vitro, BM cells were cultured for 9 days in the presence of AhR ligands I3S or I3C. AhR activation by I3S and I3C showed almost no effect on the total number of cells generated from BM precursors (Fig. 1D). However, I3S and I3C significantly reduced the proportion of CD11c+B220+cells (5.2%, p=0.004 and 4.1%, p=0.001, respectively), compared with PBS and DMSO controls (6.6% and 7.1%, respectively) (Fig. 1E and F).
Figure 1. I3S and I3C inhibit the development of CD11c+B220+ pDCs. BM cells were induced to differentiate into CD11c+B220+ DCs with Flt3L for 9 days. Then, cells were harvested and analyzed for cell surface expression of CD11c and B220 (A) mRNA expression of AhR and Cyp1A1 (B, C) and the effects of AhR on cell growth (D), and the proportion of CD11c+B220+ cells (E). For AhR expression in CD11c+B220+ pDCs, CD11c+B220+ cells, which were sorted from the 9-day old BM cultures, were subjected to RT-PCR (B). The results for the effects of AhR on the development of CD11c+B220+ cells were analyzed by the paired Student's t-test and presented as mean±standard deviation (n=3 experiments) (F).
*p<0.05 compared with DMSO.
Reduction of IFN-α expression in Flt3L-cultured BM cells treated with I3S and I3C
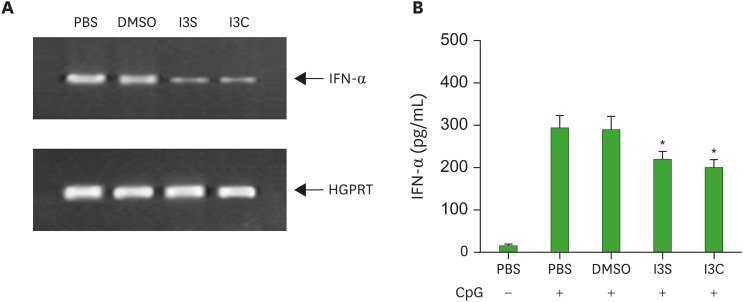
The pDCs secrete large amounts of type I IFNs in response to viral antigens through TLR9. Thus, we examined if AhR activation alters IFN-α/β producing ability of pDCs by treating BM cultures with CpG oligodeoxyribonucleotides (CpG 2216), a synthetic ligand for TLR9 (37). Twenty-four h after CpG administration, cells were harvested and assayed for IFN-α by RT-PCR and ELISA. IFN-α mRNA expression was reduced by I3S and I3C (Fig. 2A). Accordingly, the amount of IFN-α in the culture supernatant was also deceased in BM cultures treated with I3S and I3C, compared with PBS and DMSO controls (Fig. 2B).
Figure 2. I3S and I3C downregulate IFN-α production from Flt3L-treated BM cultures. BM cells were induced to differentiate into CD11c+B220+ DCs with Flt3L for 8 days and activated with CpG oligodeoxyribonucleotides (CpG 2216) for 24 h. Then, cells were harvested and analyzed for mRNA expression of IFN-α by RT-PCR and supernatants were assayed for IFN-α by ELISA.
Downregulation of the expression of STAT3 and E2-2 by AhR activated with I3S and I3C
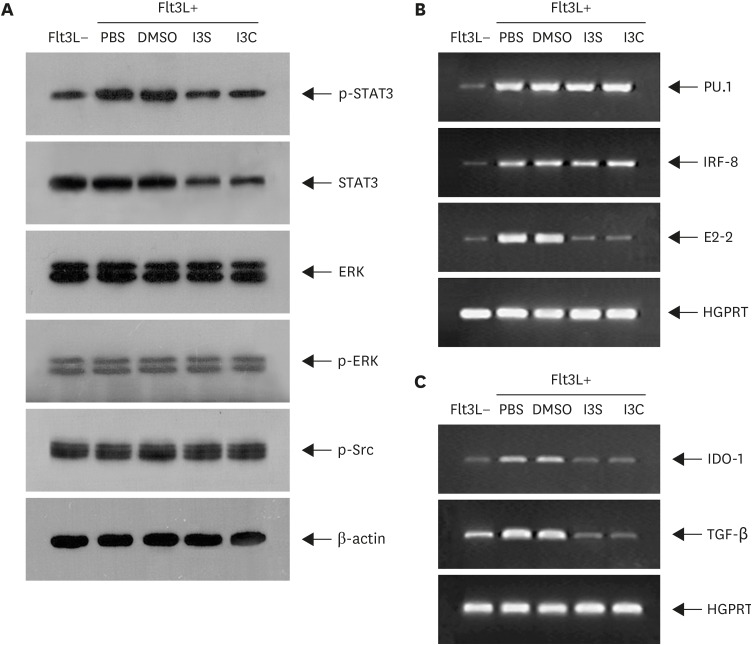
Flt3L play a key role in pDC development (38). Flt3L promotes pDC development by activating STAT3, which upregulates the expression of E2-2. E2-2 (Tcf4), which is the master transcription factor for pDC development, in turn regulates genes associated with function and development of pDC (6). Thus, we first investigated effects of AhR on the phosphorylation of STAT3 in BM cells treated with Flt3L for 1 h (Fig. 3A). Phosphorylation of STAT3, which was increased by Flt3L, was reduced by I3S or I3C. Whether the downregulation of STAT3 phosphorylation by I3S and I3C is due to transcriptional or post-transcriptional regulation, STAT3 was quantified. Interestingly, the level of STAT3 protein, which was unchanged by Flt3L, was decreased by I3S and I3C, suggesting that AhR activation downregulates the expression of STAT3. AhR also functions by interacting with other signals, altering activation of those signals such as receptor tyrosine kinases and mitogen-activated protein kinase (28). Thus, we examined if AhR activation by I3S and I3C regulates activation of ERK and c-Src. AhR showed little effect both on the activation of ERK and c-Src and the expression of ERK, suggesting that AhR works specifically on STAT3. Next, we investigated effects of AhR on the expression of transcription factors required for pDC development in BM cells treated with Flt3L for 9 days (Fig. 3B). Expression of PU1, IRF8, and E2-2 was increased in Flt3L-treated BM cells compared with BM cells. Of the 3 genes, only E2-2 expression was downregulated by I3S and I3C. pDC promotes Treg differentiation by expressing indoleamine 2,3-dioxygenase-1 (IDO-1) and TGF-β (6). Thus, effects of AhR activation on immunosuppressive functions of pDC were examined in BM cells treated with Flt3L for 9 days. Expression of IDO-1 and TGF-β, which was increased by Flt3L treatment, was reduced by I3S and I3C (Fig. 3C), suggesting that AhR activation by I3S and I3C inhibits immunosuppressive function of pDC.
Figure 3. I3S and I3C regulates expression of genes associated with development and functions of pDCs. BM cells, which were cultured in the presence of Flt3L for 1 h, were harvested and analyzed by western blotting (A) or RT-PCR (B). For analysis of genes associated with pDC functions, BM cells cultured supplemented with Flt3L for 9 days were analyzed by RT-PCR.
Impairment of oral tolerance to DNFB in mice administrated with I3S or I3C accompanied by a decrease in the proportion of CD11c+B220+ cells in mesenteric lymph nodes
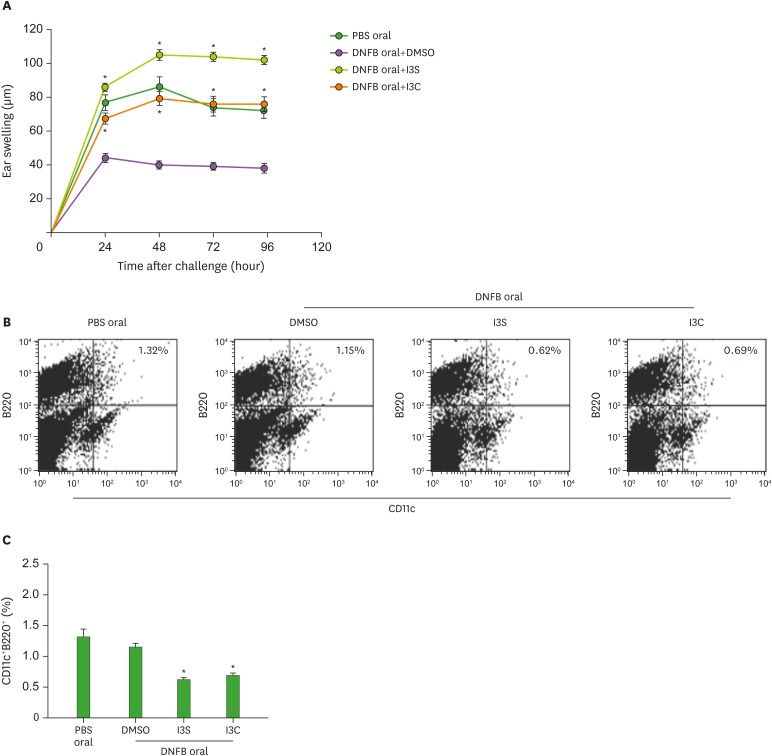
Oral tolerance, which is the immunological mechanism by which the mucosal immune system protects the host from deleterious T-cell mediated reactions to self and exogenous dietary and environmental antigens, is mainly mediated by IL-10 and/or TGF-β-producing Tregs (39). Treg development in the mucosa is promoted by tolerogenic DCs, including CD103+ DCs and pDCs (40,41). Effects of AhR on oral tolerance seem to be bipolar, depending on ligands used. In studies of the induction of oral tolerance against ovalbumin, TCDD impaired oral tolerance whereas I3C supported oral tolerance (42,43). Thus, we examined if oral tolerance is affected by AhR activated by I3S and I3C. Mice, which were fed I3S and I3C and then DNFB 7 days before skin sensitization with DNFB, were ear-challenged with DNFB 5 days later. Contact hypersensitivity was determined by the mouse ear swelling test (Fig. 4A). Control mice orally injected with PBS developed ear swelling response which lasted several days. Oral injection of DMSO (vehicle) inhibited contact hypersensitivity to DNFB. When DNFB was co-injected with I3S or I3C, contact hypersensitivity to DNFB was not suppressed, suggesting that induction of oral tolerance to DNFB is suppressed by AhR activated by I3S and I3C. One type of tolerogenic DCs in the mucosal immune system is pDC. Thus, we sacrificed mice on the fourth day after the ear-challenging of DNFB, collected mesenteric lymph nodes, and analyzed total cells for the expression of CD11c/B220. In mice treated with I3S and I3C, the proportion of CD11c+B220+ cells was greatly reduced (0.62%, p=0.00009 and 0.69%, p=0.00009, respectively), compared with PBS oral or DMSO control (1.32% and 1.15%, respectively) (Fig. 4B and C).
Figure 4. I3S and I3C impair oral tolerance to DNFB and reduce the proportion of CD11c+B220+ cells in the mesenteric lymph nodes. Mice, which were fed I3S or I3C, were given a single intragastric administration of 0.3 ml of 0.1% DNFB in acetone/olive oil (1/10, v/v). Seven days later, the mice were sensitized epicutaneously onto 2 cm2 of shaved abdominal skin with 25 μl of 0.5% DNFB in acetone-olive oil (4:1, v/v) and challenged on day 5 with 4 μl of 0.2% DNFB, applied onto each side of the right ear. Ear thickness was measured before and after challenge (A). Results of (A) are presented as mean±standard deviation (n=5 mice per group). At 96 h after the challenge of DNFB, mesenteric lymph nodes were collected, stained with flurochrome-conjugated ant-CD11 Ab and anti-B220 Ab, and analyzed for expression of CD11c and B220 (B). Results of (B) are statistically analyzed and presented as mean±standard deviation (n=5 mice per group) in (C).
*p<0.05 compared with DMSO.
DISCUSSION
This study demonstrates that AhR ligands I3S and I3C inhibit the development of pDCs from BM cells stimulated with Flt3L in vitro, downregulating the expression of STAT3 and E2-2. Intragastric administration of I3S and I3C abrogated oral tolerance to DNFB characterized by suppression of DTH responses to DNFB and reduced the proportion of CD11c+B220+ cells in the mesenteric lymph nodes.
In the present study, we observed that phosphorylation of STAT3, which was enhanced by the addition of Fl3tL, was inhibited by I3S and I3C. However, expression and activation of ERK and activation of c-Src were found not to be modulated by I3S and I3C, suggesting that effects of I3S and I3C on signaling are specific on STAT3. Interestingly, the level of STAT3 protein itself was reduced by AhR activation, suggesting that AhR could regulate the expression of STAT3, resulting in a proportional decrease of the phosphorylation of STAT3. There are several potential underlyng mechanisms for the downregulated expression of STAT3 protein. One possibility is the ubiquitin-proteasome system-mediated protein degradation. AhR is a ligand-dependent E3 ubiquitin ligase, degrading target proteins, including estrogen receptor-α and STAT1 and STAT4, as early as 1 h after AhR activation (44,45). In the present study, BM cells were treated with AhR ligands 1 h before the addition of Flt3L, supporting the role of the ubiquitin-proteasome system in STAT3 degradation. Alternatively, AhR, which usually functions as a transcriptional activator, also suppresses gene expression (46,47,48). Although AhR repressor, which is upregulated by AhR signaling, functions as a negative feedback regulator of AhR signaling (49), it could regulate gene expression by working as a transcriptional repressor of genes including peroxisome proliferator-activated receptor γ (48) or by interacting with other signaling pathways such as nuclear factor κB (NF-κB) (47). AhR also regulates gene expression by interacting with other signals including NF-κB and c-Src (28,50). How STAT3 expression is regulated by AhR activation remains to be investigated.
In the present study, we demonstrated that oral tolerance induced by gastric administration of DNFB was impaired by I3S and I3C. In other studies, effects of AhR on oral tolerance were variable, depending on AhR ligands used; TCDD impaired oral tolerance whereas I3C supported it (42,43). Furthermore, I3C showed different effects on oral tolerance induced by different antigens. The model of contact hypersensitivity to DNFB used to study effects of AhR on oral tolerance in the present study is mediated by effector CD8+ T cells (51) and CD4+CD25+ Tregs play a major role in the induction of oral tolerance to DNFB (39). In an oral tolerance model to ovalbumin, oral tolerance was determined by the level of serum antibodies to ovalbumin (43). In studies related to effects of AhR on CD4 T cell differentiation, activation of AhR by I3S promotes Th17 differentiation, while inhibiting Th2 differentiation and having little effect on Th1 differentiation, suggesting that AhR functions in a lineage-specific manner (52,53). AhR can bind and be activated or inhibited by a wide variety of structurally dissimilar compounds via its ligand binding domain (LBD) (28). Differential binding of structurally diverse ligands within the AhR LBD could lead to ligand-dependent differences in the overall structure of the activated AhR, which may contribute to its ligand-dependent functional specificity by differentially recruiting coactivator or repressor and thus modulating transcriptional activity. AhR signaling could also be regulated by proteosomal degradation and AhR repressor. The AhR protein is rapidly downregulated following ligand binding, which occurs via ubiquitin-mediated 26S proteasome pathway (54). Interestingly, the kinetics of AhR degradation are different among ligands used (55,56). Depletion of AhR persisted for at least 72 h after TCDD exposure, whereas treatment with 3-methylcholanthrane or β-napthoflavone caused a transient drop of AhR protein followed by a recovery of AhR to near pretreatment levels within 72 h, suggesting that the level of AhR protein remains downregulated when cells are treated with a metabolically stable ligand such as TCDD. AhR repressor, which regulates AhR signaling by acting as a transcriptional repressor (49), is transcriptionally induced by AhR activated by various ligands, including TCDD, 3-methylcholeanthrene, and benzo[a]pyrene. However, the degree of induction is variable depending on cell types and ligands used (57,58). How AhR functions in a ligand-specific way remains to be investigated.
In the present study, we demonstrated that the population of CD11c+ B220+ pDCs in mesenteric lymph nodes of oral tolerance-induced mice was reduced by II3S or I3C. The induction and maintenance of oral tolerance is largely mediated by the combination of commensals, Treg, and tolerogenic DCs (59). In addition, CX3CR1+ macrophages, IL-22 producing innate lymphoid cells, and intestinal epithelial cells are also associated with oral tolerance induction (60,61,62). Interestingly, these cells and CD103+ DCs also express the AhR protein (13,24,63,64). Thus, it remains to be tested whether AhR impairs oral tolerance by regulating the differentiation and function of those cells.
AhR activated by TCDD reduced survival rate of mice infected with influenza A virus (65) and viral titers in AhR-deficient mouse embryonic fibroblasts (MEFs) after infection with vesicular stomatitis virus (VSV) and influenza A virus increased (66). In mice exposed to TCDD and infected with influenza A virus, the proliferation and differentiation of CD8+ cytotoxic T cells were suppressed, which could be mediated by DCs regulated by AhR (65,67). IFN-β production in AhR-deficient MEF after infection with VSV virus and influenza A virus increased compared with wild-type MEF and intraperitoneal pretreatment with FICZ resulted in a marked reduction in serum IFN-β protein at 12 h after VSV infection (66). How AhR regulates IFN-β production was elucidated. AhR upregulates expression of the ADP-ribosylase TCDD-inducible poly(ADP-ribose)polymerase, which in turn caused the downregulation of type 1 IFN response. In the present study, we demonstrated that AhR activated by I3S and I3C inhibited the differentiation of type 1 IFN-producing pDCs, suggesting that AhR could suppress the innate immune response against viral infection by regulating the development of DCs.
ACKNOWLEDGEMENTS
This study was supported by Changwon National University grants (2017-2018).
Abbreviations
- AhR
aryl hydrocarbon receptor
- APC
antigen presenting cell
- BM
bone marrow
- BSA
bovine serum albumin
- DC
dendritic cell
- DNFB
dinitrofluorobenzene
- DTH
delayed type hypersensitivity
- FICZ
6-formylindolo[3,2-b]carbazole
- Flt3L
FMS-like tyrosine kinase 3 ligand
- I3C
indole-3-carbinol
- I3S
indoxyl 3-sulfate
- IDO-1
indoleamine 2,3-dioxygenase-1
- IFN
interferon
- LBD
ligand binding domain
- MEF
mouse embryonic fibroblast
- NF-κB
nuclear factor B
- pDC
plasmacytoid dendritic cell
- PBS
phosphate buffered saline
- STAT3
signal transducer and activator of transcription 3
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TGF
transforming growth factor
- Th
T helper
- TLR
toll-like receptor
- Treg
regulatory T cell
- VSV
vesicular stomatitis virus
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
Author Contributions: Conceptualization: Park JH; Data curation: Hwang WB, Kim DJ, Oh GS; Investigation: Hwang WB, Kim DJ, Oh GS; Writing - original draft: Hwang WB, Kim DJ, Oh GS; Writing - review & editing: Park JH.
References
- 1.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo . Nat Rev Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 2.Zhou H, Wu L. The development and function of dendritic cell populations and their regulation by miRNAs. Protein Cell. 2017;8:501–513. doi: 10.1007/s13238-017-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 4.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 5.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 6.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watowich SS, Liu YJ. Mechanisms regulating dendritic cell specification and development. Immunol Rev. 2010;238:76–92. doi: 10.1111/j.1600-065X.2010.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salio M, Palmowski MJ, Atzberger A, Hermans IF, Cerundolo V. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J Exp Med. 2004;199:567–579. doi: 10.1084/jem.20031059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol. 2004;36:189–204. doi: 10.1016/s1357-2725(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 10.Swanson HI, Bradfield CA. The AH-receptor: genetics, structure and function. Pharmacogenetics. 1993;3:213–230. doi: 10.1097/00008571-199310000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Rushmore TH, Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990;265:14648–14653. [PubMed] [Google Scholar]
- 12.van Leeuwen FX, Feeley M, Schrenk D, Larsen JC, Farland W, Younes M. Dioxins: WHO's tolerable daily intake (TDI) revisited. Chemosphere. 2000;40:1095–1101. doi: 10.1016/s0045-6535(99)00358-6. [DOI] [PubMed] [Google Scholar]
- 13.Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30:447–454. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Simones T, Shepherd DM. Consequences of AhR activation in steady-state dendritic cells. Toxicol Sci. 2011;119:293–307. doi: 10.1093/toxsci/kfq354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castañeda AR, Pinkerton KE, Bein KJ, Magaña-Méndez A, Yang HT, Ashwood P, Vogel CF. Ambient particulate matter activates the aryl hydrocarbon receptor in dendritic cells and enhances Th17 polarization. Toxicol Lett. 2018;292:85–96. doi: 10.1016/j.toxlet.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jux B, Kadow S, Esser C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J Immunol. 2009;182:6709–6717. doi: 10.4049/jimmunol.0713344. [DOI] [PubMed] [Google Scholar]
- 17.Goudot C, Coillard A, Villani AC, Gueguen P, Cros A, Sarkizova S, Tang-Huau TL, Bohec M, Baulande S, Hacohen N, et al. Aryl hydrocarbon receptor controls monocyte differentiation into dendritic cells versus macrophages. Immunity. 2017;47:582–596.e6. doi: 10.1016/j.immuni.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Platzer B, Richter S, Kneidinger D, Waltenberger D, Woisetschläger M, Strobl H. Aryl hydrocarbon receptor activation inhibits in vitro differentiation of human monocytes and Langerhans dendritic cells. J Immunol. 2009;183:66–74. doi: 10.4049/jimmunol.0802997. [DOI] [PubMed] [Google Scholar]
- 19.Thordardottir S, Hangalapura BN, Hutten T, Cossu M, Spanholtz J, Schaap N, Radstake TR, van der Voort R, Dolstra H. The aryl hydrocarbon receptor antagonist StemRegenin 1 promotes human plasmacytoid and myeloid dendritic cell development from CD34+ hematopoietic progenitor cells. Stem Cells Dev. 2014;23:955–967. doi: 10.1089/scd.2013.0521. [DOI] [PubMed] [Google Scholar]
- 20.Hauben E, Gregori S, Draghici E, Migliavacca B, Olivieri S, Woisetschläger M, Roncarolo MG. Activation of the aryl hydrocarbon receptor promotes allograft-specific tolerance through direct and dendritic cell-mediated effects on regulatory T cells. Blood. 2008;112:1214–1222. doi: 10.1182/blood-2007-08-109843. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence BP, Denison MS, Novak H, Vorderstrasse BA, Harrer N, Neruda W, Reichel C, Woisetschläger M. Activation of the aryl hydrocarbon receptor is essential for mediating the anti-inflammatory effects of a novel low-molecular-weight compound. Blood. 2008;112:1158–1165. doi: 10.1182/blood-2007-08-109645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, Weiner HL. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010;107:20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takenaka MC, Quintana FJ. Tolerogenic dendritic cells. Semin Immunopathol. 2017;39:113–120. doi: 10.1007/s00281-016-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JH, Choi AJ, Kim SJ, Jeong SY. 3,3′-diindolylmethane inhibits Flt3L/GM-CSF-induced-bone marrow-derived CD103(+) dendritic cell differentiation regulating phosphorylation of STAT3 and STAT5. Immune Netw. 2015;15:278–290. doi: 10.4110/in.2015.15.6.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Ramachandran I, Gabrilovich DI. Regulation of plasmacytoid dendritic cell development in mice by aryl hydrocarbon receptor. Immunol Cell Biol. 2014;92:200–203. doi: 10.1038/icb.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976;251:4936–4946. [PubMed] [Google Scholar]
- 27.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. 2011;124:1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rannug A, Rannug U, Rosenkranz HS, Winqvist L, Westerholm R, Agurell E, Grafström AK. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J Biol Chem. 1987;262:15422–15427. [PubMed] [Google Scholar]
- 30.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 31.Wheeler JL, Martin KC, Resseguie E, Lawrence BP. Differential consequences of two distinct AhR ligands on innate and adaptive immune responses to influenza A virus. Toxicol Sci. 2014;137:324–334. doi: 10.1093/toxsci/kft255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96:3029–3039. [PubMed] [Google Scholar]
- 34.Desvignes C, Etchart N, Kehren J, Akiba I, Nicolas JF, Kaiserlian D. Oral administration of hapten inhibits in vivo induction of specific cytotoxic CD8+ T cells mediating tissue inflammation: a role for regulatory CD4+ T cells. J Immunol. 2000;164:2515–2522. doi: 10.4049/jimmunol.164.5.2515. [DOI] [PubMed] [Google Scholar]
- 35.Brawand P, Fitzpatrick DR, Greenfield BW, Brasel K, Maliszewski CR, De Smedt T. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs. J Immunol. 2002;169:6711–6719. doi: 10.4049/jimmunol.169.12.6711. [DOI] [PubMed] [Google Scholar]
- 36.Gasiewicz TA, Singh KP, Bennett JA. The Ah receptor in stem cell cycling, regulation, and quiescence. Ann N Y Acad Sci. 2014;1310:44–50. doi: 10.1111/nyas.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krug A, Rothenfusser S, Hornung V, Jahrsdörfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, Hartmann G. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 38.Schmid MA, Kingston D, Boddupalli S, Manz MG. Instructive cytokine signals in dendritic cell lineage commitment. Immunol Rev. 2010;234:32–44. doi: 10.1111/j.0105-2896.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- 39.Dubois B, Chapat L, Goubier A, Papiernik M, Nicolas JF, Kaiserlian D. Innate CD4+CD25+ regulatory T cells are required for oral tolerance and inhibition of CD8+ T cells mediating skin inflammation. Blood. 2003;102:3295–3301. doi: 10.1182/blood-2003-03-0727. [DOI] [PubMed] [Google Scholar]
- 40.Scott CL, Aumeunier AM, Mowat AM. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol. 2011;32:412–419. doi: 10.1016/j.it.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chmill S, Kadow S, Winter M, Weighardt H, Esser C. 2,3,7,8-tetrachlorodibenzo-p-dioxin impairs stable establishment of oral tolerance in mice. Toxicol Sci. 2010;118:98–107. doi: 10.1093/toxsci/kfq232. [DOI] [PubMed] [Google Scholar]
- 43.Hammerschmidt-Kamper C, Biljes D, Merches K, Steiner I, Daldrup T, Bol-Schoenmakers M, Pieters RH, Esser C. Indole-3-carbinol, a plant nutrient and AhR-ligand precursor, supports oral tolerance against OVA and improves peanut allergy symptoms in mice. PLoS One. 2017;12:e0180321. doi: 10.1371/journal.pone.0180321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H, Takahashi S, Kouzmenko A, Nohara K, Chiba T, et al. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature. 2007;446:562–566. doi: 10.1038/nature05683. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka T, Soriano MA, Grusby MJ. SLIM is a nuclear ubiquitin E3 ligase that negatively regulates STAT signaling. Immunity. 2005;22:729–736. doi: 10.1016/j.immuni.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Liu PC, Phillips MA, Matsumura F. Alteration by 2,3,7,8-tetrachlorodibenzo-p-dioxin of CCAAT/enhancer binding protein correlates with suppression of adipocyte differentiation in 3T3-L1 cells. Mol Pharmacol. 1996;49:989–997. [PubMed] [Google Scholar]
- 47.Vogel CFA, Haarmann-Stemmann T. The aryl hydrocarbon receptor repressor - more than a simple feedback inhibitor of AhR signaling: clues for its role in inflammation and cancer. Curr Opin Toxicol. 2017;2:109–119. doi: 10.1016/j.cotox.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishihara Y, Tsuji M, Vogel CF. Suppressive effects of aryl-hydrocarbon receptor repressor on adipocyte differentiation in 3T3-L1 cells. Arch Biochem Biophys. 2018;642:75–80. doi: 10.1016/j.abb.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hahn ME, Allan LL, Sherr DH. Regulation of constitutive and inducible AHR signaling: complex interactions involving the AHR repressor. Biochem Pharmacol. 2009;77:485–497. doi: 10.1016/j.bcp.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vogel CF, Matsumura F. Interaction of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) with induced adipocyte differentiation in mouse embryonic fibroblasts (MEFs) involves tyrosine kinase c-Src. Biochem Pharmacol. 2003;66:1231–1244. doi: 10.1016/s0006-2952(03)00404-0. [DOI] [PubMed] [Google Scholar]
- 51.Bour H, Peyron E, Gaucherand M, Garrigue JL, Desvignes C, Kaiserlian D, Revillard JP, Nicolas JF. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol. 1995;25:3006–3010. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- 52.Hwang SJ, Hwang YJ, Yun MO, Kim JH, Oh GS, Park JH. Indoxyl 3-sulfate stimulates Th17 differentiation enhancing phosphorylation of c-Src and STAT3 to worsen experimental autoimmune encephalomyelitis. Toxicol Lett. 2013;220:109–117. doi: 10.1016/j.toxlet.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 53.Hwang YJ, Yun MO, Jeong KT, Park JH. Uremic toxin indoxyl 3-sulfate regulates the differentiation of Th2 but not of Th1 cells to lessen allergic asthma. Toxicol Lett. 2014;225:130–138. doi: 10.1016/j.toxlet.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 54.Pollenz RS. The mechanism of AH receptor protein down-regulation (degradation) and its impact on AH receptor-mediated gene regulation. Chem Biol Interact. 2002;141:41–61. doi: 10.1016/s0009-2797(02)00065-0. [DOI] [PubMed] [Google Scholar]
- 55.Giannone JV, Li W, Probst M, Okey AB. Prolonged depletion of AH receptor without alteration of receptor mRNA levels after treatment of cells in culture with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochem Pharmacol. 1998;55:489–497. doi: 10.1016/s0006-2952(97)00493-0. [DOI] [PubMed] [Google Scholar]
- 56.Swanson HI, Perdew GH. Half-life of aryl hydrocarbon receptor in Hepa 1 cells: evidence for ligand-dependent alterations in cytosolic receptor levels. Arch Biochem Biophys. 1993;302:167–174. doi: 10.1006/abbi.1993.1195. [DOI] [PubMed] [Google Scholar]
- 57.Karchner SI, Franks DG, Powell WH, Hahn ME. Regulatory interactions among three members of the vertebrate aryl hydrocarbon receptor family: AHR repressor, AHR1, and AHR2. J Biol Chem. 2002;277:6949–6959. doi: 10.1074/jbc.M110779200. [DOI] [PubMed] [Google Scholar]
- 58.Baba T, Mimura J, Gradin K, Kuroiwa A, Watanabe T, Matsuda Y, Inazawa J, Sogawa K, Fujii-Kuriyama Y. Structure and expression of the Ah receptor repressor gene. J Biol Chem. 2001;276:33101–33110. doi: 10.1074/jbc.M011497200. [DOI] [PubMed] [Google Scholar]
- 59.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1+ macrophages to CD103+ dendritic cells. Immunity. 2014;40:248–261. doi: 10.1016/j.immuni.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 61.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 63.Chng SH, Kundu P, Dominguez-Brauer C, Teo WL, Kawajiri K, Fujii-Kuriyama Y, Mak TW, Pettersson S. Ablating the aryl hydrocarbon receptor (AhR) in CD11c+ cells perturbs intestinal epithelium development and intestinal immunity. Sci Rep. 2016;6:23820. doi: 10.1038/srep23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park JH, Choi AJ, Kim SJ, Cheong SW, Jeong SY. AhR activation by 6-formylindolo[3,2-b]carbazole and 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibit the development of mouse intestinal epithelial cells. Environ Toxicol Pharmacol. 2016;43:44–53. doi: 10.1016/j.etap.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Warren TK, Mitchell KA, Lawrence BP. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) suppresses the humoral and cell-mediated immune responses to influenza A virus without affecting cytolytic activity in the lung. Toxicol Sci. 2000;56:114–123. doi: 10.1093/toxsci/56.1.114. [DOI] [PubMed] [Google Scholar]
- 66.Yamada T, Horimoto H, Kameyama T, Hayakawa S, Yamato H, Dazai M, Takada A, Kida H, Bott D, Zhou AC, et al. Constitutive aryl hydrocarbon receptor signaling constrains type I interferon-mediated antiviral innate defense. Nat Immunol. 2016;17:687–694. doi: 10.1038/ni.3422. [DOI] [PubMed] [Google Scholar]
- 67.Jin GB, Winans B, Martin KC, Paige Lawrence B. New insights into the role of the aryl hydrocarbon receptor in the function of CD11c+ cells during respiratory viral infection. Eur J Immunol. 2014;44:1685–1698. doi: 10.1002/eji.201343980. [DOI] [PMC free article] [PubMed] [Google Scholar]