Abstract
The circadian rhythm (CR) is a set of autonomous endogenous oscillators. Exposure to the 24-hour day-night cycle synchronizes our CR system, maintaining homeostasis and human health. Several mechanisms for the CR system have been proposed, including those underlying the function (transcriptional-translational negative-feedback loops, or TTFLs), mechanisms regulating the TTFLs, and the mechanism by which the “server clock” is synchronized to environmental time. Several pathways downstream of the “server clock” perform well-characterized biological functions. However, the synchronization between the “server clock” (the endogenous master clock seated in the suprachiasmatic nucleus within the hypothalamus) and the “client clock” (imbedded in nearly every cell in the form of interlocking TTFLs) is difficult to explain with current theories. Extracellular vesicles (EVs), which are involved in intercellular communication and have recently been found to participate in regulation of the “client clock”, might be the answer to this question. In this review, we summarize the current knowledge of CRs, TTFLs, and EVs, examine research findings about the functions of EVs in the CR system, and discuss the issues requiring attention in future research.
Keywords: circadian rhythms, extracellular vesicles, exosomes, transcriptional-translational negative-feedback loops, post-translational modifications, noncoding RNAs
1. Introduction
Almost every life-form on Earth is exposed to environmental changes over the 24-hour day-night cycle (environmental time), resulting in the evolution of circadian rhythms (CRs) to adapt to daily changes 1. A wide variety of physiological processes are influenced by CRs, which are essential for maintaining the health of mammals, including humans 2. In addition to the sleep-wake cycle—the most conspicuous output of CRs—there are diurnal variations in other physiological systems, including the cardiovascular system, digestive system, endocrine system, body temperature regulation, metabolism, and immune functions 3-7.
The molecular-level core of the CR system consists of oscillating clock-related genes that constitute the transcriptional-translational negative-feedback loops (TTFLs) 8. Although the oscillators of the CR system are endogenous, exposure to the normal light/dark cycle is essential for the CR system to synchronize with environmental time and keep the body functioning normally by adapting to the environment 9-11. However, in modern society, sun-free environments in the daytime and the use of artificial light at night have become commonplace, as these technologies have allowed human lifestyles to become increasingly flexible—people can eat, sleep, work, and exercise whenever they want 2, 9-11. These lifestyle changes correlate with rising rates of several disorders, including heart disease 12, ulcers 13, cancer 14, somnipathy 15, diabetes 16, depression 17, and cognitive disorders 18. There is solid evidence that night-shift work increases the risk of malignant tumours, diabetes, and cardiomyopathy 19-25. Thus, a thorough understanding of the operating mechanism of the CR system is essential for disease prevention, early diagnosis, and effective intervention.
The endogenous CR system is mainly composed of two parts: the endogenous master clock (or “server clock”) and subordinate clocks (or “client clocks”). The endogenous “server clock” of the CR system is seated in the suprachiasmatic nucleus (SCN) in the hypothalamus 26, 27. Through specialized retinal ganglion cells, the “server clock” is calibrated by environmental time rather than simply obeying the endogenous rhythms generated by TTFLs 28, 29. The “client clocks” are imbedded in nearly every cell in the form of interlocking TTFLs, which are composed of clock genes that exert their biological effects via target genes (clock-controlled genes) 30, 31. Hence, understanding of the synchronization mechanisms between “client clock” and “server clock” is crucial for a complete understanding of the CR system.
Recently, post-translational modifications (PTMs) and noncoding RNAs (ncRNAs) have been found to regulate the physiological processes of CRs 2, 32-35. In addition, extracellular vesicles (EVs), important intercellular couriers of proteins and ncRNAs, have attracted increasing attention 36. EVs might carry the synchronization signal to correct the “client clocks” in accordance with the “server clock”.
In this review, we will first introduce the molecular mechanism of CRs. Then, we will highlight the current knowledge concerning EVs and the regulatory mechanisms in the CR system. Finally, we will discuss how EV-mediated gene regulation could regulate the CR system. Given their emerging potential in diagnostics and even therapeutics, a thorough understanding of the role of EVs in the CR system could help tackle CR-related diseases.
2. Circadian Rhythms
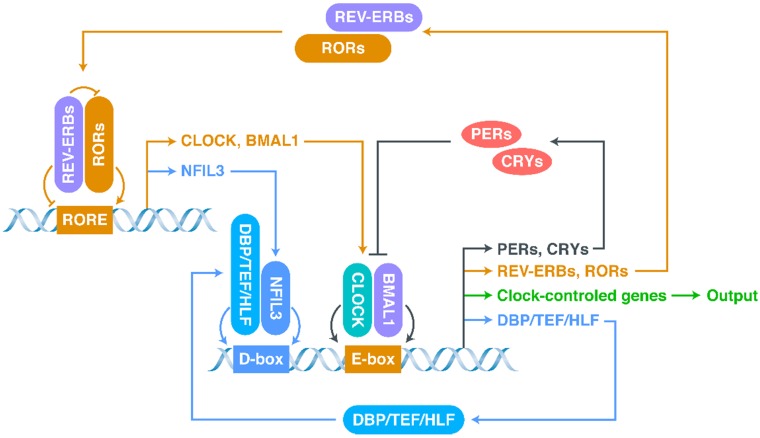
The most basic structure of the CR system is the “client clock”, a cell-autonomous circadian oscillator that exists in all cells, which is composed of clock-related proteins and is described as a network of interlocking TTFLs (Figure 1) 21, 37-40. The core loop of TTFLs is constituted by both transcriptional activator genes [CLOCK, Brain and muscle Arnt-like protein 1 (BMAL1)] and repressor genes [Period-1 (PER1), PER2, PER3, Cryptochrome-1 (CRY1) and CRY2] 21. The CLOCK-BMAL1 complex binds to the E-box-containing regulatory elements in repressor genes 41-43. The working mechanism of the core loop, in brief, is that CLOCK-BMAL1 transactivation activates the transcription of repressor genes (including CRYs and PERs) together with the output genes (clock-controlled genes), and then, after translation, the accumulated CRY and PER proteins interact with each other to suppress CLOCK-BMAL1 activation (negative feedback) 38. As the repression progresses, the protein levels of repressors, which have short half-lives and can be degraded by proteasomes, decrease, allowing a new CR cycle to begin 33, 44.
Figure 1.
The network of interlocking TTFLs.
In addition to the core loop, there are two sub-loops, which are coupled to the core loop to consummate the oscillation. The first sub-loop consists of retinoic acid receptor-related orphan receptors (RORs) and nuclear receptors called REV-ERBs (including REV-ERBα and REV-ERBβ) 21, 38, 39, 45-47. REV-ERBs compete with RORs for binding to ROR-binding elements (RORE): REV-ERBs repress, whereas RORs activate, CLOCK and BMAL1 39, 45. The second sub-loop consists of D-box-related proteins, including D‐box binding protein (DBP), thyrotroph embryonic factor (TEF), and hepatic leukaemia factor (HLF) 39, 48, 49. These proteins interact with nuclear factor interleukin-3-regulated protein (NFIL3), a downstream protein of RORs/REV-ERBs, at D-boxes 39, 48, 49. This molecular-level oscillator network is the foundation of the CR system.
The “server clock” seated in the SCN is the master of the CR system in mammals 50—it receives the input signal (light from the external environment) via the retinohypothalamic tract to synchronize the “server clock” to environmental time 51. The “server clock” then communicates with the wider central nervous system (CNS) and non-CNS organs 52. Ablation of the SCN leads to dysregulation of clock genes in the “client clock” of most tissues, causing arhythmicity of behaviours and physiological functions 8. Thus, the duty of the “server clock” is to synchronize the “client clock” to environmental time 53.
There are three classical neuroendocrine pathways governing the peripheral effects induced by the “server clock”. First, the “server clock” induces the release of epinephrine/norepinephrine via the nerve endings of the autonomic nervous system 54. Second, it induces the release of glucocorticoids by the adrenal gland via the hypothalamic-pituitary-adrenal axis 55. Third, it induces melatonin release by activating the pineal gland 56. These neuroendocrine pathways and their downstream effects can explain many of the peripheral functions of the “server clock” 56. However, these pathways cannot explain how the “client clocks” synchronize with the “server clock”.
There is mounting evidence that disruption of the CR system is intimately implicated in the pathology of neurodegenerative diseases 57-59, metabolic disorders 60, chronic inflammatory diseases 61, cardiovascular disease 62, and malignant tumours 19. The peripheral blood cells of Parkinson's disease patients have abnormal clock gene expression 63, 64. Kim et al. found that deletion of the secretory vesicle proteins IA-2 and IA-2β, which have also recently been found to be closely related to EV secretion, affects the CR system 65, 66. These findings suggest that the pathogenesis of CR-related diseases is complicated and multi-dimensional (at the level of the “server clock”, the “client clock”, or in the signal transduction between them, for example).
3. Regulatory Mechanisms of the TTFLs
Recent research has been focused on the multi-level regulatory mechanisms of TTFLs, which can be classified into groups depending on their main level of action: PTMs and ncRNAs. Both the “server clock” and the “client clock” are precisely regulated by PTMs 33-35.
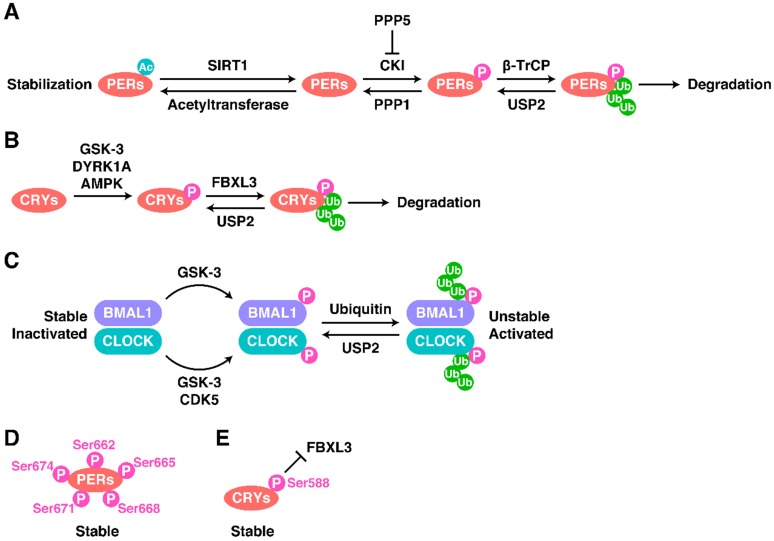
Both the total level and the phosphorylated level of PER proteins dramatically influence the oscillations of the CR system 38, 67. The main modification of PERs are shown in Figure 2A. In most cases, PER1-3 are phosphorylated by casein kinase I (CKI) and then poly-ubiquitinated by the Skp-Cullin-F-box (SCF) complex containing the F-box type E3 ligase β-TrCP1 or β-TrCP2, before degradation by the proteasome 38, 68, 69. PER2 is phosphorylated at Ser53 by CKII and then degraded 38. However, the different phosphorylation sites on PER2 seem to have different effects on the stability of PER2 and thus on oscillations. Phosphorylation of Ser662 (and the four downstream serines Ser665, Ser668, Ser671, and Ser674) leads to the stabilization of PER2 (Figure 2D) 38, 70, 71.
Figure 2.
Regulatory Mechanisms of the TTFLs. The modification of (A) PERs, (B) CRYs and (C) BMAL1-CLOCK as well as some special cases (D and E).
The opposite of phosphorylation is de-phosphorylation. Phosphoprotein phosphatase 1 (PPP1) can de-phosphorylate PERs and thus antagonize the CKI-mediated degradation of PERs 38, 72. PPP5 regulates the stabilization of PERs and the CR cycle by preventing the auto-phosphorylation of CKI 72, 73. Moreover, de-ubiquitination and acetylation can also stabilize proteins. For example, BMAL1, PER1, and CRY1-2 can be de-ubiquitinated by ubiquitin-specific protease 2 (USP2), stabilizing them 72, 74, 75. SIRT1 binds to PER2, inducing its de-acetylation and promoting its degradation 76, 77.
The phosphorylation and ubiquitination of CRY proteins is also important, and a diagram showing the main modification of CRYs is shown in Figure 2B. AMP-activated protein kinase (AMPK) phosphorylates CRY1 at Ser71 to induce the degradation of CRY1 by recruiting FBXL3 78. The FBXL3-containing SCF complex induces the ubiquitination of CRY proteins and terminates their transcriptional repression activity by promoting their degradation 79, 80. Dual-specificity tyrosine-phosphorylation-regulated kinase 1A (DYRK1A) phosphorylates Ser557 on the C-terminus of CRY2 81, and glycogen synthase kinase 3 (GSK-3) induces the secondary phosphorylation of CRY2 at Ser553 81, 82. After this two-step phosphorylation, CRY2 is degraded by an unknown E3 ligase 81. The phosphorylation of CRY1 at Ser588 blocks the interaction between FBXL3 and CRY1, stabilizing CRY1 (Figure 2E), but the responsible kinase remains unknown 83.
The mechanisms regulating CLOCK and BMAL1 act in a completely different way to those just described (Figure 2C). First, the total protein levels of CLOCK and BMAL1 are relatively stable, but their phosphorylation levels are circadian 67, 84. Phosphorylation decreases the stability of CLOCK and BMAL1 but promotes their activity 85, 86. Cyclin-dependent kinase 5 (CDK5) phosphorylates CLOCK at Thr451 and Thr461 87. GSK-3 phosphorylates CLOCK at Ser427 and BMAL1 at Ser17 85, 86.
Noncoding RNAs are also involved in the regulation of the CR system. Researchers have developed a computational model involving PERs, CRYs, CLOCK, BMAL1, and two microRNAs (miRNAs; miR-219 and miR-132) 88-90. Using this model, it was found that the most studied CR-related miRNAs to date, miR-219 and miR-132, can activate the translation of PER1 and regulate the mammalian CR system 88, 89, 91, 92. MiR-219 in the SCN shows a rhythmicity correlated to the CR and has its peak expression level at midday 88, 93. Photo-activated expression of miR-132 requires CREB and MAPK/ERK 88, 94, 95.
In addition, circulating miR-494 and miR-142-3p can modulate CRs by targeting the clock gene BMAL1 96, 97, while miR-433 regulates the expression of PER2 and BMAL1 98. BMAL1 is also targeted by miR-27b-3p 113 and miR-155 99, while PER1 is targeted by miR-34a 100, and the miR-192/194 cluster can inhibit the PER family 101. Finally, translation of CRY1 is regulated by miR-185 102.
The lncRNA TUG-1 is required for photoreceptor differentiation, but the underlying mechanism is still unknown 88, 103. The level of the lncRNA HULC is positively correlated with the expression level of CLOCK and can upregulate its downstream output genes 104. However, it is essential to screen more potential CR-related ncRNAs, and study their mechanisms and functions in order to broaden understanding of the regulatory network of CR.
4. Basic Concepts of Extracellular Vesicles
EV secretion was initially regarded as a process to eliminate unwanted compounds from cells 105, 106. However, pioneers in the field (Raposo et al.) found, in 1996, that EVs play important roles in immune responses 107. Since then, the functions of EVs in intercellular communication have drawn increasing attention and the number of annual citations has dramatically increased, from 28 in 1996 to 24,765 in 2016 108. Almost all eukaryotic cells take up and secrete EVs, and these minute EVs contain genetic instructions (nucleic acids and proteins) that regulate the function of the recipient cells, whether under normal or pathological conditions, sometimes mildly, sometimes strongly 108. Moreover, the communication process mediated by EVs is conserved from bacteria to plants and animals 109, 110. Most interestingly, there is much evidence of cross-species communication via EVs, even between micro-organisms and mammals 111.
EVs can be roughly classified into three categories: exosomes (EXOs), microvesicles (MVs), and apoptotic bodies (ABs) 106, 112-114. EXOs and MVs are the most important EVs in intercellular communication, while ABs are rapidly eliminated by immune cells 115. EXOs (also known as inward-budding vesicles; 30-100 nm in diameter) are generated on endosomal membranes by inward budding during the maturation process of multi-vesicular bodies (MVBs) 105, 106, 116, 117. Before MVBs fuse with the plasma membrane and release EXOs, EXOs in MVBs are called intra-luminal vesicles 106, 116, 117. MVs (also known as outward-budding vesicles; 50-1,000 nm in diameter) are generated and released on the plasma membrane by outward budding 106, 113 and were initially studied for their important roles in blood coagulation 106, 118, 119. However, new research suggests MVs are important players in intercellular communication 106, 120.
Although there are differences between MVs and EXOs in terms of their biogenesis and release, most research on the biological function of EVs has not strictly accounted for the differences in their intracellular origins. Many researchers do not strictly consider small EVs, isolated by gradient centrifugation or 0.22 μm filters, as EXOs 121, 122. The mainstream view now holds that there is no need to make a detailed distinction between EXOs and MVs, if the research is focused on their pathological and physiological functions, rather than their biogenesis. In this review, we have called them both EVs, in accord with most reviews discussing EVs 123, 124.
5. Extracellular Vesicle-Mediated Intercellular Communication
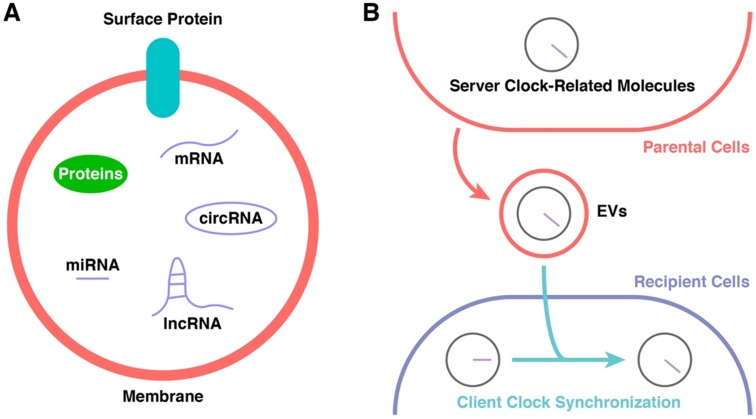
EVs contain and transport several types of molecules, including membrane proteins and cytosolic proteins, messenger RNAs, and ncRNAs (such as miRNAs, lncRNAs, and circular RNAs) (Figure 3A) 113, 115, 125. The regulatory functions of EVs in signalling pathways of recipient cells are based on either receptor-ligand interactions or direct content delivery after internalization 115, 123. Their phospholipid bilayers enable EVs to protect their cargo from the external environment, and they are found in almost all body fluids 124, 126, 127. The different surface proteins expressed on EVs give them different targeting properties 128.
Figure 3.
Potential Participants in CR Synchronization. (A) The constitution of EVs. (B) The cargoes of EVs might be the key of CR synchronization.
EVs can be derived from nearly every type of cell, both normal 129, 130 and malignant 131, 132. Normal cells use EVs to coordinate, communicate, and cooperate with their “colleagues” 115. Malignant cells use EVs to mislead normal cells and issue aberrant orders. EVs derived from malignant cells can deliver signals to establish a pre-metastatic niche, a suitable environment for metastasis 133-136. The constitution of EVs derived from malignant cells is markedly different from that of EVs derived from normal cells 137, 138, and this makes them potential biomarkers in liquid biopsies. EVs also show potential as diagnostic markers and progression markers of infectious diseases 139-141. Hence, it is also possible to choose EVs as potential markers to monitor the functions of the CR and to detect early warning signals related to dysfunction of the internal CR system.
EVs are also potential therapeutic tools to regulate and maintain the internal CR system and prevent/treat disease caused by dysfunction of the internal CR system. Stem cells once showed great potential in regenerative medicine because of their unparalleled proliferation/differentiation potential 142. However, embryonic stem cells and induced pluripotent stem (iPS) cells have shown key weaknesses, including immunological rejection, tumour formation, and circulation damage 124, 143. In recent years, EVs have become rising stars in the field of regenerative medicine for use in cell-free therapy. Increasing evidence suggests that stem/progenitor cell-derived EVs have therapeutic functions similar to or even better than their parent cells 124, 144. In addition, EVs are stable and easy to preserve because they can retain bioactivity during lyophilisation and other extreme conditions during handling, owing to the protective effect of their lipid bilayer 124, 145. Moreover, EVs can cross barriers such as the blood-brain barrier 136, so the route of administration is relatively easy. Above all, therapeutic use of EVs is safer than direct use of their parent cells because of their hypoallergenic nature and lack of oncogenic potential 124. Evidence of their hypoallergenicity includes the fact that human-derived EVs work well upon first injection and repeated injections in animal models, including rats, mice, and pigs 108. Adamiak et al. found that over half of the mice they injected with iPS cells developed teratomas, whereas mice injected with EVs derived from iPS cells (even from the same cells that had caused teratomas) did not develop teratomas 144. Stem/progenitor cell-derived EVs (or even EVs derived from fragments of cytoplasm such as platelets) regulate the biological processes of the target cells by delivering parent cell-originated nucleic acids and proteins 124, 126, 146-148.
Natural EVs cannot always meet therapeutic needs, and developing improved/modified EVs will be an exciting area of future research 124, 126, 127, 149, 150. For example, EVs derived from synovial mesenchymal stem cells enhance the proliferation and migration of chondrocytes, but reduce the formation of extracellular matrix 127. This problem can be solved by enhancing the levels of miR-140-5p in these EVs 127. Modified or optimized EVs might therefore be the future of EV-based therapeutic medicine. With advances in deep knowledge of the CR system and of the technology of EV-based therapy, preventing or reversing malfunctions, caused by modern life such as night shift work and jet lag, will become a reality.
6. Circadian Rhythms and Extracellular Vesicles
Recent research has suggested that EVs act as a bridge between the “server clock” and “client clock” (Figure 3B). Khalyfa et al. employed a mouse model of chronic nocturnal shift work and found altered intestinal flora and increased colonic cell permeability accompanied by changes in the components of plasma EVs, including clock genes 36. SIRT1, which is involved in the regulation of PER acetylation (see previous section), is regulated by EVs 151, 152. EVs also regulate the phosphorylation of GSK-3 153, which plays an important role in the regulation of CRYs and BMAL1/CLOCK (see previous section). AMPK, which participates in the regulation of CRYs (see previous section), is regulated by EVs and is probably related to ncRNAs in EVs 154-156.
MiR-132, which plays an important role in regulating PERs (see previous section), is enriched in EVs derived from fibrocytes, adipose tissue-derived stem cells, cardiac progenitor cells, neurons, serum, and umbilical cord blood 157-162. MiR-219, which participates in activating PER1 translation (see the previous section), is enriched in serum-derived EVs 163. Therefore, although direct links have yet to be shown, these results suggest that EVs in the circulatory system are communicators between the “server clock” and the “client clock”.
An online database of EVs, EVpedia (http://student4.postech.ac.kr/evpedia2_xe/xe/), could help to discover the existence of CR-related genes in EVs 164-168. The database confirmed the existence of PER2, PER3, CKI families, AMPKβ, AMPKγ, GSK-3α, and GSK-3β, as well as miR-219a, miR-219b, miR-132, miR-494, miR-142, miR-433, miR-27b-3p, miR-192, and miR-194 in EVs. Thus, EVs contain enough bioactive molecules to regulate the “client clock” of recipient cells, so it is essential to verify whether EVs participate in liaising between the “server clock” and the “client clock”. It is important not only to detect any additional CR-related molecules carried by EVs but also to verify the idiographic functions of these molecules.
Shende et al. found that several circulating miRNAs (miR-152, miR-494, and miR-142-3p) correlate with diurnal oscillation (expression peaks near midday and 8/12 h later) and participate in the regulation of clock genes in a mouse model 96. However, this study did not distinguish whether these miRNAs were free in the plasma or packaged in EVs. Circulating miR-494 and miR-142-3p can regulate CRs by targeting the clock gene BMAL1 96, but the locations of these miRNAs were not confirmed.
In blood, there are two major forms of circulating miRNAs: miRNAs as “cargos” of EVs (miRNA-EVs) and miRNAs bound to Argonaute (AGO) proteins (miRNA-AGOs) 169. It is generally believed that miRNA-AGOs cannot be internalized by recipient cells because of their size and lack of bioactivity for penetration 169-171, but miRNA-EVs can be efficiently internalized with their miRNAs by recipient cells via penetration 170, 172. When viewed from this perspective, the circulating miRNAs that regulate CR-related functions stand a good chance of being carried in EVs. Nonetheless, it is important to evaluate not only the total content of genetic material in plasma but also their specific locations (whether enclosed in EVs or not).
7. Discussion and Outlook
As outlined above, the mechanism underlying the CR appears to be a complicated, interconnected, and multi-level system. Although the fundamental structure and some regulatory mechanisms have been identified, many questions remain. In particular, current knowledge cannot explain the specific synchronization methods between “server clock” and “client clock”. Before reaching full understanding of the synchronization methods, the regulation of TTFLs at the molecular level needs to be better understood. In this review, we have summarized current understanding of the regulation of TTFLs, including by PTM and ncRNAs, in mammals. It is possible that the “server clock” transports PTM-related molecules or ncRNAs to the “client clock” through some as-yet unidentified mechanism, which may be EVs.
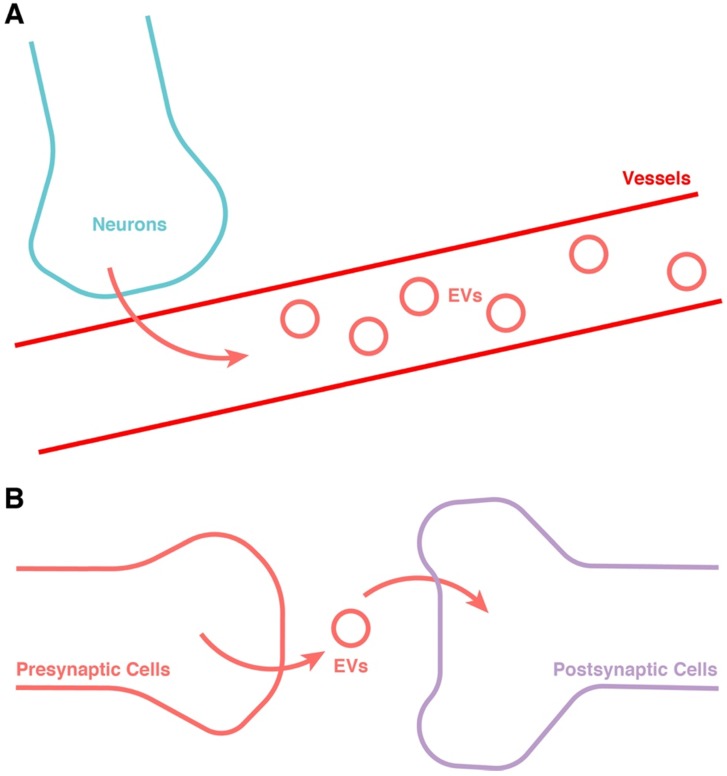
In addition to the transport of EVs in blood, the transmission of EVs between neurons is also worthy of serious attention 173 (Figure 4). The release of EVs has been found to be dependent on synaptic activity 174, and could be an important intermediary in neuron-neuron communication 175, 176. In the larvae of Drosophila, EVs have also been found to be involved in controlling the retrograde postsynaptic signal 177. The function of EVs related to synaptic activity could be explained by the function of EV-containing cargos including miRNAs and synaptic-associated proteins 178. This might be not only an important addition to the communication between “server clock” and CNS besides electroneurographic signals, but also a potential jigsaw of the entirety of CR synchronization. Hence, this research area also merits much further attention.
Figure 4.
Potential approach for the release of EVs from the “server clock”. (A) Release into the blood. (B) Transportation from neuron to neuron through the synapse.
Some evidence indicates that EVs participate in the regulation of individual cells' “client clocks”. Circulating RNAs also have important roles in the regulation of “client clocks”. Although the localization of these RNAs is not known, it is well recognized that circulating RNAs with biological effects are mainly found in EVs. It is very important to verify the localization of these circulating RNAs and their specific biological effects in the regulation of “client clocks”. An online database containing a large amount of high-throughput data such as EVpedia can greatly advance the progress of research. However, it will be necessary to establish an online database of the CR system, and carry out multi-database joint analysis to bring a breakthrough in this research area.
Acknowledgments
The National Natural Science Foundation of China [Nos. 81871834, 81802226 and 81301589] supported this work.
Author Contributions
Shi-Cong Tao planned and wrote this manuscript. Shang-Chun Guo helped with planning and writing. All authors reviewed the manuscript.
References
- 1.Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 1971;68:2112–6. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiological reviews. 2013;93:107–35. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardin PE, Hall JC, Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990;343:536–40. doi: 10.1038/343536a0. [DOI] [PubMed] [Google Scholar]
- 4.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends in cell biology. 2014;24:90–9. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA. et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science (New York, NY) 2007;318:1786–9. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 6.Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB. et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nature structural & molecular biology. 2007;14:1207–13. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science (New York, NY) 2016;354:994–9. doi: 10.1126/science.aah4965. [DOI] [PubMed] [Google Scholar]
- 8.Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science (New York, NY) 2016;354:1004–8. doi: 10.1126/science.aah4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedrosian TA, Nelson RJ. Influence of the modern light environment on mood. Molecular psychiatry. 2013;18:751–7. doi: 10.1038/mp.2013.70. [DOI] [PubMed] [Google Scholar]
- 10.Fonken LK, Nelson RJ. The effects of light at night on circadian clocks and metabolism. Endocrine reviews. 2014;35:648–70. doi: 10.1210/er.2013-1051. [DOI] [PubMed] [Google Scholar]
- 11.Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nature reviews Neuroscience. 2003;4:649–61. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 12.Kivimaki M, Virtanen M, Elovainio M, Vaananen A, Keltikangas-Jarvinen L, Vahtera J. Prevalent cardiovascular disease, risk factors and selection out of shift work. Scandinavian journal of work, environment & health. 2006;32:204–8. doi: 10.5271/sjweh.1000. [DOI] [PubMed] [Google Scholar]
- 13.Koda S, Yasuda N, Sugihara Y, Ohara H, Udo H, Otani T. et al. [Analyses of work-relatedness of health problems among truck drivers by questionnaire survey] Sangyo eiseigaku zasshi = Journal of occupational health. 2000;42:6–16. doi: 10.1539/sangyoeisei.kj00002552185. [DOI] [PubMed] [Google Scholar]
- 14.Ball LJ, Palesh O, Kriegsfeld LJ. The Pathophysiologic Role of Disrupted Circadian and Neuroendocrine Rhythms in Breast Carcinogenesis. Endocrine reviews. 2016;37:450–66. doi: 10.1210/er.2015-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trenell MI, Marshall NS, Rogers NL. Sleep and metabolic control: waking to a problem? Clinical and experimental pharmacology & physiology. 2007;34:1–9. doi: 10.1111/j.1440-1681.2007.04541.x. [DOI] [PubMed] [Google Scholar]
- 16.Morikawa Y, Nakagawa H, Miura K, Soyama Y, Ishizaki M, Kido T. et al. Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scandinavian journal of work, environment & health. 2005;31:179–83. doi: 10.5271/sjweh.867. [DOI] [PubMed] [Google Scholar]
- 17.Bildt C, Michelsen H. Gender differences in the effects from working conditions on mental health: a 4-year follow-up. International archives of occupational and environmental health. 2002;75:252–8. doi: 10.1007/s00420-001-0299-8. [DOI] [PubMed] [Google Scholar]
- 18.Cho K, Ennaceur A, Cole JC, Suh CK. Chronic jet lag produces cognitive deficits. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:Rc66. doi: 10.1523/JNEUROSCI.20-06-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. Breast cancer and circadian disruption from electric lighting in the modern world. CA: a cancer journal for clinicians. 2014;64:207–18. doi: 10.3322/caac.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breast cancer on the night shift. Lancet (London, England) 2009; 373: 1054. [DOI] [PubMed]
- 21.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science (New York, NY) 2010;330:1349–54. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS medicine. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Berg MP, Viersma JW, Beaufort-Krol GC, Bink-Boelkens MT, Bezzina CR, Veldkamp MW. et al. A large family characterised by nocturnal sudden death. Netherlands heart journal: monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. 2002;10:304–12. [PMC free article] [PubMed] [Google Scholar]
- 24.Vetter C, Devore EE, Wegrzyn LR, Massa J, Speizer FE, Kawachi I. et al. Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. Jama. 2016;315:1726–34. doi: 10.1001/jama.2016.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steptoe A. Night shift work and the cardiovascular health of medical staff. European heart journal. 2009;30:2560–1. doi: 10.1093/eurheartj/ehp310. [DOI] [PubMed] [Google Scholar]
- 26.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proceedings of the National Academy of Sciences of the United States of America. 1972;69:1583–6. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain research. 1972;42:201–6. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 28.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science (New York, NY) 2002;295:1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 29.Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM. et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science (New York, NY) 2003;301:525–7. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 30.Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nature reviews Molecular cell biology. 2010;11:764–76. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, Takumi T. Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC molecular biology. 2004;5:18. doi: 10.1186/1471-2199-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardin PE. Molecular genetic analysis of circadian timekeeping in Drosophila. Advances in genetics. 2011;74:141–73. doi: 10.1016/B978-0-12-387690-4.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nature reviews Molecular cell biology. 2007;8:139–48. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- 34.Reischl S, Kramer A. Kinases and phosphatases in the mammalian circadian clock. FEBS letters. 2011;585:1393–9. doi: 10.1016/j.febslet.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 35.Stojkovic K, Wing SS, Cermakian N. A central role for ubiquitination within a circadian clock protein modification code. Frontiers in molecular neuroscience. 2014;7:69. doi: 10.3389/fnmol.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khalyfa A, Poroyko VA, Qiao Z, Gileles-Hillel A, Khalyfa AA, Akbarpour M. et al. Exosomes and Metabolic Function in Mice Exposed to Alternating Dark-Light Cycles Mimicking Night Shift Work Schedules. Frontiers in physiology. 2017;8:882. doi: 10.3389/fphys.2017.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–90. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 38.Hirano A, Fu YH, Ptacek LJ. The intricate dance of post-translational modifications in the rhythm of life. Nature structural & molecular biology. 2016;23:1053–60. doi: 10.1038/nsmb.3326. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nature reviews Genetics. 2017;18:164–79. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annual review of genomics and human genetics. 2004;5:407–41. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP. et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science (New York, NY) 1998;280:1564–9. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 42.Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X. et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 43.Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B. et al. Interacting molecular loops in the mammalian circadian clock. Science (New York, NY) 2000;288:1013–9. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 44.Preussner M, Heyd F. Post-transcriptional control of the mammalian circadian clock: implications for health and disease. Pflugers Archiv: European journal of physiology. 2016;468:983–91. doi: 10.1007/s00424-016-1820-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U. et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–60. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 46.Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P. et al. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527–37. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Fang B, Emmett MJ, Damle M, Sun Z, Feng D. et al. GENE REGULATION. Discrete functions of nuclear receptor Rev-erbalpha couple metabolism to the clock. Science (New York, NY) 2015;348:1488–92. doi: 10.1126/science.aab3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitsui S, Yamaguchi S, Matsuo T, Ishida Y, Okamura H. Antagonistic role of E4BP4 and PAR proteins in the circadian oscillatory mechanism. Genes & development. 2001;15:995–1006. doi: 10.1101/gad.873501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gachon F, Fonjallaz P, Damiola F, Gos P, Kodama T, Zakany J. et al. The loss of circadian PAR bZip transcription factors results in epilepsy. Genes & development. 2004;18:1397–412. doi: 10.1101/gad.301404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hood S, Amir S. The aging clock: circadian rhythms and later life. The Journal of clinical investigation. 2017;127:437–46. doi: 10.1172/JCI90328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annual review of physiology. 2010;72:551–77. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dibner C, Schibler U. Circadian timing of metabolism in animal models and humans. Journal of internal medicine. 2015;277:513–27. doi: 10.1111/joim.12347. [DOI] [PubMed] [Google Scholar]
- 53.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annual review of neuroscience. 2012;35:445–62. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geiger SS, Fagundes CT, Siegel RM. Chrono-immunology: progress and challenges in understanding links between the circadian and immune systems. Immunology. 2015;146:349–58. doi: 10.1111/imm.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spies CM, Hoff P, Mazuch J, Gaber T, Maier B, Strehl C. et al. Circadian rhythms of cellular immunity in rheumatoid arthritis: a hypothesis-generating study. Clinical and experimental rheumatology. 2015;33:34–43. [PubMed] [Google Scholar]
- 56.Paganelli R, Petrarca C, Di Gioacchino M. Biological clocks: their relevance to immune-allergic diseases. Clinical and molecular allergy: CMA. 2018;16:1. doi: 10.1186/s12948-018-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kondratova AA, Kondratov RV. The circadian clock and pathology of the ageing brain. Nature reviews Neuroscience. 2012;13:325–35. doi: 10.1038/nrn3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mattis J, Sehgal A. Circadian Rhythms, Sleep, and Disorders of Aging. Trends in endocrinology and metabolism: TEM. 2016;27:192–203. doi: 10.1016/j.tem.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abbott SM, Videnovic A. Chronic sleep disturbance and neural injury: links to neurodegenerative disease. Nature and science of sleep. 2016;8:55–61. doi: 10.2147/NSS.S78947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McFadden E, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. The relationship between obesity and exposure to light at night: cross-sectional analyses of over 100,000 women in the Breakthrough Generations Study. American journal of epidemiology. 2014;180:245–50. doi: 10.1093/aje/kwu117. [DOI] [PubMed] [Google Scholar]
- 61.Lucassen EA, Coomans CP, van Putten M, de Kreij SR, van Genugten JH, Sutorius RP. et al. Environmental 24-hr Cycles Are Essential for Health. Current biology: CB. 2016;26:1843–53. doi: 10.1016/j.cub.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 62.Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E1402–11. doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB. et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA neurology. 2014;71:589–95. doi: 10.1001/jamaneurol.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai Y, Liu S, Sothern RB, Xu S, Chan P. Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson's disease. European journal of neurology. 2010;17:550–4. doi: 10.1111/j.1468-1331.2009.02848.x. [DOI] [PubMed] [Google Scholar]
- 65.Kim SM, Power A, Brown TM, Constance CM, Coon SL, Nishimura T. et al. Deletion of the secretory vesicle proteins IA-2 and IA-2beta disrupts circadian rhythms of cardiovascular and physical activity. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2009;23:3226–32. doi: 10.1096/fj.09-132019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cianciaruso C, Phelps EA, Pasquier M, Hamelin R, Demurtas D, Alibashe Ahmed M. et al. Primary Human and Rat beta-Cells Release the Intracellular Autoantigens GAD65, IA-2, and Proinsulin in Exosomes Together With Cytokine-Induced Enhancers of Immunity. Diabetes. 2017;66:460–73. doi: 10.2337/db16-0671. [DOI] [PubMed] [Google Scholar]
- 67.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–67. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 68.Eide EJ, Woolf MF, Kang H, Woolf P, Hurst W, Camacho F. et al. Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Molecular and cellular biology. 2005;25:2795–807. doi: 10.1128/MCB.25.7.2795-2807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reischl S, Vanselow K, Westermark PO, Thierfelder N, Maier B, Herzel H. et al. Beta-TrCP1-mediated degradation of PERIOD2 is essential for circadian dynamics. Journal of biological rhythms. 2007;22:375–86. doi: 10.1177/0748730407303926. [DOI] [PubMed] [Google Scholar]
- 70.Vanselow K, Vanselow JT, Westermark PO, Reischl S, Maier B, Korte T. et al. Differential effects of PER2 phosphorylation: molecular basis for the human familial advanced sleep phase syndrome (FASPS) Genes & development. 2006;20:2660–72. doi: 10.1101/gad.397006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shanware NP, Hutchinson JA, Kim SH, Zhan L, Bowler MJ, Tibbetts RS. Casein kinase 1-dependent phosphorylation of familial advanced sleep phase syndrome-associated residues controls PERIOD 2 stability. The Journal of biological chemistry. 2011;286:12766–74. doi: 10.1074/jbc.M111.224014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gallego M, Kang H, Virshup DM. Protein phosphatase 1 regulates the stability of the circadian protein PER2. The Biochemical journal. 2006;399:169–75. doi: 10.1042/BJ20060678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Partch CL, Sancar A. Cryptochromes and circadian photoreception in animals. Methods in enzymology. 2005;393:726–45. doi: 10.1016/S0076-6879(05)93038-3. [DOI] [PubMed] [Google Scholar]
- 74.Scoma HD, Humby M, Yadav G, Zhang Q, Fogerty J, Besharse JC. The de-ubiquitinylating enzyme, USP2, is associated with the circadian clockwork and regulates its sensitivity to light. PloS one. 2011;6:e25382. doi: 10.1371/journal.pone.0025382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y, Duguay D, Bedard N, Rachalski A, Baquiran G, Na CH. et al. Regulation of behavioral circadian rhythms and clock protein PER1 by the deubiquitinating enzyme USP2. Biology open. 2012;1:789–801. doi: 10.1242/bio.20121990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F. et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–28. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 77.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D. et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–40. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF. et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science (New York, NY) 2009;326:437–40. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Godinho SI, Maywood ES, Shaw L, Tucci V, Barnard AR, Busino L. et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science (New York, NY) 2007;316:897–900. doi: 10.1126/science.1141138. [DOI] [PubMed] [Google Scholar]
- 80.Busino L, Bassermann F, Maiolica A, Lee C, Nolan PM, Godinho SI. et al. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science (New York, NY) 2007;316:900–4. doi: 10.1126/science.1141194. [DOI] [PubMed] [Google Scholar]
- 81.Kurabayashi N, Hirota T, Sakai M, Sanada K, Fukada Y. DYRK1A and glycogen synthase kinase 3beta, a dual-kinase mechanism directing proteasomal degradation of CRY2 for circadian timekeeping. Molecular and cellular biology. 2010;30:1757–68. doi: 10.1128/MCB.01047-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harada Y, Sakai M, Kurabayashi N, Hirota T, Fukada Y. Ser-557-phosphorylated mCRY2 is degraded upon synergistic phosphorylation by glycogen synthase kinase-3 beta. The Journal of biological chemistry. 2005;280:31714–21. doi: 10.1074/jbc.M506225200. [DOI] [PubMed] [Google Scholar]
- 83.Gao P, Yoo SH, Lee KJ, Rosensweig C, Takahashi JS, Chen BP. et al. Phosphorylation of the cryptochrome 1 C-terminal tail regulates circadian period length. The Journal of biological chemistry. 2013;288:35277–86. doi: 10.1074/jbc.M113.509604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoshitane H, Takao T, Satomi Y, Du NH, Okano T, Fukada Y. Roles of CLOCK phosphorylation in suppression of E-box-dependent transcription. Molecular and cellular biology. 2009;29:3675–86. doi: 10.1128/MCB.01864-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spengler ML, Kuropatwinski KK, Schumer M, Antoch MP. A serine cluster mediates BMAL1-dependent CLOCK phosphorylation and degradation. Cell cycle (Georgetown, Tex) 2009;8:4138–46. doi: 10.4161/cc.8.24.10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone-Corsi P. Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PloS one. 2010;5:e8561. doi: 10.1371/journal.pone.0008561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kwak Y, Jeong J, Lee S, Park YU, Lee SA, Han DH. et al. Cyclin-dependent kinase 5 (Cdk5) regulates the function of CLOCK protein by direct phosphorylation. The Journal of biological chemistry. 2013;288:36878–89. doi: 10.1074/jbc.M113.494856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhadra U, Patra P, Pal-Bhadra M. Cardinal Epigenetic Role of non-coding Regulatory RNAs in Circadian Rhythm. Molecular neurobiology; 2017. [DOI] [PubMed] [Google Scholar]
- 89.Liu K, Wang R. MicroRNA-mediated regulation in the mammalian circadian rhythm. Journal of theoretical biology. 2012;304:103–10. doi: 10.1016/j.jtbi.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 90.Kojima S, Green CB. Circadian genomics reveal a role for post-transcriptional regulation in mammals. Biochemistry. 2015;54:124–33. doi: 10.1021/bi500707c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng HY, Obrietan K. Revealing a role of microRNAs in the regulation of the biological clock. Cell cycle (Georgetown, Tex) 2007;6:3034–5. doi: 10.4161/cc.6.24.5106. [DOI] [PubMed] [Google Scholar]
- 92.Cheng HY, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP. et al. microRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54:813–29. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun E, Shi Y. MicroRNAs: Small molecules with big roles in neurodevelopment and diseases. Experimental neurology. 2015;268:46–53. doi: 10.1016/j.expneurol.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 94.Jagannath A, Butler R, Godinho SI, Couch Y, Brown LA, Vasudevan SR. et al. The CRTC1-SIK1 pathway regulates entrainment of the circadian clock. Cell. 2013;154:1100–11. doi: 10.1016/j.cell.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hansen KF, Sakamoto K, Obrietan K. MicroRNAs: a potential interface between the circadian clock and human health. Genome medicine. 2011;3:10. doi: 10.1186/gm224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shende VR, Goldrick MM, Ramani S, Earnest DJ. Expression and rhythmic modulation of circulating microRNAs targeting the clock gene Bmal1 in mice. PloS one. 2011;6:e22586. doi: 10.1371/journal.pone.0022586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shende VR, Kim SM, Neuendorff N, Earnest DJ. MicroRNAs function as cis- and trans-acting modulators of peripheral circadian clocks. FEBS letters. 2014;588:3015–22. doi: 10.1016/j.febslet.2014.05.058. [DOI] [PubMed] [Google Scholar]
- 98.Smith SS, Dole NS, Franceschetti T, Hrdlicka HC, Delany AM. MicroRNA-433 Dampens Glucocorticoid Receptor Signaling, Impacting Circadian Rhythm and Osteoblastic Gene Expression. The Journal of biological chemistry. 2016;291:21717–28. doi: 10.1074/jbc.M116.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF. et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:7231–6. doi: 10.1073/pnas.1501327112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han Y, Meng F, Venter J, Wu N, Wan Y, Standeford H. et al. miR-34a-dependent overexpression of Per1 decreases cholangiocarcinoma growth. Journal of hepatology. 2016;64:1295–304. doi: 10.1016/j.jhep.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nagel R, Clijsters L, Agami R. The miRNA-192/194 cluster regulates the Period gene family and the circadian clock. The FEBS journal. 2009;276:5447–55. doi: 10.1111/j.1742-4658.2009.07229.x. [DOI] [PubMed] [Google Scholar]
- 102.Lee KH, Kim SH, Lee HR, Kim W, Kim DY, Shin JC. et al. MicroRNA-185 oscillation controls circadian amplitude of mouse Cryptochrome 1 via translational regulation. Molecular biology of the cell. 2013;24:2248–55. doi: 10.1091/mbc.E12-12-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Young TL, Matsuda T, Cepko CL. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Current biology: CB. 2005;15:501–12. doi: 10.1016/j.cub.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 104.Cui M, Zheng M, Sun B, Wang Y, Ye L, Zhang X. A long noncoding RNA perturbs the circadian rhythm of hepatoma cells to facilitate hepatocarcinogenesis. Neoplasia (New York, NY) 2015;17:79–88. doi: 10.1016/j.neo.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) The Journal of biological chemistry. 1987;262:9412–20. [PubMed] [Google Scholar]
- 106.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nature reviews Molecular cell biology; 2018. [DOI] [PubMed] [Google Scholar]
- 107.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ. et al. B lymphocytes secrete antigen-presenting vesicles. The Journal of experimental medicine. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marban E. The Secret Life of Exosomes: What Bees Can Teach Us About Next-Generation Therapeutics. Journal of the American College of Cardiology. 2018;71:193–200. doi: 10.1016/j.jacc.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO reports. 2015;16:24–43. doi: 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Robinson DG, Ding Y, Jiang L. Unconventional protein secretion in plants: a critical assessment. Protoplasma. 2016;253:31–43. doi: 10.1007/s00709-015-0887-1. [DOI] [PubMed] [Google Scholar]
- 111.Di Liegro CM, Schiera G, Di Liegro I. Extracellular Vesicle-Associated RNA as a Carrier of Epigenetic Information. Genes; 2017. p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. The Journal of cell biology. 1983;97:329–39. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of cell biology. 2013;200:373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current protocols in cell biology; 2006. Chapter 3: Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 115.Tao SC, Guo SC, Zhang CQ. Platelet-derived Extracellular Vesicles: An Emerging Therapeutic Approach. International journal of biological sciences. 2017;13:828–34. doi: 10.7150/ijbs.19776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. European journal of cell biology. 1984;35:256–63. [PubMed] [Google Scholar]
- 117.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. The Journal of cell biology. 1985;101:942–8. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Satta N, Toti F, Feugeas O, Bohbot A, Dachary-Prigent J, Eschwege V. et al. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. Journal of immunology (Baltimore, Md: 1950) 1994;153:3245–55. [PubMed] [Google Scholar]
- 119.Sims PJ, Faioni EM, Wiedmer T, Shattil SJ. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. The Journal of biological chemistry. 1988;263:18205–12. [PubMed] [Google Scholar]
- 120.Antonyak MA, Cerione RA. Microvesicles as mediators of intercellular communication in cancer. Methods in molecular biology (Clifton, NJ) 2014;1165:147–73. doi: 10.1007/978-1-4939-0856-1_11. [DOI] [PubMed] [Google Scholar]
- 121.Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. Journal of extracellular vesicles; 2013. p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B. et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E968–77. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226–32. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 124.Tao SC, Guo SC, Zhang CQ. Modularized Extracellular Vesicles: The Dawn of Prospective Personalized and Precision Medicine. Advanced Science; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo J. et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8:169–84. doi: 10.7150/thno.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tao SC, Rui BY, Wang QY, Zhou D, Zhang Y, Guo SC. Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug delivery. 2018;25:241–55. doi: 10.1080/10717544.2018.1425774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180–95. doi: 10.7150/thno.17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature biotechnology. 2011;29:341–5. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 129.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P. et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–56. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 130.Chen CY, Rao SS, Ren L, Hu XK, Tan YJ, Hu Y. et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics. 2018;8:1607–23. doi: 10.7150/thno.22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3794–9. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A. et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nature cell biology. 2008;10:619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 133.Ciardiello C, Cavallini L, Spinelli C, Yang J, Reis-Sobreiro M, de Candia P. et al. Focus on Extracellular Vesicles: New Frontiers of Cell-to-Cell Communication in Cancer. International journal of molecular sciences. 2016;17:175. doi: 10.3390/ijms17020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Seminars in cancer biology. 2011;21:139–46. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 135.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–95. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 136.van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 137.Mahmoudi K, Ezrin A, Hadjipanayis C. Small extracellular vesicles as tumor biomarkers for glioblastoma. Molecular aspects of medicine. 2015;45:97–102. doi: 10.1016/j.mam.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 138.Xiang D, Shigdar S, Qiao G, Wang T, Kouzani AZ, Zhou SF. et al. Nucleic acid aptamer-guided cancer therapeutics and diagnostics: the next generation of cancer medicine. Theranostics. 2015;5:23–42. doi: 10.7150/thno.10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochimica et biophysica acta. 2012;1820:940–8. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 140.Barile L, Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacology & therapeutics. 2017;174:63–78. doi: 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 141.Schorey JS, Harding CV. Extracellular vesicles and infectious diseases: new complexity to an old story. The Journal of clinical investigation. 2016;126:1181–9. doi: 10.1172/JCI81132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ. et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–7. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hartman ME, Dai DF, Laflamme MA. Human pluripotent stem cells: Prospects and challenges as a source of cardiomyocytes for in vitro modeling and cell-based cardiac repair. Advanced drug delivery reviews. 2016;96:3–17. doi: 10.1016/j.addr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Adamiak M, Cheng G, Bobis-Wozowicz S, Zhao L, Kedracka-Krok S, Samanta A. et al. Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circulation research. 2018;122:296–309. doi: 10.1161/CIRCRESAHA.117.311769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Akers JC, Ramakrishnan V, Yang I, Hua W, Mao Y, Carter BS. et al. Optimizing preservation of extracellular vesicular miRNAs derived from clinical cerebrospinal fluid. Cancer biomarkers: section A of Disease markers. 2016;17:125–32. doi: 10.3233/CBM-160609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tao SC, Yuan T, Rui BY, Zhu ZZ, Guo SC, Zhang CQ. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics. 2017;7:733–50. doi: 10.7150/thno.17450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7:81–96. doi: 10.7150/thno.16803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guo SC, Tao SC, Yin WJ, Qi X, Sheng JG, Zhang CQ. Exosomes from Human Synovial-Derived Mesenchymal Stem Cells Prevent Glucocorticoid-Induced Osteonecrosis of the Femoral Head in the Rat. International journal of biological sciences. 2016;12:1262–72. doi: 10.7150/ijbs.16150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tao SC, Guo SC, Li M, Ke QF, Guo YP, Zhang CQ. Chitosan Wound Dressings Incorporating Exosomes Derived from MicroRNA-126-Overexpressing Synovium Mesenchymal Stem Cells Provide Sustained Release of Exosomes and Heal Full-Thickness Skin Defects in a Diabetic Rat Model. Stem cells translational medicine. 2017;6:736–47. doi: 10.5966/sctm.2016-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang ZC, Tang C, Dong Y, Zhang J, Yuan T, Tao SC. et al. Targeting the long noncoding RNA MALAT1 blocks the pro-angiogenic effects of osteosarcoma and suppresses tumour growth. International journal of biological sciences. 2017;13:1398–408. doi: 10.7150/ijbs.22249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Forterre A, Jalabert A, Chikh K, Pesenti S, Euthine V, Granjon A. et al. Myotube-derived exosomal miRNAs downregulate Sirtuin1 in myoblasts during muscle cell differentiation. Cell cycle (Georgetown, Tex) 2014;13:78–89. doi: 10.4161/cc.26808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shi XF, Wang H, Kong FX, Xu QQ, Xiao FJ, Yang YF. et al. Exosomal miR-486 regulates hypoxia-induced erythroid differentiation of erythroleukemia cells through targeting Sirt1. Experimental cell research. 2017;351:74–81. doi: 10.1016/j.yexcr.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 153.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN. et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem cell research. 2013;10:301–12. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 154.Liu L, Jin X, Hu CF, Li R, Zhou Z, Shen CX. Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2017;43:52–68. doi: 10.1159/000480317. [DOI] [PubMed] [Google Scholar]
- 155.Zhang C, Xiao X, Chen M, Aldharee H, Chen Y, Long W. Liver kinase B1 restoration promotes exosome secretion and motility of lung cancer cells. Oncology reports. 2018;39:376–82. doi: 10.3892/or.2017.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu F, Bu Z, Zhao F, Xiao D. Increased T-helper 17 cell differentiation mediated by exosome-mediated microRNA-451 redistribution in gastric cancer infiltrated T cells. Cancer science. 2018;109:65–73. doi: 10.1111/cas.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhu LL, Huang X, Yu W, Chen H, Chen Y, Dai YT. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia; 2017. [DOI] [PubMed] [Google Scholar]
- 158.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM. et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovascular research. 2014;103:530–41. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 159.Cleys ER, Halleran JL, McWhorter E, Hergenreder J, Enriquez VA, da Silveira JC. et al. Identification of microRNAs in exosomes isolated from serum and umbilical cord blood, as well as placentomes of gestational day 90 pregnant sheep. Molecular reproduction and development. 2014;81:983–93. doi: 10.1002/mrd.22420. [DOI] [PubMed] [Google Scholar]
- 160.Cervio E, Barile L, Moccetti T, Vassalli G. Exosomes for Intramyocardial Intercellular Communication. Stem cells international. 2015;2015:482171. doi: 10.1155/2015/482171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Geiger A, Walker A, Nissen E. Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochemical and biophysical research communications. 2015;467:303–9. doi: 10.1016/j.bbrc.2015.09.166. [DOI] [PubMed] [Google Scholar]
- 162.Xu B, Zhang Y, Du XF, Li J, Zi HX, Bu JW. et al. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell research. 2017;27:882–97. doi: 10.1038/cr.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pusic AD, Kraig RP. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia. 2014;62:284–99. doi: 10.1002/glia.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim DK, Lee J, Kim SR, Choi DS, Yoon YJ, Kim JH. et al. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics (Oxford, England) 2015;31:933–9. doi: 10.1093/bioinformatics/btu741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Choi DS, Kim DK, Kim YK, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–71. doi: 10.1002/pmic.201200329. [DOI] [PubMed] [Google Scholar]
- 166.Kim DK, Kang B, Kim OY, Choi DS, Lee J, Kim SR, EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. Journal of extracellular vesicles; 2013. p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Choi DS, Kim DK, Kim YK, Gho YS. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass spectrometry reviews. 2015;34:474–90. doi: 10.1002/mas.21420. [DOI] [PubMed] [Google Scholar]
- 168.Kim DK, Lee J, Simpson RJ, Lotvall J, Gho YS. EVpedia: A community web resource for prokaryotic and eukaryotic extracellular vesicles research. Seminars in cell & developmental biology. 2015;40:4–7. doi: 10.1016/j.semcdb.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 169.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome research. 2003;13:2498–504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Eguchi A, Lazaro RG, Wang J, Kim J, Povero D, Willliams B. et al. Extracellular vesicles released by hepatocytes from gastric infusion model of alcoholic liver disease contain a MicroRNA barcode that can be detected in blood. Hepatology (Baltimore, Md) 2017;65:475–90. doi: 10.1002/hep.28838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P. et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic acids research. 2010;38:W214–20. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Negash AA, Gale M Jr. Hepatitis regulation by the inflammasome signaling pathway. Immunological reviews. 2015;265:143–55. doi: 10.1111/imr.12279. [DOI] [PubMed] [Google Scholar]
- 173.Zappulli V, Friis KP, Fitzpatrick Z, Maguire CA, Breakefield XO. Extracellular vesicles and intercellular communication within the nervous system. The Journal of clinical investigation. 2016;126:1198–207. doi: 10.1172/JCI81134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ghidoni R, Paterlini A, Albertini V, Glionna M, Monti E, Schiaffonati L. et al. Cystatin C is released in association with exosomes: a new tool of neuronal communication which is unbalanced in Alzheimer's disease. Neurobiology of aging. 2011;32:1435–42. doi: 10.1016/j.neurobiolaging.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chivet M, Javalet C, Hemming F, Pernet-Gallay K, Laulagnier K, Fraboulet S. et al. Exosomes as a novel way of interneuronal communication. Biochemical Society transactions. 2013;41:241–4. doi: 10.1042/BST20120266. [DOI] [PubMed] [Google Scholar]
- 176.Edelstein L, Smythies J. The role of epigenetic-related codes in neurocomputation: dynamic hardware in the brain. Philosophical transactions of the Royal Society of London Series B, Biological sciences; 2014. p. 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Korkut C, Li Y, Koles K, Brewer C, Ashley J, Yoshihara M. et al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013;77:1039–46. doi: 10.1016/j.neuron.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Goldie BJ, Dun MD, Lin M, Smith ND, Verrills NM, Dayas CV. et al. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic acids research. 2014;42:9195–208. doi: 10.1093/nar/gku594. [DOI] [PMC free article] [PubMed] [Google Scholar]