Abstract
The incidence of type 2 diabetes increases with age. The age‐dependent decline in functional β‐cell mass contributes to the increased risk of onset of diabetes, reflecting the central role of pancreatic β‐cells in glucose homeostasis. Indeed, the replication rate of human and rodent β‐cells is known to decline sharply with age, and such a characteristic of β‐cells might explain the increased onset of type 2 diabetes in the older population. The molecular mechanism involved in the age‐dependent decline of β‐cell proliferation has been extensively studied, mainly using rodents and in vitro culture systems, but its molecular basis is still largely unknown. A mechanism by which glucagon‐like peptide‐1 receptor activation induces human β‐cell proliferation only within a restricted time window was recently suggested in a study in which human islets were grafted into immunodeficient mice. The authors found that the mitogenic effects of exendin‐4 require calcineurin/nuclear factor of activated T‐cells signaling, and that only in juvenile islets, exendin‐4 induced the expression of nuclear factor of activated T‐cells signaling components, as well as downstream target genes that facilitate β‐cell proliferation. These findings provide a mechanistic explanation as to why glucagon‐like peptide 1 exerts mitogenic effects only in juvenile human β‐cells.
The replication rate of human pancreatic β‐cells is believed to be at 3–4% during the first several years of the postnatal period, and then after 10 years‐of‐age it dramatically declines to a low level, at 0.05–0.1%. In adulthood, the replication rate of β‐cells remains low, at 0.01–0.1%. Previously, the lifespan of rat β‐cells was assessed by the observation of lipofuscin granule accumulation using electron microscopy. The authors estimated the half‐life of β‐cells in 12‐month‐old rats to be 30–60 days. In contrast to rats, the lifespan of human pancreatic β‐cells was estimated to be >20 years, consistent with the lower replication rate of human β‐cells. However, human adult β‐cells show active proliferation when an individual is pregnant or has liver disease. The presence of obesity increases insulin resistance, and thus an excess secretion of insulin is required to maintain normal glucose tolerance. Therefore, obese individuals require hypertrophy of their β‐cells to maintain normal glucose tolerance. Indeed, the β‐cell volume density of obese non‐diabetic Caucasians was shown to be increased by 1.5‐fold compared with lean non‐diabetic control individuals. Mechanistic analysis of β‐cell proliferation largely depends on data from rodents, as it has been very difficult to experimentally reconstitute human β‐cell proliferation, and systems that enable the assessment of proliferation potency of human β‐cells have been greatly anticipated.
Whereas an age‐associated decline in human β‐cell proliferation has been previously reported, its molecular basis has never been tested in vivo, and no mechanistic explanation has been provided to support the idea. An age‐associated increase in p16INK4A has been implicated in the impaired proliferation of aged β‐cells. P16INK4A is a tumor suppressor protein and a cyclin kinase inhibitor that prevents phosphorylation of the retinoblastoma protein by cyclin‐dependent kinase 4, thus preventing E2F transcription factors from activating the cell division cycle. The expression of p16INK4A in β‐cells is suppressed by the polycomb genes, Ezh2 and Bmi1, both of which decline with age. The postnatal decline of Ezh2 and Bmi1 appears to be mediated by the decline of signaling through the platelet‐derived growth factor receptor.
Glucagon‐like peptide‐1 (GLP‐1) has a well‐known role in stimulating insulin secretion, which is known as the incretin effect, in addition to enhancing insulin biosynthesis and preventing β‐cell apoptosis1. GLP‐1 and its analogs, such as Ex‐4, have been reported to induce mouse β‐cell proliferation2. Previous studies investigating whether GLP‐1 stimulates human β‐cell proliferation have provided conflicting results.
A recent study by Dai et al.3 at Vanderbilt University reported the establishment of a system that enables the assessment of human β‐cell replication in vivo. In this system, human pancreatic islets were grafted into a renal capsule from immunodeficient non‐obese diabetic scid gamma mice, these mice were treated with Ex‐4 for 2 weeks using osmotic pumps, and Ex‐4‐induced β‐cell replication was assessed by Ki67 immunostaining. They found that Ex‐4 shows mitogenic effects on juvenile human β‐cell grafts (from 0.5 to 7 years‐of‐age), but not on adult β‐cell grafts (>20 years‐of‐age). Here, another important finding was that Ex‐4 enhanced glucose‐stimulated insulin secretion similarly in juvenile human β‐cell grafts and in adult human β‐cells. Accordingly, these results indicated that the Ex‐4‐induced promotion of β‐cell proliferation was age‐dependent. Their results also suggested that both β‐cell proliferation, as well as insulin secretion induced by GLP‐1 receptor (GLP‐1R) activation, are regulated by two distinct signaling pathways3.
Then, what is the molecular mechanism that mediates the age‐dependent human β‐cell proliferation induced by GLP‐1R agonists? To address this question, ribonucleic acid sequencing analysis was carried out and identified 1,440 genes showing enhanced expression in juvenile human islet grafts stimulated by Ex‐4. Gene ontogeny analysis showed that genes involved in the calcineurin/nuclear factor of activated T cells (NFAT) signaling pathway were on the top of the gene list, which were preferentially expressed in juvenile islet grafts stimulated by Ex‐4. Among the NFAT family members, the messenger ribonucleic acid of NFAT1, NFAT2 and NFAT4 is abundantly expressed in juvenile human islets, but is markedly declined in adult islets. Furthermore, in juvenile human islets, Ex‐4 significantly induced the expression of the target genes of NFAT, including cyclin A, cyclin‐dependent kinase and Forkhead box protein M1, which are involved in stimulating β‐cell proliferation. A crucial role for the calcineurin/NFAT pathway in Ex‐4‐induced proliferation of human β‐cells was shown by the observation that the addition of FK‐506, a strong inhibitor of the NFAT signaling pathway, almost completely eliminated Ex‐4‐induced β‐cell proliferation, concomitantly with the disruption of Ex‐4‐induced activation of downstream effectors. Taken together, the authors concluded that the calcineurin/NFAT pathway mediates the age‐dependent human β‐cell proliferation induced by GLP‐1R activation (Figure 1)3.
Figure 1.
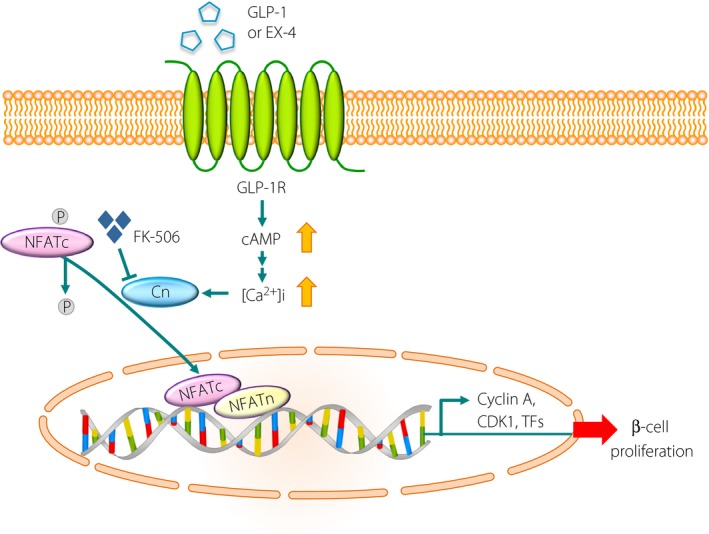
Model of glucagon‐like peptide‐1 (GLP‐1) receptor‐induced mitogenesis of juvenile human β‐cells through the calcineurin/nuclear factor of activated T cells (NFAT) pathway. Binding of GLP‐1 (or exendin‐4 [Ex‐4]) to the GLP‐1 receptor (GLP‐1R) increases intracellular calcium concentrations through cyclic adenosine monophosphate (cAMP) production. An increase in intracellular calcium concentration potentiates calcineurin (Cn), a calcium‐sensitive phosphatase. Activated Cn dephosphorylates the cytoplasmic component of NFAT (NFATc), which triggers its rapid entry into the nucleus. In the nucleus, NFATc proteins assemble on deoxyribonucleic acid with partner proteins termed NFATn, thereby activating target genes, including cell cycle activators (cyclin A and CDK1) and proliferation‐activating transcription factors (TFs), such as Forkhead box protein M1, which are all produced at low levels in adult islets. FK‐506, a well‐known inhibitor of calcineurin, inhibits β‐cell proliferation and thus might predispose individuals to diabetes. CDK, cyclin dependent kinase; P, phosphorylation.
GLP‐1R agonists, when used chronically, have been shown to inhibit bodyweight gain and enhance liver insulin sensitivity, and thereby decrease insulin demand of the whole body4. A previous study2 reported that 4 weeks of Ex‐4 administration at the same dose as that used in the report by Dai et al.3 (300 pmol/kg bodyweight/day) to 8‐week‐old C57BL/6 mice did not stimulate any β‐cell proliferation, although 3 days of Ex‐4 administration significantly stimulated β‐cell proliferation assessed by Ki67 immunostaining. The observed difference in 3 days vs 4 weeks of administration might be explained by the fact that 4 weeks of Ex‐4 administration significantly suppressed bodyweight gain in C57BL/6 mice compared with control phosphate‐buffered saline‐treated groups2. Therefore, the results obtained from graft experiments reported by Dai et al.3 showing that the 4‐week‐administration of Ex‐4 did not induce proliferation of adult human β‐cells needs to be interpreted with caution; the possibility cannot be ruled out that mitogenic effects of Ex‐4 on adult human β‐cells might have been underestimated by the decreased demand of insulin secretion as a result of the increased insulin sensitivity of host non‐obese diabetic scid gamma mice achieved by 4 weeks of treatment with Ex‐43. It is of special importance that juvenile human β‐cells showed significant proliferation under the same condition.
It has been reported that β‐cell proliferation induced by GLP‐1 is dependent on the function of pdx1. The stimulation of insulin‐1 (INS‐1) insulinoma cells and human pancreatic cells with GLP‐1 results in induction of the pdx1 gene, and mouse β‐cells deficient for pdx1 cannot proliferate in response to GLP‐1. Insulin receptor substrate 2 (IRS2), a crucial adaptor molecule for β‐cell survival and proliferation, is also implicated in GLP‐1‐induced β‐cell replication. Signaling through insulin‐like growth factor, which is activated by GLP‐1, contributes to GLP‐1‐induced β‐cell proliferation. It is well known that activation of the GLP‐1R provokes Ca2+ signaling. A previous study reported the crucial role for calcineurin signaling in controlling human β‐cell survival5. Although the authors detected no human β‐cell replication in the islet culture system, treatment with the calcineurin inhibitor, tacrolimus, significantly induced human β‐cell apoptosis. Furthermore, a crucial role for calcineurin/NFAT signaling in human β‐cell survival has been suggested by the observation that 10–30% of patients treated with the immunosuppressant, FK‐506, developed diabetes mellitus6. It is therefore intriguing that the study by Dai et al.3 directly connects the Ca2+ signaling elicited by GLP‐1R activation to human β‐cell proliferation by using a novel human islet graft technique. The development of an engraftment system that enables the assessment of human β‐cell replication in vivo will significantly facilitate prospective analyses of the proliferative potency of human β‐cells, and might eventually provide avenues towards discovering novel therapeutic strategies for functional β‐cell expansion in humans.
Disclosure
The author declares no conflict of interest.
Acknowledgments
This work was supported by MEXT/JSPS KAKENHI. Yoshio Fujitani has received research grants from Astellas Pharma, Takeda Pharmaceutical Company, MSD K.K. and Novartis.
(J Diabetes Investig 10.1111/jdi.12861, 2018)
References
- 1. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 2013; 17: 819–837. [DOI] [PubMed] [Google Scholar]
- 2. Arakawa M, Ebato C, Mita T, et al Effects of exendin‐4 on glucose tolerance, insulin secretion, and beta‐cell proliferation depend on treatment dose, treatment duration and meal contents. Biochem Biophys Res Commun 2009; 390: 809–814. [DOI] [PubMed] [Google Scholar]
- 3. Dai C, Hang Y, Shostak A, et al Age‐dependent human β cell proliferation induced by glucagon‐like peptide 1 and calcineurin signaling. J Clin Invest 2017; 127: 3835–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dhir G, Cusi K. Glucagon like peptide‐1 receptor agonists for the management of obesity and non‐alcoholic fatty liver disease: a novel therapeutic option. J Investig Med 2018; 66: 7–10. [DOI] [PubMed] [Google Scholar]
- 5. Oetjen E, Baun D, Beimesche S, et al Inhibition of human insulin gene transcription by the immunosuppressive drugs cyclosporin A and tacrolimus in primary, mature islets of transgenic mice. Mol Pharmacol 2003; 63: 1289–1295. [DOI] [PubMed] [Google Scholar]
- 6. Goodyer WR, Gu X, Liu Y, et al Neonatal β cell development in mice and humans is regulated by calcineurin/NFAT. Dev Cell 2012; 23: 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


