Abstract
The androgen receptor drives the growth of metastatic castration-resistant prostate cancer. This has led to the development of multiple novel drugs targeting this hormone-regulated transcription factor, such as enzalutamide – a potent androgen receptor antagonist. Despite the plethora of possible treatment options, the absolute survival benefit of each treatment separately is limited to a few months. Therefore, current research efforts are directed to determine the optimal sequence of therapies, discover novel drugs effective in metastatic castration-resistant prostate cancer and define patient subpopulations that ultimately benefit from these treatments. Molecular studies provide evidence on which pathways mediate treatment resistance and may lead to improved treatment for metastatic castration-resistant prostate cancer. This review provides, firstly a concise overview of the clinical development, use and effectiveness of enzalutamide in the treatment of advanced prostate cancer, secondly it describes translational research addressing enzalutamide response vs resistance and lastly highlights novel potential treatment strategies in the enzalutamide-resistant setting.
Keywords: enzalutamide, treatment resistance, biomarkers, androgen receptor, mutations, prostate cancer, mCRPC, androgen deprivation therapy, docetaxel
Introduction
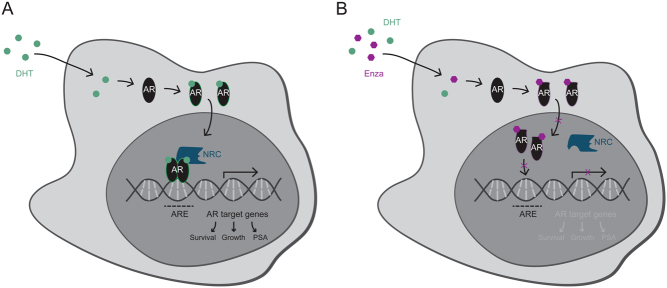
Ever since the discovery that prostate cancer (PCa) growth after androgen deprivation therapy (ADT) remains dependent on androgen receptor (AR) signaling, researchers have been looking for new effective ways to block the action of this hormone-dependent transcription factor (Attard et al. 2009, Tran et al. 2009, Scher et al. 2012). Upon stimulation with androgens, the AR dissociates from its molecular chaperones and translocates to the nucleus, where it binds to thousands of sites throughout the human genome to regulate transcription of directly responsive genes, including pro-mitotic genes involved in tumor cell proliferation (Fig. 1A) (Brinkmann et al. 1999, Itkonen & Mills 2012, Mills 2014).
Figure 1.
AR signaling axis and mechanism of action of enzalutamide. (A) Upon dihydrotestosterone (DHT) binding, the AR dimerizes and translocates to the nucleus, where it binds to AR-response elements (ARE) and recruits nuclear receptor coregulators (NRC), so-called coactivators or corepressors, to regulate transcription of directly responsive genes involved in cell proliferation and survival. (B) Enzalutamide (Enza) binding to the ligand-binding pocket of the AR results in a conformational change, rendering the receptor incapable of forming an active transcriptional complex. Further, enzalutamide blocks AR nuclear translocation and the enzalutamide-bound AR is impaired in its DNA-binding ability, ultimately preventing AR-dependent gene expression.
Inhibiting androgen signaling through ADT initially results in tumor regression in the vast majority of cases, but inevitably the tumor cells adapt to low androgen levels, leading to disease progression, which is known as castration resistance (Harris et al. 2009, Massard & Fizazi 2011, Karantanos et al. 2013).
Potent antiandrogens, that either target the AR directly through physical competition with the receptor’s natural ligand dihydrotestosterone (DHT) or indirectly via inhibition of androgen biosynthesis, are among the treatment options for metastatic castration-resistant prostate cancer (mCRPC) (Helsen et al. 2014).
At the moment, enzalutamide (MDV-3100) is the most frequently prescribed compound for treatment of mCRPC (Sanford 2013). This drug belongs to the class of direct androgen receptor inhibitors and tackles the AR pathway at multiple nodes: by preventing ligand binding, by blocking AR nuclear translocation and by inhibiting DNA transactivation, ultimately abrogating the expression of androgen-responsive genes (Fig. 1B) (Tran et al. 2009, van Soest et al. 2013). The multiple stage actions of enzalutamide on AR signaling are considered the main reason for its superior clinical activity over other direct AR inhibitors, such as flutamide, bicalutamide and nilutamide (Antonarakis 2013).
However, due to inter-patient heterogeneity of PCa, which is widely recognized as a major drawback for therapy efficacy, treatment responses to enzalutamide vary between patients (Boyd et al. 2012). Whereas some patients do not have a substantial clinical benefit from enzalutamide therapy, others who do benefit, start progressing after a certain period of time, which is also dependent on therapy sequencing (Scher et al. 2012, Beer et al. 2014, Merseburger et al. 2015).
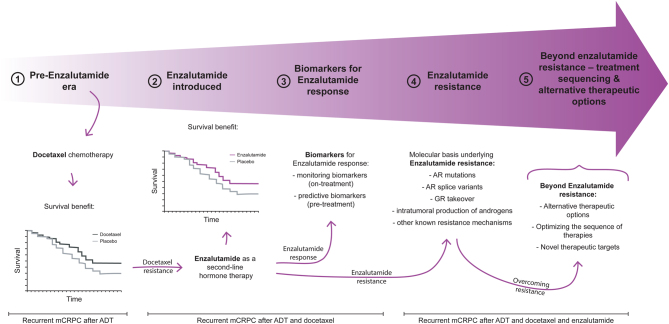
This review, of which the content is illustrated in Fig. 2 (1–5), will firstly provide a comprehensive insight into the use of enzalutamide in the treatment of advanced PCa – spanning from treatment options in the pre-enzalutamide era (1) to its preclinical development and the landmark studies that led to its FDA approval for mCRPC (2). Thereupon, we discuss translational research directed at tackling unmet clinical needs in the treatment of advanced PCa using enzalutamide. This includes having on-treatment and predictive biomarkers for treatment response (3); a better understanding of molecular mechanisms underlying enzalutamide resistance (4); and lastly, the development of novel therapeutic approaches aimed to overcome therapy resistance (5).
Figure 2.
Graphical summary capturing the topics discussed in this review. Docetaxel has been the first agent showing a survival benefit in mCRPC patients (1). Despite initial responses upon docetaxel chemotherapy, patients eventually progress, whereby enzalutamide has been shown to be effective in such a docetaxel-resistant mCRPC setting (2). Current translational research efforts are aimed at developing biomarkers for enzalutamide response (3), understanding molecular underpinnings of enzalutamide-resistant mCRPC (4) and optimizing treatment strategies to overcome enzalutamide resistance (5).
The pre-enzalutamide era
Androgen deprivation therapy
ADT has been the standard of care for patients with symptomatic metastatic PCa since the forties of the last century (Merseburger et al. 2016). However, despite initial response to ADT, eventually resistance emerges in practically every patient, which is mediated by AR-dependent or -independent pathways (Scher & Sawyers 2005). Initially, two retrospective studies have shown a limited survival benefit of continued androgen suppression with luteinizing hormone-releasing hormone (LHRH) analogs in the mCRPC setting (Taylor et al. 1993, Hussain et al. 1994). Based on these findings, all mCRPC patients enrolled in the trials discussed further below continue androgen suppression therapy. Although data are limited, the benefits of continuing androgen deprivation outweighed the potential risks of discontinuing the therapy.
Chemotherapy
In 2004, the TAX-327 trial initiated a transition in systemic mCRPC treatment (Tannock et al. 2004). In this phase III study, 1006 patients with mCRPC were randomized to receive prednisone either in combination with mitoxantrone (a chemotherapy that provides palliation, but does not lead to an improvement in survival for patients with castration-refractory PCa (Tannock et al. 1996) or with docetaxel (a chemotherapy that has been reported in phase II studies to successfully reduce serum prostate-specific antigen (PSA) levels Beer et al. 2001, Berry et al. 2001).
Whereas mitoxantrone, as a type II topoisomerase inhibitor that intercalates between DNA bases and thereby disrupts DNA synthesis and repair (Nitiss 2009, Pommier et al. 2010), is not directly linked to AR biology, docetaxel is. It belongs to the taxane class of chemotherapeutic agents that bind to tubulin and hyperstabilize microtubules, which ultimately leads to impairments of the mitotic cell cycle and AR signaling by preventing its nuclear translocation (Kuroda et al. 2009, Zhu et al. 2010, Darshan et al. 2011, Fitzpatrick & de Wit 2014).
The TAX-327 study identified docetaxel as the first chemotherapeutic drug that showed a modest overall survival (OS) benefit compared to mitoxantrone (Table 1) (Berthold et al. 2008). Based on these results, docetaxel was established as a first-line therapy option for both, symptomatic as well as asymptomatic mCRPC.
Table 1.
Clinical trials of systemic treatments for mCRPC that improve overall survival.
| Trial (registration number) | Study intervention | Median overall survival (95% CI) | Hazard ratio (95% CI; P-value) | References | Sequence | |||
|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | |||||
| Chemotherapy | TAX-327a | Docetaxel + Prednisone | Mitoxantrone + Prednisone | 19.2 months (17.5–21.3) | 16.3 months (14.3–17.9) | 0.76 (0.62–0.94; P = 0.009) | Berthold et al. (2008), Tannock et al. (2004) | Progression after ADT without chemotherapy |
| Hormonal therapy | PREVAIL (Nbib1212991) | Enzalutamide | Placebo | 35.3 months (32.2–not yet reached) | 31.3 months (28.8–34.2) | 0.77 (0.67–0.88; P = 0.0002) | Beer et al. (2017, 2014) | Progression after ADT without chemotherapy |
| AFFIRM (Nbib974311) | Enzalutamide | Placebo | 18.4 months (17.3–not yet reached) | 13.6 months (11.3–15.8) | 0.63 (0.53–0.75; P < 0.001) | Scher et al. (2012) | Progression after ADT and docetaxel | |
aNo trial registration number available for TAX-327.
ADT, androgen-deprivation therapy; CI, confidence interval.
Docetaxel resistance
As described earlier, mCRPC patients treated with docetaxel-based chemotherapy have a modest OS benefit implying most patients will progress rather rapidly. In patients with a good initial response to docetaxel therapy, re-challenging with the same chemotherapeutic agent results in a PSA response in up to 60% of patients with a median time to progression of 6 months (Beer et al. 2004). As this response is less profound as compared to the therapeutic effect in the first round, it could also be hypothesized that the efficacy of docetaxel re-challenge will keep decreasing until its effect becomes negligible. Mechanisms underlying this docetaxel resistance in the mCRPC setting can be diverse (Seruga et al. 2011). On the one hand, those include rather general mechanisms associated with resistance to taxanes, including an altered microtubule composition affecting docetaxel binding (such as upregulation of certain isotypes Ranganathan et al. 1998 or mutations Yin et al. 2010), a reduced intracellular drug accumulation due to overexpression of drug efflux pumps (such as P-glycoprotein Zhu et al. 2013) or an impaired drug distribution due to aberrant angiogenesis (Marignol et al. 2008). On the other hand, resistance can also develop due to mechanisms intrinsic to the biology of mCRPC like continued AR signaling which stimulates PCa growth and inhibits apoptosis (Seruga et al. 2011) or due to the activation of compensatory oncogenic pathways (such as PI3K/AKT or MAPK/ERK Zhu & Kyprianou 2008) which are themselves associated with proliferation and survival. As a result of taxane resistance, new therapeutic approaches tackling docetaxel-resistant mCRPC were needed and much sought-after.
Enzalutamide as a second-line hormone therapy
Preclinical development
Ever since molecular profiling studies have revealed that many CRPC tumors remain AR driven, there has been great interest in identifying novel and potent strategies to better block the AR signaling axis (Chen et al. 2004). Such next-generation antiandrogens should – unlike their first-generation counterparts (e.g. bicalutamide and flutamide) – preferably possess greater AR-binding affinities without any agonistic effects (Chen et al. 2004, Bambury & Scher 2015). In their search for such improved antiandrogens, Tran et al. (2009) screened nearly 200 thiohydantoin derivatives of RU59063 – a non-steroidal AR agonist with a relatively high affinity and selectivity over other nuclear hormone receptors – for retained activity in human PCa cells that overexpressed the AR protein, which is also clinically observed in the castration-resistant disease setting. RD162 and MDV3100 (now enzalutamide) were selected as the lead compounds for additional biological validation, and importantly, both antiandrogens led to tumor regression in xenograft models (Tran et al. 2009). Due to its favorable drug-like properties, such as oral bioavailability and longer serum half-life, enzalutamide was selected for further clinical development (Bambury & Scher 2015).
Clinical testing
The preclinically demonstrated antitumor activity of enzalutamide was subsequently validated in a phase I/II trial, in which patients with progressive mCRPC were enrolled in dose-escalation cohorts, ultimately demonstrating its safety and tolerability, along with antitumor effect at all tested doses (Scher et al. 2010).
In 2012, the preliminary analysis of the AFFIRM trial was published, being the first phase III study on enzalutamide in the mCRPC setting (Scher et al. 2012). In this trial, 1199 mCRPC patients who progressed on docetaxel therapy were randomized to receive either enzalutamide or placebo. Enzalutamide treatment significantly improved patient outcome after docetaxel therapy compared to the placebo control group (Table 1).
The efficacy of enzalutamide and its limited toxicity as compared to chemotherapy could not only be achieved in mCRPC patients who were previously treated with docetaxel, but also in the chemotherapy-naïve setting, as addressed by the PREVAIL study (Beer et al. 2014). This was a randomized phase III trial including 1717 chemo-naïve mCRPC men comparing enzalutamide therapy to a placebo. Again, enzalutamide therapy resulted in a significant improvement in OS and radiographic progression-free survival (rPFS) (Table 1) (Beer et al. 2017).
Moreover, the results of the randomized phase III PROSPER trial were recently published. Therein, the addition of enzalutamide or placebo to continued ADT was tested with regards to its potential to delay metastasis formation in men with non-metastasized CRPC who are at high risk for developing distant lesions. In this setting, enzalutamide therapy led to a 71% lower risk of metastasis or death compared to placebo (Hussain et al. 2018).
Based on these results, enzalutamide is now a primary treatment option for metastasis-free CRPC and asymptomatic mCRPC, whereas docetaxel is mainly used in men with symptomatic metastasized disease and acquired resistance to first-line therapeutics (Ryan et al. 2013, Beer et al. 2014, Hussain et al. 2018).
Biomarkers for enzalutamide response
The readout of PSA levels as a diagnostic biomarker was already introduced in the 1980s, but has also been questioned since then, mainly due to its non-specificity as a marker for cancerous lesions (Oesterling 1991, Salman et al. 2015). However, PSA measurements as a monitoring biomarker for either treatment response or resistance following PCa diagnosis and corresponding interventions, are routinely used in the clinic. PSA declines of at least 30% after 4 weeks and >30% or >50% after 12 weeks of treatment have been shown to correlate with a survival advantage especially in patients treated with AR-targeting compounds, whereas stable or increased PSA levels correlated with poorer outcome (Scher et al. 2008, Brasso et al. 2015, Fuerea et al. 2016, Rescigno et al. 2016). Moreover, circulating tumor cells (CTCs) seem to be a promising tool to predict a treatment-induced survival benefit. It has been observed that patients with a decline in number of CTCs (>30%) after 4 weeks of therapy have a better prognosis (Scher et al. 2015, Lorente et al. 2016, Heller et al. 2017, Prekovic et al. 2018a ). Consequently, CTCs could be a better marker for treatment resistance in tumors progressing without an obvious PSA rise, taking into account that further validation is warranted before it can be recommended in daily clinical practice. These on-treatment readouts, however, solely allow monitoring of a patient’s response to for example, enzalutamide therapy. Whereas some men do respond exceptionally well and continue treatment for several years, others progress within months or even do not show any response at all (Attard & Antonarakis 2016). Thus, biomarkers that enable the identification of patient subpopulations that benefit from enzalutamide treatment are urgently needed to improve the management of PCa patients.
Especially in the primary disease setting, tissue biopsies have proved to be highly informative. Besides classification systems based on clinical parameters (such as Gleason score, PSA and clinical staging) (D’Amico et al. 1998), genomic analyses may provide risk-assessment biomarkers that stratify patients with PCa on outcome (Irshad et al. 2013, Knezevic et al. 2013, Stelloo et al. 2015). However, the bone-predominant metastatic landscape of CRPC renders them rather impractical in routine clinical practice and current approaches almost exclusively focus on minimally invasive biomarkers from blood (Wyatt et al. 2016). Until now, several studies have shown that the profiling of CTCs or cell-free tumor DNA (cfDNA) in liquid biopsies enables the detection of AR splice variants, AR copy number gains and AR mutations, all of whom are at least associated with enzalutamide resistance and poorer prognosis (Schwarzenbach et al. 2009, Antonarakis et al. 2014, Diaz & Bardelli 2014, Salvi et al. 2016, Wyatt et al. 2016, Conteduca et al. 2017). Nonetheless, no such biomarker is implemented and routinely used in the clinic thus far, and further studies that robustly validate each biomarker in a prospective fashion are required for a potential practice change (Attard & Antonarakis 2016).
Molecular basis underlying enzalutamide resistance
The AFFIRM and PREVAIL trials clearly demonstrated the advantages of enzalutamide treatment. However, 46 (AFFIRM) and 22% (PREVAIL) of patients with mCRPC did not respond to second- or first-line treatment with enzalutamide, meaning that their PSA levels did not decline ≥50% from baseline. The remaining 54 and 78% of enzalutamide-treated patients responded initially, but PSA progression could be observed after a median time of 8.3 months (AFFIRM) and 11.2 months (PREVAIL) (Scher et al. 2012, Beer et al. 2014).
The mechanisms underlying this pre-existent or acquired resistance to enzalutamide are still not fully elucidated, but several possible mechanisms have been proposed (Claessens et al. 2014). In the next section, we will briefly discuss such potential mechanisms, which are elaborately discussed in Prekovic et al. (2018b ).
AR mutations
Gain-of-function mutations in the AR gene, especially within the exon 7 (encoding for the ligand-binding domain), have been found in 5–30% of CRPC patients (Taplin et al. 1999, Coutinho et al. 2016, Kumar et al. 2016, Rathkopf et al. 2017, Pal et al. 2018). These genomic alterations do not only permit receptor activation by various circulating steroids next to testosterone (such as H875Y or T878A), but may also alter the responsiveness of the AR to antiandrogens, resulting in antagonist-to-agonist switching (Grossmann et al. 2001, Nadal et al. 2017, Prekovic et al. 2018a ). This is exemplified by the F877L/T878A and M896V/S889G double mutants, which were associated with resistance (Lallous et al. 2016, Prekovic et al. 2016) and have recently been found in cfDNA extracted from plasma of mCRPC patients progressing on enzalutamide therapy (Azad et al. 2015, Wyatt et al. 2016).
AR splice variants
Alternatively spliced AR variants, especially AR-V7, have been reported to be implicated in resistance to AR-targeting drugs, including enzalutamide. AR-V7 is an AR isoform that lacks the ligand-binding domain (LBD), causing the variant to be constitutively active and resistant to LBD-targeting inhibitors (Guo et al. 2009, Hu et al. 2009, Antonarakis et al. 2016). Multiple studies have demonstrated that AR-V7 expression is a biomarker for resistance to AR-targeting drugs in CRPC (Li et al. 2013, Scher et al. 2016, Antonarakis et al. 2017, Del Re et al. 2017, Qu et al. 2017, Todenhofer et al. 2017), but it remains to date unclear whether AR-V7 is driving the resistance or whether it merely is a manifestation of treatment-induced selective pressure without being the key-driver to therapy failure.
Glucocorticoid receptor takeover
The glucocorticoid receptor (GR) has been reported to be upregulated or re-expressed after AR blockade, indicating a complex cross-talk between AR and GR biology. Due to great similarities in the mechanism of action between nuclear receptors, GR is suggested to take over the role of AR by driving the expression of a subset of androgen-responsive genes, thus enabling the tumor to progress even in presence of the AR-selective antagonist enzalutamide (Arora et al. 2013, Kach et al. 2015, Li et al. 2017, Shah et al. 2017, Puhr et al. 2018).
Intratumoral production of androgens
In addition, reactivation of the AR can occur via intratumoral production of androgens, enabling the prostate cancers to progress despite ongoing androgen deprivation (Locke et al. 2008). The expression of one of the essential enzymes in androgen biosynthesis, AKR1C3, was significantly increased in enzalutamide-resistant cells and xenograft tumors as well as in clinical specimens of advanced PCa, making it an attractive therapeutic target (Wako et al. 2008, Pfeiffer et al. 2011, Hamid et al. 2013, Liu et al. 2015, 2017). Inhibition of AKR1C3 as a novel therapeutic strategy is currently under investigation in a clinical trial (Nbib2935205) studying its potential benefit in combination with enzalutamide therapy in mCRPC (Pan et al. 2018).
Other known resistance mechanisms
Next to the aforementioned AR-related underpinnings of enzalutamide resistance, several additional mechanisms have been described to give rise to therapy resistance, but are not within the scope of this review. Among those are very diverse adaptations, such as metabolic changes (e.g. shifting to aerobic glycolysis Cui et al. 2014 or alterations in the hexosamine biosynthetic pathway Itkonen et al. 2016), but also autophagy (Nguyen et al. 2014) or activation of certain signaling pathways (such as Wnt Lee et al. 2015 or interleukin 6 Liu et al. 2014b ) – all of whom are addressed in depth in Prekovic et al. (2018b).
Beyond enzalutamide resistance – therapy sequencing and alternative therapeutic options
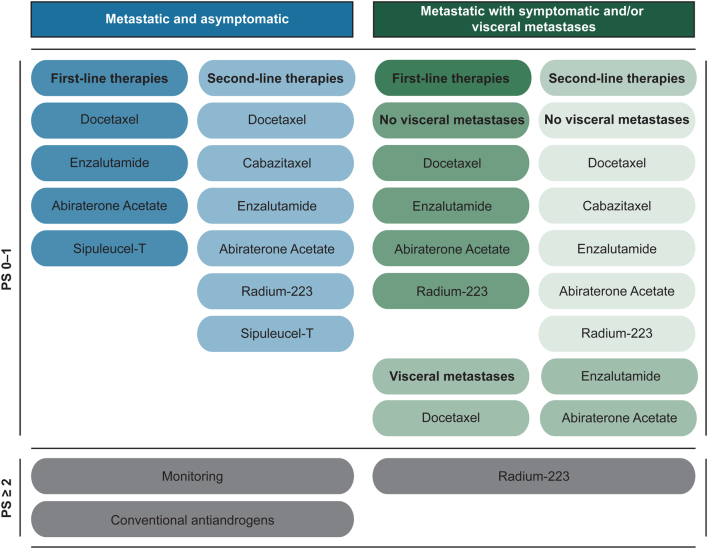
Scheduling of enzalutamide treatment in mCRPC patients can differ greatly; depending on a patient’s PCa stage, overall health status, treatment history and personal preference (Fig. 3). The mechanisms behind enzalutamide resistance (or other AR antagonists) may therefore also differ as these may depend on the settings in which the drug was administered. Over the last decade, several treatments have been developed, even though the optimal sequence of therapies still remains to be determined. This is especially the case, since none of the available therapeutic options described in Table 2 have yet been compared head-to-head in clinical trials (Sartor & Gillessen 2014).
Figure 3.
Schematic representation of treatment options in the mCRPC setting according to current standards of care. Therapeutic options are subdivided in first- and second-line therapies based on the clinical setting of the disease (asymptomatic vs symptomatic or visceral metastases). Treatment options are also determined by the overall performance status (PS) of the patient (PS 0–1: normal activity or some symptoms, but almost entirely ambulatory; PS ≥2: symptomatic patients <50% of daytime in bed up until completely bedridden).
Table 2.
Multiple large clinical trials of alternative therapies that improve survival of patients with mCRPC.
| Trial (registration number) | Study intervention | Median overall survival (95% CI) | Hazard ratio (95% CI; P-value) | References | Sequence | |||
|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | |||||
| Hormonal therapy | COU-AA-302 (Nbib887198) | Abiraterone + Prednisone | Placebo + Prednisone | 34.7 months (32.7–36.8) | 30.3 months (28.7–33.3) | 0.81 (0.70–0.93; P = 0.0033) | Ryan et al. (2013, 2015), Rathkopf et al. (2014) | Progression after ADT without chemotherapy |
| COU-AA-301 (Nbib638690) | Abiraterone + Prednisone | Placebo + Prednisone | 15.8 months (14.8–17.0) | 11.2 months (10.4–13.1) | 0.74 (0.64–0.86; P < 0.0001) | De Bono et al. (2011), Fizazi et al. (2012) | Progression after ADT and docetaxel | |
| Chemotherapy | TROPIC (Nbib417079) | Cabazitaxel + Prednisone | Mitoxantrone + Prednisone | 15.1 months (14.1–16.3) | 12.7 months (11.6–13.7) | 0.70 (0.59–0.83; P < 0.0001) | de Bono et al. (2010), Bahl et al. (2013) | Progression after ADT and docetaxel |
| Immunotherapy | IMPACT (Nbib65442) | Sipuleucel-T | Placebo | 25.8 months (22.8–27.7) | 21.7 months (17.7–23.8) | 0.78 (0.61–0.98; P = 0.03) | Kantoff et al. (2010) | Progression after ADT, unspecified docetaxel status |
| Alpha-particle Therapy | ALSYMPCA (Nbib699751) | Radium-223 | Placebo | 14.9 months (13.9–16.1) | 11.3 months (10.1–12.8) | 0.70 (0.58–0.83; P < 0.001) | Parker et al. (2013) | Progression after ADT, unspecified docetaxel status |
ADT, androgen-deprivation therapy; CI, confidence interval.
Available therapeutic options in clinical practice
For mCRPC patients responding to enzalutamide, there is no doubt that outcomes have improved significantly. Nevertheless, despite the survival benefits, patients are still progressing and improvements in absolute survival rates are rather disappointing. Besides enzalutamide, several other therapeutic options with proven benefit for mCRPC patients have been developed in the past 10 years, which are summarized in Table 2 and will be briefly discussed hereafter.
Cabazitaxel
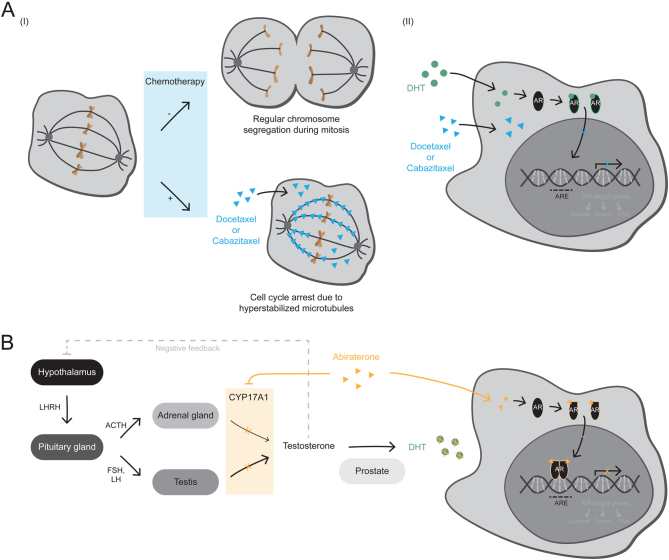
Cabazitaxel is – like docetaxel – a taxane, which stabilizes microtubules and consequently impairs mitotic cell division (Fig. 4A) (Fitzpatrick and de Wit 2014, Quinn et al. 2017). However, the drug shows antitumor activity in docetaxel-resistant models, potentially due to the fact that cabazitaxel is a poor substrate for the drug efflux pump P-glycoprotein, which is reported to contribute to docetaxel resistance (Paller & Antonarakis 2011). In line with this, cabazitaxel has been shown to improve OS in mCRPC patients with progressive disease on or after docetaxel-based intervention (de Bono et al. 2010, Bahl et al. 2013).
Figure 4.
Mechanisms of action of taxane chemotherapeutics and the antiandrogen abiraterone acetate. (A) Taxane chemotherapeutics, such as docetaxel and cabazitaxel, act by hyperstabilizing microtubules, which – due to the microtubules' role in chromosome segregation during mitosis – causes a cell cycle arrest in metaphase followed by apoptosis. Moreover, taxanes directly affect AR signaling by inhibiting the microtubule-dependent AR nuclear translocation in response to androgen stimulation. (AR, androgen receptor; ARE, AR-response element; DHT, dihydrotestosterone). (B) Abiraterone is a cytochrome P450 17A1 (CYP17A1) inhibitor that leads to androgen deprivation by inhibiting the intracellular biosynthesis of androgens in the testis and adrenal glands. Androgens are produced via the hypothalamic-pituitary-testis and to a small degree also via the hypothalamic-pituitary-adrenal axis. Within these axes, CYP17A1 is responsible for converting cholesterol to androgens, such as testosterone, which gets reduced to the potent AR agonist DHT in the prostate. In addition to androgen deprivation, abiraterone is capable of directly interacting with the AR and thereby blocks the signaling of this hormone-responsive transcription factor. (ACTH, adrenocorticotropic hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; LHRH, luteinizing hormone-releasing hormone; NRC, nuclear receptor coregulator).
Abiraterone acetate
Abiraterone acetate (hereafter referred to as abiraterone) is targeting the AR signaling axis by inhibiting cytochrome P450 17A1 (CYP17A1) – an enzyme involved in intracellular biosynthesis of androgens that enables prostate cancer cells to bypass androgen deprivation (Fig. 4B) (Montgomery et al. 2008, Attard et al. 2009, Helsen et al. 2014). In addition, it has been demonstrated that abiraterone and one of its metabolic derivatives are able to directly bind to the AR and thereby inhibit the signaling of this ligand-dependent transcription factor (Richards et al. 2012, Soifer et al. 2012, van Soest et al. 2013, Li et al. 2015). Several large clinical trials have shown its efficacy in the hormone-naïve metastatic PCa setting (Fizazi et al. 2017, James et al. 2017) as well as in the chemo-naïve (Ryan et al. 2015) and post-docetaxel (Fizazi et al. 2012) mCRPC setting.
Sipuleucel-T
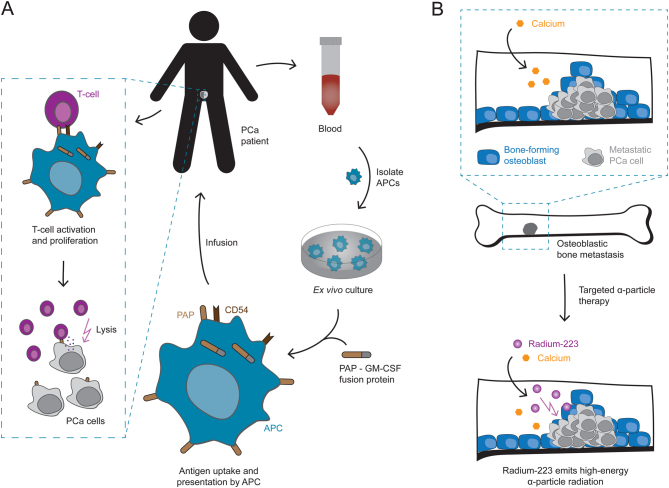
Sipuleucel-T is an autologous cell-based cancer immunotherapy, in which the patient’s immune system is reprogrammed to recognize and eradicate cancer cells (Higano et al. 2010). During the procedure, antigen-presenting cells are isolated from blood and primed ex vivo to recognize prostatic acid phosphatase – an enzyme overexpressed in prostate cancers – after which the activated immune cells are reinfused into the patient (Fig. 5A) (Di Lorenzo et al. 2011, Handy & Antonarakis 2018). In a phase III trial, this therapeutic cancer vaccine has prolonged OS of mCRPC patients with asymptomatic or minimally symptomatic disease, making it the first immunotherapeutic approach shown to improve survival in PCa (Kantoff et al. 2010). However, Sipuleucel-T administration has thus far only been tested with concurrent or prior to enzalutamide therapy (Nbib1981122), where both treatment schedules seem to result in similarly robust immune responses with no differences in median OS (Antonarakis et al. 2018, Petrylak et al. 2018). Until now, Sipuleucel-T is therefore considered as a therapeutic option prior to docetaxel and enzalutamide, as recommended by a European Expert Consensus Panel, unless further studies demonstrate its effectiveness in the enzalutamide-resistant mCRPC setting (Fitzpatrick et al. 2014).
Figure 5.
Mechanisms of action of Sipuleucel-T and Radium-223. (A) Sipuleucel-T is a cancer immunotherapy, which makes use of autologous antigen-presenting cells (APCs) to activate the patient’s immune system against PCa cells. Dendritic cells, the most efficient APCs, are isolated from blood samples and cultured ex vivo together with a recombinant fusion protein, composed of prostatic acid phosphatase (PAP) – an enzyme overexpressed in PCa; and granulocyte-macrophage colony-stimulating factor (GM-CSF) – a cytokine that enhances immune responses. The APCs take up these antigens, present them on their surface, and upon activation express the surface marker Cluster of Differentiation 54 (CD54) – a glycoprotein that is involved in APC – T-cell interactions. The activated APCs are then reinfused into the patient to trigger a T-cell response against PAP-expressing PCa cells. (B) Targeted alpha-particle therapy with Radium-223 is a treatment option in symptomatic mCRPC patients with skeletal metastasis. Radium-223 is a radioactive calcium analog that – like calcium itself – gets incorporated by osteoblasts into the bone matrix. This especially occurs at sites of increased bone formation, as found in bone metastasis from PCa, where Radium-223 emits high-energy alpha-particle radiation that causes severe DNA damage in nearby cells.
Radium-223
In symptomatic mCRPC patients with skeletal metastases, Radium-223 dichloride (Radium-223) is an additional therapeutic option that improves OS (Parker et al. 2013). Radium-223 is a bone-seeking calcium mimetic that concentrates at areas of increased bone turnover, as found in osteoblastic bone metastases from prostate cancer, where it emits high-energy alpha-particle radiation that causes severe DNA damage in nearby cells (Fig. 5B) (Henriksen et al. 2002, Bruland et al. 2006, Parker et al. 2013, Deshayes et al. 2017).
Therapeutics in clinical development
The antiandrogens apalutamide (ARN-509) (Dellis & Papatsoris 2018) and darolutamide (ODM-201) (Shore 2017) are two novel therapeutics, which are currently under clinical investigation. Whereas apalutamide’s structure is highly similar to enzalutamide’s, darolutamide is structurally distinct. Nevertheless, both novel antiandrogens possess a higher affinity for the AR LBD and less passage through the blood–brain barrier compared to enzalutamide. This should reduce the risk of seizures – a common side-effect of non-steroidal antiandrogens, potentially due to an off-target binding to GABAA receptors in the brain (Imamura & Sadar 2016), which in the initial phase I/II dose-escalation study occurred in about 2.1% of enzalutamide-treated patients (3 of 140), all of whom, however, received doses that were more than twice as high as the later on approved dosage of 160 mg/day (Scher et al. 2010, Higano et al. 2015). The results of a placebo-controlled phase III trial showed significantly improved metastasis-free survival and time to symptomatic progression upon apalutamide treatment in men with non-metastatic CRPC (Smith et al. 2018). Similarly, a study investigating the efficacy of darolutamide in this setting is presently running (Nbib2200614).
Another drug that is currently being studied with regards to overcoming enzalutamide resistance is niclosamide. It is an FDA-approved anthelminthic drug, which has been identified as a potent AR-V7 inhibitor in PCa cells, resulting in PCa cell growth inhibition in vitro and tumor growth inhibition in vivo. Further, if administered in combination with enzalutamide, niclosamide could re-sensitize enzalutamide-resistant tumors to the antiandrogen (Liu et al. 2014a ). Currently, the safety and pharmacokinetics of the combination therapy are being tested in phase I trials (Nbib2532114, Nbib3123978), in which the poor oral bioavailability of niclosamide has recently been reported to limit its efficacy (Schweizer et al. 2018). Therefore, the current oral formulation of niclosamide might not be effective enough as a mCRPC intervention, demonstrating the importance of further clinical testing along with the development of niclosamide analogs with improved pharmacokinetic and antitumor properties (Schweizer et al. 2018).
Taken together, there are – at least theoretically – several alternative treatment options for mCRPC patients whose disease progressed on or after enzalutamide treatment. However, while choosing an appropriate subsequent therapeutic option, possible cross-resistance needs to be considered – especially among the next-generation antiandrogens. Moreover, a potential attenuation in a drug’s clinical efficiency may occur if used as a second- or third-line intervention, emphasizing the importance of optimal treatment scheduling.
Optimizing the sequence of therapies
The introduction of the aforementioned novel effective therapies has added an additional dimension to the complex therapeutic landscape of mCRPC. As all of them have proven survival advantages, diverse scenarios of therapeutic interventions could be generated, but it still remains elusive how best to sequence and/or combine these treatment options.
Clinical and translational research exploring enzalutamide scheduling
Since it is out of the scope of this review to discuss all ongoing clinical studies with enzalutamide (co)treatment, we have compiled a non-exhaustive list of clinical trials registered on clinicaltrials.gov (Supplementary Table 1, see section on supplementary data given at the end of this article). Herein, we will focus on the limited number of studies that compare the efficacy of the various treatment options with the aim to identify an optimal sequence of treatments. One such trial is the ongoing OSTRICh study, in which patients with poor prognostic features who progressed on docetaxel therapy are randomized between cabazitaxel and either enzalutamide or abiraterone (Nbib3295565).
Sequential treatment with different AR-targeting agents has shown limited efficacy as exemplified by modest PSA responses when sequentially treated with enzalutamide and abiraterone or vice versa (Loriot et al. 2013, Noonan et al. 2013, Bianchini et al. 2014, Schrader et al. 2014, Brasso et al. 2015, Cheng et al. 2015, Petrelli et al. 2015, Badrising et al. 2016a ). Furthermore, it is suggested that docetaxel has a reduced activity after prior therapy with enzalutamide or abiraterone (Mezynski et al. 2012, Aggarwal et al. 2013, Suzman et al. 2014).
A possible combinatorial treatment regimen is currently being tested in trials that evaluate the efficacy of enzalutamide in combination with taxane-based chemotherapeutics for the treatment of mCRPC. Such chemohormonal therapies have proven benefit in the metastatic non-castrate PCa setting, prior to developing hormone insensitivity. Therein, the CHAARTED (Sweeney et al. 2015, 2016, Kyriakopoulos et al. 2018) and STAMPEDE (James et al. 2016) trials showed that upfront addition of docetaxel chemotherapy to ADT at diagnosis of treatment-naïve metastatic PCa improves OS as compared to standard-of-care ADT. Based on these results, upfront docetaxel combined with ADT is considered to be a treatment option in men with de novo metastatic hormone-naïve PCa (Gillessen et al. 2018a ). However, its benefit in the mCRPC setting remains elusive, as such chemohormonal combinations (such as enzalutamide + docetaxel (Nbib1565928) or enzalutamide + cabazitaxel (Nbib2522715)) have thus far only been tested in phase I/II trials with relatively small sample sizes and consequently require further study in a larger population (Morris et al. 2016, Sternberg 2016).
Up to now, the consensus on therapy sequencing in the mCRPC setting is mostly based on small retrospective studies that are unable to give a clear answer. Recently, a post-registration study evaluated the efficacy and safety of enzalutamide treatment in patients with mCRPC who had previously progressed on abiraterone. Therein, enzalutamide therapy was beneficial in some patients, whereas the majority presented cross-resistance between the two hormonal agents (de Bono et al. 2018). Similar results were shown in a retrospective study, in which the response to enzalutamide was associated with a longer interval between end of abiraterone and start of enzalutamide treatment, suggesting that over time the chance for a subsequent enzalutamide response potentially increases (Badrising et al. 2016b ).
On the basis of the observed cross-resistance, it is important to evaluate which of the endocrine treatment options is more effective as first-line therapy for patients with mCRPC. This issue is currently being addressed in the ENABLE study for prostate cancer, a phase III multicenter randomized controlled trial, in which the efficacies of enzalutamide and abiraterone will be compared head to head (Izumi et al. 2017).
Additionally, a randomized controlled trial (Nbib2125357) is currently being performed, which assesses PSA response rates in therapy-naïve mCRPC patients being sequentially treated with abiraterone and enzalutamide or vice versa (Chi et al. 2017).
Combining different AR-targeting drugs simultaneously might improve efficacy as compared to consecutive treatment. This is being investigated in patients treated with enzalutamide or abiraterone alone vs a combination therapy consisting of both antiandrogens (Nbib1949337, Nbib1995513). Furthermore, although in the hormone-naïve setting, the result update of the STAMPEDE trial is awaited with high expectations, as it includes an arm with such a combination therapy (Nbib268476, Arm J).
Another approach to re-challenge enzalutamide-resistant mCRPC has been described by Schweizer et al. (2015) and is referred to as bipolar androgen therapy (BAT). BAT is exploiting the adaptive increase of AR levels in CRPC, allowing the tumor cells to cope with castrate levels of testosterone, by rapidly cycling between androgen stimulation and deprivation. A subsequent phase II study of BAT in mCRPC patients that progressed on enzalutamide showed successful re-sensitization to the drug, when the patients were re-challenged with the antiandrogen upon progression on testosterone therapy (Teply et al. 2018).
Current consensus guidelines for enzalutamide treatment and therapy sequencing
The St. Gallen Advanced Prostate Cancer Consensus Conference (APCCC) assists clinicians in their therapeutic decision-making regarding the management of patients with advanced prostate cancer (Gillessen et al. 2018a ,b ). The recommendations most relevant to this review have been summarized in Supplementary Table 2. Accordingly, enzalutamide is considered as a first-line treatment in patients with asymptomatic mCRPC, regardless of whether they had received ADT alone or in combination with docetaxel in the castration-sensitive setting. Similarly, enzalutamide is a first-line option for symptomatic men who received docetaxel in the castration-naïve setting; whereas either docetaxel, abiraterone or enzalutamide treatment are the therapies of choice for symptomatic patients who did not receive docetaxel in this setting. Furthermore, there was consensus that both, asymptomatic as well as symptomatic mCRPC patients, progressing on or after first-line docetaxel chemotherapy should receive either enzalutamide or abiraterone as a second-line agent.
Novel therapeutic targets
In addition to the clinically used enzalutamide alternatives described earlier, there are currently several treatment strategies in development. The studies with the most promising (pre-) clinical data and/or ongoing clinical trials are discussed hereafter.
In clinical development
Recently whole-exome and transcriptome analysis of advanced PCa revealed that 89% of 150 mCRPC patients had clinically targetable aberrations (Robinson et al. 2015). Next to well-known frequently occurring aberrations (AR, ETS, TP53 and PTEN), new genomic alterations were found to be highly enriched in mCRPC patients, including PIK3CA/B, R-spondin, BRAF/RAF1, APC, β-catenin and ZBTB16/PLZF. Furthermore, genes involved in DNA damage repair (BRCA2, BRCA1 and ATM) were altered more frequently than expected (Robinson et al. 2015). More recently, Pritchard et al. (2016) have found that 11.8% of patients with metastatic PCa have inherited germline mutations in DNA damage repair genes, which seem to be effectively treatable with the PARP-inhibitor olaparib (Mateo et al. 2015). In consequence of the identification of these genomic alterations in mCRPC, there is a great interest in the design of clinical trials targeting these pathways in combination with enzalutamide treatment. Trials that are currently running include PI3K/AKT/mTOR pathway inhibition using LY3023414 (Nbib2407054), TGF-β receptor I pathway inhibition using galunisertib (Nbib2452008) and IGF1 pathway inhibition using xentuzumab (Nbib2204072).
mCRPC is also characterized by changes in the epigenetic and chromatin status like altered histone acetylation or DNA methylation, based on which chromatin readers/modifiers are regarded as potential therapeutic targets (Li et al. 2005, Metzger et al. 2005, Spans et al. 2016, Bennett & Licht 2018). Therefore, the BET family of proteins which recognize and bind acetylated histones and are implicated in transcriptional regulation processes are potential therapeutic targets (Padmanabhan et al. 2016). In particular, BRD4, a conserved member of the BET family of chromatin readers, has a crucial role in global RNA polymerase II (RNA-Pol II)-mediated transcription (Jang et al. 2005, Asangani et al. 2014). Inhibition of BRD4 recruitment to active chromatin results in displacement of RNA-Pol II from its target genes and eventually leads to growth inhibitory effects in CRPC xenograft models (Filippakopoulos et al. 2010, Asangani et al. 2014, 2016, Welti et al. 2018). Besides, BRD4 can physically interact with the N-terminal domain of the AR to mediate its transcriptional signaling (Yang et al. 2005, Asangani et al. 2014, Urbanucci et al. 2017). Hence, clinical trials have been initiated that investigate safety, pharmacodynamics, pharmacokinetics and clinical responses to a BET inhibitor (GSK525762) as monotherapy (Nbib1587703) or in combination with antiandrogens (Nbib3150056) in men with chemo-naïve or chemo-treated CRPC.
Different types of immunotherapy, e.g. anti-PD-L1 antibodies, are being examined in nearly all types of cancer, showing most efficacy in tumors with a high mutational load and an immunologically ‘hot’ tumor microenvironment (Hodi et al. 2010, Powles et al. 2014, Rizvi et al. 2015, Spranger 2016). However, PCa is generally characterized by a relatively ‘cold’ microenvironment with little cytotoxic T-cell infiltration (Wargo et al. 2016). Moreover, the mutational frequency is comparatively low, possibly restricting successful immunotherapy-mediated interventions to prostate tumors with deficient DNA damage repair (Sartor & de Bono 2018). Recently, Zehir et al. (2017) reported a patient case with castration- and enzalutamide-resistant PCa, who responded exceptionally well to anti-PD-L1 immunotherapy. Prospective clinical sequencing of the patient’s tumor and blood samples revealed a DNA mismatch-repair (MMR) deficiency signature in the cancerous tissue without a clear underlying somatic or germline MMR pathway lesion (Zehir et al. 2017). Hence, clinical trials are currently investigating the safety and efficacy of PD-L1 checkpoint inhibition as a monotherapy (using Avelumab) in patients with metastatic neuroendocrine-like PCa (Nbib3179410), and as a combinatorial treatment (using Atezolizumab and enzalutamide) in patients with mCRPC (Nbib3016312).
In the preclinical phase
Besides targeting the AR itself, translational research has focused over the last couple of years on finding treatment options interfering with molecules that are associated with the AR signaling pathway and thus required for proper AR action. By now, hundreds of these AR regulators and interactors have been identified, all of which could be of interest for future drug development (Heemers & Tindall 2007, Paltoglou et al. 2017, Stelloo et al. 2018). In the following, we briefly discuss therapeutic intervention strategies with promising preclinical results that target a subset of AR coregulators and thus serve as a proof of principle.
Recently, several inhibitors of the histone acetyltransferases E1A-binding protein (p300) and cAMP response element-binding protein (CREB)-binding protein (CBP) have been developed, such as GNE-049 (Jin et al. 2017), A-485 (Lasko et al. 2017) and CCS1477 (Pegg et al. 2017). CBP and p300 are two closely related and known transcriptional AR coactivators that have been suggested to play an important role in PCa progression (Debes et al. 2003, Heemers et al. 2007). In preclinical studies p300/CBP inhibitors block the AR transcriptional program and PCa cell proliferation in cell lines as well as castration-resistant xenograft models (Jin et al. 2017, Lasko et al. 2017, Pegg et al. 2017), supporting their potential clinical impact, which needs to be further validated in clinical trials. Very recently, a phase I/II trial assessing the safety and biological activity of the p300/CBP inhibitor CCS1477 as monotherapy or in combination with abiraterone or enzalutamide in mCRPC patients has been initiated (Nbib3568656).
Other AR coactivators that are currently being studied regarding their potential as therapeutic targets are the p160 steroid receptor coactivators SRC-1, SRC-2 and SRC-3 (Lonard & O’Malley 2016). SRC-1 and SRC-3 have been reported to be overexpressed in PCa cell lines and clinical specimens, where their expression levels have been associated with tumor grade and disease-specific survival (Gnanapragasam et al. 2001, Lonard & O’Malley 2016). Moreover, SRC-3 knockdown experiments in mice have shown decreased tumor growth, indicating its importance in prostate cancer proliferation and progression (Zhou et al. 2005, 2010). SRC-2 has been suggested as an PCa oncogene on the basis of integrated genomic profiling of 218 prostate tumors, illustrating SRC-2 gene amplifications, mutations or overexpression to occur in 8% of primary and 37% of metastatic PCa lesions (Taylor et al. 2010). Rather recently, a novel potent small-molecule inhibitor for SRCs (SI-2) has been developed, which is setting the stage for further (pre-)clinical validation (Song et al. 2016). Paradoxically, not only SRC inhibition, but also hyper-stimulation can be exploited to selectively induce cancer cell death and in vivo tumor growth inhibition. A high-throughput screen identified a small molecule (MCB-613), which over-activates SRC transcriptional programs, leading to excessive cellular stress in cancer cells that highly rely on proper SRC functioning (Wang et al. 2015).
While most of the above-mentioned novel therapeutic approaches represent systemic treatments, interventions that specifically interfere with acquired features in PCa and thus would limit off-target effects, are of prime interest. A fusion of the androgen-responsive transmembrane protease serine 2 (TMPRSS2) and the v-ets erythroblastosis virus E26 oncogene homolog (ERG) is found in approximately 50% of prostate cancer cases, making it the most common genetic aberration in PCa (Tomlins et al. 2005, Cancer Genome Atlas Research 2015). TMPRSS2-ERG gene fusions lead to overexpression of the usually lowly expressed ERG master transcription factor driven by the androgen-regulated promoter of TMPRSS2. This is considered an early event in PCa development and phenotypically results in increased PCa cell migration, invasion and incomplete differentiation compared to benign prostate epithelial cells due to an altered transcriptional profile (Tomlins et al. 2008, Mounir et al. 2015, Kron et al. 2017). Thus far, three preclinical approaches have been published using either a peptide-based vaccine to prime the patient’s immune system to recognize the TMPRSS2-ERG fusion as an antigen (Kissick et al. 2013); liposomal nanovectors containing TMPRSS2-ERG-specific siRNAs (Shao et al. 2012) or cell-permeable ERG inhibitory peptides that specifically block ERG-mediated transcription by interacting with its DNA-binding domain (Wang et al. 2017). However, much more preclinical validations and targeting strategies have to be explored until this therapeutic approach could potentially move to the clinic.
Although AR action remains essential in mCRPC, this is not the only targetable molecule driving this complex disease. Indeed, increasing evidence suggests that a subset of antiandrogen-resistant tumors show neuroendocrine features, which seem to be a consequence of treatment-induced adaptation of adenocarcinomas with genomic and epigenomic drivers associated with decreased AR activity and epithelial plasticity (Epstein et al. 2014, Beltran et al. 2016). Efficacy of platinum-based chemotherapy has been suggested in small-cell neuroendocrine PCa before and a trial (Nbib2208583) is currently investigating this, based on the molecular phenotype of mCRPC (Aparicio et al. 2013).
Conclusion and future perspectives
The introduction of enzalutamide as a second-line hormonal therapy for patients with mCRPC has led to significant improvements in the management of the disease. Due to tumor heterogeneity, the duration of benefit to enzalutamide interventions varies between patients. While some men do respond extremely well and continue treatment for several years, others progress rapidly as a result of treatment resistance. The increasing number of ongoing clinical trials reflects the successful preclinical advances in understanding enzalutamide resistance mechanisms and in discovering novel therapeutic targets to maximize clinical outcome. However, the disease continues to be terminal and current treatment options, including enzalutamide and its alternatives, have only a modest impact on survival, highlighting that many aspects of the disease remain poorly understood. Only by understanding which mechanisms underlie treatment resistance, robust molecular or clinical biomarkers can be developed to guide therapeutic decision-making and to identify patient subpopulations that benefit thereof mostly. That way, well thought-out therapeutic strategies can be designed, comprising optimal patient-tailored therapy sequencing and combination.
Supplementary Material
Declaration of interest
Van der Poel H G, Bergman A M and Zwart W receive grant support from Astellas Pharma.
Funding
This work is supported by grants from the Alpe d’HuZes/Dutch Cancer Society KWF (10084), Movember (NKbib1), and the Dutch Organization for scientific research NWO (91716401).
Acknowledgements
The authors would like to thank Thomas van den Broeck, Steven Joniau and Frank Claessens for their contributions and input on the initial draft of the manuscript.
References
- Aggarwal R, Halabi S, Kelly WK, George D, Mahoney JF, Millard F, Stadler WM, Morris MJ, Kantoff P, Monk JP, et al. 2013. The effect of prior androgen synthesis inhibition on outcomes of subsequent therapy with docetaxel in patients with metastatic castrate-resistant prostate cancer: results from a retrospective analysis of a randomized phase 3 clinical trial (CALGB 90401) (Alliance). Cancer 119 3636–3643. ( 10.1002/cncr.28285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, et al. 2014. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. New England Journal of Medicine 371 1028–1038. ( 10.1056/NEJMoa1315815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis ES. 2013. Enzalutamide: the emperor of all anti-androgens. Translational Andrology and Urology 2 119–120. ( 10.3978/j.issn.2223-4683.2012.09.04) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis ES, Armstrong AJ, Dehm SM, Luo J. 2016. Androgen receptor variant-driven prostate cancer: clinical implications and therapeutic targeting. Prostate Cancer and Prostatic Diseases 19 231–241. ( 10.1038/pcan.2016.17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Zhu Y, Silberstein JL, Taylor MN, Maughan BL, Denmeade SR, et al. 2017. Clinical significance of androgen receptor splice Variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. Journal of Clinical Oncology 35 2149–2156. ( 10.1200/JCO.2016.70.1961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis ES, Small EJ, Petrylak D, Quinn DI, Kibel AS, Chang N, Dearstyne E, Harmon M, Campogan D, Haynes H, et al. 2018. Antigen-specific CD8 lytic phenotype induced by Sipuleucel-T in hormone-sensitive or castration-resistant prostate cancer and association with overall survival. Clinical Cancer Research [epub]. ( 10.1158/1078-0432.CCR-18-0638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio AM, Harzstark AL, Corn PG, Wen S, Araujo JC, Tu SM, Pagliaro LC, Kim J, Millikan RE, Ryan C, et al. 2013. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clinical Cancer Research 19 3621–3630. ( 10.1158/1078-0432.CCR-12-3791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, Shah N, Cai L, Efstathiou E, Logothetis C, et al. 2013. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 155 1309–1322. ( 10.1016/j.cell.2013.11.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asangani IA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R, Escara-Wilke J, Wilder-Romans K, Dhanireddy S, Engelke C, et al. 2014. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature 510 278–282. ( 10.1038/nature13229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asangani IA, Wilder-Romans K, Dommeti VL, Krishnamurthy PM, Apel IJ, Escara-Wilke J, Plymate SR, Navone NM, Wang S, Feng FY, et al. 2016. BET bromodomain inhibitors enhance efficacy and disrupt resistance to AR antagonists in the treatment of prostate cancer. Molecular Cancer Research 14 324–331. ( 10.1158/1541-7786.MCR-15-0472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard G, Antonarakis ES. 2016. Prostate cancer: AR aberrations and resistance to abiraterone or enzalutamide. Nature Reviews. Urology 13 697–698. ( 10.1038/nrurol.2016.212) [DOI] [PubMed] [Google Scholar]
- Attard G, Reid AH, A’Hern R, Parker C, Oommen NB, Folkerd E, Messiou C, Molife LR, Maier G, Thompson E, et al. 2009. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. Journal of Clinical Oncology 27 3742–3748. ( 10.1200/JCO.2008.20.0642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad AA, Volik SV, Wyatt AW, Haegert A, Le Bihan S, Bell RH, Anderson SA, McConeghy B, Shukin R, Bazov J, et al. 2015. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clinical Cancer Research 21 2315–2324. ( 10.1158/1078-0432.CCR-14-2666) [DOI] [PubMed] [Google Scholar]
- Badrising SK, van der Noort V, Hamberg P, Coenen JL, Aarts MJ, van Oort IM, van den Eertwegh AJ, Los M, van den Berg HP, Gelderblom H, et al. 2016a. Enzalutamide as a fourth- or fifth-line treatment option for metastatic castration-resistant prostate cancer. Oncology 91 267–273. ( 10.1159/000448219) [DOI] [PubMed] [Google Scholar]
- Badrising SK, van der Noort V, van den Eertwegh AJ, Hamberg P, van Oort IM, van den Berg HP, Los M, Aarts MJ, Coenen JL, Gelderblom H, et al. 2016b. Prognostic parameters for response to enzalutamide after docetaxel and abiraterone treatment in metastatic castration-resistant prostate cancer patients; a possible time relation. Prostate 76 32–40. ( 10.1002/pros.23094) [DOI] [PubMed] [Google Scholar]
- Bahl A, Oudard S, Tombal B, Ozguroglu M, Hansen S, Kocak I, Gravis G, Devin J, Shen L, de Bono JS, et al. 2013. Impact of cabazitaxel on 2-year survival and palliation of tumour-related pain in men with metastatic castration-resistant prostate cancer treated in the tropic trial. Annals of Oncology 24 2402–2408. ( 10.1093/annonc/mdt194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambury RM, Scher HI. 2015. Enzalutamide: development from bench to bedside. Urologic Oncology 33 280–288. ( 10.1016/j.urolonc.2014.12.017) [DOI] [PubMed] [Google Scholar]
- Beer TM, Pierce WC, Lowe BA, Henner WD. 2001. Phase II study of weekly docetaxel in symptomatic androgen-independent prostate cancer. Annals of Oncology 12 1273–1279. ( 10.1023/A:1012258723075) [DOI] [PubMed] [Google Scholar]
- Beer TM, Garzotto M, Henner WD, Eilers KM, Wersinger EM. 2004. Multiple cycles of intermittent chemotherapy in metastatic androgen-independent prostate cancer. British Journal of Cancer 91 1425–1427. ( 10.1038/sj.bjc.6602198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, et al. 2014. Enzalutamide in metastatic prostate cancer before chemotherapy. New England Journal of Medicine 371 424–433. ( 10.1056/NEJMoa1405095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer TM, Armstrong AJ, Rathkopf D, Loriot Y, Sternberg CN, Higano CS, Iversen P, Evans CP, Kim CS, Kimura G, et al. 2017. Enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer: extended analysis of the Phase 3 PREVAIL Study. European Urology 71 151–154. ( 10.1016/j.eururo.2016.07.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, Marotz C, Giannopoulou E, Chakravarthi BV, Varambally S, et al. 2016. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nature Medicine 22 298–305. ( 10.1038/nm.4045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RL, Licht JD. 2018. Targeting epigenetics in cancer. Annual Review of Pharmacology and Toxicology 58 187–207. ( 10.1146/annurev-pharmtox-010716-105106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry W, Dakhil S, Gregurich MA, Asmar L. 2001. Phase II trial of single-agent weekly docetaxel in hormone-refractory, symptomatic, metastatic carcinoma of the prostate. Seminars in Oncology 28 8–15. [DOI] [PubMed] [Google Scholar]
- Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. 2008. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. Journal of Clinical Oncology 26 242–245. ( 10.1200/JCO.2007.12.4008) [DOI] [PubMed] [Google Scholar]
- Bianchini D, Lorente D, Rodriguez-Vida A, Omlin A, Pezaro C, Ferraldeschi R, Zivi A, Attard G, Chowdhury S, de Bono JS. 2014. Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone. European Journal of Cancer 50 78–84. ( 10.1016/j.ejca.2013.08.020) [DOI] [PubMed] [Google Scholar]
- Boyd LK, Mao X, Lu YJ. 2012. The complexity of prostate cancer: genomic alterations and heterogeneity. Nature Reviews. Urology 9 652–664. ( 10.1038/nrurol.2012.185) [DOI] [PubMed] [Google Scholar]
- Brasso K, Thomsen FB, Schrader AJ, Schmid SC, Lorente D, Retz M, Merseburger AS, von Klot CA, Boegemann M, de Bono J. 2015. Enzalutamide antitumour activity against metastatic castration-resistant prostate cancer previously treated with docetaxel and abiraterone: a multicentre analysis. European Urology 68 317–324. ( 10.1016/j.eururo.2014.07.028) [DOI] [PubMed] [Google Scholar]
- Brinkmann AO, Blok LJ, de Ruiter PE, Doesburg P, Steketee K, Berrevoets CA, Trapman J. 1999. Mechanisms of androgen receptor activation and function. Journal of Steroid Biochemistry and Molecular Biology 69 307–313. [DOI] [PubMed] [Google Scholar]
- Bruland OS, Nilsson S, Fisher DR, Larsen RH. 2006. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clinical Cancer Research 12 6250s–6257s. ( 10.1158/1078-0432.CCR-06-0841) [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research N 2015. The molecular taxonomy of primary prostate cancer. Cell 163 1011–1025. ( 10.1016/j.cell.2015.10.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. 2004. Molecular determinants of resistance to antiandrogen therapy. Nature Medicine 10 33–39. ( 10.1038/nm972) [DOI] [PubMed] [Google Scholar]
- Cheng HH, Gulati R, Azad A, Nadal R, Twardowski P, Vaishampayan UN, Agarwal N, Heath EI, Pal SK, Rehman HT, et al. 2015. Activity of enzalutamide in men with metastatic castration-resistant prostate cancer is affected by prior treatment with abiraterone and/or docetaxel. Prostate Cancer and Prostatic Diseases 18 122–127. ( 10.1038/pcan.2014.53) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi KN, Annala M, Sunderland K, Khalaf D, Finch D, Oja CD, Vergidis J, Zulfiqar M, Beja K, Vandekerkhove G, et al. 2017. A randomized phase II cross-over study of abiraterone + prednisone (ABI) vs enzalutamide (ENZ) for patients (pts) with metastatic, castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology 35 5002 ( 10.1200/JCO.2017.35.15_suppl.5002) [DOI] [Google Scholar]
- Claessens F, Helsen C, Prekovic S, Van den Broeck T, Spans L, Van Poppel H, Joniau S. 2014. Emerging mechanisms of enzalutamide resistance in prostate cancer. Nature Reviews. Urology 11 712–716. ( 10.1038/nrurol.2014.243) [DOI] [PubMed] [Google Scholar]
- Conteduca V, Wetterskog D, Sharabiani MTA, Grande E, Fernandez-Perez MP, Jayaram A, Salvi S, Castellano D, Romanel A, Lolli C, et al. 2017. Androgen receptor gene status in plasma DNA associates with worse outcome on enzalutamide or abiraterone for castration-resistant prostate cancer: a multi-institution correlative biomarker study. Annals of Oncology 28 1508–1516. ( 10.1093/annonc/mdx155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho I, Day TK, Tilley WD, Selth LA. 2016. Androgen receptor signaling in castration-resistant prostate cancer: a lesson in persistence. Endocrine-Related Cancer 23 T179–T197. ( 10.1530/ERC-16-0422) [DOI] [PubMed] [Google Scholar]
- Cui Y, Nadiminty N, Liu C, Lou W, Schwartz CT, Gao AC. 2014. Upregulation of glucose metabolism by NF-kappaB2/p52 mediates enzalutamide resistance in castration-resistant prostate cancer cells. Endocrine-Related Cancer 21 435–442. ( 10.1530/ERC-14-0107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, et al. 1998. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280 969–974. ( 10.1001/jama.280.11.969) [DOI] [PubMed] [Google Scholar]
- Darshan MS, Loftus MS, Thadani-Mulero M, Levy BP, Escuin D, Zhou XK, Gjyrezi A, Chanel-Vos C, Shen R, Tagawa ST, et al. 2011. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Research 71 6019–6029. ( 10.1158/0008-5472.CAN-11-1417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, et al. 2010. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376 1147–1154. ( 10.1016/S0140-6736(10)61389-X) [DOI] [PubMed] [Google Scholar]
- De Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr, Saad F. 2011. Abiraterone and increased survival in metastatic prostate cancer. New England Journal of Medicine 364 1995–2005. ( 10.1056/NEJMoa1014618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono JS, Chowdhury S, Feyerabend S, Elliott T, Grande E, Melhem-Bertrandt A, Baron B, Hirmand M, Werbrouck P, Fizazi K. 2018. Antitumour activity and safety of enzalutamide in patients with metastatic castration-resistant prostate cancer previously treated with abiraterone acetate plus prednisone for ≥24 weeks in Europe. European Urology 74 37–45. ( 10.1016/j.eururo.2017.07.035) [DOI] [PubMed] [Google Scholar]
- Debes JD, Sebo TJ, Lohse CM, Murphy LM, Haugen DA, Tindall DJ. 2003. p300 in prostate cancer proliferation and progression. Cancer Research 63 7638–7640. [PubMed] [Google Scholar]
- Del Re M, Biasco E, Crucitta S, Derosa L, Rofi E, Orlandini C, Miccoli M, Galli L, Falcone A, Jenster GW, et al. 2017. The detection of androgen receptor splice variant 7 in plasma-derived exosomal RNA strongly predicts resistance to hormonal therapy in metastatic prostate cancer patients. European Urology 71 680–687. ( 10.1016/j.eururo.2016.08.012) [DOI] [PubMed] [Google Scholar]
- Dellis AE, Papatsoris AG. 2018. Apalutamide: the established and emerging roles in the treatment of advanced prostate cancer. Expert Opinion on Investigational Drugs 27 553–559. ( 10.1080/13543784.2018.1484107) [DOI] [PubMed] [Google Scholar]
- Deshayes E, Roumiguie M, Thibault C, Beuzeboc P, Cachin F, Hennequin C, Huglo D, Rozet F, Kassab-Chahmi D, Rebillard X, et al. 2017. Radium 223 dichloride for prostate cancer treatment. Drug Design, Development and Therapy 11 2643–2651. ( 10.2147/DDDT.S122417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo G, Buonerba C, Kantoff PW. 2011. Immunotherapy for the treatment of prostate cancer. Nature Reviews. Clinical Oncology 8 551–561. ( 10.1038/nrclinonc.2011.72) [DOI] [PubMed] [Google Scholar]
- Diaz LA, Jr, Bardelli A. 2014. Liquid biopsies: genotyping circulating tumor DNA. Journal of Clinical Oncology 32 579–586. ( 10.1200/JCO.2012.45.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JI, Amin MB, Beltran H, Lotan TL, Mosquera JM, Reuter VE, Robinson BD, Troncoso P, Rubin MA. 2014. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. American Journal of Surgical Pathology 38 756–767. ( 10.1097/PAS.0000000000000208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, et al. 2010. Selective inhibition of BET bromodomains. Nature 468 1067–1073. ( 10.1038/nature09504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick JM, de Wit R. 2014. Taxane mechanisms of action: potential implications for treatment sequencing in metastatic castration-resistant prostate cancer. European Urology 65 1198–1204. ( 10.1016/j.eururo.2013.07.022) [DOI] [PubMed] [Google Scholar]
- Fitzpatrick JM, Bellmunt J, Fizazi K, Heidenreich A, Sternberg CN, Tombal B, Alcaraz A, Bahl A, Bracarda S, Di Lorenzo G, et al. 2014. Optimal management of metastatic castration-resistant prostate cancer: highlights from a European Expert Consensus Panel. European Journal of Cancer 50 1617–1627. ( 10.1016/j.ejca.2014.03.010) [DOI] [PubMed] [Google Scholar]
- Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ, Saad F, et al. 2012. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncology 13 983–992. ( 10.1016/S1470-2045(12)70379-0) [DOI] [PubMed] [Google Scholar]
- Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Ozguroglu M, Ye D, Feyerabend S, Protheroe A, et al. 2017. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. New England Journal of Medicine 377 352–360. ( 10.1056/NEJMoa1704174) [DOI] [PubMed] [Google Scholar]
- Fuerea A, Baciarello G, Patrikidou A, Albiges L, Massard C, Di Palma M, Escudier B, Fizazi K, Loriot Y. 2016. Early PSA response is an independent prognostic factor in patients with metastatic castration-resistant prostate cancer treated with next-generation androgen pathway inhibitors. European Journal of Cancer 61 44–51. ( 10.1016/j.ejca.2016.03.070) [DOI] [PubMed] [Google Scholar]
- Gillessen S, Attard G, Beer TM, Beltran H, Bossi A, Bristow R, Carver B, Castellano D, Chung BH, Clarke N, et al. 2018a. Management of patients with advanced prostate cancer: the report of the advanced prostate cancer consensus conference APCCC 2017. European Urology 73 178–211. ( 10.1016/j.eururo.2017.06.002) [DOI] [PubMed] [Google Scholar]
- Gillessen S, de Bono JS, Sartor O, Omlin AG. 2018b. Reply to Finn E Von Eyben, Irene Virgolini and Giandomenico Roviello’s Letter to the Editor Re: Gillessen S, Attard G, Beer TM, Beltran H, Bossi A, Bristow R, Carver B, Castellano D, Chung BH, Clarke N, et al. 2018 Management of patients with advanced prostate cancer: the report of the advanced prostate cancer consensus conference APCCC 2017 2017. Eur Urol 2018;73:178-211. European Urology 73 e32–e33. ( 10.1016/j.eururo.2017.06.002) [DOI] [PubMed] [Google Scholar]
- Gnanapragasam VJ, Leung HY, Pulimood AS, Neal DE, Robson CN. 2001. Expression of RAC 3, a steroid hormone receptor co-activator in prostate cancer. British Journal of Cancer 85 1928–1936. ( 10.1054/bjoc.2001.2179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann ME, Huang H, Tindall DJ. 2001. Androgen receptor signaling in androgen-refractory prostate cancer. Journal of the National Cancer Institute 93 1687–1697. ( 10.1093/jnci/93.22.1687) [DOI] [PubMed] [Google Scholar]
- Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, et al. 2009. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Research 69 2305–2313. ( 10.1158/0008-5472.CAN-08-3795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid AR, Pfeiffer MJ, Verhaegh GW, Schaafsma E, Brandt A, Sweep FC, Sedelaar JP, Schalken JA. 2013. Aldo-keto reductase family 1 member C3 (AKR1C3) is a biomarker and therapeutic target for castration-resistant prostate cancer. Molekuliarna Meditsina 18 1449–1455. ( 10.2119/molmed.2012.00296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handy CE, Antonarakis ES. 2018. Sipuleucel-T for the treatment of prostate cancer: novel insights and future directions. Future Oncology 14 907–917. ( 10.2217/fon-2017-0531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WP, Mostaghel EA, Nelson PS, Montgomery B. 2009. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nature Clinical Practice. Urology 6 76–85. ( 10.1038/ncpuro1296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemers HV, Tindall DJ. 2007. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocrine Reviews 28 778–808. ( 10.1210/er.2007-0019) [DOI] [PubMed] [Google Scholar]
- Heemers HV, Sebo TJ, Debes JD, Regan KM, Raclaw KA, Murphy LM, Hobisch A, Culig Z, Tindall DJ. 2007. Androgen deprivation increases p300 expression in prostate cancer cells. Cancer Research 67 3422–3430. ( 10.1158/0008-5472.CAN-06-2836) [DOI] [PubMed] [Google Scholar]
- Heller G, Fizazi K, McCormack R, Molina A, MacLean D, Webb IJ, Saad F, de Bono JS, Scher HI. 2017. The added value of circulating tumor cell enumeration to standard markers in assessing prognosis in a metastatic castration-resistant prostate cancer population. Clinical Cancer Research 23 1967–1973. ( 10.1158/1078-0432.CCR-16-1224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsen C, Van den Broeck T, Voet A, Prekovic S, Van Poppel H, Joniau S, Claessens F. 2014. Androgen receptor antagonists for prostate cancer therapy. Endocrine-Related Cancer 21 T105–T118. ( 10.1530/ERC-13-0545) [DOI] [PubMed] [Google Scholar]
- Henriksen G, Breistol K, Bruland OS, Fodstad O, Larsen RH. 2002. Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Research 62 3120–3125. [PubMed] [Google Scholar]
- Higano CS, Small EJ, Schellhammer P, Yasothan U, Gubernick S, Kirkpatrick P, Kantoff PW. 2010. Sipuleucel-T. Nature Reviews. Drug Discovery 9 513–514. ( 10.1038/nrd3220) [DOI] [PubMed] [Google Scholar]
- Higano CS, Beer TM, Taplin ME, Efstathiou E, Hirmand M, Forer D, Scher HI. 2015. Long-term safety and antitumor activity in the Phase 1–2 study of enzalutamide in pre- and post-docetaxel castration-resistant prostate cancer. European Urology 68 795–801. ( 10.1016/j.eururo.2015.01.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine 363 711–723. ( 10.1056/NEJMoa1003466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, et al. 2009. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Research 69 16–22. ( 10.1158/0008-5472.CAN-08-2764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M, Wolf M, Marshall E, Crawford ED, Eisenberger M. 1994. Effects of continued androgen-deprivation therapy and other prognostic factors on response and survival in phase II chemotherapy trials for hormone-refractory prostate cancer: a Southwest Oncology Group report. Journal of Clinical Oncology 12 1868–1875. ( 10.1200/JCO.1994.12.9.1868) [DOI] [PubMed] [Google Scholar]
- Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, Ivashchenko P, Demirhan E, Modelska K, Phung, et al. 2018. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. New England Journal of Medicine 378 2465–2474. ( 10.1056/NEJMoa1800536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y, Sadar MD. 2016. Androgen receptor targeted therapies in castration-resistant prostate cancer: bench to clinic. International Journal of Urology 23 654–665. ( 10.1111/iju.13137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irshad S, Bansal M, Castillo-Martin M, Zheng T, Aytes A, Wenske S, Le Magnen C, Guarnieri P, Sumazin P, Benson MC, et al. 2013. A molecular signature predictive of indolent prostate cancer. Science Translational Medicine 5 202ra122 ( 10.1126/scitranslmed.3006408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itkonen H, Mills IG. 2012. Chromatin binding by the androgen receptor in prostate cancer. Molecular and Cellular Endocrinology 360 44–51. ( 10.1016/j.mce.2011.09.037) [DOI] [PubMed] [Google Scholar]
- Itkonen HM, Gorad SS, Duveau DY, Martin SE, Barkovskaya A, Bathen TF, Moestue SA, Mills IG. 2016. Inhibition of O-GlcNAc transferase activity reprograms prostate cancer cell metabolism. Oncotarget 7 12464–12476. ( 10.18632/oncotarget.7039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi K, Mizokami A, Namiki M, Inoue S, Tanaka N, Yoshio Y, Ishibashi K, Kamiyama M, Kawai N, Enokida H, et al. 2017. Enzalutamide versus abiraterone as a first-line endocrine therapy for castration-resistant prostate cancer (ENABLE study for PCa): a study protocol for a multicenter randomized phase III trial. BMC Cancer 17 677 ( 10.1186/s12885-017-3661-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, Ritchie AW, Parker CC, Russell JM, Attard G, et al. 2016. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387 1163–1177. ( 10.1016/S0140-6736(15)01037-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, Ritchie AWS, Amos CL, Gilson C, Jones RJ, et al. 2017. Abiraterone for prostate cancer not previously treated with hormone therapy. New England Journal of Medicine 377 338–351. ( 10.1056/NEJMoa1702900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. 2005. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Molecular Cell 19 523–534. ( 10.1016/j.molcel.2005.06.027) [DOI] [PubMed] [Google Scholar]
- Jin L, Garcia J, Chan E, de la Cruz C, Segal E, Merchant M, Kharbanda S, Raisner R, Haverty PM, Modrusan Z, et al. 2017. Therapeutic targeting of the CBP/p300 bromodomain blocks the growth of castration-resistant prostate cancer. Cancer Research 77 5564–5575. ( 10.1158/0008-5472.CAN-17-0314) [DOI] [PubMed] [Google Scholar]
- Kach J, Conzen SD, Szmulewitz RZ. 2015. Targeting the glucocorticoid receptor in breast and prostate cancers. Science Translational Medicine 7 305ps319 ( 10.1126/scitranslmed.aac7531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. 2010. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New England Journal of Medicine 363 411–422. ( 10.1056/NEJMoa1001294) [DOI] [PubMed] [Google Scholar]
- Karantanos T, Corn PG, Thompson TC. 2013. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 32 5501–5511. ( 10.1038/onc.2013.206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissick HT, Sanda MG, Dunn LK, Arredouani MS. 2013. Development of a peptide-based vaccine targeting TMPRSS2:ERG fusion-positive prostate cancer. Cancer Immunology Immunotherapy 62 1831–1840. ( 10.1007/s00262-013-1482-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezevic D, Goddard AD, Natraj N, Cherbavaz DB, Clark-Langone KM, Snable J, Watson D, Falzarano SM, Magi-Galluzzi C, Klein EA, et al. 2013. Analytical validation of the Oncotype DX prostate cancer assay – a clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genomics 14 690 ( 10.1186/1471-2164-14-690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron KJ, Murison A, Zhou S, Huang V, Yamaguchi TN, Shiah YJ, Fraser M, van der Kwast T, Boutros PC, Bristow RG, et al. 2017. TMPRSS2-ERG fusion co-opts master transcription factors and activates NOTCH signaling in primary prostate cancer. Nature Genetics 49 1336–1345. ( 10.1038/ng.3930) [DOI] [PubMed] [Google Scholar]
- Kumar A, Coleman I, Morrissey C, Zhang X, True LD, Gulati R, Etzioni R, Bolouri H, Montgomery B, White T, et al. 2016. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nature Medicine 22 369–378. ( 10.1038/nm.4053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K, Liu H, Kim S, Guo M, Navarro V, Bander NH. 2009. Docetaxel down-regulates the expression of androgen receptor and prostate-specific antigen but not prostate-specific membrane antigen in prostate cancer cell lines: implications for PSA surrogacy. Prostate 69 1579–1585. ( 10.1002/pros.21004) [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, Shevrin DH, Dreicer R, Hussain M, Eisenberger M, et al. 2018. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized Phase III E3805 CHAARTED trial. Journal of Clinical Oncology 36 1080–1087. ( 10.1200/JCO.2017.75.3657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallous N, Volik SV, Awrey S, Leblanc E, Tse R, Murillo J, Singh K, Azad AA, Wyatt AW, LeBihan S, et al. 2016. Functional analysis of androgen receptor mutations that confer anti-androgen resistance identified in circulating cell-free DNA from prostate cancer patients. Genome Biology 17 10 ( 10.1186/s13059-015-0864-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasko LM, Jakob CG, Edalji RP, Qiu W, Montgomery D, Digiammarino EL, Hansen TM, Risi RM, Frey R, Manaves V, et al. 2017. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature 550 128–132. ( 10.1038/nature24028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Ha S, Logan SK. 2015. Divergent androgen receptor and beta-catenin signaling in prostate cancer cells. PLoS ONE 10 e141589 ( 10.1371/journal.pone.0141589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LC, Carroll PR, Dahiya R. 2005. Epigenetic changes in prostate cancer: implication for diagnosis and treatment. Journal of the National Cancer Institute 97 103–115. ( 10.1093/jnci/djbib10) [DOI] [PubMed] [Google Scholar]
- Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. 2013. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Research 73 483–489. ( 10.1158/0008-5472.CAN-12-3630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Bishop AC, Alyamani M, Garcia JA, Dreicer R, Bunch D, Liu J, Upadhyay SK, Auchus RJ, Sharifi N. 2015. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature 523 347–351. ( 10.1038/nature14406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Alyamani M, Zhang A, Chang KH, Berk M, Li Z, Zhu Z, Petro M, Magi-Galluzzi C, Taplin ME, et al. 2017. Aberrant corticosteroid metabolism in tumor cells enables GR takeover in enzalutamide resistant prostate cancer. eLife 6 e20183 ( 10.7554/eLife.20183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Lou W, Zhu Y, Nadiminty N, Schwartz CT, Evans CP, Gao AC. 2014a. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clinical Cancer Research 20 3198–3210. ( 10.1158/1078-0432.CCR-13-3296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhu Y, Lou W, Cui Y, Evans CP, Gao AC. 2014b. Inhibition of constitutively active Stat3 reverses enzalutamide resistance in LNCaP derivative prostate cancer cells. Prostate 74 201–209. ( 10.1002/pros.22741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Lou W, Zhu Y, Yang JC, Nadiminty N, Gaikwad NW, Evans CP, Gao AC. 2015. Intracrine androgens and AKR1C3 activation confer resistance to enzalutamide in prostate cancer. Cancer Research 75 1413–1422. ( 10.1158/0008-5472.CAN-14-3080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Armstrong CM, Lou W, Lombard A, Evans CP, Gao AC. 2017. Inhibition of AKR1C3 activation overcomes resistance to abiraterone in advanced prostate cancer. Molecular Cancer Therapeutics 16 35–44. ( 10.1158/1535-7163.MCT-16-0186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, Ettinger SL, Gleave ME, Nelson CC. 2008. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Research 68 6407–6415. ( 10.1158/0008-5472.CAN-07-5997) [DOI] [PubMed] [Google Scholar]
- Lonard DM, O’Malley BW. 2016. Molecular pathways: targeting steroid receptor coactivators in cancer. Clinical Cancer Research 22 5403–5407. ( 10.1158/1078-0432.CCR-15-1958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente D, Olmos D, Mateo J, Bianchini D, Seed G, Fleisher M, Danila DC, Flohr P, Crespo M, Figueiredo I, et al. 2016. Decline in circulating tumor cell count and treatment outcome in advanced prostate cancer. European Urology 70 985–992. ( 10.1016/j.eururo.2016.05.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loriot Y, Bianchini D, Ileana E, Sandhu S, Patrikidou A, Pezaro C, Albiges L, Attard G, Fizazi K, De Bono JS, et al. 2013. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Annals of Oncology 24 1807–1812. ( 10.1093/annonc/mdt136) [DOI] [PubMed] [Google Scholar]
- Marignol L, Coffey M, Lawler M, Hollywood D. 2008. Hypoxia in prostate cancer: a powerful shield against tumour destruction? Cancer Treatment Reviews 34 313–327. ( 10.1016/j.ctrv.2008.01.006) [DOI] [PubMed] [Google Scholar]
- Massard C, Fizazi K. 2011. Targeting continued androgen receptor signaling in prostate cancer. Clinical Cancer Research 17 3876–3883. ( 10.1158/1078-0432.CCR-10-2815) [DOI] [PubMed] [Google Scholar]
- Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N, et al. 2015. DNA-repair defects and olaparib in metastatic prostate cancer. New England Journal of Medicine 373 1697–1708. ( 10.1056/NEJMoa1506859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merseburger AS, Haas GP, von Klot CA. 2015. An update on enzalutamide in the treatment of prostate cancer. Therapeutic Advances in Urology 7 9–21. ( 10.1177/1756287214555336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merseburger AS, Alcaraz A, von Klot CA. 2016. Androgen deprivation therapy as backbone therapy in the management of prostate cancer. Onco Targets and Therapy 9 7263–7274. ( 10.2147/OTT.S117176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger E, Wissmann M, Yin N, Muller JM, Schneider R, Peters AH, Gunther T, Buettner R, Schule R. 2005. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 437 436–439. ( 10.1038/nature04020) [DOI] [PubMed] [Google Scholar]
- Mezynski J, Pezaro C, Bianchini D, Zivi A, Sandhu S, Thompson E, Hunt J, Sheridan E, Baikady B, Sarvadikar A, et al. 2012. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Annals of Oncology 23 2943–2947. ( 10.1093/annonc/mds119) [DOI] [PubMed] [Google Scholar]
- Mills IG. 2014. Maintaining and reprogramming genomic androgen receptor activity in prostate cancer. Nature Reviews. Cancer 14 187–198. ( 10.1038/nrc3678) [DOI] [PubMed] [Google Scholar]
- Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. 2008. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Research 68 4447–4454. ( 10.1158/0008-5472.CAN-08-0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MJ, Rathkopf DE, Novotny W, Gibbons JA, Peterson AC, Khondker Z, Ouatas T, Scher HI, Fleming MT. 2016. Phase Ib study of enzalutamide in combination with docetaxel in men with metastatic castration-resistant prostate cancer. Clinical Cancer Research 22 3774–3781. ( 10.1158/1078-0432.CCR-15-2638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounir Z, Lin F, Lin VG, Korn JM, Yu Y, Valdez R, Aina OH, Buchwalter G, Jaffe AB, Korpal M, et al. 2015. TMPRSS2:ERG blocks neuroendocrine and luminal cell differentiation to maintain prostate cancer proliferation. Oncogene 34 3815–3825. ( 10.1038/onc.2014.308) [DOI] [PubMed] [Google Scholar]
- Nadal M, Prekovic S, Gallastegui N, Helsen C, Abella M, Zielinska K, Gay M, Vilaseca M, Taules M, Houtsmuller AB, et al. 2017. Structure of the homodimeric androgen receptor ligand-binding domain. Nature Communications 8 14388 ( 10.1038/ncomms14388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HG, Yang JC, Kung HJ, Shi XB, Tilki D, Lara PN, Jr, DeVere White RW, Gao AC, Evans CP. 2014. Targeting autophagy overcomes Enzalutamide resistance in castration-resistant prostate cancer cells and improves therapeutic response in a xenograft model. Oncogene 33 4521–4530. ( 10.1038/onc.2014.25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss JL. 2009. Targeting DNA topoisomerase II in cancer chemotherapy. Nature Reviews. Cancer 9 338–350. ( 10.1038/nrc2607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan KL, North S, Bitting RL, Armstrong AJ, Ellard SL, Chi KN. 2013. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Annals of Oncology 24 1802–1807. ( 10.1093/annonc/mdt138) [DOI] [PubMed] [Google Scholar]
- Oesterling JE. 1991. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. Journal of Urology 145 907–923. ( 10.1016/S0022-5347(17)38491-4) [DOI] [PubMed] [Google Scholar]
- Padmanabhan B, Mathur S, Manjula R, Tripathi S. 2016. Bromodomain and extra-terminal (BET) family proteins: new therapeutic targets in major diseases. Journal of Bio-Science 41 295–311. ( 10.1007/s12038-016-9600-6) [DOI] [PubMed] [Google Scholar]
- Pal SK, Patel J, He M, Foulk B, Kraft K, Smirnov DA, Twardowski P, Kortylewski M, Bhargava V, Jones JO. 2018. Identification of mechanisms of resistance to treatment with abiraterone acetate or enzalutamide in patients with castration-resistant prostate cancer (CRPC). Cancer 124 1216–1224. ( 10.1002/cncr.31161) [DOI] [PubMed] [Google Scholar]
- Paller CJ, Antonarakis ES. 2011. Cabazitaxel: a novel second-line treatment for metastatic castration-resistant prostate cancer. Drug Design, Development and Therapy 5 117–124. ( 10.2147/DDDT.S13029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltoglou S, Das R, Townley SL, Hickey TE, Tarulli GA, Coutinho I, Fernandes R, Hanson AR, Denis I, Carroll JS, et al. 2017. Novel androgen receptor coregulator GRHL2 exerts both oncogenic and antimetastatic functions in prostate cancer. Cancer Research 77 3417–3430. ( 10.1158/0008-5472.CAN-16-1616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C-x, Lara P, Evans CP, Parikh M, de Vere White R, Dall’era M, Liu C, Robles D, Gao A. 2018. A phase Ib/II trial of indomethacin and enzalutamide to treat castration-resistant prostate cancer (CRPC). Journal of Clinical Oncology 36 ( 10.1200/JCO.2018.36.6_suppl.TPS394) [DOI] [Google Scholar]
- Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, Chodacki A, Wiechno P, Logue J, Seke M, et al. 2013. Alpha emitter radium-223 and survival in metastatic prostate cancer. New England Journal of Medicine 369 213–223. ( 10.1056/NEJMoa1213755) [DOI] [PubMed] [Google Scholar]
- Pegg N, Brooks N, Worthington J, Young B, Prosser A, Jordan L, Taddei D, Brown R, Harbottle G, Shannon J, et al. 2017. Characterisation of CCS1477: a novel small molecule inhibitor of p300/CBP for the treatment of castration resistant prostate cancer. Journal of Clinical Oncology 35 11590–11590. ( 10.1200/JCO.2017.35.15_suppl.11590) [DOI] [Google Scholar]
- Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, Barni S. 2015. Enzalutamide after docetaxel and abiraterone acetate treatment in prostate cancer: a pooled analysis of 10 case series. Clinical Genitourinary Cancer 13 193–198. ( 10.1016/j.clgc.2014.10.006) [DOI] [PubMed] [Google Scholar]
- Petrylak DP, Drake CG, Pieczonka CM, Corman JM, Garcia JA, Dunshee C, Van Mouwerik T, Tyler RC, Chang NN, Quinn D. 2018. Overall survival and immune responses with sipuleucel-T and enzalutamide: STRIDE study. Journal of Clinical Oncology 36 246–246. ( 10.1200/JCO.2018.36.6_suppl.246) [DOI] [Google Scholar]
- Pfeiffer MJ, Smit FP, Sedelaar JP, Schalken JA. 2011. Steroidogenic enzymes and stem cell markers are upregulated during androgen deprivation in prostate cancer. Molekuliarna Meditsina 17 657–664. ( 10.2119/molmed.2010.00143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y, Leo E, Zhang H, Marchand C. 2010. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chemico-Biological 17 421–433. ( 10.1016/j.chembiol.2010.04.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et al. 2014. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515 558–562. ( 10.1038/nature13904) [DOI] [PubMed] [Google Scholar]
- Prekovic S, van Royen ME, Voet AR, Geverts B, Houtman R, Melchers D, Zhang KY, Van den Broeck T, Smeets E, Spans L, et al. 2016. The effect of F877L and T878A mutations on androgen receptor response to enzalutamide. Molecular Cancer Therapeutics 15 1702–1712. ( 10.1158/1535-7163.MCT-15-0892) [DOI] [PubMed] [Google Scholar]
- Prekovic S, Van den Broeck T, Moris L, Smeets E, Claessens F, Joniau S, Helsen C, Attard G. 2018a. Treatment-induced changes in the androgen receptor axis: liquid biopsies as diagnostic/prognostic tools for prostate cancer. Molecular and Cellular Endocrinology 462 56–63. ( 10.1016/j.mce.2017.08.020) [DOI] [PubMed] [Google Scholar]
- Prekovic S, Van den Broeck T, Linder S, van Royen ME, Houtsmuller AB, Handle F, Joniau S, Zwart W, Claessens F. 2018b. Molecular underpinnings of enzalutamide resistance. Endocrine-Related Cancer 25 R545–R557. ( 10.1530/ERC-17-0136) [DOI] [PubMed] [Google Scholar]
- Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, Garofalo A, Gulati R, Carreira S, Eeles R, et al. 2016. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. New England Journal of Medicine 375 443–453. ( 10.1056/NEJMoa1603144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhr M, Hoefer J, Eigentler A, Ploner C, Handle F, Schaefer G, Kroon J, Leo A, Heidegger I, Eder I, et al. 2018. The glucocorticoid receptor is a key player for prostate cancer cell survival and a target for improved antiandrogen therapy. Clinical Cancer Research 24 927–938. ( 10.1158/1078-0432.CCR-17-0989) [DOI] [PubMed] [Google Scholar]
- Qu F, Xie W, Nakabayashi M, Zhang H, Jeong SH, Wang X, Komura K, Sweeney CJ, Sartor O, Lee GM, et al. 2017. Association of AR-V7 and prostate-specific antigen RNA levels in blood with efficacy of abiraterone acetate and enzalutamide treatment in men with prostate cancer. Clinical Cancer Research 23 726–734. ( 10.1158/1078-0432.CCR-16-1070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn DI, Sandler HM, Horvath LG, Goldkorn A, Eastham JA. 2017. The evolution of chemotherapy for the treatment of prostate cancer. Annals of Oncology 28 2658–2669. ( 10.1093/annonc/mdx348) [DOI] [PubMed] [Google Scholar]
- Ranganathan S, Benetatos CA, Colarusso PJ, Dexter DW, Hudes GR. 1998. Altered beta-tubulin isotype expression in paclitaxel-resistant human prostate carcinoma cells. British Journal of Cancer 77 562–566. ( 10.1038/bjc.1998.91) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathkopf DE, Smith MR, Ryan CJ, Berry WR, Shore ND, Liu G, Higano CS, Alumkal JJ, Hauke R, Tutrone RF, et al. 2017. Androgen receptor mutations in patients with castration-resistant prostate cancer treated with apalutamide. Annals of Oncology 28 2264–2271. ( 10.1093/annonc/mdx283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno P, Lorente D, Bianchini D, Ferraldeschi R, Kolinsky MP, Sideris S, Zafeiriou Z, Sumanasuriya S, Smith AD, Mehra N, et al. 2016. Prostate-specific antigen decline after 4 weeks of treatment with abiraterone acetate and overall survival in patients with metastatic castration-resistant prostate cancer. European Urology 70 724–731. ( 10.1016/j.eururo.2016.02.055) [DOI] [PubMed] [Google Scholar]
- Richards J, Lim AC, Hay CW, Taylor AE, Wingate A, Nowakowska K, Pezaro C, Carreira S, Goodall J, Arlt W, et al. 2012. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Research 72 2176–2182. ( 10.1158/0008-5472.CAN-11-3980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. 2015. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348 124–128. ( 10.1126/science.aaa1348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, et al. 2015. Integrative clinical genomics of advanced prostate cancer. Cell 161 1215–1228. ( 10.1016/j.cell.2015.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, et al. 2013. Abiraterone in metastatic prostate cancer without previous chemotherapy. New England Journal of Medicine 368 138–148. ( 10.1056/NEJMoa1209096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, Miller K, Logothetis CJ, Shore ND, Small EJ, et al. 2015. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncology 16 152–160. ( 10.1016/S1470-2045(14)71205-7) [DOI] [PubMed] [Google Scholar]
- Salman JW, Schoots IG, Carlsson SV, Jenster G, Roobol MJ. 2015. Prostate specific antigen as a tumor marker in prostate cancer: biochemical and clinical aspects. In Advances in Cancer Biomarkers: From Biochemistry to Clinic for a Critical Revision, pp 93–114. Ed Scatena R. Dordrecht, Netherlands: Springer Netherlands; ( 10.1007/978-94-017-7215-0_7) [DOI] [PubMed] [Google Scholar]
- Salvi S, Casadio V, Conteduca V, Lolli C, Gurioli G, Martignano F, Schepisi G, Testoni S, Scarpi E, Amadori D, et al. 2016. Circulating AR copy number and outcome to enzalutamide in docetaxel-treated metastatic castration-resistant prostate cancer. Oncotarget 7 37839–37845. ( 10.18632/oncotarget.9341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford M. 2013. Enzalutamide: a review of its use in metastatic, castration-resistant prostate cancer. Drugs 73 1723–1732. ( 10.1007/s40265-013-0129-9) [DOI] [PubMed] [Google Scholar]
- Sartor O, Gillessen S. 2014. Treatment sequencing in metastatic castrate-resistant prostate cancer. Asian Journal of Andrology 16 426–431. ( 10.4103/1008-682X.126378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor O, de Bono JS. 2018. Metastatic prostate cancer. New England Journal of Medicine 378 645–657. ( 10.1056/NEJMra1701695) [DOI] [PubMed] [Google Scholar]
- Scher HI, Sawyers CL. 2005. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. Journal of Clinical Oncology 23 8253–8261. ( 10.1200/JCO.2005.03.4777) [DOI] [PubMed] [Google Scholar]
- Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, et al. 2008. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. Journal of Clinical Oncology 26 1148–1159. ( 10.1200/JCO.2007.12.4487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, et al. 2010. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet 375 1437–1446. ( 10.1016/S0140-6736(10)60172-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et al. 2012. Increased survival with enzalutamide in prostate cancer after chemotherapy. New England Journal of Medicine 367 1187–1197. ( 10.1056/NEJMoa1207506) [DOI] [PubMed] [Google Scholar]
- Scher HI, Heller G, Molina A, Attard G, Danila DC, Jia X, Peng W, Sandhu SK, Olmos D, Riisnaes R, et al. 2015. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. Journal of Clinical Oncology 33 1348–1355. ( 10.1200/JCO.2014.55.3487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher HI, Lu D, Schreiber NA, Louw J, Graf RP, Vargas HA, Johnson A, Jendrisak A, Bambury R, Danila D, et al. 2016. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncology 2 1441–1449. ( 10.1001/jamaoncol.2016.1828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader AJ, Boegemann M, Ohlmann CH, Schnoeller TJ, Krabbe LM, Hajili T, Jentzmik F, Stoeckle M, Schrader M, Herrmann E, et al. 2014. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. European Urology 65 30–36. ( 10.1016/j.eururo.2013.06.042) [DOI] [PubMed] [Google Scholar]
- Schwarzenbach H, Alix-Panabieres C, Muller I, Letang N, Vendrell JP, Rebillard X, Pantel K. 2009. Cell-free tumor DNA in blood plasma as a marker for circulating tumor cells in prostate cancer. Clinical Cancer Research 1032–1038. ( 10.1158/1078-0432.CCR-08-1910) [DOI] [PubMed] [Google Scholar]
- Schweizer MT, Antonarakis ES, Wang H, Ajiboye AS, Spitz A, Cao H, Luo J, Haffner MC, Yegnasubramanian S, Carducci MA, et al. 2015. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: results from a pilot clinical study. Science Translational Medicine 7 269ra262 ( 10.1126/scitranslmed.3010563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer MT, Haugk K, McKiernan JS, Gulati R, Cheng HH, Maes JL, Dumpit RF, Nelson PS, Montgomery B, McCune JS, et al. 2018. A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer. PLoS ONE 13 e0198389 ( 10.1371/journal.pone.0198389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seruga B, Ocana A, Tannock IF. 2011. Drug resistance in metastatic castration-resistant prostate cancer. Nature Reviews. Clinical Oncology 8 12–23. ( 10.1038/nrclinonc.2010.136) [DOI] [PubMed] [Google Scholar]
- Shah N, Wang P, Wongvipat J, Karthaus WR, Abida W, Armenia J, Rockowitz S, Drier Y, Bernstein BE, Long HW, et al. 2017. Regulation of the glucocorticoid receptor via a BET-dependent enhancer drives antiandrogen resistance in prostate cancer. eLife 6 e27861 ( 10.7554/eLife.27861.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Tekedereli I, Wang J, Yuca E, Tsang S, Sood A, Lopez-Berestein G, Ozpolat B, Ittmann M. 2012. Highly specific targeting of the TMPRSS2/ERG fusion gene using liposomal nanovectors. Clinical Cancer Research 18 6648–6657. ( 10.1158/1078-0432.CCR-12-2715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore ND. 2017. Darolutamide (ODM-201) for the treatment of prostate cancer. Expert Opinion on Pharmacotherapy 18 945–952. ( 10.1080/14656566.2017.1329820) [DOI] [PubMed] [Google Scholar]
- Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, Olmos D, Mainwaring PN, Lee JY, Uemura H, et al. 2018. Apalutamide treatment and metastasis-free survival in prostate cancer. New England Journal of Medicine 378 1408–1418. ( 10.1056/NEJMoa1715546) [DOI] [PubMed] [Google Scholar]
- Soifer HS, Souleimanian N, Wu S, Voskresenskiy AM, Collak FK, Cinar B, Stein CA. 2012. Direct regulation of androgen receptor activity by potent CYP17 inhibitors in prostate cancer cells. Journal of Biological Chemistry 287 3777–3787. ( 10.1074/jbc.M111.261933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Chen J, Zhao M, Zhang C, Yu Y, Lonard DM, Chow DC, Palzkill T, Xu J, O’Malley BW, et al. 2016. Development of potent small-molecule inhibitors to drug the undruggable steroid receptor coactivator-3. PNAS 113 4970–4975. ( 10.1073/pnas.1604274113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spans L, Van den Broeck T, Smeets E, Prekovic S, Thienpont B, Lambrechts D, Karnes RJ, Erho N, Alshalalfa M, Davicioni E, et al. 2016. Genomic and epigenomic analysis of high-risk prostate cancer reveals changes in hydroxymethylation and TET1. Oncotarget 7 24326–24338. ( 10.18632/oncotarget.8220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S. 2016. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. International Immunology 28 383–391. ( 10.1093/intimm/dxw014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelloo S, Nevedomskaya E, van der Poel HG, de Jong J, van Leenders GJ, Jenster G, Wessels LF, Bergman AM, Zwart W. 2015. Androgen receptor profiling predicts prostate cancer outcome. EMBO Molecular Medicine 7 1450–1464. ( 10.15252/emmm.201505424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelloo S, Nevedomskaya E, Kim Y, Hoekman L, Bleijerveld OB, Mirza T, Wessels LFA, van Weerden WM, Altelaar AFM, Bergman AM, et al. 2018. Endogenous androgen receptor proteomic profiling reveals genomic subcomplex involved in prostate tumorigenesis. Oncogene 37 313–322. ( 10.1038/onc.2017.330) [DOI] [PubMed] [Google Scholar]
- Sternberg CN. 2016. Improving survival for metastatic castrate-resistant prostate cancer: will combination therapy help us to move forward? European Urology 70 722–723. ( 10.1016/j.eururo.2016.05.030) [DOI] [PubMed] [Google Scholar]
- Suzman DL, Luber B, Schweizer MT, Nadal R, Antonarakis ES. 2014. Clinical activity of enzalutamide versus docetaxel in men with castration-resistant prostate cancer progressing after abiraterone. Prostate 74 1278–1285. ( 10.1002/pros.22844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, et al. 2015. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. New England Journal of Medicine 373 737–746. ( 10.1056/NEJMoa1503747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney C, Chen Y, Liu G, Carducci M, Jarrard D, Eisenberger M, Wong Y, Patrick-Miller L, Hahn N, Kohli M, et al. 2016. Long term efficacy and QOL data of chemohormonal therapy (C-HT) in low and high volume hormone naïve metastatic prostate cancer (PrCa): E3805 CHAARTED trial. Annals of Oncology 27 720PD ( 10.1093/annonc/mdw372.04) [DOI] [Google Scholar]
- Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, Armitage GR, Wilson JJ, Venner PM, Coppin CM, et al. 1996. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. Journal of Clinical Oncology 14 1756–1764. ( 10.1200/JCO.1996.14.6.1756) [DOI] [PubMed] [Google Scholar]
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, et al. 2004. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. New England Journal of Medicine 351 1502–1512. ( 10.1056/NEJMoa040720) [DOI] [PubMed] [Google Scholar]
- Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, Balk SP. 1999. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Research 59 2511–2515. [PubMed] [Google Scholar]
- Taylor CD, Elson P, Trump DL. 1993. Importance of continued testicular suppression in hormone-refractory prostate cancer. Journal of Clinical Oncology 11 2167–2172. ( 10.1200/JCO.1993.11.11.2167) [DOI] [PubMed] [Google Scholar]
- Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, et al. 2010. Integrative genomic profiling of human prostate cancer. Cancer Cell 18 11–22. ( 10.1016/j.ccr.2010.05.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teply BA, Wang H, Luber B, Sullivan R, Rifkind I, Bruns A, Spitz A, DeCarli M, Sinibaldi V, Pratz CF, et al. 2018. Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: an open-label, phase 2, multicohort study. Lancet Oncology 19 76–86. ( 10.1016/S1470-2045(17)30906-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todenhofer T, Azad A, Stewart C, Gao J, Eigl BJ, Gleave ME, Joshua AM, Black PC, Chi KN. 2017. AR-V7 Transcripts in whole blood RNA of patients with metastatic castration resistant prostate cancer correlate with response to abiraterone acetate. Journal of Urology 197 135–142. ( 10.1016/j.juro.2016.06.094) [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, et al. 2005. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310 644–648. ( 10.1126/science.1117679) [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, Helgeson BE, Cao Q, Prensner JR, Rubin MA, Shah RB, et al. 2008. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia 10 177–188. ( 10.1593/neo.07822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, et al. 2009. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324 787–790. ( 10.1126/science.1168175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanucci A, Barfeld SJ, Kytola V, Itkonen HM, Coleman IM, Vodak D, Sjoblom L, Sheng X, Tolonen T, Minner S, et al. 2017. Androgen receptor deregulation drives bromodomain-mediated chromatin alterations in prostate cancer. Cell Reports 19 2045–2059. ( 10.1016/j.celrep.2017.05.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soest RJ, van Royen ME, de Morree ES, Moll JM, Teubel W, Wiemer EA, Mathijssen RH, de Wit R, van Weerden WM. 2013. Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castration-resistant prostate cancer. European Journal of Cancer 49 3821–3830. ( 10.1016/j.ejca.2013.09.026) [DOI] [PubMed] [Google Scholar]
- Wako K, Kawasaki T, Yamana K, Suzuki K, Jiang S, Umezu H, Nishiyama T, Takahashi K, Hamakubo T, Kodama T, et al. 2008. Expression of androgen receptor through androgen-converting enzymes is associated with biological aggressiveness in prostate cancer. Journal of Clinical Pathology 61 448–454. ( 10.1136/jcp.2007.050906) [DOI] [PubMed] [Google Scholar]
- Wang L, Yu Y, Chow DC, Yan F, Hsu CC, Stossi F, Mancini MA, Palzkill T, Liao L, Zhou S, et al. 2015. Characterization of a steroid receptor coactivator small molecule stimulator that overstimulates cancer cells and leads to cell stress and death. Cancer Cell 28 240–252. ( 10.1016/j.ccell.2015.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Qiao Y, Asangani IA, Ateeq B, Poliakov A, Cieslik M, Pitchiaya S, Chakravarthi B, Cao X, Jing X, et al. 2017. Development of peptidomimetic inhibitors of the ERG gene fusion product in prostate cancer. Cancer Cell 31 844–847. ( 10.1016/j.ccell.2017.05.001) [DOI] [PubMed] [Google Scholar]
- Wargo JA, Reddy SM, Reuben A, Sharma P. 2016. Monitoring immune responses in the tumor microenvironment. Current Opinion in Immunology 41 23–31. ( 10.1016/j.coi.2016.05.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welti J, Sharp A, Yuan W, Dolling D, Nava Rodrigues D, Figueiredo I, Gil V, Neeb A, Clarke M, Seed G, et al. 2018. Targeting bromodomain and extra-terminal (BET) family proteins in castration-resistant prostate cancer (CRPC). Clinical Cancer Research 24 3149–3162. ( 10.1158/1078-0432.CCR-17-3571) [DOI] [PubMed] [Google Scholar]
- Wyatt AW, Azad AA, Volik SV, Annala M, Beja K, McConeghy B, Haegert A, Warner EW, Mo F, Brahmbhatt S, et al. 2016. Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. JAMA Oncology 2 1598–1606. ( 10.1001/jamaoncol.2016.0494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, Zhou Q. 2005. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Molecular Cell 19 535–545. ( 10.1016/j.molcel.2005.06.029) [DOI] [PubMed] [Google Scholar]
- Yin S, Bhattacharya R, Cabral F. 2010. Human mutations that confer paclitaxel resistance. Molecular Cancer Therapeutics 9 327–335. ( 10.1158/1535-7163.MCT-09-0674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, et al. 2017. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nature Medicine 23 703–713. ( 10.1038/nm.4333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HJ, Yan J, Luo W, Ayala G, Lin SH, Erdem H, Ittmann M, Tsai SY, Tsai MJ. 2005. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Research 65 7976–7983. ( 10.1158/0008-5472.CAN-04-4076) [DOI] [PubMed] [Google Scholar]
- Zhou XE, Suino-Powell KM, Li J, He Y, Mackeigan JP, Melcher K, Yong EL, Xu HE. 2010. Identification of SRC3/AIB1 as a preferred coactivator for hormone-activated androgen receptor. Journal of Biological Chemistry 285 9161–9171. ( 10.1074/jbc.M109.085779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ML, Kyprianou N. 2008. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocrine-Related Cancer 15 841–849. ( 10.1677/ERC-08-0084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ML, Horbinski CM, Garzotto M, Qian DZ, Beer TM, Kyprianou N. 2010. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Research 70 7992–8002. ( 10.1158/0008-5472.CAN-10-0585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Liu C, Nadiminty N, Lou W, Tummala R, Evans CP, Gao AC. 2013. Inhibition of ABCB1 expression overcomes acquired docetaxel resistance in prostate cancer. Molecular Cancer Therapeutics 12 1829–1836. ( 10.1158/1535-7163.MCT-13-0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a