Abstract
Infection by blood flukes Hapalotrema mistroides and Neospirorchis sp. (Digenea: Spirorchiidae) has been recently reported in Caretta caretta (Linnaeus, 1758) in the Mediterranean Sea. Observations of post mortem lesions are generally used to assess disease severity, and few attempts have been made to standardize the evaluation of the parasitic burden from tissue egg counts. Faeces and spleen homogenates of 105 loggerheads from the northwestern Adriatic Sea were submitted to a sedimentation-flotation technique for the research of spirorchiid eggs; molecular techniques were used for unequivocal identification. Egg quantification for positive faeces and spleen samples was achieved using a modified McMaster method. Spleen samples were also submitted to quantification through the only method cited in the literature for similar purposes, which involves preventive chemical digestion. Correlations between splenic counts obtained from the two different methods and between faecal and splenic egg burdens were calculated using Spearman's rho test. Concordance between studies on eggs in faeces and spleen tissue was also calculated. Eggs of H. mistroides and Neogen-11 were found in spleen and faecal samples. Strong correlations were found between splenic egg burdens calculated from the two methods for H. mistroides, demonstrating that the modified McMaster method can be used for quantification. A multiplying factor must be used before drawing comparisons, as egg burdens are higher in value when measured after chemical digestion. High concordance was obtained from a qualitative examination of faeces and spleen tissue of H. mistroides, showing that copromicroscopic examination can be used for in vivo diagnosis. As weak correlations were found between faecal and splenic egg counts, faecal burden cannot be regarded as indicative of disease severity. For Neogen-11, low concordance was found between faeces and spleen tissue, likely reflecting lower levels of egg embolization in organs.
Keywords: Sea turtles, Spirorchiidae, McMaster, Egg burden
Graphical abstract
Highlights
-
•
A method for quantifying splenic spirorchiid egg burden in sea turtles is proposed.
-
•
The new method reliably esteems tissue egg burden, and has easy implementation.
-
•
Fecal eggs output is also quantified and compared to splenic egg burden.
-
•
Fecal egg output does not realistically esteem disease severity.
-
•
Eggs of the different genera show different degree of embolization in the spleen.
1. Introduction
Infections by blood flukes of the family Spirorchiidae (Digenea) have been recently re-described in loggerhead sea turtles from different regions of the Mediterranean Sea (Cribb et al., 2017; Santoro et al., 2017; Marchiori et al., 2017). The presence of Hapalotrema mistroides Monticelli, 1896 in the basin is historically recognized (Monticelli, 1896; Looss, 1899, 1902; Gohar, 1934, 1935) while Neospirorchis sp. Neogen-11 and Amphiorchis sp. have been found in the region for the first time in recent years (Marchiori et al., 2017; Cribb et al., 2017). Epidemiological studies characterizing distributions of different genera and effective impacts of the disease on sea turtle populations in the Mediterranean Sea are still lacking. Spirorchiidiasis is globally found in sea turtles with varying degrees of severity: fatal disease is observed with asymptomatic infections, appearing as incidental findings during necropsy (Gordon et al., 1998; Flint et al., 2010; Stacy et al., 2010a; Santoro et al., 2017; Marchiori et al., 2017). Different risk factors for the acquisition of the infection and for disease severity are presented in the literature (e.g., geographical area, host species and parasites involved) (Work et al., 2005; Flint et al., 2010; Stacy et al., 2010a; Chapman et al., 2017). The severity of the disease is typically defined based on observations of gross and microscopic lesions during post mortem examination; few attempts to standardize the evaluation of parasitic burden have been made, generating an impact score based on one or more criteria: the number of granulomas per high power field (40×) (Flint et al., 2010; Santoro et al., 2017) or the number of adults and egg emboli and the presence and forms of organ injury (Stacy et al., 2010a), leaving room for subjectivity in operator expertise and in carcass preservation conditions. Complexities of such evaluations are compounded by incomplete knowledge of the site tropism of the different spirorchiid species. Quantifications of tissue egg burden are reported in only one work (Work et al., 2005) wherein the spleen is used as target tissue for such measurements while obtained values expressed as eggs per gram of spleen (epgs) are considered in an epidemiological analysis. Nevertheless, this method is limited by the cost of pepsin and by time-consuming procedures required for chemical digestion.
Copromicroscopic exams have also been used to detect spirorchiid infection (Wolke et al., 1982; Greiner, 2013) and are reported among methods used for ante mortem diagnosis, as this efficient and simple method can be easily applied in vivo in sea turtles rescue facilities. Nevertheless, faecal egg counts and infection sizes have been speculated to be unrelated as suggested by studies on schistosomiasis (De Bont et al., 2002) even though no specific studies on this matter have been carried out in relation to spirorchiids.
The aim of this study is to propose and validate an efficient alternative means to quantify spirorchiid egg burdens on infested loggerhead spleen tissue and to compare derived results with those obtained with the only method published in the literature thus far (Work et al., 2005). To evaluate the feasibility of quantifying faecal egg output as an indicator, its correlations with egg burdens in spleen tissue are also investigated.
2. Materials and methods
2.1. Sampling and laboratory analyses
In total, 105 dead loggerhead sea turtles were found stranded along northwestern Adriatic coast from 2013 to 2017 and were subjected to necropsy and successive analyses. During necropsy, stool samples taken from the rectum and spleen tissues were collected and stored frozen at −20 °C at the Department of Animal Medicine, Productions and Health of Padova University for parasitological examination.
A qualitative analysis was performed on the spleens of all turtles for the detection of spirorchiid eggs through the use of a concentration-flotation technique described by Marchiori et al. (2017). Eggs were classified as type 1, 2 or 3 depending on their morphology according to Wolke et al. (1982).
Tissue egg burden quantification was applied to positive spleen samples using a new method based on mechanical homogenization (MH) and based on principles of the Mc Master technique, which is commonly used for faecal examination in different species of animals (Mc. Gordon and Whitlock, 1939). For this purpose, 2 g of randomly selected splenic parenchyma were diced and homogenized in a blender in 6 ml of high specific gravity (s.g.) solution (sodium nitrate, sodium thiosulphate and sucrose, s.g. 1.450). Part of the obtained solution (0.30 ml) was then used to fill McMaster chambers. Egg counts were calculated as the number of eggs per gram of spleen (epgs) as follows: epgs = number of counted eggs X 10.
To compare quantitative methods, a representative number of spleen samples (n = 25) was selected and subjected to a second means of quantifying of tissue egg burdens through chemical homogenization (CH) as previously described by Work et al. (2005) with minimal modifications and as briefly described here. Two grams of randomly selected spleen tissue were diced and left in PBS solution for 24 h at 37 °C. Homogenates were then digested in 2% (w/v) pepsin dissolved in 1% NaCl solution and in 0.03% HCl in a warm water bath (37 °C) fitted to a shaker for 24 h. The digested solutions were then filtered with coarse (100 μm) and fine (40 μm) mesh filters to recover spirorchiid eggs. The filters were then rinsed extensively with tap water to remove eggs from the meshes. The solutions were then centrifuged (3500 rpm × 5 min) and sediment was resuspended in 2000 μl of physiological solution. Egg counts were performed in an aliquot of 100 μl of the obtained solution and their total volumes were calculated.
The filtered pepsin-solution was also centrifuged at 3500 rpm × 5 min and the sediment was observed to exclude the passage of type 3 eggs through filter meshes.
Faecal samples from the turtles were subjected to qualitative copromicroscopic examination by means of a common concentration-flotation technique using the same high s.g. solution applied to the spleen to identify spirorchiid eggs. Positive faecal samples were subjected to quantitative analysis by use of the McMaster method using two grams of faeces in 6 ml of solution to provide an estimation of egg output in terms of eggs per gram (epg).
Molecular analyses were carried out on positive samples to identify spirorchiid species while targeting the ITS-2 spacer of rDNA as described by Stacy et al. (2010b).
2.2. Statistical analyses
The correlation between the two research methods (MH and CH methods) for the estimation of egg burdens in the spleen derived from quantitative estimation was investigated using Spearman's rho test. For both methods, a value of 5 epgs was arbitrarily assigned to samples deemed negative through quantitative estimation but positive through qualitative analysis while assuming a random distribution between the negative sample (=0 epgs) and threshold (=10 epgs). To evaluate the repeatability of the MH method, three samples with low, moderate and high egg counts were selected to repeat the same quantitative procedure on 8 different portions of the organ, including peripheral and central areas. The repeatability of the newly developed quantitative method on the spleen was assessed by investigating the CV (Coefficient of Variance expressed as a percentage).
To compare results derived using two matrixes (spleen and faeces), concordance between the copromicroscopic examination and the detection of eggs in spleen tissue from qualitative results (pos/neg) was calculated as the number of samples with same result of the total number of samples examined (% concordance). This was evaluated using kappa-type statistics (Landis and Koch, 1977), which express the proportion of agreement determined beyond chance with a value (parameter k) of 0 (no agreement) to 1 (perfect agreement).
Correlations found between quantitative estimations of the faeces and spleen samples (assessed via the MH method) were investigated by means of Spearman's rho test. In this case, the mean 5 epg value was assigned to positive samples under a threshold for quantitative analysis.
The overall level of statistical significance was set to p < 0.05. Statistical analyses were performed using the Excel® 14.7.1. and IBM SPSS Statistics 20 software programs.
3. Results
Only spirorchiid eggs of types 1 and 3 were identified in both faeces and spleen tissue (Fig. 1) with overall prevalence values of 27,6% (29/105) and 12,3% (13/105) for the two types, respectively. Molecular analyses carried out on positive samples identified eggs of type 1 as Hapalotrema mistroides (GenBank accession number: LT617052) and eggs of type 3 as Neospirorchis Neogen-11 (GenBank accession number: LT617053) in all positive samples. We refer to eggs of type 1 as H. mistroides and to eggs of type 3 as Neogen-11 from here onward. The number of samples tested and the number of positive samples tested are reported in Table 1 for qualitative and quantitative analyses of both matrixes. Comparisons of quantitative results derived from individuals tested with both methodologies and of both matrixes are shown in Table 2, Table 3, respectively.
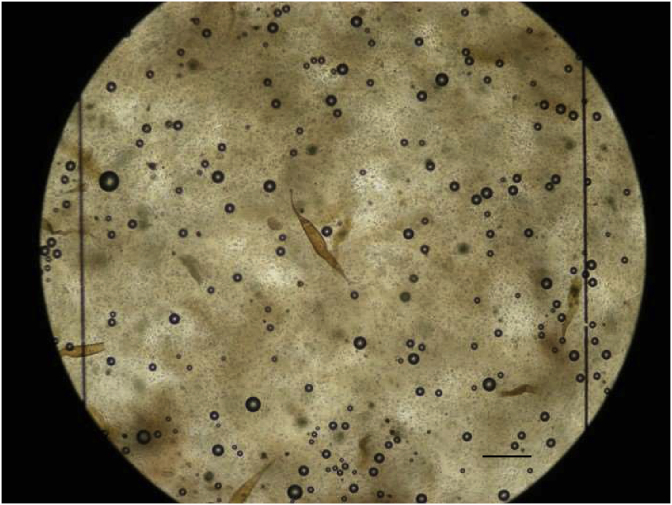
Fig. 1.
Eggs of H. mistroides in the McMaster chamber for quantification of splenic egg burden (Scale bar: 200 μm).
Table 1.
Results of qualitative and quantitative analyses in spleen and feces samples.
| Tested individuals | Matrix | Analysis | Positive | Eggs type 1 (Hapalotrema mistroides) |
Eggs type 3 (Neospirorchis sp. Neogen-11) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positivea | Range (epg/s) | Average | St. Dev. | Positivea | Range (epg/s) | Average | St. Dev. | ||||
| All individuals (n = 105) | Spleen | Qualitative | 27 | 26 | – | – | – | 5 | – | – | – |
| Individuals positive to qualitative analysis (n = 27) |
Spleen |
Quantitative (MH) |
– |
25 |
0-11,300 |
1065.4 |
2242.2 |
3 |
0–20 |
2.2 |
5.6 |
| All individuals (n = 105) | Feces | Qualitative | 30 | 27 | – | – | – | 13 | – | – | – |
| Individuals positive to qualitative analysis (n = 31) | Feces | Quantitative (McMaster) | – | 10 | 0–700 | 46.8 | 129.3 | 9 | 0–533 | 30.4 | 100.3 |
Samples negative at quantitative analysis, but positive at qualitative are considered negative in these columns; St. Dev., Standard deviation.
Table 2.
Comparison between results of quantitative analyses in spleen using the two methodologies (MH and CH).
| Tested individuals | Matrix | Analysis | Eggs type 1 (Hapalotrema mistroides) |
Eggs type 3 (Neospirorchis sp. Neogen-11) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Range (epg/s) | Average | St. Dev. | Positive | Range (epg/s) | Average | St. Dev. | |||
| Individuals tested by both quantitative methods (n = 25) | Spleen | Quantitative (MH) | 21 | 0-3100 | 633.4 | 947.0 | 2 | 0–20 | 1.6 | 4.5 |
| Spleen | Quantitative (CH) | 21 | 0-11,304 | 1838.8 | 2795.0 | 0 | – | – | – | |
Table 3.
Comparison between results of quantitative analyses in spleen and feces.
| Tested individuals | Matrix | Analysis | Eggs type 1 (Hapalotrema mistroides) |
Eggs type 3 (Neospirorchis sp. Neogen-11) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Range (epg/s) | Average | St. Dev. | Positive | Range (epg/s) | Average | St. Dev. | |||
| Individuals tested by both quantitative methods (n = 31) | Feces | Quantitative (McMaster) | 10 | 0–700 | 46.8 | 129.3 | 9 | 0–533 | 30.4 | 100.3 |
| Spleen | Quantitative (MH) | 25 | 0-11,300 | 927.9 | 2118.7 | 3 | 0–20 | 1.9 | 5.3 | |
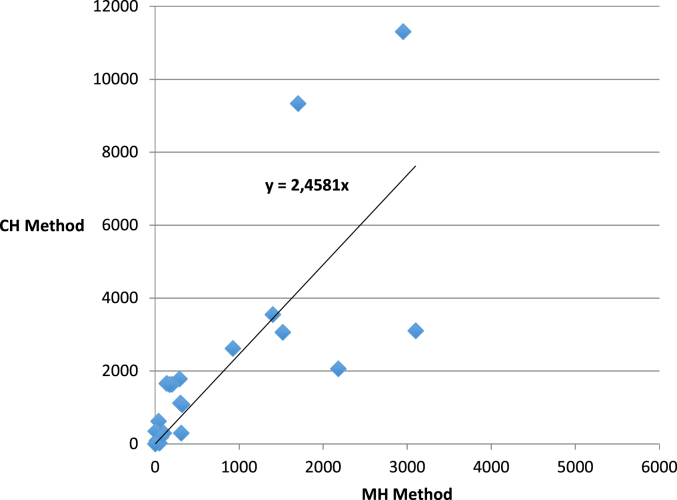
Qualitative and quantitative analyses of spleen tissues carried out with the MH method reveal tissue eggs of types 1 and 3 while the CH method did not detect any eggs of type 3. The correlation found between epgs values for spleen H. mistroides from the two quantitative methods was calculated from 25 samples (21 positive results from qualitative spleen testing; 2 samples with negative spleen results and positive results upon copromicroscopic examination; 2 samples with negative faeces and spleen results) with high Spearman's test results (rho = 0.904; p < 0.5). Epgs values estimated from the CH method are generally higher with a factor of 2.5 as shown in Fig. 2. Correlations found for Neogen-11 were not calculated due to a lack of positive samples found from the CH analysis.
Fig. 2.
Scatter plot with trend line showing the correlation between eggs counts using the two quantitative methods (MH and CH) in the spleen.
Regarding the repeatability of the MH method, average and standard deviation (SD) values of actual counts for the three series of 7/8 replications were respectively measured as 3431.3 (SD = 489.3) for sample 230/16, 525.0 (SD = 148.6) for sample 192/16 and 58.6 (SD = 20.4) for sample 194/17. It was thus estimated that samples with 100 eggs/gr would present a CV of roughly 33% (errors resulting from the methodology used allow for counts of 67–133 L1/g), that samples with 1000 eggs/gr would present a CV of approximately 20%, and that samples with 10,000 eggs/gr would present a CV of approximately 12.5%.
Qualitative copromicroscopic exam results were found to be positive for 30 animals and mostly with H. mistroides (Table 1). Due to insufficient faecal material, a quantitative analysis was not performed on 1 faecal sample positive for H. mistroides. At the same time, two samples deemed negative for both types of eggs from qualitative faecal examination were subjected to quantitative analysis, as they belonged to animals with positive spleen results.
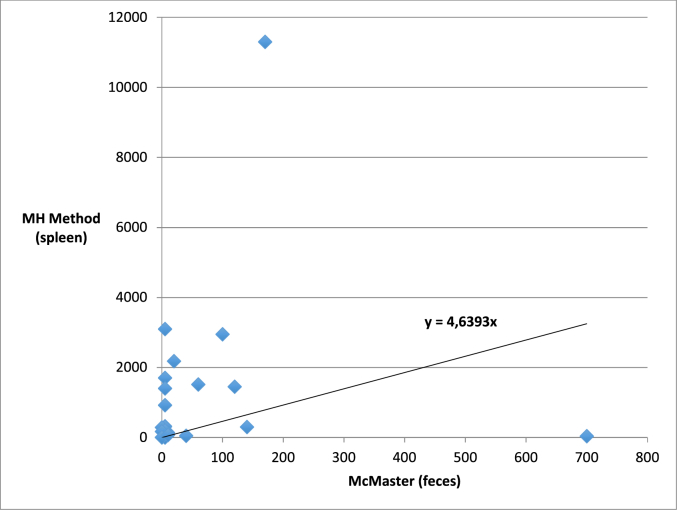
Concordance between qualitative copromicroscopic and qualitative exam results for spleen tissue (n = 105) was observed at 95% (parameter k = 0.87) and 92% (parameter k = 0.52) for Hapalotrema mistroides and Neogen-11, respectively (Table 4, Table 5). The correlation between the number of eggs of H. mistroides found in the same individuals (n = 31) with modified McMaster levels in faeces and spleen tissue (MH method) was found to be low (rho = 0.428; p = 0.016) as shown in Fig. 3. The same correlation was not investigated for Neogen-11 due to the limited number of positive samples observed.
Table 4.
Concordance matrix for Eggs type 1 (H. mistroides) detected by qualitative exam in feces and spleen.
| Feces |
Total | |||
|---|---|---|---|---|
| Negative | Positive | |||
| Spleen | Negative | 76 | 3 | 79 |
| Positive | 2 | 24 | 26 | |
| Total | 78 | 27 | 105 | |
Table 5.
Concordance matrix for Eggs type 3 (Neospirorchis Neogen-11) detected by qualitative exam in feces and spleen.
| Feces |
Total | |||
|---|---|---|---|---|
| Negative | Positive | |||
| Spleen | Negative | 92 | 8 | 100 |
| Positive | 0 | 5 | 5 | |
| Total | 92 | 13 | 105 | |
Fig. 3.
Scatter plot with trend line showing the correlation between fecal egg output and splenic egg burden calculated by MH Method.
4. Discussion
Attempts to standardize evaluations of the severity of spirorchiidiasis in sea turtles have been made based mostly on observations of gross and microscopic lesions (Stacy et al., 2010a; Flint et al., 2010). The only quantitative method published (Work et al., 2005) evaluates the intensity of infection based on egg burdens expressed as eggs per gram of tissue while using the spleen as a target organ for such evaluation. The spleen indeed has been described as one of the most common sites of spirorchiid egg accumulation due to its role in blood filtering (Flint et al., 2009, 2010). Nevertheless, the CH method (Work et al., 2005) takes considerable processing and mostly due to the duration of sample digestion with pepsin, which lasts up to 24 h. The recovery of eggs from filters after digestion also necessitates a very careful and time-expensive procedure to guarantee the measurement of all eggs. Finally, economic costs of the procedure must take into account the cost of materials (pepsin solution, and filters) needed to carry it out. Through the present study we developed a new means of identifying spirorchiid eggs in spleen tissue that is as sensitive as the aforementioned method in detecting spirorchiid eggs in spleen tissue. Therefore, mechanical homogenization appears to be a sufficient means of releasing eggs from splenic parenchyma and is more efficient and cost effective because it exploits materials commonly used in laboratories. However, lower tissue egg burden values were detected through the mechanical process than through the chemical digestion of tissues when employing the CH method, suggesting that the proteolytic action of pepsin enhances the availability of eggs. Nevertheless, the strong correlation found between egg counts obtained from the two methods (rho = 0,904) shows that the two methods assess parasitic burdens of splenic tissue to similar degrees. However, a multiplying factor must be used to draw comparisons, as epgs values estimated using the CH method are nearly three times higher than those derived from the MH method.
According to our evaluation of the repeatability of the MH method, the CV method presented a decreasing trend with higher counts. The high percentage of CV found (roughly 30% in samples with roughly 100 epgs) may depend on variability intrinsic to the method in terms of a non-homogeneous distribution of eggs in the spleen with different parenchymal portions of the organ harbouring different egg burdens. In the future the precise definition of the specific part of the spleen to be sampled may improve the repeatability of the method. However, errors resulting from the methodology appear to be sufficiently limited when compared to the overall variability of epgs values that can be obtained from different turtles, which spans over thousands of units (in this study, from 0 to 11,300 epgs). As a consequence, this method appears to be reasonably repeatable for use in epidemiological studies.
Regarding Neospirorchis Neogen-11, eggs of this genus were not found using the CH method in all samples deemed positive from the MH method. The filtered solution, after the digestion of the sample with pepsin, was also checked for the presence of Neospirorchis eggs, which are very similar in size to filters with the smallest meshes (i.e., 40 μm), but this procedure never generated positive results, proving that eggs were not present in the sample after digestion. The absence of type 3 eggs observed when using the CH method is difficult to explain but may be due to the degeneration of such eggs during chemical digestion. Our results suggest that this method is not adapted to detect round eggs typical of genus Neospirorchis.
Comparisons between different matrixes for the examination of spirorchiid eggs in sea turtles were drawn in this study to determine the reliability of copromicroscopic exams for detecting and quantifying parasite burdens. Copromicroscopy has rarely been used for the detection of spirorchiid eggs during surveys of sea turtles (Wolke et al., 1982; Greiner, 2013; Santoro et al., 2017), as the observation of gross and microscopic lesions in post mortem exams in combination with the application of sensitive molecular tools (Chapman et al., 2016) allows for the detection of spirorchiidiasis without the need to rely on such an exam. However, this approach can complement findings derived from other techniques to identify other stages of parasites or associated lesions. Copromicroscopy is another efficient and inexpensive diagnostic method that can be used in vivo, as ante mortem diagnosis remains difficult to achieve and as research on antibodies directed towards surface antigens is to date the only diagnostic tool available (Herbst et al., 1998; Graczyk et al., 1995; Work et al., 2005). Attempts to amplify DNA of spirorchiid flukes from blood samples have been recently made with negative results (Chapman, 2016).
Carcass decomposition and egg elimination through the decomposition of tissues have been hypothesized as potential means through which spirorchiids emit their eggs into the environment and mostly for species with tropism for organs seemingly remote from access to the external environment (i.e., CNS and endocrine organs) (Stacy et al., 2010a, 2017). Nevertheless, shedding by faecal route is very probable for all those species exhibiting a gastrointestinal localization of eggs and/or adults, including H. mistroides and the Neospirorchis genotype examined here (Stacy et al., 2010a; Chapman et al., 2017; Marchiori et al., 2017). The presence of eggs of H. mistroides in the spleen with that in feces revealed high concordance values, demonstrating the potential usefulness of copromicroscopy for the detection of H. mistroides infections. The high likelihood of detecting H. mistroides eggs in spleen tissue may be explained by the localization of adult flukes, which are typically found in the left aorta or cardiac chamber and which likely release their eggs into the arterial system. Being the spleen a filter of arterial blood, this could account for eggs emboli to be held in this organ (Flint et al., 2009). Further studies on egg distribution dynamics in the host's body could help confirm this hypothesis.
Quantitative estimations of parasitic burden in terms of epgs and epg are poorly correlated. This can be attributed to different dynamics of egg accumulation in the two matrixes. As suggested by Work et al. (2005), splenic egg burdens likely reflect chronic accumulation and infection by spirorchiids rather than adult spirorchiid burdens. The correlation observed between faecal counts and the number of adult worms is also difficult to assess and not necessarily existent as demonstrated in studies on schistosomiasis (Gryseels and de Vlas, 1996; De Bont et al., 2002). Difficulties associated with finding adult spirorchiids due to poor animal preservation conditions and to the well-known cryptic nature of these flukes prevented us from determining the value of this correlation in the examined animals by counting adult worms. Nevertheless, as pathological effects of spirorchiidiasis in turtles are known to be directly related to the presence of egg granulomas in tissues, splenic egg burdens appear to be more reliable than faecal egg counts in assessing disease severity in turtles; faecal egg burdens appear to have no utility in the assessment of disease effects on live sea turtles as speculated by Chapman (2016).
Regarding Neogen-11 we found low concordance between the two matrixes, and the limited number of positive samples observed prevented us from assessing this correlation through quantitative estimation. This finding may be justified by different migration dynamics observed within the definitive host of this parasite in comparison to those observed for Hapalotrema. In fact, adult flukes of genotype Neogen-11 deposit their eggs locally in the submucosa of the intestine, showing limited signs of embolization (Stacy et al., 2017) and thus explaining the bypassing of systemic circulation and of the spleen. This hypothesis is supported by low epgs values found for this species through spleen analysis compared to those derived via copromicroscopy.
Studies on the correlation between splenic and egg burdens of other organs are lacking in the literature, and the presence of preferential embolization sites in the host's body remains to be studied in relation to different spirorchiids species (Stacy et al., 2017). Studies of correlations between splenic egg burdens and other post-mortem data related to pathological effects of each spirorchiid species should be conducted to characterize the meaning of splenic egg burdens as a predictor of the impacts of infection on the host's health.
5. Conclusions
The proposed method, which is based on the mechanical homogenization of splenic parenchyma and a modified McMaster technique, is here demonstrated to serve as a good means of detecting spirorchiids infection and calculating tissue egg burdens. The proposed method presents advantages over methods described in the literature (e.g., its efficient implementation and workability).
Research on H. mistroides eggs in the faeces of loggerhead turtles could be used to diagnose infections of this species in vivo. Nevertheless, faecal egg counts should not be regarded as indicative of the total parasitic burden and consequently of the severity of a disease affecting an animal.
The determination of parasitic burden expressed as epgs may be used as a starting point for future epidemiological studies assessing risk factors and disease impacts of different spirorchiid species found in the Mediterranean area.
Declarations of interest
None.
Acknowledgments
We thank anonymous reviewers for their detailed revisions and helpful comments. We are grateful to Maddalena Nardo (University of Camerino) for her support in the experimental work; Dr. Cinzia Centelleghe (Department of Comparative Biomedicine and Food Science, University of Padova) for performing sampling during necropsies; Dr. Giuseppe Palmisano, Emanuele Zanetti and Dr. Michele Povinelli for their logistic support. This work was financially supported by a Research Project of Padova University (Prot. CPDA149521/14).
Contributor Information
Erica Marchiori, Email: erica.marchiori@phd.unipd.it.
Rudi Cassini, Email: rudi.cassini@unipd.it.
Irene Ricci, Email: irene.ricci@unicam.it.
Federica Marcer, Email: federica.marcer@unipd.it.
References
- Chapman P.A. University of Queensland; 2016. Diversity, Impacts and Diagnosis of Pathogenic Parasites in Sea Turtles from Queensland, Australia. PhD Thesis. [Google Scholar]
- Chapman P.A., Traub R.J., Kyaw-Tanner M.T., Owen H., Flint M., Cribb T.H., Mills P.C. Terminal restriction fragment length polymorphism for the identification of spirorchiid ova in tissues from the green sea turtle, Chelonia mydas. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0162114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P.A., Owen H., Flint M., Soares Magalhães R.J., Traub R.J., Cribb T.H., Kyaw-Tanner M.T., Mills P.C. Molecular epidemiology and pathology of spirorchiid infection in green sea turtles (Chelonia mydas) Int. J. Parasitol. Parasites and Wildlife. 2017;6:39–47. doi: 10.1016/j.ijppaw.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribb T.H., Crespo-Picazo J.L., Cutmore S.C., Stacy B.A., Chapman P.A., Garcìa-Pàrraga D. Elucidation of the first definitively identified life cycle for a marine turtle blood fluke (Trematoda: Spirorchidae) enables informed control. Int. J. Parasitol. 2017;47(1):61–67. doi: 10.1016/j.ijpara.2016.11.002. [DOI] [PubMed] [Google Scholar]
- De Bont J., Shaw D.J., Vercruysse J. The relationship between faecal egg counts, worm burden and tissue egg counts in early Schistosoma mattheei infections in cattle. Acta Trop. 2002;81:63–76. doi: 10.1016/s0001-706x(01)00198-x. [DOI] [PubMed] [Google Scholar]
- Flint M., Patterson-Kane J.C., Limpus C.J., Work T.M., Blair D., Mills P.C. Postmortem diagnostic investigation of disease in free-ranging marine turtle populations: a review of common pathologic findings and protocols. J. Vet. Diagn. Invest. 2009;21(6):733–759. doi: 10.1177/104063870902100601. [DOI] [PubMed] [Google Scholar]
- Flint M., Patterson-Kane J.C., Limpus C.J., Mills P.C. Health surveillance of stranded Green Turtles in Southern Queensland, Australia (2006–2009): an epidemiological analysis of causes of disease and mortality. EcoHealth. 2010;7:135–145. doi: 10.1007/s10393-010-0300-7. [DOI] [PubMed] [Google Scholar]
- Gohar N. Liste des trematodes parasites et de leurs hotes vertebres signales dans la Vallee du Nil. Ann. Parasitol. Hum. Comp. 1934;12:322–331. [Google Scholar]
- Gohar N. Liste des trematodes parasites et de leurs hotes vertebres signales dans la Vallee du Nil. (Suite et fin. Ann. Parasitol. Hum. Comp. 1935;13:80–90. [Google Scholar]
- Gordon A.N., Kelly W.R., Cribb T.H. Lesions caused by cardiovascular flukes (Digenea: Spirorchiidae) in stranded green turtles (Chelonia mydas) J. Vet. Pathol. 1998;35:21–30. doi: 10.1177/030098589803500102. [DOI] [PubMed] [Google Scholar]
- Graczyk T.K., Aguirre A.A., Balazs G.H. Detection by Elisa of circulating anti-blood fluke (Carettacola, Hapalotrema and Learedius) immunoglobulins in Hawaiian green turtles (Chelonia mydas) J. Parasitol. 1995;81(3):416–421. [PubMed] [Google Scholar]
- Greiner E.C. Parasites of marine turtles. In: Wyneken J., Lohmann K.J., Lutz J.A., editors. vol. 3. CRC Press; USA: 2013. pp. 427–446. (The Biology of Sea Turtles). [Google Scholar]
- Gryseels B., de Vlas S.J. Worm burdens in schistosome infections. Parasitol. Today. 1996;12(3):115–119. doi: 10.1016/0169-4758(96)80671-5. [DOI] [PubMed] [Google Scholar]
- Herbst L.H., Greiner E.C., Ehrhart L.M., Bagley D.A., Klein P.A. Serological association between spirorchidiasis, Herpesvirus infection and Fibropapillomatosis in green turtles from Florida. J. Wildl. Dis. 1998;34(3):496–507. doi: 10.7589/0090-3558-34.3.496. [DOI] [PubMed] [Google Scholar]
- Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Looss A. Weitere Beiträge zur Kenntniss der Trematoden Fauna Aegyptens, Zugleich Versuch einer natürlichen Gliederung des Genus Distomum Retzius. Zool. Jb-r. Abt. Syst. 1899;12:521–784. [Google Scholar]
- Looss A. Ueber neue und bekannte Trematoden aus Seeschildkröten. Nebst Erörterungen zur Systematik und Nomenclatur. Zool. Jb-r. Abt. Syst., Oekol. Geogr. Tiere. 1902;16:411–894. [Google Scholar]
- McGordon H., Whitlock H.V. A new technique for counting nematode eggs in sheep faeces. J. Counc. Sci. Ind. Res. 1939;12:50–52. [Google Scholar]
- Marchiori E., Negrisolo E., Cassini R., Garofalo L., Poppi L., Tessarin C., Marcer F. Cardiovascular flukes (Trematoda: Spirorchiidae) in Caretta caretta Linneaeus, 1758 from the Mediterranean Sea. Parasites Vectors. 2017;10(467):1–14. doi: 10.1186/s13071-017-2396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli F.S. Di un ematozoo della Thalassochelys caretta Linn. Int. Mschr. Anat. Physiol. 1896;13:141–172. [Google Scholar]
- Santoro M., Di Nocera F., Iaccarino D., Lawton S.P., Cerrone A., Degli Uberti B., D'Amore M., Affuso A., Hochscheid S., Maffucci F., Galiero G. Pathology and molecular analysis of Hapalotrema mistroides (Digenea: Spirorchiidae) infecting a Mediterranean loggerhead turtle Caretta caretta. Dis. Aquat. Org. 2017;124:101–108. doi: 10.3354/dao03117. [DOI] [PubMed] [Google Scholar]
- Stacy B.A., Foley A.M., Greiner E., Herbst L.H., Bolten A., Klein P., Manire C., Jacobson E.R. Spirorchiidiasis in stranded loggerhead Caretta caretta and green turtles Chelonia mydas in Florida (USA): host pathology and significance. Dis. Aquat. Org. 2010;89(3):237–259. doi: 10.3354/dao02195. [DOI] [PubMed] [Google Scholar]
- Stacy B.A., Frankovich T., Greiner E., Alleman A.R., Herbst L.H., Klein P., Bolten A., McIntosh A., Jacobson E.R. Detection of spirorchiid Trematodes in gastropods tissues by polymerase chain reaction: preliminary identification of an intermediate host of Learedius learedi. J. Parasitol. 2010;96(4):752–757. doi: 10.1645/GE-2382.1. [DOI] [PubMed] [Google Scholar]
- Stacy B.A., Chapman P.A., Foley A.M., Greiner E.C., Herbst L.H., Bolten A.B., Klein P.A., Manire C.A., Jacobson E.R. Evidence of diversity, site and host specificity of sea turtle blood flukes (Digenea: Schistosomatoidea: “Spirorchidae”): a molecular prospecting study. J. Parasitol. 2017;103(6):756–767. doi: 10.1645/16-31. [DOI] [PubMed] [Google Scholar]
- Wolke R.E., Brooks D.R., George A. Spirorchidiasis in loggerhead sea turtles (Caretta caretta): pathology. J. Wildl. Dis. 1982;18:175–185. doi: 10.7589/0090-3558-18.2.175. [DOI] [PubMed] [Google Scholar]
- Work T.M., Balazs G.H., Schumacher J.L., Marie A. Epizootiology of spirorchiid infection in green turtles (Chelonia mydas), in Hawaii. J. Parasitol. 2005;91:871–876. doi: 10.1645/GE-454R.1. [DOI] [PubMed] [Google Scholar]