Abstract
Aims/Introduction
To examine the magnitude and pattern of cognitive dysfunction in children with type 1 diabetes, and the possible effects associated with other disease variables, such as early onset diabetes, severe hypoglycemia and hyperglycemia.
Materials and Methods
We carried out a meta‐analysis using the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis guidelines. We searched MedLine, Embase and PsycINFO to identify studies on cognitive function in children with type 1 diabetes that were published up until 30 September 2016. Effect sizes understood as the standardized mean differences between groups with diabetes and control groups (i.e., Hedges’ g) were calculated to quantify the extent of cognitive dysfunction in those groups consisting of children with diabetes.
Results
A total of 19 studies met our inclusion criteria, comprising 1,355 participants with type 1 diabetes and 696 controls. Compared with non‐diabetic controls, children with type 1 diabetes showed a significantly poorer cognitive performance overall (g = −0.46), as well as specific deficits in full‐scale intelligence (g = −1.06), attention (g = −0.60) and psychomotor speed (g = −0.46). Glycemic extremes were associated with poorer overall cognition (g = −0.18), as well as slightly lower performance in memory (g = −0.27).
Conclusions
We found that type 1 diabetes was associated with cognitive dysfunction characterized by a lowered intelligence, diminished attention and a slowing of psychomotor speed. Glycemic extremes, which are described as a period of high glucose levels and severe hypoglycemia, were related to cognitive dysfunction in children with type 1 diabetes.
Keywords: Children, Cognitive function, Type 1 diabetes
Introduction
The incidence of type 1 diabetes varies from 0.1 to 36.8 per 100,000 and is increasing worldwide1. Many studies have shown that type 1 diabetes affects cognitive development. Children with type 1 diabetes, compared with non‐diabetic controls, have somewhat lower cognitive performance across most cognitive domains; principally, intelligence, memory, executive function, attention and psychomotor speed2, 3, 4, 5, 6, 7, 8, 9. Not all studies, however, have shown such cognitive dysfunctions in children with type 1 diabetes when compared with non‐diabetic controls10, 11. Debate continues regarding the presence and extent of both general cognitive dysfunction and deficits in specific domains of functioning. These inconsistencies might be due to the differences in study design, measures for assessment of cognitive function, sampling procedures and the heterogeneity of glycemic variability in type 1 diabetes12.
Several studies have documented the risk factors associated with cognitive deficits in children with type 1 diabetes3, 4, 5. Recent years have seen the publication of several meta‐analyses on cognitive function in children with type 1 diabetes2, 13, 14, yielding several important findings. For example, Gaudieri reported that in children with early‐onset diabetes (EOD) was associated with poorer performance across most cognitive domains2. Tonoli et al.14 reported severe hypoglycemia, chronic hyperglycemia and EOD could be significant factors impacting cognitive performance in patients with type 1 diabetes.
To summarize, one consistent finding is EOD is associated with lower cognitive performance. Many studies have shown that severe hypoglycemic episodes or chronic hyperglycemia was associated with cognitive deficits, but the associations are less consistent3, 15, 16, 17, 18. Furthermore, through clinical observation, we found that the illness‐related risks, such as early‐onset diabetes, severe hypoglycemia and chronic hyperglycemia did not exist alone; that is, individual participants might possess more than one risk factor, and these factors might have synergistic effects. Most studies mainly explored the independent impact of illness‐related risk factors on cognitive function, without considering the possible synergistic effect between different risk factors15, 16, 17. It remains unclear whether cognitive impairment is more obvious among young people with more than one risk factor.
Using meta‐analysis to summarize data across studies, the primary aim of the current meta‐analysis was to investigate the pattern and magnitude of cognitive dysfunction in children with type 1 diabetes compared with non‐diabetic controls. The secondary aim was to evaluate the possible synergistic effects of severe hypoglycemia and chronic hyperglycemia on cognitive deficits in children with type 1 diabetes. In addition, due to the decreased ability to recognize and report symptoms, and behavioral issues, children with EOD have a relatively high risk of severe hypoglycemic episodes. So we also examined the possible effects of EOD and severe hypoglycemia on cognitive outcomes.
Materials and Methods
The present meta‐analysis was guided and reported in accordance with the Preferred Reporting Items for Systematic Reviews and meta‐analysis guidelines.
Data sources and searches
PubMed (MEDLINE), EMBASE and PsycINFO electronic databases were searched for relevant articles published between 1 January 1985 and 30 September 2016, using MeSH and Entrez terms and keywords. Search terms included: ‘diabetes mellitus,’ ‘diabetes,’ ‘hyperglycemia,’ ‘hypoglycemia,’ ‘children,’ ‘adolescents,’ ‘youths,’ ‘cognition,’ ‘cognitive function,’ ‘executive functioning,’ and ‘learning and memory.’ We also considered related reviews to identify additional studies.
Study selection
Two authors examined all titles and abstracts, and reviewed potential eligible studies independently. Any disagreements between abstracts and the whole text were adjudicated by a third reviewer, who made the decision to either include or exclude the study. We included studies that fulfilled the following criteria: (i) results were published in English before 30 September 2016; (ii) participants were children aged <18 years without any other physiological and mental disease; (iii) the study protocol included at least one measure of cognitive function, such as intelligence, memory, attention, psychomotor speed and so on; (iv) inclusion of a control group matched for at least age; and (v) the published manuscript provided sufficient data for the calculation of effect sizes, or the authors were willing to provide these data. If the same dataset was published in several articles, we opted to select the one with the most complete information.
Data extraction and quality assessment
For each included study, the following data were extracted: study characteristics (publication year, name of author, country); study design; sample characteristics (sample size, sex, age, country, age of diabetes onset); and raw scores of cognitive function tests, including means and standard deviations of the diabetes and non‐diabetes samples. We classified cognitive function into more specific cognitive domains of intelligence (full scale intelligence, verbal intelligence, performance intelligence); memory (verbal memory, visual memory and spatial memory); attention; executive function; and psychomotor speed. Additionally, some studies included a cognitive test not fitting any of these domains; these were allocated to a category of other cognitive functions. When the raw scores, including means and standard deviations, were not reported in the published articles, authors of these studies were contacted to supply those results. Three articles did not report means and standard deviations, and the authors did not reply to our contact efforts. As such, these data were excluded.
Selection of measures of cognition
We used a recursive strategy to select measures of cognition in the articles19. First, all studies were included if they met the inclusion criteria. Next, we categorized the cognitive tasks into specific domains, which we selected for this meta‐analysis: intelligence (full scale intelligence, verbal intelligence, performance intelligence); memory (verbal memory, visual memory and spatial memory); attention; executive function; and psychomotor speed (see Table S1). In some studies, multiple cognitive tasks were used to measure the same cognitive dimension. Following Cheung20, we selected one variable per independent sample per cognitive dimension and achieved maximum homogeneity in each dimension in selecting variables. In order to avoid the ceiling effects in the measures, and taking into account the best discrimination between the groups, we selected the parameters with higher difficulty. When we faced a choice of selection between error rates and reaction times in some studies, we chose reaction times for the meta‐analysis following Salthouse20.
Quality assessment
We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines21 and used Agency for Health‐care Research and Quality to assess the quality of the studies included in the meta‐analysis. Specifically, we evaluated whether each study: (i) defined the source of information; (ii) listed inclusion and exclusion criteria for the group with diabetes and healthy control group; (iii) described assessments undertaken for quality assurance purposes; (iv) summarized the response rates of patients and the integrity of the data collection; (v) clarified what follow up, if any, was expected, and the percentage of patients for which incomplete data or follow up was obtained; and (vi) used appropriate statistical methods. Characteristics of studies, and inclusion and exclusion criteria for the diabetes group and control group are presented in Table 1.
Table 1.
Characteristics of 19 studies in children with type 1 diabetes and control groups
| First author (ref.) | n | Females (%) | Ethnicity (%) (W/H/A/O) | Age (years) | HbA1c (%) | Included complications/characteristics | Exclusion criteria/absent characteristics | Participants matched for |
|---|---|---|---|---|---|---|---|---|
| Group 1/group 2 | Group 1/group 2 | Group 1/group 2 | Group 1/group 2 | Group 1/group 2 | ||||
| Ryan38 | 125/83 | 46.4/51.8 | 100 | 15.0 ± 2.1/14.7 ± 2.4 | NR | Hy | NR | A, G, E, SES |
| Reich37 | 24/14 | 54.2/57.1 | NR | 11.20/11.2 | NR | Hy | CI, NI | A, G, SES |
| Bjørgaas36 | 28/28 | NR | NR | 12.9 ± 2.00/13.00 ± 2.0 | 8.5 ± 1.2 | Hy | P, NI, MI | A, G,E, I |
| Rovet40 | 16/0 | 37.5 | NR | 12.1 ± 2.6 | 8.4 | Hy | NR | A |
| Hershey35 | 25/16 | NR | NR | 14.5 ± 2.7/14.7 ± 2.8 | 9.1 | Hy | NR | A, PE |
| Kaufman34 | 55/15 | 49.1/60.0 | NR | 7.9 ± 1.6/7.5 ± 1.8 | 7.8 ± 1.1 | Hy | CI | A, G |
| Northam4 | 90/84 | 50.0/52.4 | NR | 12.1 ± 2.9/12.1 ± 2.8 | 4.5–5.7 | Hy | CI, NI, NES | A, SES |
| Hannonen5 | 21/10 | 57.1/50.0 | NR | 9.5 ± 2.1/9.3 ± 1.8 | 8.3 ± 1.9 | Hy, DKA | NR | A, G, PE |
| Hershey6 | 50/32 | NR | NR | 11.8 ± 2.8/11.5 ± 2.8 | NR | Hy, Hp | R, Ne, N, P, MI | A, G, PE |
| Wysocki41 | 142/0 | 44.4 | NR | 11.6 ± 2.7 | 8.1 ± 1.0 | Hy | NR | A |
| Strudwick33 | 84/0 | NR | NR | 10.3 ± 2.5 | 8.3 ± 1.0 | Hy | P, NI, MI | A |
| Hershey10 | 103/60 | NR | NR | 12.8 ± 3.0/12.8 ± 3.0 | 8.3 ± 0.9 | Hy | CI, MI, NI | A, G, E, SES |
| Perantie3 | 117/58 | 44.4/48.3 | NR | 12.1 ± 3.0/11.4 ± 3.2 | 8.5 ± 0.6 | Hy | R, Ne, N, P, CI | A,PE |
| Patiño‐Fernández32 | 36/32 | 58.3/43.8 | 40/49/6/5 | 4.7 ± 1.5/4.1 ± 1.2 | 8.4 | Hy, | P,MI | A,G,E |
| Aye31 | 28/17 | 50.0/41.2 | NR | 7.0 ± 1.4/7.2 ± 1.6 | 7.6 ± 1.0 | Hy | ND | A |
| Lin39 | 106/75 | 49.0/51.0 | NR | 8.5 ± 4.3/9.0 ± 3.8 | 9.2 ± 1.8 | Hy, Hp | CNS, T | A, G, SES |
| Cato30 | 144/72 | 46.0/47.0 | 80/7/4/7; 86/6/6/3 | 7.0 ± 1.7/6.9 ± 1.8 | 7.9 ± 0.9 | Hy, DKA | PB, L, P, NI | A, G, PE, I |
| Lin42 | 95/67 | 48.4/50.7 | NR | 8.6 ± 3.3/9.1 ± 3.4 | 9.0 | Hy, DKA | NI | A, G, SES |
| Semenkovich16 | 66/33 | 42.0/48.0 | NR | 11.9 ± 2.6/11.9 ± 2.7 | 6.6 ± 0.9 | Hy, DKA | P, Hyp, PB | A, G, SES |
Data are mean ± standard deviation, unless stated otherwise.
A, age; CI, chronic illness; CNS, center nervous system disease; DKA, diabetic ketoacidosis; E, education; G, gender; Group 1/group 2, type 1 diabetes group/control group; HbA1c, glycated hemoglobin; Hp, hyperglycemia; Hy, hypoglycemic episodes; Hyp, hypertension; I, income level; L, low birthweight; MI, mental illness; N, neuropathy; Ne, nephropathy; NES, non‐English Speaking; NR, not reported; P, psychological disorder; PB, premature birth <36 weeks; PE, parents’ education; NI, nervous illness; R, retinopathy; SES, socioeconomic status; T, trauma; W/H/A/O, white, Hispanic, African American, other.
Statistical analysis
Statistical analyses were carried out using stata 12.0 software (Statacrop LP, College Station, TX, USA). The most used measurement was the standardized mean difference, which showed the difference in cognitive function between the group with diabetes and control group for each study21. We estimate the standardized mean difference using Hedges’ g 22, 23.
We used a random effects model in which each study is weighted by the inverse of its variance to pool the individual effect size24. The direction of the effect size showed that high scores mean good performance or high scores mean bad performance. In the subgroup meta‐analysis, given the limited number of studies referring to the relationship between diabetes‐related variables and cognitive function, we combined the cognitive domains of verbal memory, visual memory and spatial memory into the overall domain of memory25. Examination of the effect size (Hedges’ g) determines the magnitude of differences across cognitive domains.
Heterogeneity across the studies was measured by I² statistics. I² is defined as the percentage of variation of the total variation across studies that are due to heterogeneity rather than sampling error26. If the test of heterogeneity is significant, we will use subgroup analyses or sensitivity analyses to explore the sources of the heterogeneity26. Publication bias was measured by Egger's test27 and Begg's test28. All cognitive tests were carried out with a Benjamini and Hochberg false discovery rate correction for P‐value (P < 0.05)29. A P‐value of <0.05 was set as statistically significant.
Results
Study characteristics
Following our search strategy, 134 potential studies were detected by screening. However, 115 studies were excluded because their participants were aged >18 years, lacked control groups, did not report standard scores for the cognitive function tests or the same dataset was published elsewhere (Figure 1).
Figure 1.

Flow chart of the screening process.
A total of 19 published studies met the inclusion criteria. The combined sample size of these studies was 2,031 (group with diabetes: n = 1,355, control group without diabetes: n = 696). The age of the participants ranged from 4 to 18 years, and the age of the control groups had been matched with the groups with diabetes. Studies were carried out in six countries: the USA (n = 12); Australia (n = 4); Canada (n = 1); Finland (n = 1); and Norway (n = 1). Cross‐sectional studies were the most common3, 4, 5, 10, 18, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39. There were four longitudinal studies6, 40, 41, 42 that studied the relationship between type 1 diabetes and cognitive decline over time. The average age at evaluation across all participants was approximately 12 years, and the average age at diabetes onset was 6 years.
Study characteristics are presented in Tables 1 and 2. Four studies reported ethnic minorities; 14 studies matched participants for age, sex and education; and four studies matched for age only. Most studies in Table 1 showed heterogeneity of disease variables, including duration of diabetes, and diabetes complications (hypoglycemic episodes or hyperglycemia). Three studies did not report the duration of diabetes. Table S1 describes the most commonly reported tests in each cognitive domain.
Table 2.
Characteristics of 10 studies in participants with severe hypoglycemia and without severe hypoglycemia
| First author (ref.) | n | Age (years) | Age at onset (years) | Duration (years) | HbA1c (%) | Included complications/characteristics | Exclusion criteria/absent characteristics | Participants matched for |
|---|---|---|---|---|---|---|---|---|
| Group 1/group 2 | Group 1/group 2 | Group 1/group 2 | Group 1/group 2 | Group 1/group 2 | ||||
| Bjørgaas36 | 15/13 | 12.9 ± 2.0/13.0 ± 2.0 | 6.9 ± 2.3/10 ± 2.0 | 6.0 ± 2.3/2.9 ± 1.9 | 9.0 ± 1.5/8.0 ± 0.9 | Hy | P, NI, MI | A, G, E, I |
| Rovet40 | 9/7 | 12.1 ± 2.6 | 4.5 ± 3.0 | 6.5 ± 2.8 | 8.4 | Hy | NR | NR |
| Kaufman34 | 8/47 | 7.9 ± 1.6 | 4.5 ± 2.1 | 2.6 ± 2.0 | 7.8 ± 1.1 | Hy | CI | A |
| Hannonen5 | 11/10 | 9.6 ± 2.2/9.1 ± 1.9 | 3.32 ± 1.45/5.40 ± 2.38 | 6.2 ± 2.5/3.7 ± 1.6 | 8.3 ± 1.9/8.4 ± 2.0 | Hy, DKA | NR | A,G, PE |
| Hershey6 | 34/16 | 11.6 ± 2.7/12.1 ± 2.9 | 6.0 ± 3.2/9.1 ± 3.1 | 5.6 ± 3.0/3 ± 3.0 | NR | Hy, Hp | R, Ne, N, P, MI | A, G, PE |
| Wysocki41 | 58/84 | 11.6 ± 2.7 | 6.6 ± 2.8 | 5.0 ± 2.9 | 8.1 ± 1.0 | Hy | NR | A |
| Hershey10 | 63/40 | 12.5 ± 2.3/13.4 ± 2.8 | 7.1 ± 3.1/10.2 ± 3.6 | 5.4 ± 2.8/3.3 ± 1.6 | 8.6 ± 0.8/8.1 ± 1.0 | Hy | CI, MI, NI | A, G, E, SES |
| Strudwick33 | 41/43 | 10.3 ± 2.5/10.0 ± 2.4 | 3.1 ± 1.1/3.4 ± 1.5 | 7.3 ± 2.9/6.6 ± 2.5 | 8.3 ± 1.0/8.0 ± 0.9 | Hy | P, NI, MI | A |
| Perantie3 | 67/50 | 12.5 ± 3.0/11.7 ± 2.9 | 6.2 ± 3.0/7.7 ± 3.1 | 6.3 ± 3.0/4.0 ± 3.0 | 8.5 ± 0.6/8.4 ± 0.8 | Hy | R, Ne, N, P, CI | A, PE |
| Lin42 | 41/54 | 8.6 ± 3.3 | NR | NR | 9.0 | Hy, DKA | NI | A, SES |
Data are mean ± standard deviation, unless stated otherwise. A, age; CI, chronic illness; CNS, center nervous system disease; DKA, diabetic ketoacidosis; E, education; G, gender; Group 1/group 2, hypoglycemia group/no hypoglycemia group; HbA1c, glycated hemoglobin; Hp, hyperglycemia; Hyp, hypertension; I, income level; L, low birthweight; MI, mental illness; N, neuropathy; Ne, nephropathy; NI, nervous illness; NES, non‐English Speaking; NR, not reported; P, psychological disorder; PB, premature birth <36 weeks; PE, parents education; R, retinopathy; SES, socioeconomic status.
We report effect sizes for each comparison involving >250 children with type 1 diabetes. The results showed that on each domain there is a difference between the group with diabetes and the healthy control group. Figure 2 shows the forest plots from the meta‐analysis. Compared with non‐diabetic controls, the children with type 1 diabetes showed a significantly poorer performance on overall cognition (g = −0.46, P < 0.01), which included general intelligence (full‐scale intelligence quotient [FSIQ], verbal intelligence quotient [VIQ] and performance intelligence quotient [PIQ]), memory, attention, executive function and psychomotor speed. The effect sizes for FSIQ (g = −1.08, P = 0.030) approached a large difference (i.e., g >−0.90). The effect sizes for attention (g = −0.60, P = 0.047) and psychomotor speed (g = −0.46, P = 0.020) were in moderate range (i.e., −0.80 < g < −0.20), in contrast to VIQ (g = −0.95, P = 0.101), PIQ (g = −0.50, P = 0.134), verbal memory (g = −0.09, P = 0.649), visual memory (g = 0.34, P = 0.418), spatial memory (g = −0.81, P = 0.195) and executive function (g = 0.02, P = 0.943), where there was almost no difference between two groups.
Figure 2.
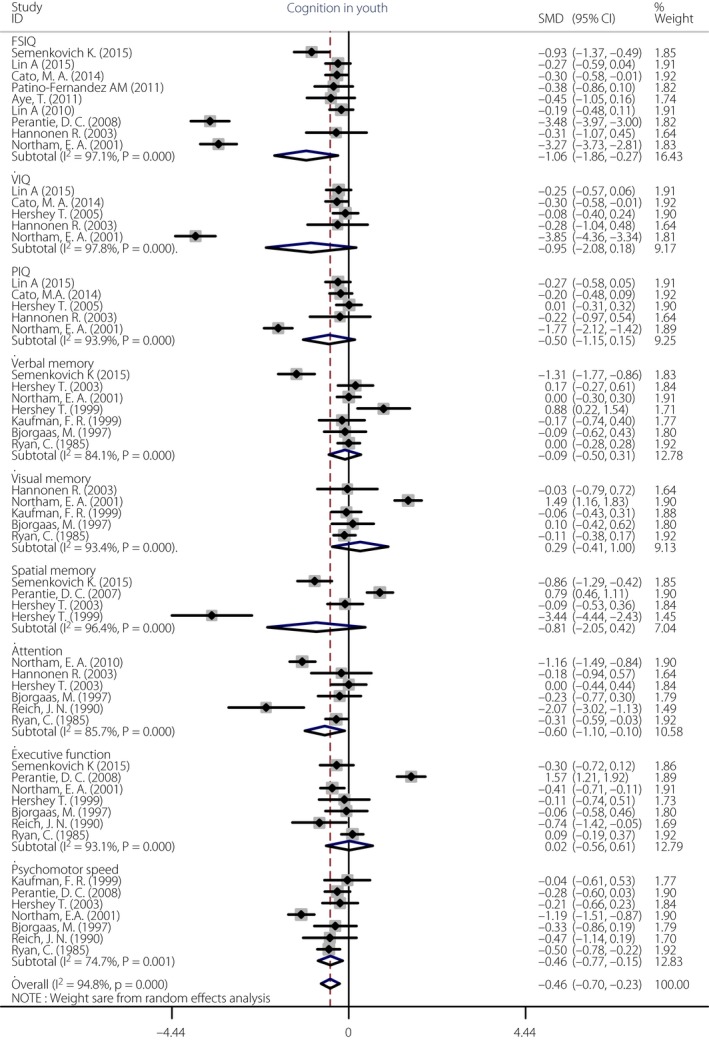
Forest plot of the cognitive domains in children with type 1 diabetes compared with healthy control groups.
The test of overall heterogeneity was significant (I² = 94.8%, P < 0.001), so we used sensitivity analysis to explore the source of heterogeneity. The results of sensitivity analysis showed that four relevant studies3, 4, 16, 34 were the source of heterogeneity. When we removed these studies and repeated the analysis, the test for heterogeneity was no longer statistically significant (I² = 32.9%, P = 0.033). After removing these studies, overall cognition (g = −0.19, P < 0.01), as well as FSIQ (g = −0.280, P < 0.01) and psychomotor speed (g = −0.413, P < 0.01), the effect sizes were still significant. In other cognitive domains, such as PIQ (g = −0.161, P = 0.065), memory (g = 0.033, P = 0.689), attention (g = −0.433, P = 0.066), executive function (g = −0.114, P = 0.479), the effects were not significant (Figure S1).
Subgroup meta‐analysis
Severe hypoglycemia group vs non‐hypoglycemia group
Subgroup meta‐analysis was carried out for the difference of cognitive domains between children with severe hypoglycemic episodes compared with children without hypoglycemic episodes in the included studies. Severe hypoglycemic episodes in this meta‐analysis were defined as events with neurological dysfunction including seizures, loss of consciousness, inability to arouse from sleep and/or those that required the assistance of someone other than the patient for treatment41. A total of 10 studies described the impact of severe hypoglycemic episodes on cognitive function3, 5, 6, 10, 33, 34, 36, 40, 41, 42. The severe hypoglycemia group included those who had at least one severe hypoglycemic episode, and the non‐hypoglycemia group included those with no hypoglycemic episodes. The performance of type 1 diabetes participants with severe hypoglycemia (n = 347) was compared with those without hypoglycemic episodes (n = 364). Figure 3 shows that children with severe hypoglycemic episodes were somewhat more impaired in overall cognition (g = −0.18, P = 0.020) than children without hypoglycemia, and showed slightly lower performance in memory (g = −0.25, P = 0.032). In other cognitive domains, such as FSIQ (g = −0.09, P = 0.170), VIQ (g = −0.04, P = 0.378), PIQ (g = −0.01, P = 0.834), attention (g = −0.10, P = 0.901), executive function (g = −0.43, P = 0.366) and psychomotor speed (g = −0.10, P = 0.688), the effects were not significant. The test of overall heterogeneity was significant (I² = 52.3%, P < 0.001). When we used sensitivity analysis to remove the study3 and repeated the analysis, the test for heterogeneity was no longer significant (I² = 31.8%, P > 0.05). The differences of cognition between the two groups were still significant (g = −0.123, P = 0.028).
Figure 3.
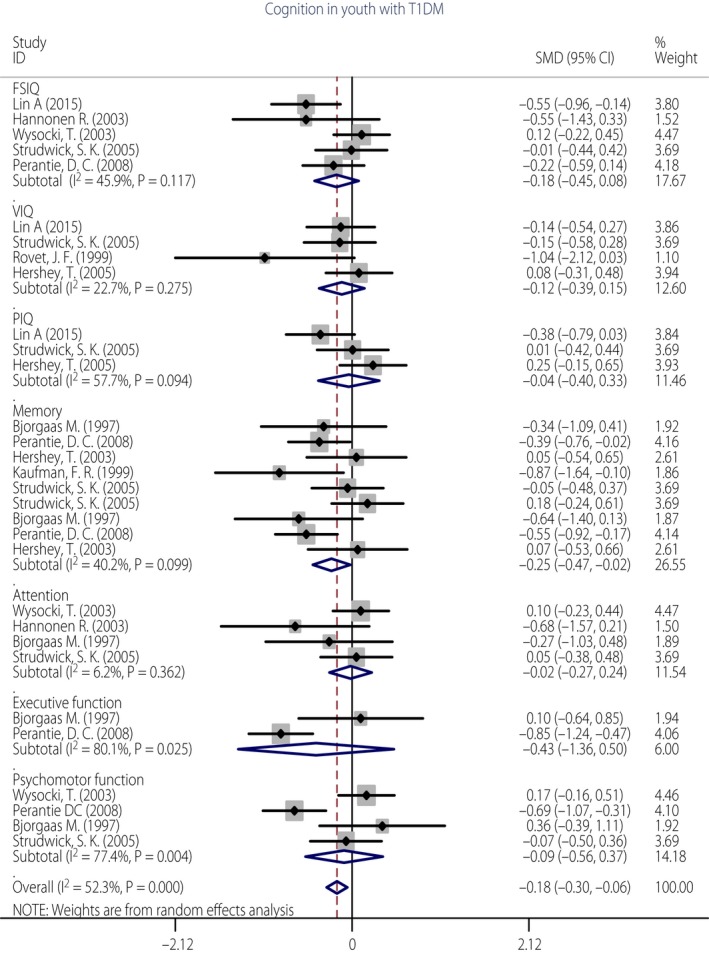
Forest plot of the association of severe hypoglycemia in cognitive domains of children with type 1 diabetes.
Effect of glycemic extremes
Glycated hemoglobin (HbA1c) is a measure of blood glucose index over the preceding 6–8 weeks and is used as an index of chronic hyperglycemia. Higher HbA1c values indicate average higher glucose levels, with values >8% generally taken as an indication of poor control43. A period of high glucose levels with severe hypoglycemic episodes could be a confounding factor, which is described as glycemic extremes. A separate analysis was carried out for the differences on cognitive domains between groups with high levels of HbA1c (>8.0%) with and without hypoglycemic episodes. In eight studies included in this research3, 5, 10, 33, 36, 40, 41, 42, the diabetes group was classified into two groups according to the presence of hypoglycemic episodes and the average of HbA1c values. The performance of type 1 diabetes participants with high levels of HbA1c (>8.0%) experiencing hypoglycemic episodes (n = 305) was compared with those with high levels of HbA1c (>8.0%) not experiencing hypoglycemic episodes (n = 301). As shown in the subgroup comparison of cognitive domains in Figure 4, hypoglycemic episodes in the presence of higher blood glucose levels (HbA1c >8.0%) were associated associated with poorer overall cognition (g = −0.18, P = 0.020), as well as slightly lower performance in memory (g = −0.27, P = 0.041). In other cognitive domains, such as FSIQ (g = −0.09, P = 0.170), VIQ (g = −0.04, P = 0.378), PIQ (g = −0.01, P = 0.834), attention (g = −0.10, P = 0.901), executive function (g = −0.44, P = 0.366) and psychomotor speed (g = −0.10, P = 0.688), the effects were not significant. The test of overall heterogeneity was significant (I² = 53.8%, P < 0.001). When we removed the study3 and repeated the analysis, the test for heterogeneity was no longer significant (I² = 34.0%, P > 0.05). The differences of cognition between the two groups were still significant (g = −0.123, P = 0.028).
Figure 4.
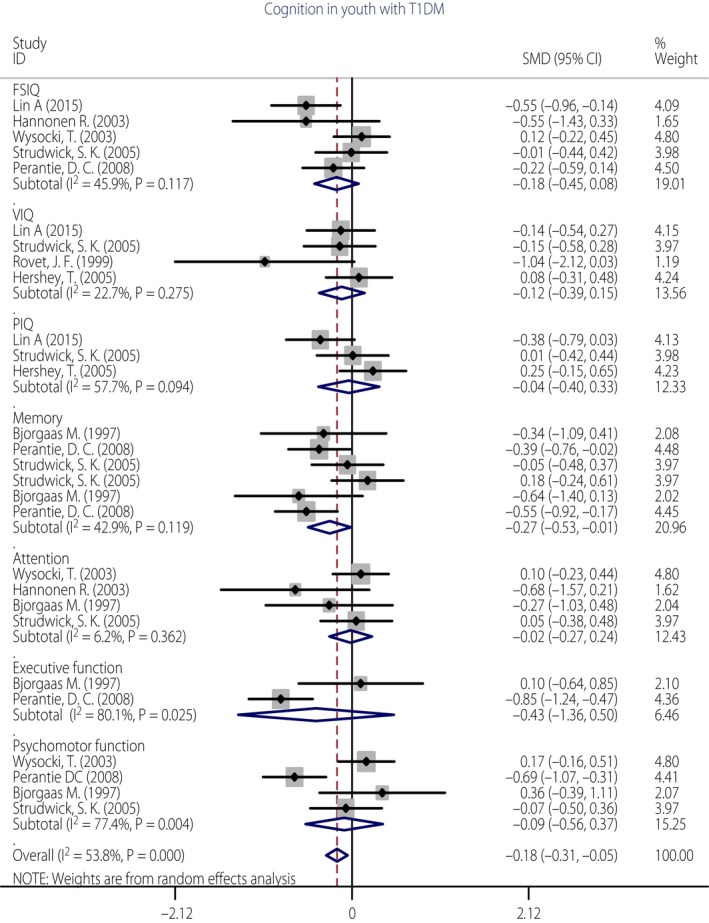
Forest plot of the association of glycemic extremes in cognitive domains of children with type 1 diabetes.
Early‐onset severe hypoglycemia vs late‐onset severe hypoglycemia
An additional meta‐analysis was carried out for the difference in cognitive domains between children with early‐onset severe hypoglycemia compared with late‐onset hypoglycemia in four studies3, 33, 36, 40. The early‐onset severe hypoglycemia group included those who had severe hypoglycemia before the age of 7 years, and the late‐onset severe hypoglycemia group included those who had the first severe hypoglycemic episode after the age of 7 years. The cognitive performance of type 1 diabetes participants with early severe hypoglycemia (n = 45) was compared with late hypoglycemic episodes (n = 87). Figure S2 shows that children with early‐onset severe hypoglycemia performed more poorly than children with late‐onset severe hypoglycemia in overall cognition (g = −0.28, P = 0.012). In each cognitive domain, such as FSIQ (g = −0.35, P = 0.09), memory (g = −0.31, P = 0.175), attention (g = −0.57, P = 0.301) and psychomotor speed (g = −0.17, P = 0.506), the effects were not significant. The test of overall heterogeneity was not significant (I² = 6.9%, P = 0.378).
Publication bias
Egger's linear regression test (t = −0.71, P = 0.488, 95% CI −10.60−5.29) and Begg's rank correlation test (z = 1.47, P = 0.142) suggested that there was no significant publication bias (Figure S3).
Discussion
In the present, we used meta‐analysis to synthesize assessment results across studies in order to investigate cognitive dysfunction in children with type 1 diabetes compared with healthy control groups. We calculated effect sizes (Hedges’ g) to show the magnitude of differences between people with diabetes and non‐diabetic controls in cognitive function13. Specifically, we derived effect sizes for the most commonly reported cognitive domains, including intelligence (FSIQ, VIQ and PIQ), memory (verbal memory, visual memory and spatial memory), executive function, attention and psychomotor speed. In addition, we systematically quantified the effects of severe hypoglycemia and chronic hyperglycemia on cognitive function in children with type 1 diabetes.
The main findings of the present meta‐analysis support the hypothesis that type 1 diabetes is associated with cognitive dysfunction in children. Children with type 1 diabetes performed lower than control participants on measures of intelligence and on a broad range of specific neuropsychological functions, such as memory, attention, executive function and psychomotor speed. Score differences were found in all broad cognitive domains except verbal memory and visual memory. The effect size of overall cognition (−0.33) was moderate in magnitude for children with diabetes vs controls. In each cognitive domain, children with type 1 diabetes showed significantly lower performance than non‐diabetic controls in full scale intelligence (−1.08), attention (−0.60) and psychomotor speed (−0.46; Figure 2). These findings were similar to previous studies reporting that cognitive dysfunction emerged early in the disease course and tended to have very circumscribed effects, particularly on intelligence, attention and psychomotor speed16, 44. Although the differences of magnitude across most cognitive domains were modest (i.e., effect size g < 0.5) and there were no significant abnormalities in clinical manifestation, the modest forms of cognitive dysfunction might potentially hamper children's daily activities, especially in more demanding situations, such as study and academic skill tests. Therefore, the modest forms of cognitive dysfunction should be taken seriously.
The subgroup analysis in the present meta‐analysis mainly examines the effects of glycemic extremes on cognitive function in young people with type 1 diabetes. The results indicated that children with severe hypoglycemia showed significantly poorer performance in overall cognition. These findings are consistent with previous reviews and developmental cognitive studies in children with recurrent hypoglycemic episodes6, 10, 14. A neuroimaging study found that severe hypoglycemic events were associated with a lower density of gray matter in brain regions responsible for language processing and memory45. In this analysis, we tried to use subgroup analysis to calculate statistics of the two different measures (cross‐sectional study and longitudinal study). Cross‐sectional studies showed that the effects of severe hypoglycemia on cognitive dysfunction were significant (g = −0.22, P = 0.005); however, longitudinal studies suggested that severe hypoglycemia episodes were not associated with cognitive dysfunction (g = −0.096, P = 0.354; Figure S4). It is worth noting that among the three longitudinal studies, two studies40, 43 suggested that severe hypoglycemic episodes were significantly associated with negative change in VIQ, and another study41 reported that severe hypoglycemic episodes did not have adverse effects on cognition over 18 months. Lin and Rovet's studies had a longer follow‐up period (12 and 7 years vs 18 months), and the negative effects of severe hypoglycemia were significant. Hence, we thought that severe hypoglycemia might be a plausible cause of cognitive decline, and the hypoglycemia might have long‐term effects on cognitive dysfunction in children with type 1 diabetes. However, the currently available evidence is not sufficient to fully address the long‐term effect of severe hypoglycemia on cognitive dysfunction. More longitudinal study will need to be carried out in the future.
In the present study, we combined elevated HbA1c level and history of hypoglycemic episodes into a confounding factor, which is described as glycemic extremes. We found glycemic extremes were associated with poorer overall cognition, as well as slightly lower performance in memory. These findings indicate that children with a large glucose variable range might be more prone to cognitive dysfunction than children with consistently high glucose levels. A recent study showed that glucose variability might have a greater adverse impact on the developing brain than either prolonged high or low glucose levels46. There were two possible mechanisms of blood glucose variability to develop cognitive dysfunction according to previous studies. One mechanism showed that glycemic variability was associated with an increased production of reactive oxygen species that could damage the central nervous system47, 48. Another mechanism showed that oscillating glucose could have a more toxic impact on oxidative stress generation than constant high glucose, which might lead to mitochondrial dysfunction and neurons cell damage49, 50. However, in many studies, the effect of glycemic variability on cognitive function in children with type 1 diabetes was neglected. Thus, this kind of synergistic effect might further strengthen the insight into the impacts of glycemic variability on cognitive function. We thought this might be a new perspective to consider the effects of glycemic extremes that could have important implications.
Another important issue is the interaction between early age of diabetes onset and severe hypoglycemia. In particular, children with type 1 diabetes onset before the age of 7 years could be more sensitive to the effects of severe hypoglycemic episodes. Indeed, three studies3, 36, 40 showed that children with an earlier age of diabetes onset and a history of severe hypoglycemia had poorer performance of overall cognition (g = −0.39) than children with late age of diabetes onset and a history of severe hypoglycemia. These findings indicate that severe hypoglycemia experienced early in development might be more harmful to cognitive performance than severe hypoglycemia later in life.
Type 1 diabetes most often emerges in childhood or adolescence (<18 years), and the complications can increase with age. Glycemic extremes occur commonly in children with type 1 diabetes, which is due to the inadequacies of insulin replacement therapy. A recent review reported that developing brains, which need higher energy for brain growth and neural pruning, might be more sensitive to glycemic extremes50. Although diabetes and EOD states cannot be changed, the negative effects of glycemic extremes can be minimized by metabolic control and diabetes management. These findings might have clinical applications for type 1 diabetes management of young children, and could provide the impetus for further studies. More prospective cohort studies are required to fully evaluate the intricate relationship between glycemic variability and cognitive function.
The present meta‐analysis had several limitations. First, most of the studies included in the meta‐analysis were cross‐sectional studies in which the direction of causality was uncertain. Longitudinal studies with more strict design and continuous monitoring of blood glucose to track glucose excursions would be a marked improvement. Second, though we attempted to classify published data to measure each cognitive domain, specific cognitive tests to measure a particular function varied across these studies, contributing a large source of heterogeneity. Specific cognitive tests used, testing age of patients and duration of illness were possible sources of heterogeneity in this study. Third, some studies in the systematic literature search were not included in the meta‐analysis, as they did not report test scores or reported non‐standardized results. The consequence was to reduce the sample of available studies. Fourth, frequencies of severe hypoglycemia might also be associated with cognitive dysfunction. However, available studies of the impact of frequencies of severe hypoglycemia on cognitive function were limited, so the effects have not been inspected in this meta‐analysis. Fifth, most results were still statistically significant adjusting for multiple comparisons, except the memory scores (Table S2). Therefore, we could not report that glycemic extremes were significantly associated with memory deficits, but there was a trend. Sixth, in the present meta‐analysis, we applied the Begg's test and Egger's test to estimate the putative publication bias. Although we have used two methods to evaluate the publication bias, we cannot totally rule out the possibility that the regression lines that appeared did not adequately represent the true estimates, as there might be some possible bias that cannot be detected by these proposed methods. Finally, it is important to note that most studies included in this meta‐analysis were carried out with Caucasian participants, except for two studies that were carried out with minority groups30, 32. We must, therefore, be cautious when generalizing the results of the current meta‐analysis to other ethnic groups.
The present findings show that type 1 diabetes is associated with cognitive dysfunction in young people, which is characterized by a lowered intelligence, diminished attention and a slowing of psychomotor speed. We have suggested that glycemic extremes are related to cognitive dysfunction in young people. To examine the effect of type 1 diabetes and glycemic extremes on cognitive deficits more clearly, more longitudinal studies combined with neuroimaging will need to be carried out in the future.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 Forest plot for the cognitive domains in young people after excluding the four studies contributing the heterogeneity.
Figure S2 Forest plot of the cognitive domains in participants with early hypoglycemic episodes compared with participants with late hypoglycemic episodes.
Figure S3 Egger's publication bias plot and Begg's funnel plot.
Figure S4 Forest plot of the association of severe hypoglycemia on cognitive domains in children with type 1 diabetes (classified by longitudinal study and cross‐sectional study).
Table S1 Overview of studies examining the association of type 1 diabetes with each cognitive domain.
Table S2 The P‐value and correction P‐value (false discovery rate correction).
Acknowledgments
We declare that there was no funding for this work, and no honorarium, grant or other form of payment was given to anyone to produce the manuscript.
J Diabetes Investig. 2018
References
- 1. Maahs DM, West NA, Lawrence JM, et al Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010; 39: 481–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaudieri PA, Chen R, Greer TF, et al Cognitive function in children with type 1 diabetes a meta‐analysis. Diabetes Care 2008; 31: 1892–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perantie DC, Lim A, Wu J, et al Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatr Diabetes 2008; 9: 87–95. [DOI] [PubMed] [Google Scholar]
- 4. Northam EA, Anderson PJ, Jacobs R, et al Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care 2001; 24: 1541–1546. [DOI] [PubMed] [Google Scholar]
- 5. Hannonen R, Tupola S, Ahonen T, et al Neurocognitive functioning in children with type‐1 diabetes with and without episodes of severe hypoglycaemia. Dev Med Child Neurol 2003; 45: 262–268. [DOI] [PubMed] [Google Scholar]
- 6. Hershey T, Lillie R, Sadler M, et al Severe hypoglycemia and long‐term spatial memory in children with type 1 diabetes mellitus: a retrospective study. J Int Neuropsychol Soc 2003; 9: 740–750. [DOI] [PubMed] [Google Scholar]
- 7. Duke DC, Harris MA. Executive function, adherence, and glycemic control in adolescents with type 1 diabetes: a literature review. Curr DiabRep 2014; 14: 1–10. [DOI] [PubMed] [Google Scholar]
- 8. Rovet J, Alvarez M. Attentional functioning in children and adolescents with IDDM. Diabetes Care 1997; 20: 803–810. [DOI] [PubMed] [Google Scholar]
- 9. Ryan CM. Diabetes, aging, and cognitive decline. Neurobiol Aging 2005; 26: 21–25. [DOI] [PubMed] [Google Scholar]
- 10. Hershey T, Perantie DC, Warren SL, et al Frequency and timing of severe hypoglycemia affects spatial memory in children with type 1 diabetes. Diabetes Care 2005; 28: 2372–2377. [DOI] [PubMed] [Google Scholar]
- 11. Musen G, Jacobson AM, Ryan CM, et al Impact of diabetes and its treatment on cognitive function among adolescents who participated in the Diabetes Control and Complications Trial. Diabetes Care 2008; 31: 1933–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Desrocher M, Rovet J. Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychol 2004; 10: 36–52. [DOI] [PubMed] [Google Scholar]
- 13. Naguib JM, Kulinskaya E, Lomax CL, et al Neuro‐cognitive performance in children with type 1 diabetes – a meta‐analysis. J Pediatr Psychol 2009; 34: 271–282. [DOI] [PubMed] [Google Scholar]
- 14. Tonoli C, Heyman E, Roelands B, et al Type 1 diabetes‐associated cognitive decline: a meta‐analysis and update of the current literature. J Diabetes 2014; 6: 499–513. [DOI] [PubMed] [Google Scholar]
- 15. Cameron FJ. The impact of diabetes on brain function in childhood and adolescence. Pediatr Clin North Am 2015; 62: 911–927. [DOI] [PubMed] [Google Scholar]
- 16. Semenkovich K, Bischoff A, Doty T, et al Clinical presentation and memory function in youth with type 1 diabetes. Pediatr Diabetes 2015; 17: 492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wessels AM, Scheltens P, Barkhof F, et al Hyperglycaemia as a determinant of cognitive decline in patients with type 1 diabetes. Eur J Pharmacol 2008; 585: 88–96. [DOI] [PubMed] [Google Scholar]
- 18. Aye T, Barnea‐Goraly N, Ambler C, et al White matter structural differences in young children with type 1 diabetes: a diffusion tensor imaging study. Diabetes Care 2012; 35: 2167–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ettinger U, Merten N, Kambeitz J. Meta‐analysis of the association of the SLC6A3 3′‐UTR VNTR with cognition. Neurosci Biobehav Rev 2016; 60: 72–81. [DOI] [PubMed] [Google Scholar]
- 20. Cheung MWL. Modeling dependent effect sizes with three‐level meta‐analyses: a structural equation modeling approach. Psychol Methods 2014; 19: 211. [DOI] [PubMed] [Google Scholar]
- 21. Von Elm E, Altman DG, Egger M, et al The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med 2007; 45: 247–251. [DOI] [PubMed] [Google Scholar]
- 22. Hedges LV. Distribution theory for Glass's estimator of effect size and related estimators. J Educ Behav Stat 1981; 6: 107–128. [Google Scholar]
- 23. Hedges LV, Olkin I. Statistical Methods for Meta‐analysis. San Diego, CA: Academic Press, 2014. [Google Scholar]
- 24. Borenstein M, Hedges LV, Higgins J, et al Front Matter. Chichester: John Wiley & Sons Ltd, 2009. [Google Scholar]
- 25. Brands AMA, Biessels GJ, De Haan EHF, et al The effects of type 1 diabetes on cognitive performance – a meta‐analysis. Diabetes Care 2005; 28: 726–735. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JPT, Thompson SG, Deeks JJ, et al Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Egger M, Smith GD, Schneider M, et al Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1088; 1994: 50. [PubMed] [Google Scholar]
- 29. Benjamini Y, Hochberg Y. Controlling the false discovery rate – a practical and powerful approach to multiple testing. J R Stat Soc Ser C Appl Stat 1995; 57: 289–300. [Google Scholar]
- 30. Cato MA, Mauras N, Ambrosino J, et al Cognitive functioning in young children with type 1 diabetes. J Int Neuropsychol Soc 2014; 20: 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aye T, Reiss AL, Kesler S, et al The feasibility of detecting neuropsychologic and neuroanatomic effects of type 1 diabetes in young children. Diabetes Care 2011; 34: 1458–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patiño‐Fernández AM, Delamater AM, Applegate EB, et al Neurocognitive functioning in preschool‐age children with type 1 diabetes mellitus. Pediatr Diabetes 2010; 11: 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Strudwick SK, Carne C, Gardiner J, et al Cognitive functioning in children with early onset type 1 diabetes and severe hypoglycemia. J Pediatr 2005; 147: 680–685. [DOI] [PubMed] [Google Scholar]
- 34. Kaufman FR, Epport K, Engilman R, et al Neurocognitive functioning in children diagnsed with diabetes before age 10 years. J Diabetes Complications 1999; 13: 31–38. [DOI] [PubMed] [Google Scholar]
- 35. Hershey T, Bhargava N, Sadler M, et al Conventional versus intensive diabetes therapy in children with type 1 diabetes: effects on memory and motor speed. Diabetes Care 1999; 22: 1318–1324. [DOI] [PubMed] [Google Scholar]
- 36. Bjørgaas M, Gimse R, Vik T, et al Cognitive function in type 1 diabetic children with and without episodes of severe hypoglycaemia. Acta Paediatr 1997; 86: 148–153. [DOI] [PubMed] [Google Scholar]
- 37. Reich JN, Kaspar JC, Puczynski MS, et al Effect of a hypoglycemic episode on neuropsychological functioning in diabetic children. J Clin Exp Neuropsychol 1990; 12: 613–626. [DOI] [PubMed] [Google Scholar]
- 38. Ryan C, Vega A, Drash A. Cognitive deficits in adolescents who developed diabetes early in life. Pediatrics 1985; 75: 921–927. [PubMed] [Google Scholar]
- 39. Lin A, Northam EA, Rankins D, et al. Neuropsychological profiles of young people with type 1 diabetes 12 yr after disease onset. Pediatric Diabetes 2010; 11: 235–243. [DOI] [PubMed] [Google Scholar]
- 40. Rovet JF, Ehrlich RM. The effect of hypoglycemic seizures on cognitive function in children with diabetes: a 7‐year prospective study. J Pediatr 1999; 134: 503–506. [DOI] [PubMed] [Google Scholar]
- 41. Wysocki T, Harris MA, Mauras N, et al Absence of adverse effects of severe hypoglycemia on cognitive function in school‐aged children with diabetes over 18 months. Diabetes Care 2003; 26: 1100–1105. [DOI] [PubMed] [Google Scholar]
- 42. Lin A, Northam EA, Werther GA, et al Risk factors for decline in IQ in youth with type 1 diabetes over the 12 years from diagnosis/illness onset. Diabetes Care 2015; 38: 236–242. [DOI] [PubMed] [Google Scholar]
- 43. Diabetes Control and Complications Trial . Long‐term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007; 2007: 1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schwartz DD, Axelrad ME, Anderson BJ. Neurocognitive functioning in children and adolescents at the time of type 1 diabetes diagnosis: associations with glycemic control 1 year after diagnosis. Diabetes Care 2014; 37: 2475–2482. [DOI] [PubMed] [Google Scholar]
- 45. Musen G, Lyoo IK, Sparks CR, et al Effects of type 1 diabetes on gray matter density as measured by voxel‐based morphometry. Diabetes 2006; 55: 326–333. [DOI] [PubMed] [Google Scholar]
- 46. Northam EA, Cameron FJ. Understanding the diabetic brain: new technologies but old challenges. Diabetes 2013; 62: 341–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813–820. [DOI] [PubMed] [Google Scholar]
- 48. Piconi L, Quagliaro L, Assaloni R, et al Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes Metab Res Rev 2006; 22: 198–203. [DOI] [PubMed] [Google Scholar]
- 49. Ceriello A, Esposito K, Piconi L, et al Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008; 57: 1349. [DOI] [PubMed] [Google Scholar]
- 50. Arbelaez AM, Semenkovich K, Hershey T. Glycemic extremes in youth with T1DM: effects on the developing brain's structural and functional integrity. Pediatr Diabetes 2013; 14: 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Forest plot for the cognitive domains in young people after excluding the four studies contributing the heterogeneity.
Figure S2 Forest plot of the cognitive domains in participants with early hypoglycemic episodes compared with participants with late hypoglycemic episodes.
Figure S3 Egger's publication bias plot and Begg's funnel plot.
Figure S4 Forest plot of the association of severe hypoglycemia on cognitive domains in children with type 1 diabetes (classified by longitudinal study and cross‐sectional study).
Table S1 Overview of studies examining the association of type 1 diabetes with each cognitive domain.
Table S2 The P‐value and correction P‐value (false discovery rate correction).


