Abstract
Aims/Introduction
Previous studies have shown that glucose peak time during the oral glucose tolerance test varies in type 2 diabetes patients; however, characteristics of this heterogeneity remain unclear. This research aimed to investigate the characteristics of delayed glucose peak time in type 2 diabetes.
Materials and Methods
A total of 178 participants who underwent the oral glucose tolerance test were divided into five groups according to glucose peak time.
Results
A total of 25 participants with normal glucose tolerance had a glucose peak at 30 min. Among participants with type 2 diabetes, 28 had a glucose peak at 60 min, 48 at 90 min, 45 at 120 min and 32 at 150 min. With the glucose peak time delayed, glycated hemoglobin, area under the glucose curve and homeostatic model assessment of insulin resistance increased gradually (P = 0.038, P < 0.0001, P < 0.0001, respectively), and oral glucose insulin sensitivity, homeostatic model assessment of β‐cell function, insulinogenic index, modified β‐cell function index and disposition indices decreased (P < 0.0001 for all). On multinominal logistic regression, insulinogenic index (odds ratio 0.73, 95% confidence interval 0.57–0.93, P = 0.01), modified β‐cell function index (odds ratio 0.67, 95% confidence interval 0.47–0.94, P = 0.023) and oral glucose insulin sensitivity (odds ratio 0.91, 95% confidence interval 0.87–0.96, P < 0.0001) were independently correlated with delayed glucose peak time.
Conclusions
Delay in glucose peak time indicated an increase in blood glucose and a decrease in insulin sensitivity and secretion. Furthermore, insulinogenic index, modified β‐cell function index and oral glucose insulin sensitivity contributed to delayed glucose peak time.
Keywords: Glucose peak time, Insulin sensitivity, Pancreatic β‐cell function
Introduction
The oral glucose tolerance test (OGTT) is a common method of evaluating glucose tolerance status. The OGTT provides information about insulin resistance and secretion assessment both directly and indirectly through surrogate indices, such as the homeostatic model assessment of insulin resistance (HOMA‐IR) and homeostatic model assessment of beta‐cell function (HOMA‐β)1, the Matsuda index2, quantitative insulin sensitivity check index3 and the Stumvoll index4. The gold standard for assessing insulin secretion and sensitivity is the hyperinsulinemic‐euglycemic clamp5. Previous studies suggest that OGTT‐derived indices used for evaluating insulin resistance and secretion are highly related to the clamp tests1, 2, 6. However, OGTT‐derived indices are difficult to implement in routine clinical practice and epidemiological studies because they require complicated calculations, and are not practical for different ethnicities, regions, ages and disease courses7, 8, 9, 10. Recently, there has been increased interest in the discovery and validation of novel markers that can evaluate insulin secretion and resistance9, 11. A marker of particular interest is the shape of the glucose curve during an OGTT.
Limited studies have focused on the possible information captured from the shape of the OGTT glucose curve. In 1994, a study in Japanese (with an English abstract) classified the curve as ‘upward,’ ‘domed’ or ‘biphasic,’ and found that the prevalence of upward and domed curves was higher in type 2 diabetes mellitus patients12. Subsequently, other studies offered different classifications of OGTT curve shapes, such as monophasic, biphasic, triphasic and unclassified, or 4/5‐phases, and showed various metabolic phenotypes of insulin secretion and sensitivity found in the different shapes13, 14, 15. Additionally, the OGTT curve shape has been used to assess future type 2 diabetes mellitus risk in individuals with impaired fasting glucose and impaired glucose tolerance16. OGTT curve shapes have also been investigated in adolescents, obese children and women with gestational diabetes17, 18, 19. In such studies, complex shapes have been found to be related to better glucose tolerance and metabolic status. Although there is limited research on the monophasic curve, one of its characteristics is the delayed glucose peak time, which predicts a high risk of prediabetes or diabetes in individuals with normal glucose tolerance (NGT)20. However, delayed glucose peak time in type 2 diabetes mellitus requires further study.
Therefore, the present study aimed to investigate the characteristics and indications of the delayed glucose peak time in type 2 diabetes mellitus patients, and to elucidate the relationship between glucose peak time and insulin secretion, insulin sensitivity, and metabolic characteristics.
Methods
Participants
Individuals with monophasic OGTT glucose curves were included in the present study. The NGT participants were confirmed on the basis of OGTT results (fasting plasma glucose [FPG] <5.6 mmol/L [100 mg/dL] and 2‐h plasma glucose [2hPG] <7.8 mmol/L [140 mg/dL])21. Type 2 diabetes mellitus patients underwent OGTT for classification of glucose curve shape. All diabetes patients previously received oral hypoglycemic agents and discontinued the antihyperglycemic treatments for 3 days before OGTT to control the variation. The study population comprised 153 type 2 diabetes mellitus patients and 25 individuals with NGT from the Endocrinology Internal Medicine Ward and Clinic of the Affiliated Hospital of Nantong University, Nantong, China, between February 2009 and January 2010. The exclusion criteria were hepatic disease, kidney disease, cardiac disease, pregnancy, infection, injury, surgery, progressive muscular atrophy and acute diabetic complications. Before participation, all participants provided informed consent. The study protocol was approved by the ethics committee of the hospital, and was carried out in line with the ethical rules of the Helsinki Declaration.
Anthropometric measurements
Height and weight were measured before the OGTT. Waist circumference was determined at the narrowest part of the torso, and participants were weighed wearing only underwear. Body mass index (BMI) was calculated by dividing weight by the square of height (BMI = kg/m2). Blood pressure was measured by an electronic sphygmomanometer (Omron®, Omron Healthcare, IL, USA).
Blood sampling and OGTT, insulin and C‐peptide release test
Participants reported to the clinic at 07.00 h after a 10–12‐h overnight fast. Fasting blood samples were collected for measurement of total cholesterol (TC), triglycerides (TG) and glycated hemoglobin (HbA1c). Blood lipids were measured using the enzyme method (ADVIA® 2400; Siemens, Berlin, Germany). HbA1c was measured by high‐performance liquid chromatography (VARIANT™ II; Bio‐Rad, Hercules, CA, USA), and presented consistent with the recommendations of the National Glycohemoglobin Standardization Program. Inter‐assay and intra‐assay variations were ≤5%.
Oral glucose tolerance test was carried out with a 75‐g glucose load, and blood samples were collected from an antecubital vein through a small polyethylene catheter at baseline, 30, 60, 90, 120, 150 and 180 min for measurements of glucose, insulin and C‐peptide. Plasma glucose was measured using the glucose oxidation method (ADVIA® 2400; Siemens), insulin and C‐peptide concentrations were measured using the chemiluminescence method (Cobas e411 analyzer; Roche, Basel, Switzerland).
Classification of glucose peak time
A glucose threshold of 0.25 mmol/L (4.5 mg/dL) has been reported to minimize fluctuations in blood glucose concentrations; therefore, the fluctuations could be attributed to the method of glucose analysis rather than physiological reasons13. The glucose curve was defined by plotting glucose concentrations during the 3‐h OGTT. A monophasic curve was characterized by a gradual rise in plasma glucose concentrations until a maximum value was reached followed by a subsequent decline in glucose of ≥0.25 mmol/L (4.5 mg/dL). A second rise in the glucose concentrations of ≥0.25 mmol/L (4.5 mg/dL) after the decline in glucose, or the glucose concentrations after glucose load continuously increased until 180 min, were defined as the biphasic or unclassified curve, which were excluded in our study13, 18, 22.
Based on different OGTT glucose peaks at 60, 90, 120 and 150 min, the monophasic glucose curves of the type 2 diabetes mellitus patients were classified as P60, P90, P120 and P150, respectively.
Evaluation for insulin sensitivity, resistance and secretion
The areas under the curve for glucose (AUCG0–180 min), insulin (AUCINS0–180 min) and C‐peptide levels (AUCC‐peptide0–180 min) were calculated by the trapezoid rule. Oral glucose insulin sensitivity (OGIS)6 was calculated (see http://webmet.pd.cnr.it/ogis/index.php) to measure insulin sensitivity. The homeostatic model assessment of insulin resistance (HOMA‐IR)1 was calculated using the following equation to measure insulin sensitivity: fasting glucose (mmol/L) × fasting insulin (mIU/L)/22.5. Insulin secretion was defined by the HOMA of β‐cell function (HOMA‐β)1, insulinogenic index (IGI)23, and modified β‐cell function index (MBCI)24. The following formulas were used to calculate the insulin secretion parameters: HOMA‐β = (20 × fasting insulin [mIU/L])/(fasting glucose [mmol/L] − 3.5), IGI was calculated as the ratio of the increment of insulin to that of glucose at 30 min after glucose ingestion. MBCI = (fasting insulin [mIU/L] × fasting glucose [mmol/L])/(2‐h plasma glucose [mmol/L] + 1‐h plasma glucose [mmol/L] − 7).
Disposition indices
To evaluate the relationship between pancreatic β‐cell function and insulin sensitivity, the disposition indices were calculated as IGI × OGIS and MBCI × OGIS25.
Statistical analysis
Statistical analyses were completed in SPSS version 20.0 (IBM, Chicago, IL, USA) and SAS version 9.2 (SAS Institute, Cary, NC, USA). Data were presented as mean ± standard error. Data that did not meet the normal distribution were log10 transformed. The means were compared using one‐way analysis of variance (anova). The post‐hoc Student–Newman–Keuls test was used for multiple comparisons. Univariate analysis was used to investigate the association between glucose peak time and variables such as age, sex, disease course, family history, BMI, waist‐to‐height ratio, systolic blood pressure, diastolic blood pressure, FPG, TC, TG, HbA1c, HOMA‐β, IGI, MBCI, HOMA‐IR and OGIS. Variables significantly associated with glucose peak time were entered into a multinominal logistic regression model, including age, HbA1c, IGI, MBCI and OGIS. Statistical significance was considered at P < 0.05.
Results
Prevalence of glucose peak time
During the OGTT, 25 participants with NGT had a glucose peak at 30 min. For the type 2 diabetes mellitus patients, 28 (18.30%) showed a glucose peak at 60 min (P60), 48 (31.37%) at 90 min (P90), 45 (29.41%) at 120 min (P120) and 32 (20.92%) at 150 min (P150; Figure 1a).
Figure 1.
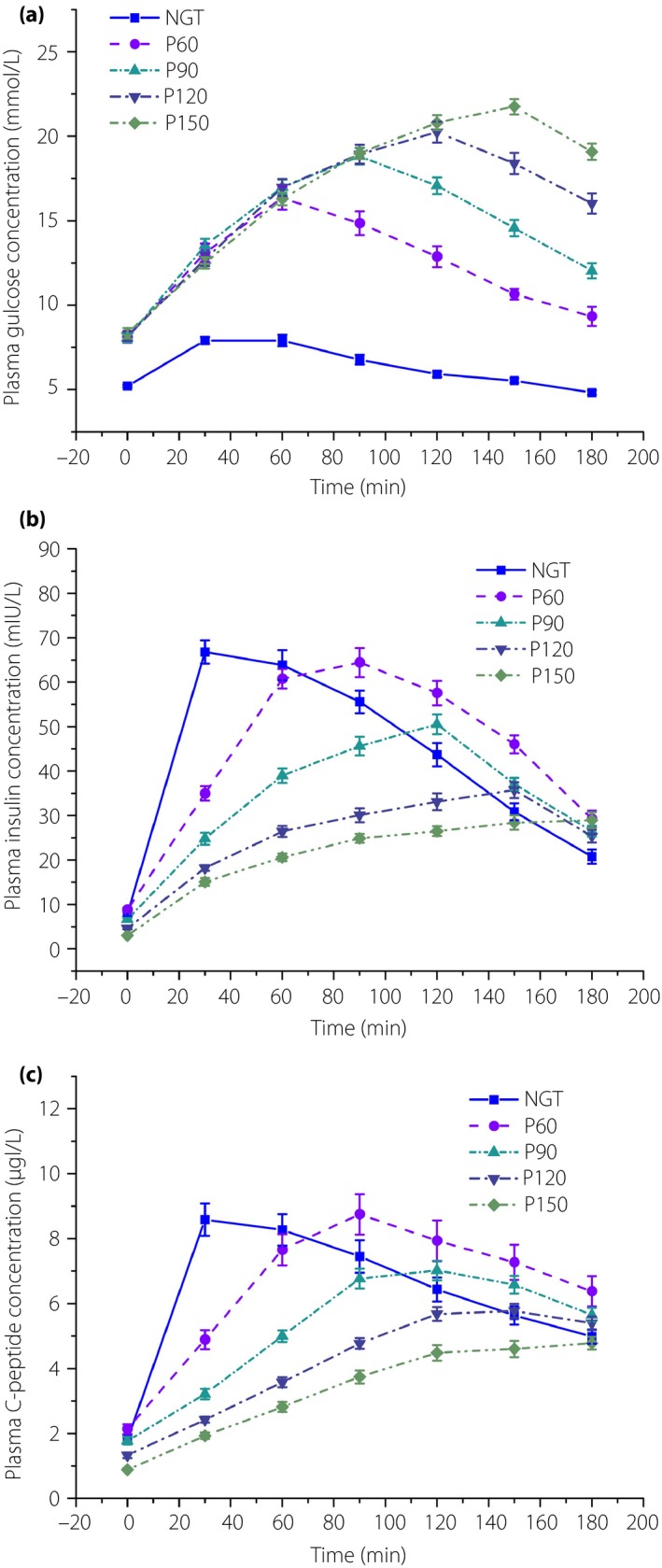
(a) Glucose, (b) insulin and (c) C‐peptide response curve during the oral glucose tolerance test of five groups. NGT, normal glucose tolerance; P60, glucose peak at 60 min; P90, glucose peak at 90 min; P120, glucose peak at 120 min; P150, glucose peak at 150 min.
Clinical characteristics
The participants’ clinical characteristics are presented in Table 1. HbA1c increased progressively in the five groups (NGT, P60, P90, P120, P150; P = 0.038). Weight and FBG were statistically significant, but had no tendency for increase or decrease. No statistically significant differences were found for age, disease course (comparison between four diabetes groups), systolic blood pressure, systolic blood pressure, BMI, waist‐to‐height ratio, TC and TG.
Table 1.
Clinical characteristics of the five groups of participants
| NGT | P60 | P90 | P120 | P150 | P | |
|---|---|---|---|---|---|---|
| Total (male/female) | 25 (10/15) | 28 (16/12) | 48 (35/13) | 45 (23/22) | 32 (16/16) | – |
| Age (years) | 53.08 ± 2.77 | 55.11 ± 2.16 | 53.77 ± 1.62 | 51.11 ± 1.97 | 53.62 ± 1.86 | 0.689 |
| Disease course (years) | – | 5.41 ± 0.10 | 5.19 ± 0.13 | 6.33 ± 0.15 | 6.22 ± 0.49 | 0.112 |
| Weight (kg) | 63.22 ± 1.53 | 71.78 ± 1.90 | 72.1 ± 1.33 | 70.19 ± 1.61 | 66.67 ± 1.57 | 0.003 |
| WHR | 0.82 ± 0.004 | 0.91 ± 0.009 | 0.92 ± 0.007 | 0.91 ± 0.009 | 0.90 ± 0.012 | 0.201 |
| SBP (mmHg) | 121.50 ± 3.04 | 129.50 ± 3.34 | 130.00 ± 2.46 | 135.00 ± 2.93 | 130.00 ± 3.41 | 0.46 |
| DBP (mmHg) | 70.00 ± 1.63 | 78.00 ± 1.98 | 80.00 ± 1.58 | 83.00 ± 1.30 | 80.00 ± 1.92 | 0.97 |
| BMI (kg/m2) | 25.01 ± 1.23 | 24.98 ± 2.05 | 25.27 ± 2.48 | 25.22 ± 2.64 | 25.02 ± 2.54 | 0.97 |
| FBG(mmol/L) | 5.21 ± 0.08 | 8.10 ± 0.33 | 8.06 ± 0.25 | 8.17 ± 0.29 | 8.34 ± 0.30 | <0.0001 |
| TC (mmol/L) | 4.63 ± 0.75 | 5.18 ± 0.86 | 5.10 ± 0.58 | 5.19 ± 0.72 | 4.92 ± 0.59 | 0.176 |
| TG (mmol/L) | 1.38 ± 0.26 | 2.29 ± 0.73 | 2.04 ± 0.77 | 1.98 ± 0.39 | 1.86 ± 0.33 | 0.383 |
| HbA1c (%) | 5.10 ± 0.09 | 7.98 ± 0.37 | 9.59 ± 0.35 | 10.04 ± 0.25 | 10.24 ± 0.40 | 0.038 |
Data are presented as mean ± standard error. Parameters among the five groups were compared with anova. BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; NGT, normal glucose tolerance; P60, glucose peak at 60 min; P90, glucose peak at 90 min; P120, glucose peak at 120 min; P150, glucose peak at 150 min; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; WHR, waist‐to‐hip ratio.
Glucose tolerance, insulin and C‐peptide variation trends
The present results showed that the glucose peak of the NGT group occurred at 30 min, and the glucose peak times of the four diabetes groups (P60, P90, P120, P150) gradually increased compared with those of the control group. Furthermore, AUCG0–180 min increased (P < 0.0001), whereas AUCINS0–180 min and AUCC‐peptide0–180 min decreased gradually in the five groups (NGT, P60, P90, P120, P150; P < 0.0001 for both; Table 2). As shown in Figure 1, the peak times of insulin and C‐peptide were all delayed, and lagged behind their own glucose peak times in P60, P90, P120 and P150.
Table 2.
Glucose and insulin metabolism of the five groups of participants
| NGT | P60 | P90 | P120 | P150 | P | |
|---|---|---|---|---|---|---|
| AUCG0–180 min | 39.14 ± 0.82* | 76.53 ± 3.17** | 91.01 ± 2.39* | 99.35 ± 2.82**** | 104.07 ± 2.06 | <0.0001 |
| AUCINS0–180 min | 293.42 ± 5.74* | 282.78 ± 6.67** | 214.06 ± 4.89*** | 158.04 ± 3.47**** | 135.97 ± 5.29 | <0.0001 |
| AUCC‐peptide0–180 min | 41.28 ± 2.19* | 41.16 ± 2.32** | 32.29 ± 1.92*** | 25.58 ± 1.59**** | 20.49 ± 1.56 | <0.0001 |
| HOMA‐β | 64.92 ± 4.96* | 49.59 ± 3.45 | 45.69 ± 2.25 | 42.23 ± 2.35 | 35.46 ± 3.12 | <0.0001 |
| IGI | 11.57 ± 0.55* | 4.14 ± 0.21***** | 3.10 ± 0.13**** | 1.82 ± 0.08**** | 1.21 ± 0.11 | <0.0001 |
| MBCI | 22.74 ± 1.12* | 6.59 ± 0.51***** | 4.98 ± 0.16 | 4.2 ± 0.19 | 3.22 ± 0.26 | <0.0001 |
| HOMA‐IR | 1.76 ± 0.11* | 2.72 ± 0.16 | 3.09 ± 0.11 | 3.42 ± 0.12 | 3.93 ± 0.09 | <0.0001 |
| OGIS | 438.36 ± 8.41* | 340.93 ± 8.75** | 318.81 ± 6.40*** | 295.92 ± 6.34**** | 272.62 ± 6.57 | <0.0001 |
| IGI × OGIS | 5036.01 ± 219.06* | 1276.84 ± 70.78** | 1024.87 ± 50.15*** | 581.44 ± 23.92**** | 326.49 ± 28.35 | <0.0001 |
| MBCI × OGIS | 9937.38 ± 181.46* | 2226.71 ± 62.99***** | 1527.76 ± 57.28 | 1263.19 ± 15.58 | 876.75 ± 14.40 | <0.0001 |
Data are presented as mean ± standard error. Parameters among five groups were compared with anova. Post‐hoc SNK test was used in multiple comparison. *P < 0.05, normal glucose tolerance (NGT) vs glucose peak at 60 min (P60), glucose peak at 90 min (P90), glucose peak at 120 min (P120), glucose peak at 150 min (P150). **P < 0.05, P60 vs P90, P120, P150. ***P < 0.05, P90 vs P120, P150. ****P < 0.05, P120 vs P150. *****p < 0.05, P60 vs P120, P150. AUC, area under the curve; HOMA‐β, homeostatic model assessment of β‐cell function; IGI, insulinogenic index; MBCI, modified β‐cell index; HOMA‐IR, homeostatic model assessment of insulin resistance; OGIS, oral glucose insulin sensitivity.
The peak glucose values of five groups (NGT, P60, P90, P120, P150) gradually increased (P < 0.0001; Figure 1a). The peak glucose values of P60, P90 and P150, when compared with their own groups’ 2hPG, were statistically significant (P < 0.0001 for all).
Insulin sensitivity, resistance and secretion
Homeostatic model assessment of insulin resistance was lowest in the NGT participants and presented an increasing trend in the five groups (NGT, P60, P90, P120, P150; P < 0.0001). However, OGIS showed an opposite trend and decreased gradually (P < 0.0001). The indices of insulin secretion, HOMA‐β, IGI and MBCI tended to decrease in the five groups (P < 0.0001). However, only OGIS and IGI were statistically significant according to the results of the anova post‐hoc Student–Newman–Keuls test (Table 2).
Disposition indices
The disposition indices of IGI × OGIS and MBCI × OGIS were highest in the NGT group, and decreased gradually in the four diabetes groups (P60, P90, P120, P150; P < 0.0001). However, IGI × OGIS was statistically significant according to the results of the anova post‐hoc Student–Newman–Keuls test (Table 2).
Multinominal logistic regression
All variables included age, sex, disease course, family history, BMI, waist‐to‐height ratio, systolic blood pressure, diastolic blood pressure, FPG, TC, TG, FPG, HbA1c, HOMA‐β, IGI, MBCI, HOMA‐IR and OGIS. On the multinominal logistic regression, glucose peak time was considered as a dependent variable. IGI (odds ratio [OR] 0.73, 95% confidence interval [CI] 0.57–0.93, P = 0.01), MBCI (OR 0.67, 95% CI 0.47–0.94, P = 0.023) and OGIS (OR 0.91, 95% CI 0.87–0.96, P < 0.0001) were found to be independent negative predictors after adjustment for age, disease course and blood pressure. Age was the only covariate associated with delayed glucose peak time (OR 1.05, 95% CI 1.02–1.08, P < 0.0001).
Discussion
The present study aimed to evaluate the characteristics and indications of the delay in OGTT glucose peak time in type 2 diabetes mellitus patients. Analysis showed that the peak times of glucose, insulin and C‐peptide were significantly different between NGT and type 2 diabetes mellitus participants. NGT participants had glucose, insulin and C‐peptide peaks at 30 min. Type 2 diabetes mellitus patients had glucose peaks at 60, 90, 120 and 150 min, whereas insulin and C‐peptide peak times did not synchronize with the glucose variation trend (Figure 1a–c). Takahara et al.26 described an inverted U‐shape of delayed peak insulin levels indicating glucose intolerance in the Japanese population. The results of the present study showed similar characteristics existing in type 2 diabetes mellitus patients.
Studies have shown that OGTT glucose curve shapes are correlated with glucose tolerance status, insulin sensitivity and pancreatic β‐cell function12, 13, 14, 15, 16, 17, 18, 19. Kim et al.22 investigated the non‐diabetic obese adolescents, wherein monophasic and biphasic groups were categorized according to a 120‐min OGTT. Although the two groups had similar fasting and 2‐h glucose and insulin concentrations, the monophasic group had significantly larger AUCs for glucose, insulin, C‐peptide and free fatty acid, lower insulin sensitivity, and more impaired insulin secretion, compared with the biphasic group22. Hence, the monophasic OGTT glucose curve was the predictor of lower glucose tolerance, insulin sensitivity and insulin secretion in individuals without diabetes. To investigate the significance of the monophasic OGTT glucose curve in type 2 diabetes mellitus patients, we studied the delay in glucose peak time, a characteristic of the monophasic curve. Consistent with the results of Kim et al.22, the delayed glucose peak time in type 2 diabetes mellitus in the present study was also caused by increasing insulin resistance and failure of pancreatic β‐cell function. A study investigating patients with early type 2 diabetes mellitus showed similar results, and glucose peak time appeared to have reliable reproducibility and was found to be associated with pancreatic β‐cell function27. The β‐cell function was lower in patients with early type 2 diabetes mellitus with a glucose peak at ≥90 min than in those with a peak at ≤60 min, consistent with the present findings. However, the OGTT duration was 120 min, and the present study prolonged the test duration to 180 min, wherein a glucose peak at 150 min was observed and compared. We analyzed the relationships between the different glucose peak times and HOMA‐IR, OGIS, HOMA‐β, IGI, MBCI and disposition indices in participants with NGT and type 2 diabetes mellitus patients with an average disease course of 4–6 years, and found that IGI, MBCI and OGIS contributed to the delay of glucose peak time. Therefore, we not only clarified the definition of delayed of glucose peak time in diabetes patients with an average disease duration of 4–6 years, but also elucidated the more profound pathophysiological implications of the different glucose peak times in our research.
As mentioned, on multinomial logistic regression, IGI, MBCI and OGIS were independently correlated with delayed glucose peak time. IGI represents early‐phase insulin secretion23, 28. Previous research suggests that enhancing early‐phase insulin secretion improves glucose tolerance status, not only existing in NGT and impaired glucose tolerance, but also in type 2 diabetes mellitus29, 30. Studies have reported that early‐phase insulin secretion related to hepatic glucose output and accompanied by disturbed glucagon secretion is responsible for postprandial hyperglycemia31, 32. MBCI is an OGTT‐derived index that comprehensively assesses the overall postprandial reaction of pancreatic β‐cells24. Meanwhile, we administered the 3‐h OGTT version of OGIS, which shows the insulin sensitivity after glucose tolerance6. OGIS is highly linked to peripheral insulin sensitivity33, including insulin sensitivity in the liver, muscle and adipocytes. With delayed glucose peak time indicating decreased insulin sensitivity and secretion, slower rates of decrease in glucose and increase in insulin and C‐peptide thus indicate impaired pancreatic β‐cell function and aggravated insulin resistance. Therefore, early‐phase insulin secretion, pancreatic β‐cell reaction to postprandial glucose tolerance, and peripheral insulin sensitivity play an important role in delaying glucose peak time and gradually increasing 2hPG during OGTT.
Otherwise, age was the only covariate correlated with the delayed glucose peak time in our research. Studies have shown that first‐ and second‐phase insulin secretion stimulated by glucose decrease with aging, not only in individuals with NGT and impaired glucose tolerance8, but also in type 2 diabetes mellitus patients34. Thus, the failure of pancreatic β‐cell function caused by aging contributed to the delayed glucose peak time.
In the present study, the AUC of glucose and HbA1c increased gradually with delayed glucose peak time. The peak times of glucose and 2hPG of the four diabetes groups (P60, P90, P120, P150) also gradually increased, implying that glucose toxicity and excursion increased in severity in the four diabetes groups along with the delay in glucose peak time. Glucose toxicity is a glucose metabolism disorder that affects insulin sensitivity and secretion, and contributes to type 2 diabetes mellitus development24, 35, 36. Hyperglycemia is associated with chronic diabetic complications37, 38, 39, 40. Postprandial hyperglycemia and glucose excursion, which lead to inflammation, oxidative stress and endothelia dysfunction, are predictors of microvascular and macrovascular complications40, 41. Based on these and our findings, we speculated that delayed glucose peak time might be associated with the occurrence of chronic diabetic complications. Therefore, we plan to study the relationship further in a follow‐up visit of the participants.
Studies have shown that poorly controlled glucose levels in diabetes are associated with changes in gastric emptying42, 43. Diabetic gastroparesis and accelerated gastric emptying occur in early or long‐term diabetes44. Dedik et al.45 reported that gastric emptying influences the shape of the glucose curve. In their study, disturbances in glucose metabolism increased in severity along with delayed glucose peak time. Furthermore, incretin secretion can amplify early‐phase insulin secretion after OGTT, but this effect is reduced in diabetes patients46. Thus, diabetic gastroparesis, accelerated gastric emptying and decreased incretin secretion are likely correlated with delayed glucose peak time. However, the study did not investigate the influence of gastric emptying and endogenous incretin. Therefore, further research is required to elucidate the association between gastric emptying and the different glucose peak times of the monophasic curve.
The present study had some limitations. First, this is a cross‐sectional study, future longitudinal studies should be carried out to confirm our findings. Second, the study's sample size was relatively small. Finally, the biphasic and unclassified curves were not observed. Thus, we plan to investigate this further in a follow‐up study.
In conclusion, the delay in glucose peak time in individuals with NGT and type 2 diabetes mellitus patients showed gradual aggravations in glucose metabolism, and a decrease in insulin sensitivity and secretion. Furthermore, the differences in early‐phase insulin secretion and insulin sensitivity and secretion after glucose tolerance in type 2 diabetes mellitus patients contributed to the delayed glucose peak time.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors thank Jingyi Zhu for her laboratory expertise and Lihong Huang, PhD, for her help with statistical analysis. This research was supported by a grant from the Jiangsu Health and Family Planning Commission (Y2015070).
J Diabetes Investig. 2018
References
- 1. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487–1495. [DOI] [PubMed] [Google Scholar]
- 2. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462–1470. [DOI] [PubMed] [Google Scholar]
- 3. Katz A, Nambi SS, Mather K, et al Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000; 85: 2402–2410. [DOI] [PubMed] [Google Scholar]
- 4. Stumvoll M, Mitrakou A, Pimenta W, et al Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000; 23: 295–301. [DOI] [PubMed] [Google Scholar]
- 5. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214–E223. [DOI] [PubMed] [Google Scholar]
- 6. Mari A, Pacini G, Murphy E, et al A model‐based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001; 24: 539–548. [DOI] [PubMed] [Google Scholar]
- 7. Ferrannini E, Gastaldelli A, Miyazaki Y, et al Beta‐Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005; 90: 493–500. [DOI] [PubMed] [Google Scholar]
- 8. Szoke E, Shrayyef MZ, Messing S, et al Effect of aging on glucose homeostasis: accelerated deterioration of beta‐cell function in individuals with impaired glucose tolerance. Diabetes Care 2008; 31: 539–543. [DOI] [PubMed] [Google Scholar]
- 9. Stern MP, Williams K, Haffner SM. Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test? Ann Intern Med 2002; 136: 575–581. [DOI] [PubMed] [Google Scholar]
- 10. McNeely MJ, Boyko EJ, Leonetti DL, et al Comparison of a clinical model, the oral glucose tolerance test, and fasting glucose for prediction of type 2 diabetes risk in Japanese Americans. Diabetes Care 2003; 26: 758–763. [DOI] [PubMed] [Google Scholar]
- 11. Reinehr T, Wabitsch M, Kleber M, et al Parental diabetes, pubertal stage, and extreme obesity are the main risk factors for prediabetes in children and adolescents: a simple risk score to identify children at risk for prediabetes. Pediatr Diabetes 2009; 10: 395–400. [DOI] [PubMed] [Google Scholar]
- 12. Fuchigami M, Nakano H, Oba K, et al Oral glucose tolerance test using a continuous blood sampling technique for analysis of the blood glucose curve. Nihon Ronen Igakkai Zasshi 1994; 31: 518–524. [DOI] [PubMed] [Google Scholar]
- 13. Tschritter O, Fritsche A, Shirkavand F, et al Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care 2003; 26: 1026–1033. [DOI] [PubMed] [Google Scholar]
- 14. Kanauchi M, Kimura K, Kanauchi K, et al Beta‐cell function and insulin sensitivity contribute to the shape of plasma glucose curve during an oral glucose tolerance test in non‐diabetic individuals. Int J Clin Pract 2005; 59: 427–432. [DOI] [PubMed] [Google Scholar]
- 15. Tura A, Morbiducci U, Sbrignadello S, et al Shape of glucose, insulin, C‐peptide curves during a 3‐h oral glucose tolerance test: any relationship with the degree of glucose tolerance? Am J Physiol Regul Integr Comp Physiol 2011; 300: R941–R948. [DOI] [PubMed] [Google Scholar]
- 16. Abdul‐Ghani MA, Lyssenko V, Tuomi T, et al The shape of plasma glucose concentration curve during OGTT predicts future risk of type 2 diabetes. Diabetes Metab Res Rev 2010; 26: 280–286. [DOI] [PubMed] [Google Scholar]
- 17. Morbiducci U, Di Benedetto G, Gaetano L, et al Predicting the metabolic condition after gestational diabetes mellitus from oral glucose tolerance test curves shape. Ann Biomed Eng 2014; 42: 1112–1120. [DOI] [PubMed] [Google Scholar]
- 18. Kim JY, Coletta DK, Mandarino LJ, et al Glucose response curve and type 2 diabetes risk in Latino adolescents. Diabetes Care 2012; 35: 1925–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yin C, Zhang H, Xiao Y, et al Shape of glucose curve can be used as a predictor for screening prediabetes in obese children. Acta Paediatr 2014; 103: e199–e205. [DOI] [PubMed] [Google Scholar]
- 20. Retnakaran R, Qi Y, Connelly PW, et al Risk of early progression to prediabetes or diabetes in women with recent gestational dysglycaemia but normal glucose tolerance at 3‐month postpartum. Clin Endocrinol 2010; 73: 476–483. [DOI] [PubMed] [Google Scholar]
- 21. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2012; 35(Suppl 1): S64–S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim JY, Michaliszyn SF, Nasr A, et al The shape of the glucose response curve during an oral glucose tolerance test heralds biomarkers of type 2 diabetes risk in obese youth. Diabetes Care 2016; 39: 1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pratley RE, Weyer C. The role of impaired early insulin secretion in the pathogenesis of Type II diabetes mellitus. Diabetologia 2001; 44: 929–945. [DOI] [PubMed] [Google Scholar]
- 24. Zheng S, Zhou H, Han T, et al Clinical characteristics and beta cell function in Chinese patients with newly diagnosed type 2 diabetes mellitus with different levels of serum triglyceride. BMC Endocr Disord 2015; 15: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gastaldelli A, Ferrannini E, Miyazaki Y, et al Beta‐cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia 2004; 47: 31–39. [DOI] [PubMed] [Google Scholar]
- 26. Takahara M, Katakami N, Matsuoka TA, et al An inverse U‐shaped association of late and peak insulin levels during an oral glucose load with glucose intolerance in a Japanese population: a cross‐sectional study. Endocr J 2015; 62: 217–226. [DOI] [PubMed] [Google Scholar]
- 27. Kramer CK, Vuksan V, Choi H, et al Emerging parameters of the insulin and glucose response on the oral glucose tolerance test: reproducibility and implications for glucose homeostasis in individuals with and without diabetes. Diabetes Res Clin Pract 2014; 105: 88–95. [DOI] [PubMed] [Google Scholar]
- 28. Phillips DI, Clark PM, Hales CN, et al Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med 1994; 11: 286–292. [DOI] [PubMed] [Google Scholar]
- 29. Shen J, Chen Z, Chen C, et al Impact of incretin on early‐phase insulin secretion and glucose excursion. Endocrine 2013; 44: 403–410. [DOI] [PubMed] [Google Scholar]
- 30. Kahn SE, Montgomery B, Howell W, et al Importance of early phase insulin secretion to intravenous glucose tolerance in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 2001; 86: 5824–5829. [DOI] [PubMed] [Google Scholar]
- 31. Owens DR, Cozma LS, Luzio SD. Early‐phase prandial insulin secretion: its role in the pathogenesis of type 2 diabetes mellitus and its modulation by repaglinide. Diabetes Nutr Metab 2002; 15: 19–27. [PubMed] [Google Scholar]
- 32. Yabe D, Kuroe A, Watanabe K, et al Early phase glucagon and insulin secretory abnormalities, but not incretin secretion, are similarly responsible for hyperglycemia after ingestion of nutrients. J Diabetes Complications 2015; 29: 413–421. [DOI] [PubMed] [Google Scholar]
- 33. Ozhan B, Ersoy B, Kiremitci S, et al Insulin sensitivity indices: fasting versus glucose‐stimulated indices in pediatric non‐alcoholic fatty liver disease. Eur Rev Med Pharmacol Sci 2015; 19: 3450–3458. [PubMed] [Google Scholar]
- 34. U.K. Prospective Diabetes Study Group . U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes 1995; 44: 1249–1258. [PubMed] [Google Scholar]
- 35. Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta‐cell dysfunction. Endocr Rev 2008; 29: 351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Del Prato S. Role of glucotoxicity and lipotoxicity in the pathophysiology of Type 2 diabetes mellitus and emerging treatment strategies. Diabet Med 2009; 26: 1185–1192. [DOI] [PubMed] [Google Scholar]
- 37. Diabetes Prevention Program Research Group , Nathan DM, Connor EB, et al Long‐term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15‐year follow‐up: the diabetes prevention program outcomes study. Lancet Diabetes Endocrinol 2015; 3: 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kumar V, Madhu SV, Singh G, et al Post‐prandial hypertriglyceridemia in patients with type 2 diabetes mellitus with and without macrovascular disease. J Assoc Physicians India 2010; 58: 603–607. [PubMed] [Google Scholar]
- 39. Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 2005; 54: 1–7. [DOI] [PubMed] [Google Scholar]
- 40. Monnier L, Colette C. Postprandial and basal hyperglycaemia in type 2 diabetes: contributions to overall glucose exposure and diabetic complications. Diabetes Metab 2015; 41: 6s9–6s15. [DOI] [PubMed] [Google Scholar]
- 41. Ceriello A, Colagiuri S, Gerich J, et al Guideline for management of postmeal glucose. Nutr Metab Cardiovasc Dis 2008; 18: S17–S33. [DOI] [PubMed] [Google Scholar]
- 42. Halland M, Bharucha AE. Relationship between control of glycemia and gastric emptying disturbances in diabetes mellitus. Clin Gastroenterol Hepatol 2016; 14: 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bharucha AE, Kudva Y, Basu A, et al Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol 2015; 13: 466–476.e461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tomi S, Plazinska M, Zagorowicz E, et al Gastric emptying disorders in diabetes mellitus. Pol Arch Med Wewn 2002; 108: 879–886. [PubMed] [Google Scholar]
- 45. Dedik L, Durisova M, Penesova A, et al Estimation of influence of gastric emptying on shape of glucose concentration‐time profile measured in oral glucose tolerance test. Diabetes Res Clin Pract 2007; 77: 377–384. [DOI] [PubMed] [Google Scholar]
- 46. Baggio LL, Drucker DJ. Biology of incretins. GLP‐1 and GIP. Gastroenterology 2007; 132: 2131–2157. [DOI] [PubMed] [Google Scholar]


