Summary
We report a case of a 21-year-old African American female with history of pre-diabetes, and a diagnosis of a rare leukemia, blastic-plasmacytoid dendritic neoplasm (BPDCN), who developed diabetic ketoacidosis (DKA) after the third dose of PEG-asparaginase infusion. She was successfully treated with insulin. Asparaginase is a vital part of treatment protocols for acute lymphoblastic leukemia (ALL) in combination with other chemotherapeutic drugs. Asparaginase therapy has been reported to cause hyperglycemia especially when used in conjunction with glucocorticoids for the treatment of ALL in the pediatric population. Multiple mechanisms for hyperglycemia have been hypothesized which include decreased insulin secretion, impaired insulin receptor function and excess glucagon formation. Hyperglycemia is usually self-limiting but can deteriorate to diabetic ketoacidosis. DKA is a rare adverse effect with asparaginase therapy with an incidence rate of about 0.8%.
Learning points:
DKA is a rare finding following asparaginase therapy.
Hyperglycemia is most commonly seen with asparaginase treatment when used along with glucocorticoid.
Frequent blood glucose monitoring and prompt initiation of insulin treatment with hyperglycemia can prevent severe complications.
Patients and physician education on this complication can reduce morbidity due to DKA.
Background
Asparaginase is a vital part of treatment protocols for acute lymphoblastic leukemia (ALL) in combination with other chemotherapeutic drugs (1). Over the last few decades, the use of these chemotherapeutic agents has led to a decrease in mortality and disease-free survival in ALL. However, the use of these chemotherapeutic agents has also been associated with multiple side effects including allergic reactions, acute pancreatitis and hyperglycemia. Hyperglycemia is mostly noticed with asparaginase in conjunction with glucocorticoids. The reported incidence is about 10–15% (2). Hyperglycemia is usually self-limiting and resolves after treatment but it may persist and deteriorate to diabetic ketoacidosis (DKA). DKA is rare with asparaginase therapy and mostly reported in children with incidence of 0.8% (3). Here, we present a case of an adolescent presenting with DKA after PEG-asparaginase therapy.
Case presentation
A 21-year-old African American female with history of obesity, pre-diabetes, which was diagnosed on routine blood work with an Hgb A1c of 6.2% and a new diagnosis of a rare leukemia, blastic-plasmacytoid dendritic neoplasm (BPDCN), presented to the hospital with abdominal pain, nausea and vomiting after her third dose of PEG-asparaginase infusion.
Two months prior to her hospitalization, she was diagnosed with BPDCN and treated as a high-risk ALL. BPDCN is a rare and aggressive hematopoietic malignancy, which is highly responsive to chemotherapy treatment for ALL and not to CHOP therapy (cyclophosphamide, hydroxydaunorubicin, oncovin and prednisone) (4). Prior to starting treatment, patient was given a glucometer and advised to check blood glucose at least twice a day for close monitoring. She was not on any medication for diabetes mellitus prior to starting therapy. She was educated on the potential use of insulin to control hyperglycemia associated with chemotherapy especially with her underlying diagnosis of pre-diabetes (5). The induction chemotherapy regimen included intrathecal cytarabine, vincristine, daunorubicin, peg-asparaginase, methotrexate and prednisone. Her chemotherapy course was complicated by glucocorticoid-induced hyperglycemia, vincristine-induced neuropathy, hypertension, peri-rectal abscess and a moderate skin reaction to PEG-asparaginase. Hgb A1c was not measured. During chemotherapy she required insulin treatment. The insulin requirement decreased and was eventually discontinued upon stopping the prednisone treatment. At the end of her induction chemotherapy, she underwent a bone marrow evaluation, which showed relapse. She was then scheduled to undergo consolidation therapy with peg-asparaginase, methotrexate, six mercaptopurine and cytarabine.
Investigation
On her 3rd day of consolidation chemotherapy, she developed abdominal pain, nausea and vomiting. Her initial laboratory work up upon arrival to the emergency department (ED) showed a blood glucose of 693 mg/dL (normal range (NR): 60–99 mg/dL), anion gap: 28 mg/dL ((NR): 7–16 mg/dL), beta-hydroxybuterate: 4.18 mmol/L ((NR): 0.02–0.27 mmol/L) and bicarbonate: 15 ng/dL ((NR): 20–28 ng/dL) (Fig. 1). Her Hgb A1c was 8.0% ((NV): <5.7%). Patient had also been evaluated for possible underlying infection with all results coming back negative.
Figure 1.
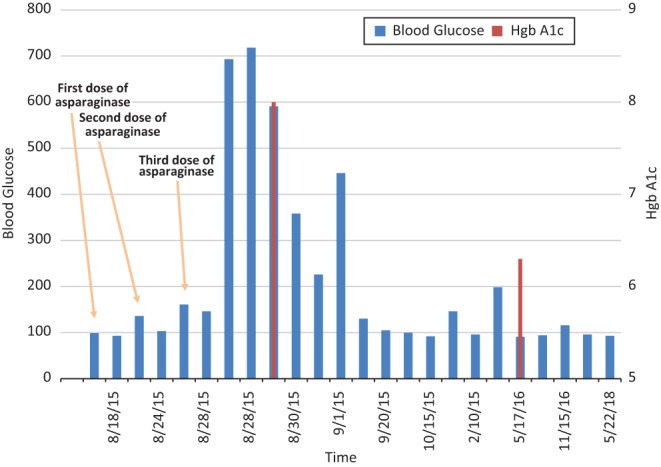
Timeline of the measurement of blood glucoses before, during and after consolidation chemotherapy.
Treatment
In the ED, she was treated with aggressive intravenous normal saline and insulin drip. She was admitted to the intensive care unit. Her blood glucose normalized and the anion gap closed on the insulin drip. She was then transitioned to a basal-bolus insulin regimen.
Outcome/follow-up
On follow-up, she refused any further treatment with PEG-asparaginase and insulin. Her blood glucose stabilized off insulin about 2 weeks after her last treatment with PEG-asparaginase. She then underwent bone marrow transplant 4 weeks later. To date, her blood glucose has been stable and no longer requires glucocorticoid treatment for bone marrow transplant.
Discussion
Asparaginase is the main stay of treatment in ALL. The two most common asparaginase preparations are l-asparaginase and PEG-asparaginase (1). Hyperglycemia is common with the use of asparaginase treatment. The incidence of hyperglycemia range from 2.5% to 23% in the pediatric population, with an incidence as high as 76% in adults with PEG-asparaginase use (1). Hyperglycemia tends to resolve within 12 days after the last dose (6).
Several mechanisms have been proposed to explain the cause of hyperglycemia (Fig. 2). The hallmark has been decreased insulin secretion, increased insulin resistance and excess glucagon formation (3, 7). Gailani et al. showed hyperglycemia associated with decreased insulin levels in patients treated with asparaginase for ALL. He postulated that asparagine is the key molecule in insulin structure as one insulin molecule has three asparagine molecules. l-Asparaginase is thought to deplete asparagine and other proteins, thus, deficiency of the substrate leads to decreased insulin synthesis (8). Another possible mechanism of decreased insulin synthesis is direct pancreatic beta cell damage due to acute pancreatitis, thus leading to hyperglycemia and possibly diabetes mellitus. The reported incidence of acute pancreatitis with asparaginase treatment is about 2–18% (9).
Figure 2.
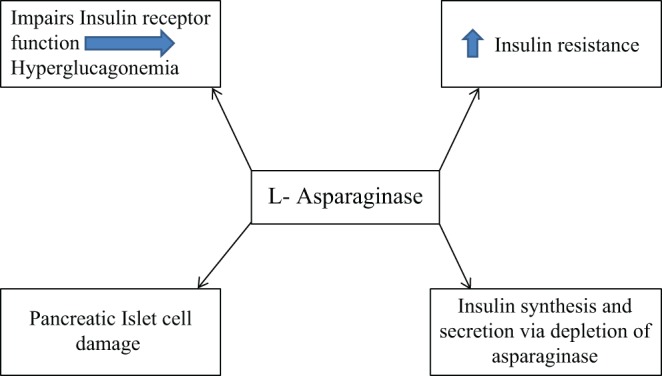
Proposed mechanism of action for hyperglycemia with l-asparaginase therapy.
Hyperglycemia with associated hyperinsulinemia was observed in animal studies. Lavine et al. showed that a single dose of l-asparaginase in rabbits acutely induced hyperglycemia and hyperinsulinemia. This was followed by an insulin tolerance test where intravenous insulin administration showed a robust decrease in glucose levels in the control rabbits as compared to those treated by l-asparaginase, thus suggesting a possible mechanism of insulin resistance. However, the same effect was not seen with long-term exposure to asparaginase treatment signifying that the duration of treatment may have a different impact on insulin function and secretion (10). This can explain our patient’s initial insulin-resistant stage of hyperglycemia following induction treatment, with later development of DKA, suggesting insulin deficiency state. Furthermore, the combination treatment with glucocorticoids, causing insulin resistance, thus another mechanism contributing to hyperglycemia in patients being treated for ALL (11).
However, hyperglycemia is not observed in all patients treated with asparaginase. Studies have identified risk factors predisposing to hyperglycemia; these include a history of impaired glucose tolerance, age >10 years, obesity, family history of diabetes mellitus and history of Down syndrome (6, 11).
Even though hyperglycemia is a common manifestation with asparaginase treatment, with or without glucocorticoid therapy, DKA is rarely seen. There are at least six case reports of DKA after asparaginase therapy treated for ALL (Table 1) (1, 2, 6, 9, 12, 13), only three of them are reported in adolescents more than 20 years old (3, 11, 14). Robertson et al. reported six cases of DKA in 797 pediatric patients treated with asparaginase for ALL (3). All patients required insulin treatment without long-term sequelae (3). DKA was observed in children older than 10 years. This is similar to our patient who was older than ten and required insulin treatment initially for management of DKA, followed by an overall clinical improvement without the need of long-term treatment. However, persistent hyperglycemia and recurrent DKA has also been reported. Wang et al. reported recurrent DKA episodes in patients being treated for ALL; however, the recurrences were related to an underlying infection in the setting of continuous asparaginase use (1).
Table 1.
Reported cases of DKA treated with asparaginase for ALL.
| Case report | Asparaginase | DKA/hyperglycemia | Risk factor* |
|---|---|---|---|
| Our patient | PEG-asparaginase | DKA | Obese; Age: 21 years |
| (1) | l-Asparaginase | 1 case of DKA | Obese; Age: 10 years |
| (6) | l-Asparaginase | 1 case of DKA | Age: 11 years |
| (9) | l-Asparaginase | DKA | Age: 16 years |
| (2) | l-Asparaginase | DKA | Age: 16 years; FH of DM |
| (12) | l-Asparaginase | DKA | Age: 25 years |
| (13) | l-Asparaginase | DKA | Age: 21 years |
*Risk factors are Obesity, Family history (FH) of DM, Age > 10 years and Down syndrome
We believe that our patient developed DKA due to persistent hyperglycemia after induction chemotherapy along with her previously known risks of pre-diabetes, family history of diabetes and obesity. Her blood glucose monitoring was poor after induction chemotherapy likely missing hyperglycemia over the last few months as reflected by Hgb A1c of 8.0% at the time of DKA.
Conclusion
Asparaginase is commonly being used for treatment of ALL. Hyperglycemia is a well-known documented complication and may develop in patients treated with asparaginase therapy, but DKA is a rare event. The occurrence of DKA does not require alteration to treatment for ALL in most cases as the hyperglycemia resolved after chemotherapy. Persistent hyperglycemia is seldom observed especially in the setting of risk factors and or underlying infection or pancreatic inflammation.
Therefore, we encourage clinicians to educate patients on symptoms of hyperglycemia and close monitoring of blood glucoses during their chemotherapy treatment to prevent development of DKA and to prompt initiation of insulin treatment when needed.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Consent was obtained from patient for publication of the submitted article.
Author contribution statement
Miriam Ahmad: She contributed in writing the paper and research of the subject. Ismat Shafiq: She also contributed to the writing of the paper and research of the paper.
References
- 1.Wang YJ, Chu HY, Shu SG, Chi CS. Hyperglycemia induced by chemotherapeutic agents used in acute lymphoblastic leukemia: report of three cases. Chinese Medical Journal 1993. 51 457–461. [PubMed] [Google Scholar]
- 2.Gifford G, Milliken S, Greenfield J. Diabetic ketoacidosis secondary to L-asparaginase in acute lymphoblastic leukaemia. Internal Medicine Journal 2013. 43 946–948. ( 10.1111/imj.12216) [DOI] [PubMed] [Google Scholar]
- 3.Roberson JR, Raju S, Shelso J, Pui CH, Howard SC. Diabetic ketoacidosis during therapy for pediatric acute lymphoblastic leukemia. Pediatric Blood and Cancer 2008. 50 1207–1212. ( 10.1002/pbc.21505) [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Wang X, Liu M, Bai O. Blastic plasmacytoid dendritic cell neoplasm: update on therapy especially novel agents. Annals of Hematology 2018. 97 563–572. ( 10.1007/s00277-018-3259-z) [DOI] [PubMed] [Google Scholar]
- 5.Earl M. Incidence and management of asparaginase-associated adverse events in patients with acute lymphoblastic leukemia. Clinical Advances in Hematology and Oncology 2009. 7 600–606. [PubMed] [Google Scholar]
- 6.Mondal R, Nandi M, Tiwari A, Chakravorti S. Diabetic ketoacidosis with L-asparaginase therapy. Indian Pediatrics 2011. 48 735–736. ( 10.1007/s13312-011-0131-9) [DOI] [PubMed] [Google Scholar]
- 7.Pui CH, Burghen GA, Bowman WP, Aur RJ. Risk factors for hyperglycemia in children with leukemia receiving L-asparaginase and prednisone. Journal of Pediatrics 1981. 99 46–50. [DOI] [PubMed] [Google Scholar]
- 8.Gailani S, Nussbaum A, Onuma T, Freeman A. Diabetes in patients treated with asparaginase. Clinical Pharmacology and Therapeutics 1971. 12 487–490. ( 10.1002/cpt1971123487) [DOI] [PubMed] [Google Scholar]
- 9.Quintanilla-Flores DL, Flores-Caballero MA, Rodriguez-Gutierrez R, Tamez-Perez HE, Gonzalez-Gonzalez JG. Acute pancreatitis and diabetic ketoacidosis following L-asparaginase/prednisone therapy in acute lymphoblastic leukemia. Case Reports in Oncological Medicine 2014. 2014 139169 ( 10.1155/2014/139169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavine RL, DiCinto DM. L-Asparaginase diabetes mellitus in rabbits: differing effects of two different schedules of L-asparaginase administration. Hormone and Metabolic Research 1984. 16 (Supplement 1) 92–96. ( 10.1055/s-2007-1014907) [DOI] [PubMed] [Google Scholar]
- 11.Lowas SR, Marks D, Malempati S. Prevalence of transient hyperglycemia during induction chemotherapy for pediatric acute lymphoblastic leukemia. Pediatric Blood and Cancer 2009. 52 814–818. ( 10.1002/pbc.21980) [DOI] [PubMed] [Google Scholar]
- 12.Lam T, Chipps D, Gunton JE. Diabetic ketoacidosis secondary to L-asparginase and dexamethasone during treatment for acute lymphoblastic leukaemia. Journal of Diabetes and Metabolism 2014. 5 422 ( 10.4172/2155-6156.1000422) [DOI] [Google Scholar]
- 13.Hsu YJ, Chen YC, Ho CL, Kao WY, Chao TY. Diabetic ketoacidosis and persistent hyperglycemia as long-term complications of L-asparaginase-induced pancreatitis. Chinese Medical Journal 2002. 65 441–445. [PubMed] [Google Scholar]
- 14.Gatzioura I, Papakonstantinou E, Dimitriadou M, Kourti M, Sidi V, Triantafyllou P, Koliouskas D, Christoforidis A. Glucose levels before the onset of asparaginase predicts transient hyperglycemia in children with acute lymphoblastic leukemia. Pediatric Blood and Cancer 2016. 63 1181–1184. ( 10.1002/pbc.25956) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a