Abstract
The majority of fatalities from poisonous mushroom ingestion are caused by amatoxins. To prevent liver failure or death, it is critical to accurately and rapidly diagnose amatoxin exposure. We have developed an liquid chromatography tandem mass spectrometry method to detect α-, β-, and γ-amanitin in urine to meet this need. Two internal standard candidates were evaluated, including an isotopically labeled 15N10-α-amanitin and a modified amanitin methionine sulfoxide synthetic peptide. Using the 15N10-α-amanitin internal standard, precision and accuracy of α-amanitin in pooled urine was ≤5.49% and between 100–106%, respectively, with a reportable range from 1–200 ng/mL. β- and γ-Amanitin were most accurately quantitated in pooled urine using external calibration, resulting in precision ≤17.2% and accuracy between 99 – 105% with calibration ranges from 2.5–200 ng/mL and 1.0–200 ng/mL, respectively. The presented urinary diagnostic test is the first method to use an isotopically labeled α-amanitin with the ability to detect and confirm human exposures to α-, β-, and γ-amanitin.
Keywords: Amanitin, amatoxin, mushroom toxin, LC-MS/MS
1. Introduction
Consumption of poisonous mushrooms is an international concern due to the prevalence of wild mushroom foraging worldwide and the easy misidentification of a poisonous mushroom as an innocuous species. Over 70% of fatalities resulting from mushroom ingestions in the United States were attributed to cyclopeptides, according to the American Association of Poison Control Centers annual reports from 2002 – 2015 [Watson, 2002, 2003; Lai, 2005; Bronstein, 2006, 2007, 2008, 2009, 2010, 2011; Mowry, 2012, 2013, 2014, 2015]. Cyclopeptide hepatotoxins, which include amatoxins, are found in many mushroom species of the genera Amanita, Lepiota, and Gallerina. Amatoxins are comprised of α-amanitin, β-amanitin, γ-amanitin, ε-amanitin, amaninamide, amanin, amanullinic acid, amanullin, and proamanullin, with the most toxic forms being α-, β-, and γ-amanitin [Bakirci, 2015]. Amanitins are structurally stable and toxicity is not reduced by routine cooking and freezing. Although α-amanitin is the most prevalent form found in Amanita phalloides, the concentration of each amanitin depends on the mushroom species and growth conditions [Bakirci, 2015; Yilmaz, 2014].
Hepatotoxicity of the amanitins is exacerbated by several factors, including rapid toxin uptake into liver cells by the OATP1B3 transport proteins and enterohepatic recirculation [Letschert, 2006]. A lethal dose will vary depending on the health of the person exposed, but as little as 0.1 mg/kg body weight of amanitin may cause death to a human adult; a single Amanita phalloides mushroom contains enough amatoxin to be fatal [Faulstich, 1980; Kaya, 2015]. Due to a 6–24 h delayed onset of poisoning symptoms, liver damage has already begun by the time exposed patients seek treatment [Santi, 2012; Broussard, 2001; Jaeger, 1993; Mengs, 2012]. Because early treatment significantly improves prognosis, patients are often aggressively treated for amatoxin poisoning without confirmation of exposure. Determination of amanitin exposure is challenging because intact mushrooms in meal remnants are few, mushroom experts to identify the mushrooms are limited, and analytical methods to detect amanitins are not readily available. Consequently, a method capable of determining amanitin exposures from a patient sample within a clinically relevant period is needed to address these problems [Santi, 2012; Broussard, 2001; Jaeger, 1993].
Amanitins have been detected intact in urine post exposure at concentrations ranging from 48.0 – 4,830 ng/mL for α-amanitin and 75.0 – 7,103 ng/mL for β-amanitin [Jaeger, 1993; Zhang, 2016]. If urine is collected within 72, and potentially up to 96 h following ingestion, the intact toxins can serve as effective biomarkers for amatoxin poisoning [Jaeger, 1993; Zhang, 2016]. Since the amount of individual amanitins will vary between mushrooms, the detection of all amatoxins in biomedical samples will provide valuable epidemiological information.
Numerous methods have been developed for the quantitation of α- and β-amanitin in human urine, but fewer have been documented for γ-amanitin. Methods previously reported for the detection or quantitation of α- or β-amanitin include enzyme-linked immunosorbent assay, radioimmunoassay, capillary zone electrophoresis, and high pressure liquid chromatography (HPLC) with electrochemical detection [Defendenti, 1998; Robinson-Fuentes, 2008; Andres, 1986; Parant, 2006]. Mass spectrometric methods used to quantify these toxins in urine include immunoaffinity extraction with liquid chromatography/mass spectrometry (LC-MS), LC with high-resolution mass spectrometry, ultra-high pressure LC-MS/MS, and UHPLC-ultra high resolution time of flight MS [Zhang, 2016; Jaurer, 2000; Helfer, 2014; Gicquel, 2014; Leite, 2013; Tomkova, 2015; Chung, 2007]. Detection limits varied for reported methods and ranged from 5 ng/mL to as low as 0.2 ng/mL; however, most methods reported did not include γ-amanitin.
A significant challenge for quantitative LC-MS methods is the enhancement or suppression of the analyte response due to salts and other co-eluting compounds contained in the matrix, known as matrix effects. Sample preparation optimization and chromatographic adjustments can reduce matrix effects, but the most effective solution is to incorporate a representative internal standard, ideally one that is isotopically labeled. Due to the complex, bicyclic amanitin structure, chemical synthesis of an isotopically labeled amanitin for use as an internal standard has been unsuccessful to date. Therefore, the majority of methods developed for the LC-MS quantitation of α-amanitin have explored a variety of surrogate internal standards. These surrogates included colchicine, fluorazepam, and tilmicosin, which although similar in mass, do not closely mimic the amanitin structure [Gicquel, 2014; Leite, 2013; Li, 2017]. To incorporate a more structurally representative internal standard with a bicyclic structure, γ-amanitin was reacted to form a methyl ether and used for quantitation with some success [Maurer, 2000]. Although synthesis has not been successful, the growth of mushrooms in a heavy nitrogen environment incorporated ten 15N atoms into α-amanitin, forming an isotopically labeled version suitable for method development [Luo, 2015].
The study presented is the first to incorporate an isotopically labeled 15N10-α-amanitin as an internal standard to quantify amanitins in urine. The method developed used an orthogonal approach with reversed phase sample preparation followed by hydrophilic interaction liquid chromatography (HILIC) separation with positive electrospray ionization MS/MS used for detection. Quantitation of α-, γ-amanitin in urine was assured through the evaluation of multiple individual and pooled urine samples.
2. Materials and methods
2.1. Reagents and supplies
α-, β-, and γ-amanitin (1 mg dried each, ≥90% purity) were purchased from Enzo Biochem, Inc. (Farmingdale, NY). 15N10-labeled α-amanitin was acquired from Michigan State University (East Lansing, MI) [Luo, 2015]. Synthetic peptide with the methionine sulfoxide (MetSSP) moiety was purchased from Battelle (Columbus, OH). Ammonium formate (10 M, crystallization grade) was purchased from Hampton Research (Aliso Viejo, CA). Formic acid (LC/MS grade, ≥ 99.5%), methanol (LC/MS grade, ≥ 99.9%), and acetonitrile (HPLC grade, ≥ 99.9%) were purchased from Fisher Scientific (Waltham, MA). Deionized (DI) water (18 MΩ-cm) was obtained from a filter system manufactured by AquaSolutions (Jasper, GA). Pooled urine and a convenient set of individual, unexposed urine samples were purchased from Tennessee Blood Services (Memphis, TN). All urine was pre-screened by the vendor in accordance with FDA regulations to be free of Hepatitis B, Syphilis, and HIV. This study used de-identified urine acquired from commercial sources, and thus the work did not meet the definition of human subjects as specified in 45 CFR 46.102 (f).
2.2. Preparation of calibration standards, quality controls, and individual urine fortifications
Stock amanitin solutions (1.0 mg/mL) were prepared by dissolving each α-, β-, and γ-amanitin purchased standard (1.0 mg) in 1.0 mL of DI water; subsequent dilutions using these solutions were prepared gravimetrically. Intermediate stock solutions containing α-, β-, and γ-amanitin were prepared in DI water at approximately 5.0, 10, 50, and 100 μg/mL. These solutions were then used to prepare eight calibration standards (1.0, 2.5, 5.0, 10, 25, 50, 100, and 200 ng/mL) and three quality control (QC) samples (7.0, 75, and 150 ng/mL) in pooled human urine. Individual solutions of 15N10-α-amanitin (700 ng/mL) and MetSSP (400 ng/mL) for use as candidate internal standards were prepared in DI water.
Urine samples from unexposed individuals, not representative of the population, were used to prepare fortified samples. Twelve individual urines were spiked at 1.0, 2.0, 2.5, 3.0, 3.5, 4.0 and 46 ng/mL with α-, β, and γ-amanitin.
2.3. Sample preparation and solid phase extraction
Calibrators, QCs, one matrix blank, and urine samples (300 μL) were combined with one or both candidate internal standards, 15N10-α-amanitin (50 μL) and MetSSP (30 μL), in a 2 mL Nunc® plate (Thermo Scientific, Rochester, NY). Each sample was diluted with 300 μL DI water. The plate was covered, briefly centrifuged to consolidate the solution, and mixed with a ThermoMixer C 96-well plate shaker (Eppendorf, Hauppauge, NY) for 10 min at 800 rpm. The entire solution was then transferred to an Oasis® Hydrophilic-Lipophilic Balance (HLB) solid phase extraction (SPE) plate (30 mg bed, 30 μm particle size) (Waters Corporation, Milford, MA) that was pre-conditioned with 1 mL of 90% acetonitrile followed by 1 mL DI water. After sample loading, the extraction plate was washed with 1 mL DI water then 1 mL of 10% methanol. The analytes were eluted into a new 2 mL Nunc® plate using 1 mL of 90% acetonitrile. The eluent was dried under nitrogen at 60 L/min and 70 °C using a TurboVap® 96 (Biotage, LLC, Charlotte, NC), and the dried samples were reconstituted using 50 μL of 90% acetonitrile/10% 10 mM ammonium formate (aq) with 0.2% formic acid (v/v). The reconstituted samples were mixed at 1000 rpm for 10 min using the ThermoMixer, then transferred to a 96-well V bottom microplate (Eppendorf, Hauppauge, NY). The microplate was then heat-sealed and placed into the instrument autosampler for analysis.
2.4. Chromatography conditions
Chromatographic separation was achieved using a 15 μL injection and a 5.0 min gradient elution on an Acquity BEH HILIC column (2.1 × 50 mm, 1.7 μm) (Waters Corporation, Milford, MA). Column temperature was maintained at 50 °C with an Agilent 1290 UHPLC system (Agilent Technologies, Santa Clara, CA). Mobile phase (A) was 75% acetonitrile and 25% 10 mM ammonium formate (aq) with 1% formic acid (v/v), and (B) was 90% acetonitrile and 10% 10 mM ammonium formate (aq) with 0.2% formic acid (v/v). The initial flow rate was 0.5 mL/min with 1% A/99% B until 1.42 min, followed by a flow rate decrease to 0.25 mL/min at 1.43 min; then the mobile phase was adjusted to 99% A/1% B by 1.55 min and held until 2.65 min. The flow rate was returned to 0.5 mL/min at 2.70 min, and the mobile phase returned to 1% A/99% B from 2.70 min until 5.00 min.
2.5. Mass spectrometry conditions
All analytes were detected using a SCIEX 6500 triple quadrupole MS with a TurboIonSpray ionization source operated in positive mode (Foster City, CA). The following conditions were applied to all analyte transitions: entrance potential (EP), 10 V; curtain gas (CUR), 20 psi; collision gas (CAD), 12 psi; ion spray voltage (IS), 4500 V; source temperature (TEM), 500 °C; ion source gas 1 (heater gas, GS1), 60 psi; and ion source gas (nebulizer gas, GS2), 20 psi. Analyte transitions and their corresponding declustering potential (DP), collision energy (CE), collision cell exit potential (CXP), and dwell time (Time) were as follows: α-amanitin (quantitation transition) m/z 919.3 → 338.9, 70 V, 67 V, 19 V, 40 ms; α-amanitin_C (confirmation transition) m/z 919.3 → 871.0, 70 V, 38 V, 21 V, 40 ms; 15N10-α-amanitin (internal standard) m/z 929.3 → 911.4, 47 V, 36 V, 24 V, 10 ms; MetSSP (internal standard) m/z 889.4 → 871.4, 130 V, 37 V, 20 V, 20 ms; β-amanitin (quantitation transition) m/z 920.3 → 644.3, 60 V, 42 V, 49 V, 10 ms; β-amanitin_C (confirmation transition) m/z 920.3 → 461.0, 60 V, 47 V, 12 V, 10 ms; γ-amanitin (quantitation transition) m/z 903.2 →855.3, 71 V, 42 V, 19 V, 60 msec; γ-amanitin_C (confirmation transition) m/z 903.2 → 243.2, 71 V, 55 V, 17 V, 60 ms.
2.6. Matrix effects and extraction efficiency
2.6.1. Post-column infusion
Matrix effects, including ion suppression, were evaluated through post-column infusion of a mixture containing MetSSP, α-, β-, γ-, and 15N10-α-amanitin prepared at 500 ng/mL each in 90% acetonitrile/10% 10 mM ammonium formate (aq) with 0.2% formic acid (v/v). The solution was infused using a syringe pump connected to a T-fitting inserted between the HPLC column and mass spectrometer. The mixture was continuously infused at 14 μL/min into the mobile phase stream, establishing a baseline response for all transitions. Individual unexposed urine samples (n=10) and pooled urine (2 different pools) were extracted and reconstituted using the sample preparation protocol, omitting internal standard addition. Once re-suspended, the extracted urine samples were injected onto the HPLC-MS/MS while the infusion continued, and the baseline was monitored for any change in signal intensity [Bonfiglio, 1999].
2.6.2. Matrix effects and extraction efficiency determination
Analyte matrix effects and extraction efficiencies were measured using pooled urine fortified at 50 ng/mL (n=4) (a) before extraction, (b) post extraction, and (c) after dry down. Internal standard matrix effects were also evaluated for both the 15N10-α-amanitin and the MetSSP internal standard solutions (n=4) using the same process at 117 ng/mL and 40 ng/mL, respectively. Solvent (mobile phase B) fortifications were prepared (n=4) at the same concentrations for the analytes (50 ng/mL) and internal standards (117 ng/mL and 40 ng/mL for 15N10-α-amanitin and MetSSP, respectively) (d). Extraction efficiency was calculated by dividing the peak areas of the pre-extraction fortified urine by the post-extraction fortified urine (a/b) x 100. The matrix effects were calculated by dividing the peak areas of the urine fortified after dry down by a fortified solvent (c/d) x 100 [Matuszewski, 2003].
2.7. Data processing and calculations
Data processing was completed using both Analyst® (Version 1.6.2) and MultiQuant™ software packages (Version 3.0.2) from SCIEX. Quantitation of all analytes using linear regression analysis with a 1/x weighting was accomplished using calibrator concentration versus the ratio of calibration standard ion area to internal standard ion area, or the calibrator ion area. All calibration curves met or exceeded established laboratory criteria with an R2 value > 0.98 and were accepted for use.
The method limits of detection (LOD) were determined by analyzing the four lowest calibrators for α-, β-, and γ-amanitin (1.0, 2.5, 5.0, and 10 ng/mL for α- and γ-amanitin (n=26), and 2.5, 5.0, 10, and 25 ng/mL for β-amanitin (n=26)). The standard deviations of the results were calculated and plotted versus expected concentrations. The extrapolation of this line to the y-intercept yielded s0, with the estimated LOD calculated as 3s0 [Taylor, 1987].
2.8. Method Validation
The method was validated by analysis of one matrix blank and two QC samples with each of the 26 replicate calibration curves. To confirm method performance at higher concentrations, an additional QC sample was characterized with ten replicates. A maximum of two curves with QC samples were evaluated per day over three months by two analysts. α-Amanitin was quantitated using 15N10-α-amanitin internal standard while β- and γ-amanitin were quantitated without the correction of internal standard. Accuracy and precision was determined for all analytes using the quantitation ion transition at concentrations of 7.0 ng/mL (n=26), 75 ng/mL (n=26), and 150 ng/mL (n=10). Specificity was evaluated by the analysis of 90 individual, unexposed urines for potential interfering peaks.
3. Results and Discussion
3.1. Method development - Analytical conditions
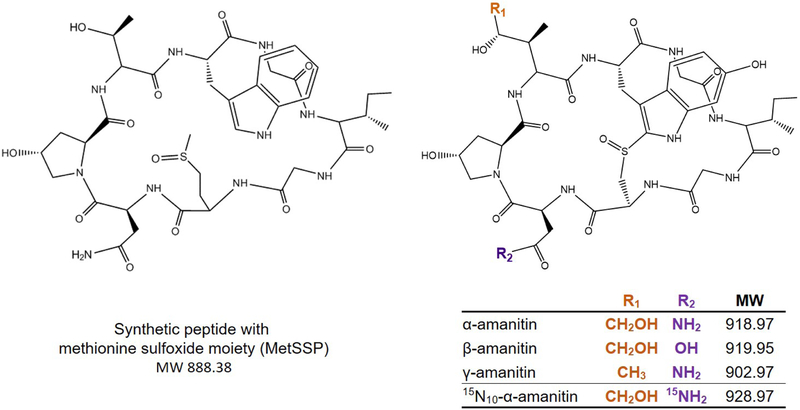
α-Amanitin was initially incorporated into an established urinary natural toxin LC-MS/MS method, using reversed phase SPE followed by separation with a reversed phase polar embedded,C18 HPLC column [Wooten, 2014; Johnson, 2005, 2009]. Previous attempts to synthesize an isotopically labeled amanitin had been unsuccessful, and in the meantime a methionine sulfoxide synthetic peptide (MetSSP) was synthesized specifically for use as an internal standard (Figure 1). This internal standard was based on a modified α-amanitin structure with a threonine substituted for the 4,5-dihydroxyisoleucine, a tryptophan for the 4-hydroxytryptophan, and a methionine sulfoxide for the cysteine, resulting in a monocyclic structure in lieu of the bicyclic amanitin structure. Both MetSSP and native α-amanitin were detected using negative ion ESI MS/MS.
Figure 1.
Structures of the methionine sulfoxide synthetic peptide (MetSSP) and the amanitins within this method.
Method evaluation between multiple instruments and analysts yielded highly variable results, indicating the need for further method optimization. Both supported liquid/liquid extraction (SLE) and normal phase and ion exchange SPE sorbents were evaluated. These approaches were not pursued further, since extraction efficiencies were extremely low (<30%). Mixed mode and reversed phase SPE sorbents were also assessed. The HLB plates had the most consistent results with the highest extraction efficiency of all sorbents tested.
Chromatographic phases, columns, and conditions were also evaluated. Normal phase and ion exchange chromatography were excluded quickly because of poor retention. Reversed phase chromatography was promising for α- and γ-amanitin; however, β-amanitin did not retain as well as the other compounds. To increase assay sensitivity and decrease matrix effects, orthogonality between sample preparation and chromatographic separation was explored by combining a reversed phase extraction coupled with HILIC chromatographic conditions. An aqueous content of 25% successfully eluted the polar amanitins off the HPLC column, and the addition of a slight gradient further sharpened the β-amanitin peak. The 1.7 μm particle size coupled with 2.1 × 5.0 mm column dimensions resulted in minimal runtime with sufficient retention, while the Ethylene Bridged Hybrid (BEH) phase selected maintained consistent retention times across various column lots throughout this study. The final HILIC chromatography resulted in improved analyte retention, peak shape, and separation of all three amanitins as compared to the initial reversed phase conditions.
In addition to sample preparation and chromatographic optimization, multiple surrogate internal standards were initially investigated, including microcystin-LR (Enzo Biochem, Inc. (Farmingdale, NY)), ciprofloxacin (Sigma-Aldrich, St. Louis, MO), a methionine sulfone synthetic peptide (Battelle, Columbus, OH), and MetSSP. Of those examined, the surrogate internal standard with greatest accuracy and precision was MetSSP. An isotopically labeled internal standard was not available at the onset of this study, but once 15N10-α-amanitin was obtained, it was incorporated for further evaluation (Figure 1) [Luo, 2015].
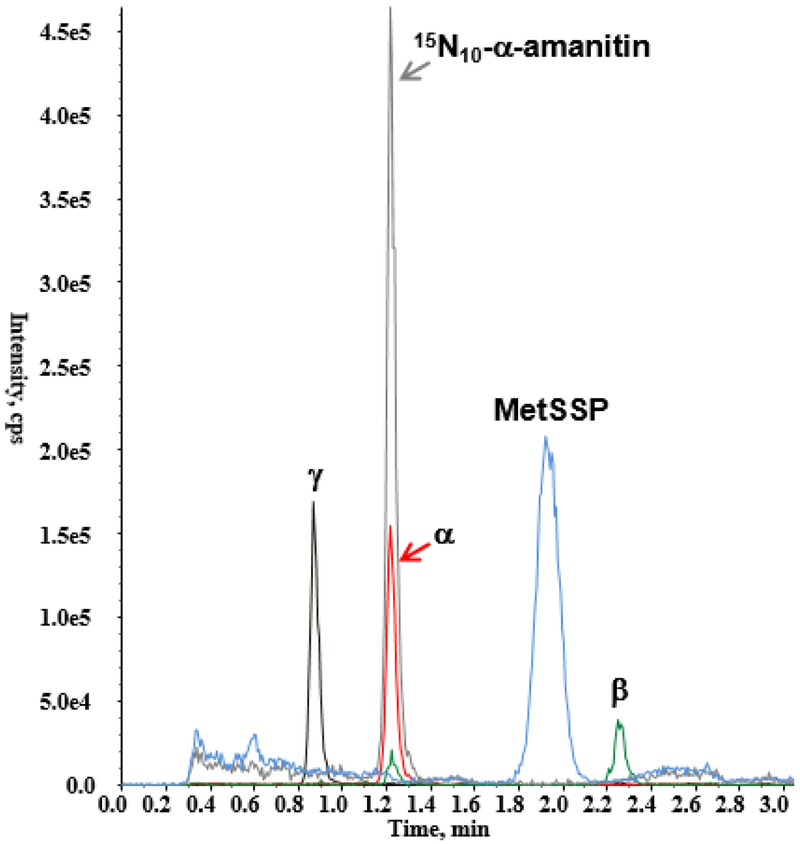
All compounds, including the two candidate internal standards, were ionized in positive mode resulting in a higher response as compared with negative mode ionization. For α-, β-, and γ-amanitin, two transitions were optimized and selected based on signal intensity and consistency of response. For each internal standard, a single transition was selected and monitored. The optimized method using HLB-SPE with HILIC chromatography resulted in consistent, resolved separation of α-, β-, and γ-amanitin, MetSSP, and 15N10-α-amanitin from urinary components (Figure 2).
Figure 2.
Representative chromatogram including the two candidate internal standards (15N10-α-amanitin and methoinine sulfoxide synthetic peptide [MetSSP]) and the three amanitins extracted from urine at 25 ng/mL.
3.2. Method development - Matrix effects and extraction efficiency
Post-column infusion was used to identify potential matrix effects from urine and to facilitate internal standard selection for each analyte. α-, β-, γ-amanitin, 15N10-α-amanitin, and MetSSP were continuously infused into the mass spectrometer during the injection and chromatographic separation of ten individual unexposed, extracted urines. The chromatographic trace was compared between analytes and internal standards (Supplemental Figure 1). The matrix effects were quite variable throughout the run for all compounds, indicating that additional chromatographic adjustments to shift analyte retention may not substantially reduce matrix effects. Despite the presence of matrix effects, the 15N10-α-amanitin chromatographic trace was nearly identical to that of α-amanitin, supporting this as an appropriate internal standard for this analyte. As this experiment only provided preliminary data on performance, a more quantitative evaluation of matrix effects was explored.
Matrix effects were significant for all analytes and internal standards, ranging between 21 – 41%, as seen in Table 1. Although the results were consistent between replicates, the matrix effects were still significant for all compounds tested. Given that a sample without matrix effects would be 100%, these values indicate a significant reduction in signal due to matrix. Because similar matrix effects were noted between α-amanitin and 15N10-α-amanitin (21 – 25%), and between β- and γ-amanitin and MetSSP (38.1, 37.4, and 41.4, respectively), further investigation of the candidate internal standards was warranted.
Table 1.
Extraction efficiencies and matrix effects for α-, β-, and γ-amanitin (50 ng/mL) and 15N10 -α-amanitin and MetSSP internal standards (100 ng/mL) (n=4).
| Extraction Efficiency and Matrix Effect | |||||
|---|---|---|---|---|---|
| % ± SD | α | β | ɣ | 15N10-α | MetSSP |
| Extraction efficiency | 97.8 ± 7.23 | 71.1 ± 1.76 | 95.4 ± 1.73 | 105 ± 6.96 | 91.3 ± 6.22 |
| Matrix Effects | 25.5 ± 2.50 | 38.1 ± 1.29 | 37.4 ± 1.73 | 21.3 ± 4.93 | 41.4 ± 0.74 |
The extraction efficiencies for α-, γ-amanitin, 15N10-α-amanitin, and MetSSP from urine (n=4) were 90% or greater; however, β-amanitin had a slightly lower extraction efficiency of 71.1% (Table 1). These extraction efficiencies were reproducible for both the analytes (50 ng/mL) and the candidate internal standards (100 ng/mL) and were deemed acceptable for this application.
3.3. Method development - Internal standard evaluation
An initial assessment of accuracy and precision for α-, β-, and γ-amanitin was determined with and without the use of the candidate internal standards. Six calibration curves were analyzed over four days using the pooled urine calibrators and QC samples. Accuracy for all analytes, regardless of internal standard used, was within 96.6–115% (Table 2).
Table 2.
Assessment of precision and accuracy of α-, β-, and γ-amanitin with and without internal standards (MetSSP and 15N10 α-amanitin) for QC Low (7.0 ng/mL) and QC Medium (75 ng/mL) in pooled urine for six calibration curves.
| α-amanitin | |||||||||
| Internal Standard | |||||||||
| None | MetSSP | 15N10-α-amanitin | |||||||
| QC Low | QC Medium | QC Low | QC Medium | QC Low | QC Medium | ||||
| Mean | 7.20 | 79.4 | 7.53 | 85.3 | 6.95 | 78.4 | |||
| CV | 13.0% | 8.75% | 17.5% | 8.01% | 3.96% | 1.51% | |||
| Accuracy | 103% | 107% | 108% | 114% | 99.3% | 105% | |||
| β-amanitin | |||||||||
| Internal Standard | |||||||||
| None | MetSSP | 15N10-α-amanitin | |||||||
| QC Low | QC Medium | QC Low | QC Medium | QC Low | QC Medium | ||||
| Mean | 6.99 | 77.3 | 7.07 | 84.5 | 6.76 | 76.4 | |||
| CV | 12.4% | 8.45% | 10.4% | 15.9% | 16.4% | 16.7% | |||
| Accuracy | 99.9% | 103% | 101% | 113% | 96.6% | 102% | |||
| ɣ-amanitin | |||||||||
| Internal Standard | |||||||||
| None | MetSSP | 15N10-α-amanitin | |||||||
| QC Low | QC Medium | QC Low | QC Medium | QC Low | QC Medium | ||||
| Mean | 7.05 | 79.2 | 7.47 | 85.6 | 6.97 | 77.6 | |||
| CV | 12.7% | 10.9% | 16.3% | 14.9% | 13.0% | 14.3% | |||
| Accuracy | 101% | 106% | 106% | 115% | 99.6% | 104% | |||
α-Amanitin using the 15N10-α-amanitin internal standard for quantitation resulted in exceptional precision (<4%). Moderate precision for β- and γ-amanitin with and without the use of internal standards for quantitation (~15%) was also noted (Table 2). Linearity of β- and γ-amanitin was significantly improved without the use of an internal standard, as determined by correlation coefficient (R2 > 0.98). These pooled urine results indicated that 15N10-α-amanitin is a suitable internal standard for α-amanitin, while β- and γ-amanitin had more consistent quantitation without the use of an internal standard.
3.4. Method characterization
Based on the results from the internal standard evaluation, the final method that was characterized incorporated the selection of the 15N10-α-amanitin internal standard for α-amanitin, while β- and γ-amanitin were quantitated without an internal standard. Method accuracy and precision were characterized through the analysis of 26 sets of pooled urine calibrators and QC samples over a period of three months. A maximum of two curves with QC samples were evaluated per day over three months by two analysts. Characterization began with two QC samples, a QC Low and QC Medium. Once the calibration range was determined (α- and γ-amanitin = 1 – 200 ng/mL, β-amanitin = 2.5 – 200 ng/mL), an additional sample (QC High = 150 ng/mL) was incorporated to have test accuracy throughout the reportable range for the final ten runs.
α-Amanitin was accurately quantitated with an accuracy within 6% of the nominal value and a precision <6% (Table 3). The accuracy for β- and γ-amanitin was within 5% of the expected values for the three QC levels (Table 3). The inter-day precision was ≤15% for all analytes with the exception of the QC Low for β-amanitin, which was near the detection limit (Table 3). These results meet or exceed FDA criteria for Bioanalytical Methods, confirming the high reproducibility and accuracy for the detection of amanitins in pooled urine [US FDA, 2018].
Table 3.
Characterized pooled urine quality control samples values mean, CV, and accuracy for QC Low and QC Medium (n=26), and QC High (n=10).
| α-amanitin | β-amanitin* | γ-amanitin* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| QC Low | QC Medium | QC High | QC Low | QC Medium | QC High | QC Low | QC Medium | QC High | |||
| Mean | 7.01 | 79.2 | 164 | 6.99 | 78.5 | 158 | 6.93 | 77.7 | 155 | ||
| CV | 5.49% | 4.25% | 4.03% | 17.2% | 14.2% | 14.8% | 8.72% | 11.8% | 10.2% | ||
| Accuracy | 100% | 106% | 106% | 99.9% | 105% | 102% | 99.0% | 104% | 101% | ||
β- and γ-amanitin characterized without internal standard.
Specificity of the method was evaluated through the preparation of over 90 individual human urine samples with no known exposure to amanitins. No peaks were detected corresponding to α-, β-, or γ-amanitin in any of the urines analyzed, indicating this method has minimal potential for false positives resulting from the matrix.
Sensitivity was assessed using results obtained from the characterization in pooled urine using the four lowest calibrators which resulted in the following calculated LODs: α-amanitin, 0.458 ng/mL; β-amanitin, 0.930 ng/mL; γ-amanitin, 0.169 ng/mL.
3.5. Method evaluation
Additional individual urines and urine pools were assessed to evaluate the method for accurate quantitation, using the characterized method (α-amanitin quantitated with the 15N10-α-amanitin internal standard and β- and γ-amanitin without an internal standard). Eleven urine samples, including nine individual urines and two different pooled urines, were fortified with α-, β-, and γ-amanitin at 46 ng/mL, extracted in duplicate, analyzed, and quantified.
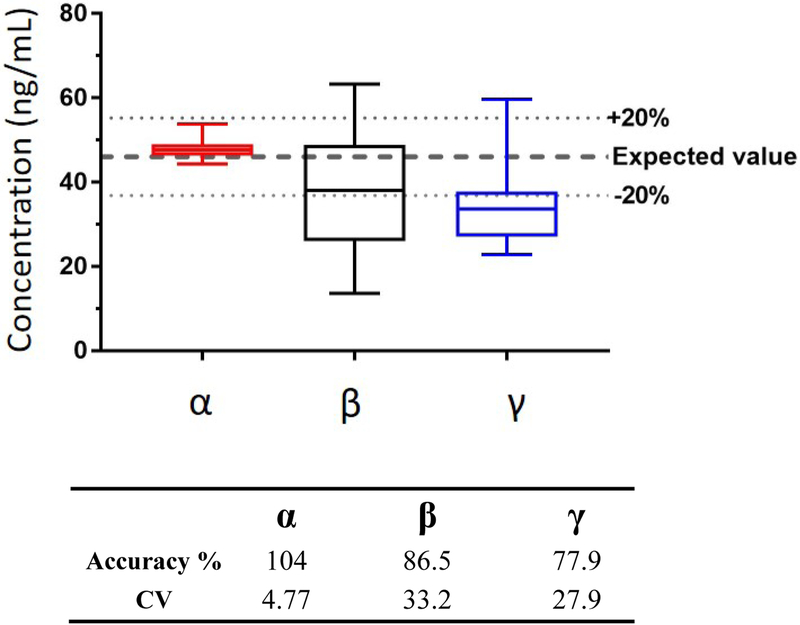
The most accurate and precise results were obtained for α-amanitin with an accuracy of 104% and a CV of 4.77% (Figure 3). The mean accuracy was within 86.5% - 104% for all analytes, with the exception of γ-amanitin with an accuracy of 77.9% (Figure 3). The CV of β- and γ-amanitin across all samples ranged from 27.9 – 54.1% (Figure 3). β-, and γ-Amanitin were also evaluated with the 15N10-α-amanitin and MetSSP for quantitation. When using the 15N10-α-amanitin as an internal standard, the R2 did not meet the 0.98 criteria for either analyte. Additionally, no improvement in the accuracy and precision of β-, and γ-amanitin was identified using these internal standards when compared with the characterized method (Data not shown).
Figure 3.
Concentrations of 11 urines fortified with α-, β-, and γ-amanitin at 46 ng/mL (9 individual urines and 2 urine pools analyzed in duplicate) using the characterized method (α-amanitin quantitated with internal standard, β- and γ-amanitin quantitated without internal standard).
These results support the efficacy of 15N10-α-amanitin to compensate for matrix effects for α-amanitin. However, the biased results for γ-amanitin and significant variability noted for both β- and γ-amanitin indicate matrix effects were still present for these two compounds. Even with sample preparation and orthogonal chromatographic separation, the surrogate internal standards evaluated in this study were unable to compensate for these variable matrix effects. Given the success of 15N10-α-amanitin as an internal standard for α-amanitin, the preparation of isotopically labeled versions of β- and γ-amanitin would likely resolve this issue but are not currently available. Instead of a highly complex chemical synthesis of this bicyclic structure, future isotopically labeled amanitins may be most easily obtained from expression (via mushrooms or yeast) in a special growth medium (15N), as was done to create the 15N10-α-amanitin [Luo, 2015]. Until those internal standards are available, this method still meets a critical need to identify exposure to amanitins and provides supportive diagnostic information to address amatoxin poisoning.
4.0. Conclusions
Typically, amanitin intoxication is identified from a history of wild mushroom ingestion and timing of symptom onset; however, a clinical means to confidently diagnose exposure to α-, β-, and γ- amanitin is needed. Consequently, an LC-MS/MS method was developed and characterized to detect α-, β- and γ-amanitin in urine. This is the first isotopic dilution method developed for amatoxins using 15N10-α-amanitin as an internal standard. The evaluation of multiple urine matrices identified significant matrix effects and supported the need for an effective internal standard, ideally, isotopic dilution, to compensate for these effects. Although an ideal internal standard for the quantitation of β- and γ-amanitin was not identified in this study, the precision and accuracy of these analytes in pooled urine without internal standard was ≤17.2%, and between 99.0–106%, respectively. Method performance could be improved once isotopically labeled versions of all analytes were incorporated; however, this method can confirm exposure to α-, β-, and γ-amanitin, providing crucial information for accurate diagnosis and treatment of amanitin toxicity.
Supplementary Material
Highlights.
First use of an isotopically labeled α-amanitin
Precise and accurate quantitation for α-amanitin using the 15N10-α-amanitin internal standard
α-, β- and γ-amanitin exposure samples can be confirmed with the developed method
Acknowledgements
The authors acknowledge Professor Jonathan Walton’s research group at Michigan State University for their efforts toward the first biosynthesis of isotopically labeled α-amanitin that was imperative for this work. The detailed development of the sample preparation protocol and chromatographic conditions completed by Alaine Garrett were crucial to the completion of this project. The authors would also like to acknowledge Christopher T. Pittman, Joe V. Wooten, Brian S. Crow, and Thomas A. Blake for their early development of a method to detect α-amanitin, which served as a starting point for much of the work contained in this paper.
Footnotes
Additional information
This work was supported by the Centers for Disease Control and Prevention, the Battelle Memorial Institute, and the Oak Ridge Institute for Science and Education. There are no competing interests or conflict of interests.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service, or the US Department of Health and Human Services.
References
- Andres RY, Frei W, Gautschi K, Vonderschmitt DJ, Radioimmunoassay for amatoxins by use of a rapid, 125I-tracer-based system. Clin Chem, 1986. 32(9): p. 1751–5. [PubMed] [Google Scholar]
- Bakirci S, Bayram R,Yilmaz I, Yaykasli KO, Bayram S, Kaya E, Purification and in vitro toxicity of gamma amanitin. Toxin Reviews, 2015. 34(4): p. 200–205. [Google Scholar]
- Bonfiglio R, King RC, Olah TV, Merkle K, The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun Mass Spectrom, 1999. 13(12): p. 1175–1185. [DOI] [PubMed] [Google Scholar]
- Bronstein AC, Spyker DA, Cantilena LR Jr, Green J, Rumack BH, Heard SE, 2006 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS). Clin Toxicol (Phila), 2007. 45(8): p. 815–917. [DOI] [PubMed] [Google Scholar]
- Bronstein AC, Spyker DA, Cantilena LR Jr, Green JL, Rumack BH, Heard SE, 2007 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 25th Annual Report. Clin Toxicol (Phila), 2008. 46(10): p. 927–1057. [DOI] [PubMed] [Google Scholar]
- Bronstein AC, Spyker DA, Cantilena LR Jr, Green JL, Rumack BH, Giffin SL, 2008 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 26th Annual Report. Clin Toxicol (Phila), 2009. 47(10): p. 911–1084. [DOI] [PubMed] [Google Scholar]
- Bronstein AC, Spyker DA, Cantilena LR Jr, Green JL, Rumack BH, Giffin SL, 2009 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 27th Annual Report. Clin Toxicol (Phila), 2010. 48(10): p. 979–1178. [DOI] [PubMed] [Google Scholar]
- Bronstein AC, Spyker DA, Cantilena LR Jr, Green JL, Rumack BH, Dart RC, 2010 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 28th Annual Report. Clin Toxicol (Phila), 2011. 49(10): p. 910–41. [DOI] [PubMed] [Google Scholar]
- Bronstein AC, Spyker DA, Cantilena LR Jr, Rumack BH, Dart RC, 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th Annual Report. Clin Toxicol (Phila), 2012. 50(10): p. 911–1164. [DOI] [PubMed] [Google Scholar]
- Broussard CN, Aggarwal A, Lacey SR, Post AB, Gramlich T, Henderson JM, Younossi ZM, Mushroom poisoning--from diarrhea to liver transplantation. Am J Gastroenterol, 2001. 96(11): p. 3195–8. [DOI] [PubMed] [Google Scholar]
- Chung WC, Tso SC, and Sze ST, Separation of polar mushroom toxins by mixed-mode hydrophilic and ionic interaction liquid chromatography-electrospray ionization-mass spectrometry. J Chromatogr Sci, 2007. 45(2): p. 104–11. [DOI] [PubMed] [Google Scholar]
- Defendenti C, Bonacina E, Mauroni M, Gelosa L, Validation of a High Performance Liquid Chromatographic Method for α-Amanitin Determination in Urine. Forensic Science International, 1998. 92: p. 59–68. [DOI] [PubMed] [Google Scholar]
- Faulstich H, Mushroom Poisoning. The Lancet, 1980: p. 794–795. [DOI] [PubMed] [Google Scholar]
- Gicquel T, Lepage S, Fradin M1 Tribut O, Duretz B, Morel I, Amatoxins (alpha- and beta-Amanitin) and phallotoxin (Phalloidin) analyses in urines using high-resolution accurate mass LC-MS technology. J Anal Toxicol, 2014. 38(6): p. 335–40. [DOI] [PubMed] [Google Scholar]
- Helfer AG, Meyer MR, Michely JA, Maurer HH, Direct analysis of the mushroom poisons alpha- and beta-amanitin in human urine using a novel on-line turbulent flow chromatography mode coupled to liquid chromatography-high resolution-mass spectrometry/mass spectrometry. J Chromatogr A, 2014. 1325: p. 92–8. [DOI] [PubMed] [Google Scholar]
- Jaeger A, Jehl F, Flesch F, Sauder P, Kopferschmitt J, Kinetics of amatoxins in human poisoning: therapeutic implications. Clinical Toxicology, 1993. 31(1): p. 63–80. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Zhou Y, Jain R, Lemire SW, Fox S, Sabourin P, Barr JR., Quantification of L-abrine in human and rat urine: a biomarker for the toxin abrin. J Anal Toxicol, 2009. 33(2): p. 77–84. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Lemire SW, Woolfitt AR, Ospina M, Preston KP, Olson CT, Barr JR, Quantification of ricinine in rat and human urine: a biomarker for ricin exposure. J Anal Toxicol, 2005. 29(3): p. 149–55. [DOI] [PubMed] [Google Scholar]
- Kaya E, Karahan S, Bayram R, Yaykasli KO, Colakoglu S, Saritas A, Amatoxin and phallotoxin concentration in Amanita phalloides spores and tissues. Toxicol Ind Health, 2015. 31(12): p. 1172–7. [DOI] [PubMed] [Google Scholar]
- Lai MW, Klein-Schwartz W, Rodgers GC, Abrams JY, Haber DA, Bronstein AC, Wruk KM, 2005 Annual Report of the American Association of Poison Control Centers’ national poisoning and exposure database. Clin Toxicol (Phila), 2006. 44(6–7): p. 803–932. [DOI] [PubMed] [Google Scholar]
- Leite M, Freitas A, Azul AM, Barbosa J, Costa S, Ramos Fl, Development, optimization and application of an analytical methodology by ultra performance liquid chromatography-tandem mass spectrometry for determination of amanitins in urine and liver samples. Anal Chim Acta, 2013. 799: p. 77–87. [DOI] [PubMed] [Google Scholar]
- Letschert K, Faulstich H, Keller D, Keppler D, Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol Sci, 2006. 91(1): p. 140–9. [DOI] [PubMed] [Google Scholar]
- Li C, Wei F, Muhammad S, Yang G, Wang S, Liu X, A cost-effective LC-MS/MS method for identification and quantification of alpha-amanitin in rat plasma: Application to toxicokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci, 2017. 1064: p. 36–39. [DOI] [PubMed] [Google Scholar]
- Luo H, DuBois B, Sgambelluri RM, Angelos ER, Li X, Holmes D, Walton JD, Production of (15)N-labeled alpha-amanitin in Galerina marginata. Toxicon, 2015. 103: p. 60–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszewski BK, Constanzer ML, Chavez-Eng CM, Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem, 2003. 75(13): p. 3019–30. [DOI] [PubMed] [Google Scholar]
- Maurer HH, Schmitt CJ, Weber AA, Kraemer T, Validated electrospray liquid chromatographic-mass spectrometric assay for the determination of the mushroom toxins alpha- and beta-amanitin in urine after immunoaffinity extraction. J Chromatogr B Biomed Sci Appl, 2000. 748(1): p. 125–35. [DOI] [PubMed] [Google Scholar]
- Mengs U, Pohl RT, and Mitchell T, Legalon(R) SIL: the antidote of choice in patients with acute hepatotoxicity from amatoxin poisoning. Curr Pharm Biotechnol, 2012. 13(10): p. 1964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowry JB, Spyker DA, Brooks DE, McMillan N, Schauben JL, 2014 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd Annual Report. Clin Toxicol (Phila), 2015. 53(10): p. 962–1147. [DOI] [PubMed] [Google Scholar]
- Mowry JB, Spyker DA, Brooks DE, Zimmerman A, Schauben JL, 2015 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd Annual Report. Clin Toxicol (Phila), 2016. 54(10): p. 924–1109. [DOI] [PubMed] [Google Scholar]
- Parant F, Peltier L, Lardet G, Pulce C, Descotes J, Moulsma M, [Phalloidin syndrome: role of Elisa-based assay for the detection of alpha- and gamma-amanitins in urine. Preliminary results]. Acta Clin Belg, 2006. 61 Suppl 1: p. 11–7. [DOI] [PubMed] [Google Scholar]
- Robinson-Fuentes VA, Jaime-Sánchez JL, García-Aguilar L, Gómez-Peralta M, Vázquez-Garcidueñas MS, Vázquez-Marrufo G, Determination of a- and b-Amanitin in Clinical Urine Samples by Capillary Zone Electrophoresis. Journal of Pharmaceutical and Biomedical Analysis, 2008. 47: p. 913–917. [DOI] [PubMed] [Google Scholar]
- Santi L, Maggioli C, Mastroroberto M, Tufoni M, Napoli L, Caraceni P, Acute Liver Failure Caused by Amanita phalloides Poisoning. Int J Hepatol, 2012. 2012: p. 487480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JK, Quality assurance of chemical measurements. 1987: Lewis Publishers; Boca Raton, FL: p. 79–82. [Google Scholar]
- Tomkova J, Ondra P, Valka I, Simultaneous determination of mushroom toxins alpha-amanitin, beta-amanitin and muscarine in human urine by solid-phase extraction and ultra-high-performance liquid chromatography coupled with ultra-high-resolution TOF mass spectrometry. Forensic Sci Int, 2015. 251: p. 209–13. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration (FDA), Bioanalytical Method Validation, Guidance for Industry, May 2018.
- Watson WA, Litovitz TL, Rodgers GC Jr, Klein-Schwartz W, Youniss J, Rose SR, Borys D, May ME, 2002 annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med, 2003. 21(5): p. 353–421. [DOI] [PubMed] [Google Scholar]
- Watson WA, Litovitz TL, Klein-Schwartz W, Rodgers GC Jr, Youniss J, Reid N, Rouse WG, Rembert RS, Borys D, 2003 annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med, 2004. 22(5): p. 335–404. [DOI] [PubMed] [Google Scholar]
- Watson WA, Litovitz TL, Rodgers GC Jr, Klein-Schwartz W, Reid N, Youniss J, Flanagan A, Wruk KM, 2004 Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med, 2005. 23(5): p. 589–666. [DOI] [PubMed] [Google Scholar]
- Wooten JV, Pittman CT, Blake TA, Thomas JD, Devlin JJ, Higgerson RA, Johnson RC., A case of abrin toxin poisoning, confirmed via quantitation of L-abrine (N-methyl-L-tryptophan) biomarker. J Med Toxicol, 2014. 10(4): p. 392–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz I, Kaya E, Sinirlioglu ZA, Bayram R, Surmen MG, Colakoglu S, Clinical importance of toxin concentration in Amanita verna mushroom. Toxicon, 2014. 87: p. 68–75. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhao Y, Li H, Zhou S, Chen D, Zhang Y, Yao Q, Sun C, A Simple and High-Throughput Analysis of Amatoxins and Phallotoxins in Human Plasma, Serum and Urine Using UPLC-MS/MS Combined with PRiME HLB muElution Platform. Toxins (Basel), 2016. 8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.