Abstract
Lysine acetyltransferases (KATs) catalyze lysine acetylation, a reversible protein modification implicated in a wide variety of disease states. Histone acetyltransferases (HATs) comprise a KAT sub-class that acetylate specific lysines in histones, hence playing an important role in the regulation of chromatin organization and function. HATs are critical regulators of signaling in many diseases, including cancer. KAT6A (also known as monocytic leukemia zinc finger protein, MOZ) and KAT6B (also known as MORF and QKF) belong to the MYST family of HATs, that comprise KAT5–KAT8. They are the targets of chromosomal translocations identified in acute myeloid leukaemia and various cancers. It seems logical therefore that inhibition of KAT6A and KAT6B may provide a therapeutic benefit in cancer. Baell et al. discovered a new class of anti-cancer drug that can put cancer cells into a permanent sleep or senescence, using high-throughput screening followed by medicinal chemistry optimization, in-cell assays, biochemical assessment of target engagement, and tumour models in mice and fish. This research showed promise in arresting tumour growth in pre-clinical models of blood and liver cancers as well as delaying or stopping relapse without damaging the cells' DNA or some harmful side-effects caused by chemotherapy and radiotherapy.
Lysine acetyltransferases (KATs) catalyze lysine acetylation, a reversible protein modification implicated in a wide variety of disease states. Histone acetyltransferases (HATs) comprise a KAT sub-class that acetylate specific lysines in histones, hence playing an important role in the regulation of chromatin organization and function [1,2]. HATs are critical regulators of signaling in many diseases, including cancer. KAT6A (also known as monocytic leukemia zinc finger protein, MOZ) and KAT6B (also known as MORF and QKF) belong to the MYST family of HATs, that comprise KAT5–KAT8 [3,4]. They are the targets of chromosomal translocations identified in acute myeloid leukaemia and various cancers. It seems logical therefore that inhibition of KAT6A and KAT6B may provide a therapeutic benefit in cancer.
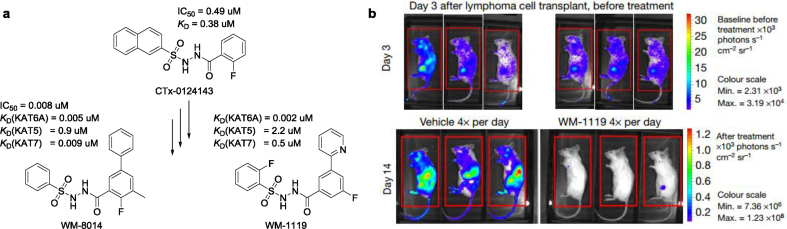
In a breakthrough article just reported in Nature [6], Baell et al. have reported the development of potent and selective first-in-class dual inhibitors of KAT6A and KAT6B with in vivo efficacy in lymphoma mouse models. As they describe, their journey began with a high throughput screening campaign testing more than 243 000 compounds [5], from which a competitive KAT6A inhibitor - the acylsulfonylhydrazide compound CTx-0124143 - was identified, with an IC50 (half-maximal inhibitory concentration) of 0.49 μM in biochemical assays. From this starting point, medicinal chemistry optimization yielded the acylsulfonylhydrazide compounds WM-8014 and WM-1119 [6](Fig. 1a), as highly potent and selective inhibitors of KAT6A and KAT6B. A pharmacological profile obtained from a panel of more than 160 diverse pharmacological targets confirmed that WM-8014 inhibits predominantly just the closely related proteins KAT6A and KAT6B (IC50 8 nM and 28 nM, respectively), as well as having desirable, drug-like physicochemical properties.
Fig. 1.
a, Medicinal chemistry optimization of CTx-0124143, WM-8014 and WM-1119. b, Bioluminescence images of EMRK1184 lymphoma cells expressing luciferase before (day 3) and after (day 14) 11 days of treatment with WM-1119 (50 mg kg−1 four times per day) or PEG400 vehicle control.
The crystal structures of a modified MYST histone acetyltransferase domain (MYSTCryst) in complex with acetyl coenzyme A or WM-8014 were elucidated. Just like the diphosphate group of acetyl-CoA, the core acylsulfonylhydrazide moiety of WM-8014 were observed to make similar hydrogen bonds to MYSTCryst. WM-8014 competed directly with acetyl-CoA in the substrate-binding domain. Baell et al. then solved the structure of MYSTCryst in complex with WM-1119, and found this to be almost identical to that of MYSTCryst–WM-8014. Biochemical and crystal structures studies confirmed that WM-8014 and WM-1119 were reversible competitors of acetyl coenzyme A and inhibited MYST-catalyzed histone acetylation. The effects of WM-8014 in a zebrafish model [7] of KRASG12V-driven hepatocellular overproliferation were examined. WM-8014 reduced significantly liver volume and hepatocyte number in S phase, but did not impair the growth of the normal liver, demonstrating WM-8014 potentiates oncogene-induced senescence but it does not affect normal hepatocyte growth. WM-8014 and WM-1119 induced cell cycle exit and cellular senescence without causing DNA damage or having a general cytotoxic effect. The effectiveness of KAT6 inhibitors WM-1119 was tested in the treatment of lymphoma in mice (Fig. 1b). All mice, with luciferase activity after the lymphoma cell transplant 3 days, were divided randomly into WM-1119-treatment and vehicle-control groups. The WM-1119-treatment group had arrested tumour growth by day 14. These results demonstrate that WM-1119 is effective in preventing the progression of lymphoma in mice, while appearing not to adversely affect healthy cells.
Baell et al. discovered a new class of anti-cancer drug that can put cancer cells into a permanent sleep or senescence, using high-throughput screening followed by medicinal chemistry optimization, in-cell assays, biochemical assessment of target engagement, and tumour models in mice and fish. This research showed promise in arresting tumour growth in pre-clinical models of blood and liver cancers as well as delaying or stopping relapse without damaging the cells' DNA or some harmful side-effects caused by chemotherapy and radiotherapy.
This paper is significant for a number of “firsts”. The compounds reported are the first dual inhibitors of KAT6A and KAT6B, the first compounds to demonstrate single digit nanomolar biochemical inhibition of these HATs or indeed any MYST HAT, the first to represent AcCoA-competitive inhibition accompanied by structural biology for any MYST HAT, and the first to demonstrate convincing on-target KAT6A and KAT6B inhibition in both a cellular and in vivo context. Another more subtle first, is the high cellular permeability in a pyrophosphate mimetic, something that is seen as a holy grail by medicinal chemists.
In summary, the research was particularly significant because the scientific community had coined the gene family ‘undruggable’. Baell et al. showed that the enzymes in question are not undruggable and this work will be the catalyst for a whole new area of scientific interest internationally. This new class of drugs is the first to target KAT6A and KAT6B proteins. Both are known to play an important role in driving cancer. KAT6A sits at number 12 on the list of genes most commonly amplified in cancers. This study, that investigated pharmacological inhibition KAT6A and KAT6B, could represent a new approach to treating cancer.
Acknowledgments
We are grateful to The Jiangsu Synergetic Innovation Center for Advanced Bio-Manufacture (No. XTE1850) for their financial support.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Verdin E., Ott M. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat Rev Mol Cell Biol. 2015;16:258–264. doi: 10.1038/nrm3931. [DOI] [PubMed] [Google Scholar]
- 2.Lee K.K., Workman J.L. Histone acetyltransferase complexes: one size doesn't fit all. Nat Rev Mol Cell Biol. 2007;8:284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 3.Allis C.D., Berger S.L., Cote J., Dent S., Jenuwien T., Kouzarides T., Zhang Y. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Voss A.K., Thomas T. MYST family histone acetyltransferases take center stage in stem cells and development. BioEssays. 2009;31:1050–1061. doi: 10.1002/bies.200900051. [DOI] [PubMed] [Google Scholar]
- 5.Falk H., Connor T., Yang H., Loft K.J., Alcindor J.L., Nikolakopoulos G.N., Street I.P. An efficient high-throughput screening method for MYST family acetyltransferases, a new class of epigenetic drug targets. J Biomol Screen. 2011;16:1196–1205. doi: 10.1177/1087057111421631. [DOI] [PubMed] [Google Scholar]
- 6.Baell J.B., Leaver D.J., Hermans S.J., Kelly G.L., Brennan M.S., Downer N.L., Thomas T. Inhibitors of histone acetyltransferases KAT6A/B induce senescence and arrest tumour growth. Nature. 2018;560:253–257. doi: 10.1038/s41586-018-0387-5. [DOI] [PubMed] [Google Scholar]
- 7.Chew T.W., Liu X.J., Spitsbergen J.M., Gong Z., Low B.C. Crosstalk of Ras and Rho: activation of RhoA abates Kras-induced liver tumorigenesis in transgenic zebrafsh models. Oncogene. 2014;33:2717–2727. doi: 10.1038/onc.2013.240. [DOI] [PubMed] [Google Scholar]