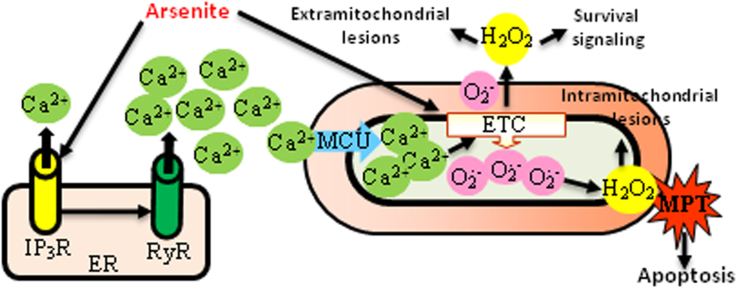
Abstract
A low concentration of arsenite (6 h), selectively stimulating the intraluminal crosstalk between the inositol-1, 4, 5-triphosphate receptor and the ryanodine receptor (RyR), increased the mitochondrial transport of RyR-derived Ca2+ through the mitochondrial Ca2+ uniporter. This event was characterized in intact and permeabilized cells, and was shown to be critical for mitochondrial superoxide (mitoO2.-) formation. Inhibition of mitochondrial Ca2+ accumulation therefore prevented the effects of arsenite, in both the mitochondrial (e.g., cardiolipin oxidation) and extramitochondrial (e.g., DNA single- strand breakage) compartments, and suppressed the Nrf2/GSH survival signaling. The effects of arsenite on Ca2+ homeostasis and mitoO2.- formation were reversible, as determined after an additional 10 h incubation in fresh culture medium and by measuring long-term viability. A 16 h continuous exposure to arsenite instead produced a sustained increase in the cytosolic and mitochondrial Ca2+ concentrations, a further increased mitoO2.- formation and mitochondrial permeability transition. These events, followed by delayed apoptosis (48 h), were sensitive to treatments/manipulations preventing mitochondrial Ca2+ accumulation. Interestingly, cells remained viable under conditions in which the deregulated Ca2+ homeostasis was not accompanied by mitoO2.-formation.
In conclusion, we report that the fraction of Ca2+ taken up by the mitochondria in response to arsenite derives from the RyR. Mitochondrial Ca2+ appears critical for mitoO2.- formation and for the triggering of both the cytoprotective and apoptotic signaling. The effects of arsenite were reversible, whereas its prolonged exposure caused a sustained increase in mitochondrial Ca2+ and mitoO2.- formation, and the prevalence of the apoptotic vs survival signaling.
Abbreviation: AA, ascorbic acid; 2-APB, 2-aminoethoxydiphenyl borate; Cf, caffeine; 7-chloro-5–2-chlorophenyl-1,5-dihydro-4,1-benzothiazepin-23H-one, CGP-37157; CsA, cyclosporin A; DHR, dihydrorhodamine 123; D-cells, differentiated cells; [Ca2+]c, cytosolic Ca2+ concentration; [Ca2+]m, mitochondrial Ca2+ concentration; GSH, glutathione; IP3R, inositol 1,4,5-trisphosphate receptor; mNCX, mitochondrial Na+/Ca2+ exchanger; mitoO2-, mitochondrial superoxide; MCU, mitochondrial Ca2+ uniporter; MPT, mitochondrial permeability transition; NAO, 10-N-nonyl acridine orange; Nrf2, nuclear factor erythroid 2 p45-related factor 2; RD-cells, respiration-deficient cells; RP-cells, respiration-proficient cells; Ry, ryanodine; RyR, ryanodine receptor; ROS, reactive oxygen species; RR, ruthenium red
Keywords: Arsenite; Ryanodine receptor; Inositol-1, 4, 5-triphosphate receptor; Mitochondrial Ca2+; Mitochondrial superoxide; Survival vs apoptotic signaling
Graphical abstract
1. Introduction
It is well established that reactive oxygen species (ROS) can be generated in multiple compartments and by different sources, which include the respiratory chain, a large family of NADPH oxidases, lipoxygenases, uncoupled nitric oxide synthase, xanthine oxidase, etc. [1], [2], [3], [4]. These diverse conditions are expected to result in different local concentrations of H2O2, depending on the site in which superoxide (O2.-) formation takes place and its dismutation to H2O2 ensues. Furthermore, ROS released by one source can eventually recruit other mechanisms leading to enhanced ROS release [5], [6]. There is for example the possibility that activation of NADPH oxidase results in enhanced H2O2 formation and in H2O2-dependent mitochondrial dysfunction, which in turn leads to additional ROS release [7], [8].
The complexity of these events is further increased by the interplay between the mechanisms involved in ROS release and Ca2+ mobilization, for at least three separate reasons. The first one is related to the fact that most of the mechanisms mediating ROS formation, in particular those based on inhibition of electron transport in the respiratory chain, or on NADPH oxidase activation, are Ca2+-dependent [6], [9]. The second reason is that ROS may effectively stimulate the release of Ca2+ from both the inositol-1, 4, 5-triphosphate receptor (IP3R) or ryanodine receptor (RyR) [9], [10], [11]. The third one is instead that Ca2+ stimulates the activity of nitric oxide synthase, thereby resulting in the formation of reactive nitrogen species, as peroxynitrite [12], [13], which effectively alter Ca2+ homeostasis [13], or eventually lead to further O2.-/H2O2 formation [12], [13], [14].
These general principles make the point that the issues of where ROS and Ca2+ are released, and how these events are interconnected, are difficult to address, and should therefore involve carefully planned experiments, using appropriate and possibly simplified approaches. For example, the cell type should be carefully selected since different cells may differentially express specific ROS sources and/or Ca2+ channels. The concentration of the agent under investigation should also be carefully chosen since toxicity and ATP depletion burst most of the above complicating factors [5], [9], [11].
Trivalent arsenic, a widely distributed environmental toxic metalloid and human carcinogen [15], [16], [17], that produces deleterious effects in diverse biomolecules located in different subcellular compartments [16], [17], represents a prototype of an agent promoting events characterized by the above complexities. Furthermore, arsenite presents an additional complication related to its ability to bind to thiol residues in proteins [16], [18], which may on the one hand directly result in ROS formation and on the other hand directly deregulate Ca2+ homeostasis, since the endoplasmic reticulum presents critical cysteines in both the IP3R and RyR [10], [19]. Not surprisingly, different studies using different cell types and arsenite concentrations reported evidence of ROS formation from different sources [16], [17] and Ca2+ mobilization through different mechanisms [20], [21], [22], [23], with the interplay between these events rarely determined [20], [23].
Understanding the details of the above effects mediated by arsenite is of critical importance since the interplay between Ca2+ and ROS is central to the regulation of events leading to the induction of skin, liver, lung, urinary tract and bladder cancers [15], [16], [17] as well as cardiovascular, hepatic, renal and nervous system disorders [16], [17]. Exposure to arsenic has a major impact on human health, as over 200 million people are exposed to arsenic, in particular through drinking water [15].
With these considerations in mind, we initially selected a cell type, U937 cells, in which a low concentration of arsenite exclusively promotes mitochondrial O2.- (mitoO2.-) formation [24], [25], [26], [27], to then address specific questions on the effect mediated by the metalloid on Ca2+ homeostasis [28]. It should be also mentioned that this cell line presents the following advantages: i) it expresses functional high affinity Na+ dependent vitamin c transporter 2 in both the plasma and mitochondrial membranes, thereby allowing the accumulation of high and low amounts of ascorbic acid (AA) in mitochondria and in the cytosol, respectively [29], [30], [31]. Mitochondrial AA can then be functional for an effective and selective scavenging of mitoO2.-. ii) this cell line does not express constitutive nitric oxide synthase and fails to express the inducible isoform in response to arsenite [24], [26], [27]. We can therefore rule out the involvement of peroxynitrite in our experiments, and hence simplify the interpretation of their outcomes; iii) this cell line can be easily manipulated to induce mitochondrial respiratory deficiency [25], [26]. iv) these cells express both the IP3R and the RyR [28], [32], [33], [34]. v) these cells can be easily differentiated to monocytes with downregulated RyR expression [34].
We recently investigated the effects of a low concentration (2.5 µM) of arsenite (6 h) using the parental U937 cell line, from now on referred to as respiration-proficient RP-cells, as well as respiration-deficient cells (RD-cells) and monocyte differentiated cells (D-cells). In this study [28], we obtained evidence for a stimulatory effect on the IP3R, based on direct binding of the metalloid on upstream targets, which however caused the release of a limited amount of Ca2+. This effect did not increase further at increasing concentrations of the metalloid, did not impair the Ca2+ response triggered by specific IP3R agonists and was ROS-independent. We also found that IP3R stimulation was associated with a very large Ca2+ release from the RyR. This effect was also ROS-independent and progressively increased at increasing arsenite concentrations. Furthermore, arsenite alone failed to release Ca2+from the RyR, or to sensitize this channel to Ca2+-induced Ca2+ release events triggered by the elevation of the cytosolic Ca2+ concentration ([Ca2+]c). Our interpretation of these findings was therefore that in addition to the direct stimulation of the IP3R signaling, the metalloid also targets the IP3R and RyR intraluminal crosstalk to increase significantly the release of Ca2+ through the second channel.
The present study addressed issues related to the regulation of mitochondrial Ca2+ uptake and accumulation, under the same conditions of arsenite exposure previously employed to investigate the effects of the metalloid on [Ca2+]c. More specifically, we asked questions related to the mechanism whereby Ca2+ is taken up by the mitochondria, as well as on the effects and consequences of this event in terms of ROS formation/regulation of both the survival and apoptotic signaling.
2. Materials and methods
2.1. Chemicals
Sodium arsenite, 2-aminoethoxydiphenyl borate (2-APB), Ry, AA, rotenone, caffeine (Cf), CaCl2, ruthenium red (RR), LaCl3, 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP-37157), Hoechst 33342, as well most of the reagent-grade chemicals were purchased from Sigma-Aldrich (Milan, Italy). Ru360 was obtained from Calbiochem (San Diego, CA). Cyclosporin A (CsA) was from Novartis (Bern, Switzerland). Fluo-4-acetoxymethyl ester, Rhod 2-acetoxymethyl ester, Dihydrorhodamine 123 (DHR), MitoSOX red, 10-N-nonyl acridine orange (NAO) and MitoTracker Red CMXRos were purchased from Molecular Probes (Leiden, The Netherlands).
2.2. Cell culture and treatment conditions
U937 human myeloid leukemia cells were cultured in suspension in RPMI 1640 medium (Sigma-Aldrich, Milan, Italy) supplemented with 10% fetal bovine serum (Euroclone, Celbio Biotecnologie, Milan, Italy), penicillin (100 units/ml) and streptomycin (100 µg/ml) (Euroclone), at 37 °C in T-75 tissue culture flasks (Corning Inc., Corning, NY, USA) gassed with an atmosphere of 95% air-5% CO2. These cells were differentiated to monocytes by a 4 days growth in culture medium supplemented with 1.3% DMSO, as previous described [35]. U937 cells were made respiration-deficient as indicated in [25].
Sodium arsenite was prepared as a 1 mM stock solution in saline A (140 mM NaCl, 5 mM KCl, 4 mM NaHCO3, and 5 mM glucose; pH 7.4) and stored at 4 °C. Cells (1.5 × 105 cells/ml) were exposed to arsenite in complete RPMI 1640 culture medium. Similar conditions were used in experiments employing permeabilized cells. Permeabilization was achieved by adding digitonin (10 μM) to a medium consisting of 0.25 M sucrose, 0.1% (w/v) BSA, 10 mM MgCl2, 10 mM K+-HEPES, and 5 mM KH2PO4 (pH 7.2) at 37 °C. Under these conditions, digitonin permeabilizes the plasma membrane but leaves mitochondrial membranes intact [36]. Treatments with AA were performed as detailed elsewhere [29].
2.3. Measurement of cytosolic and mitochondrial Ca2+levels
The cells were supplemented with either 4 µM Fluo-4-acetoxymethyl ester or 10 µM Rhod 2-acetoxymethyl ester 30 min prior to the end of the treatments. After the treatments, the cells were washed three times and fluorescence images were captured with a BX-51 microscope (Olympus, Milan, Italy), equipped with a SPOT-RT camera unit (Diagnostic Instruments, Delta Sistemi, Rome, Italy) using an Olympus LCAch 40×/0.55 objective lens. The excitation and emission wavelengths were 488 and 515 nm (Fluo-4), and 540 and 590 nm (Rhod-2) with a 5-nm slit width for both emission and excitation. The light intensity and the exposure settings were kept constant to allow quantitative comparisons of relative fluorescence intensity of cells between treatment groups. The exposure was limited to brief image acquisition intervals (≤ 5 s) to minimize photo-oxidation of Fluo-4 or Rhod-2. Fluorescence images (50 cells per sample from randomly selected fields) were digitally acquired and processed for fluorescence determination at the single cell level on a personal computer using the public domain Scion Image program. Briefly, single images were converted to 8-bit grayscale by linearly scaling from min-max to 0–255, where min and max are the two values displayed in the Image Adjust
Adjust Brightness/Contrast. The obtained images were then inverted using the tool “Invert LUT”. With inverted LUTs, pixels with a value of zero are white and pixels with a value 255 are black, the value (v) of each entry is replaced by 255-v. Mean fluorescence values were determined by averaging the fluorescence values of at least 50 cells/treatment condition/experiment.
Brightness/Contrast. The obtained images were then inverted using the tool “Invert LUT”. With inverted LUTs, pixels with a value of zero are white and pixels with a value 255 are black, the value (v) of each entry is replaced by 255-v. Mean fluorescence values were determined by averaging the fluorescence values of at least 50 cells/treatment condition/experiment.
2.4. DHR, MitoSOX red and NAO fluorescence assays
The cells were supplemented with either 10 µM DHR, 5 µM MitoSOX red or 10 µM NAO 30 min prior to the end of the treatments, washed three times with Saline A and subsequently analyzed with a fluorescence microscope. The resulting images were taken and processed as described above. The excitation and emission wavelengths were 488 and 515 nm (DHR), 510 and 580 nm (MitoSOX red), and 495 nm and 519 nm (NAO), with a 5-nm slit width for both emission and excitation. Mean fluorescence values were determined by averaging the fluorescence values of at least 50 cells/treatment condition/experiment.
2.5. Aconitase activity
The cells were washed twice with saline A, re-suspended in lysis buffer (50 mM Tris-HCl, 2 mM Na-citrate, 0.6 mM MnCl2, pH 7.4) and finally sonicated three times on ice by using the Sonicator Ultrasonic Liquid Processor XL (Heat System-Ultrasonics, Inc., NY) operating at 20 W (10 s). The resulting homogenates were centrifuged for 5 min at 18,000×g at 4 °C. Aconitase activity was determined spectrophotometrically in the supernatants at 340 nm, as described in [37].
2.6. Measurement of DNA single-strand breakage by the alkaline halo assay
DNA single-strand breakage was determined using the alkaline halo assay developed in our laboratory [38]. It is important to note that, although we refer to DNA strand scission throughout the text, the DNA nicks measured by this technique under alkaline conditions may in fact include alkali labile sites in addition to direct strand breaks. Details on the alkaline halo assay and processing of fluorescence images and on the calculation of the experimental results are also given in Ref. [38]. DNA single-strand breakage was quantified by calculating the nuclear spreading factor value, representing the ratio between the area of the halo (obtained by subtracting the area of the nucleus from the total area, nucleus + halo) and that of the nucleus, from 50 to 75 randomly selected cells/experiment/treatment condition. Results are expressed as relative nuclear spreading factor values calculated by subtracting the nuclear spreading factor values of control cells from those of treated cells.
2.7. Western blot analysis
After treatments, the cells were processed to obtain the whole cell lysates as described in [39]. Equal amounts of proteins (25 μg) were resolved in 7% sodium dodecyl sulphate polyacrylamide gel and electrotransferred to polyvinyldiene difluoride membranes. Western blot analyses were performed using antibodies against Nrf2 (1:500, D1Z9C, Cell Signaling Technology, Leiden, The Netherlands) and actin (1:1000, sc-8432, Santa Cruz, Santa Cruz, CA). Details on Western blotting apparatus and conditions are reported elsewhere [40]. Antibody against actin were used to assess equal loading of the lanes.
2.8. Measurement of GSH content by high performance liquid chromatography
The cells (1 × 106) were suspended in 100 µl of lysis buffer (0.1% Triton X-100; 0.1 M Na2HPO4; 5 mM Na-EDTA, pH 7.5), vortexed and kept for 10 min on an ice bath. Thereafter, 15 µl of 0.1 N HCl and 140 µl of precipitating solution (0.2 M glacial meta-phosphoric acid, 5 mM sodium EDTA, 5 M NaCl) were added to the samples. After centrifugation, the supernatants were collected and kept at −20 °C until the HPLC analyses. Just before analysis, 60 µl of the acid extract were supplemented with 15 µl of 0.3 M Na2HPO4 and 15 µl of a solution containing 20 mg of DTNB in 100 ml of sodium citrate (1% w/v). The mixture was stirred for 1 min at room temperature and, after 5 min, filtered through 0.22 µm pore micro-filters. The resulting samples were finally analyzed for their GSH content by an HPLC assay [41], using a 15 cm × 4.6 mm, 5 µm Supelco Discovery® C18 column (Supelco, Bellefonte, PA). The UV absorption was detected at 330 nm. The injection volume was 20 µl. The retention time of GSH was approximately 15.7 min.
2.9. Measurement of mitochondrial membrane potential
The cells received 50 nM MitoTracker Red CMXRos 30 min prior to the end of the incubation with arsenite, were washed three times with Saline A and finally analyzed with a fluorescence microscope. The resulting images were taken and processed as described above. The excitation and emission wavelengths were 545 and 610 nm, respectively, with a 5-nm slit width for both emission and excitation. Mean fluorescence values were determined by averaging the fluorescence values of at least 50 cells/treatment condition/experiment.
2.10. Immunofluorescence analysis
After treatments, the cells were suspended in 2 ml of saline A and incubated for 30 min in 35-mm tissue culture dishes containing an uncoated coverslip. Under these conditions, cells rapidly attach to the coverslip. The cells were then fixed for 1 min with 95% ethanol/5% acetic acid, washed with phosphate buffer saline (PBS, 136 mM NaCl, 10 mM Na2HPO4, 1.5 mM KH2PO4, 3 mM KCl; pH 7.4) and blocked in PBS-containing bovine serum albumin (2% w/v) (30 min at room temperature). The cells were subsequently incubated with monoclonal anti-cytochrome c antibodies (1:100 in PBS containing 2% bovine serum albumin; Santa Cruz Biotechnology) stored for 18 h at 4 °C, washed and then incubated for 3 h in the dark with fluorescein isothiocyanate (Santa Cruz Biotechnology)-conjugated secondary antibody diluted 1:100 in PBS. The cells were then analyzed with a fluorescence microscope to count the relative numbers of cells bearing a punctate fluorescence, consistent with mitochondrial localization of cytochrome c, and a diffused fluorescence, consistent with the mitochondrial loss of cytochrome. In each experiment, at least 150 cells were analyzed to calculate the percentage of cells with a diffused fluorescence, indicative of MPT-dependent loss of cytochrome c.
2.11. Fluorogenic caspase 3 assay
Caspase 3-like activity was monitored as described in Guidarelli et al. [39] Briefly, the cells were lysed and aliquots of the extract (30 μg proteins) were incubated with 12 μM Ac-DEVD-AMC, at 30 °C. Caspase 3-like activity was determined fluorometrically (excitation at 360 nm and emission at 460 nm) by quantifying the release of aminomethylcoumarin (AMC) from cleaved caspase 3 substrate (Ac-DEVD-AMC).
2.12. Analysis of apoptosis with the Hoechst 33342 assay
After treatments, the cells were incubated for 5 min with 10 μM Hoechst 33342 and then analyzed with a fluorescence microscope to assess their nuclear morphology (chromatin condensation and fragmentation). Cells with homogeneously stained nuclei were considered viable.
2.13. Statistical analysis
The results are expressed as means ± SD. Statistical differences were analyzed by one-way ANOVA followed by Dunnett's test for multiple comparison or two-way ANOVA followed by Bonferroni's test for multiple comparison. A value of P < 0.05 was considered significant.
3. Results
3.1. Arsenite promotes mitochondrial Ca2+ accumulation
RP-cells were exposed for 6 h to 2.5 µM arsenite and subsequently processed for the assessment of both the [Ca2+]c and [Ca2+]m, using respectively Fluo-4 and Rhod-2 as fluorescent probes. The results obtained in these experiments indicate that the metalloid promotes a significant increase in [Ca2+]c, as previously documented [28], as well as in the [Ca2+]m, as respectively shown in Supplementary Fig. 1A and B.
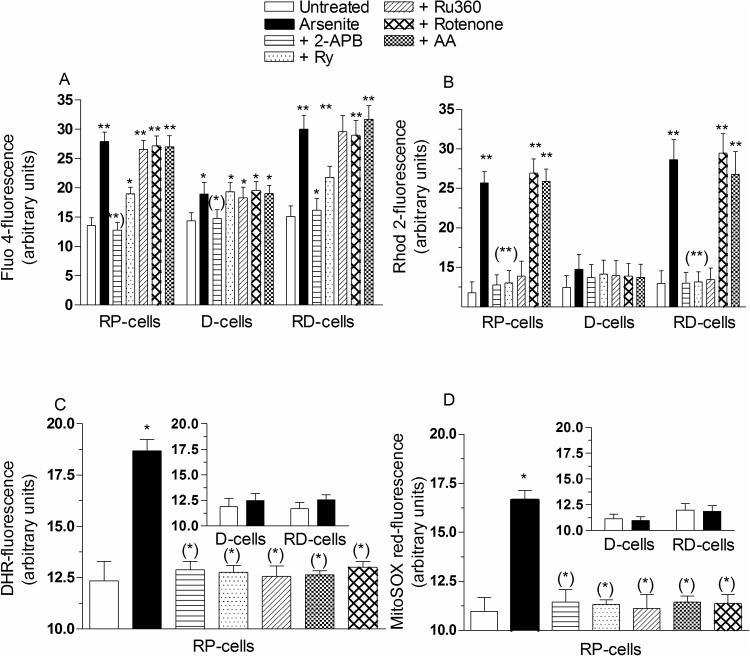
The results illustrated in Fig. 1A and B provide a quantification of the relative increases in the [Ca2+]c and [Ca2+]m and the additional information that both responses were suppressed by 2-APB, a IP3R antagonist [42]. Fig. 1B also shows that the increase in [Ca2+]m was suppressed by Ry, an antagonist of the RyR [19], and by Ru360, an inhibitor of mitochondrial Ca2+ uniporter (MCU) [43]. The increase in [Ca2+]c (Fig. 1A) was instead significantly inhibited, but not suppressed, by Ry and insensitive to Ru360.
Fig. 1.
Arsenite elevates the [Ca2+]m: the fraction of the cation cleared by the mitochondria derives from the RyR and critically mediates mitoO2.-formation. RP-, D- and RD-cells were pretreated for 15 min with 0 or 50 μM 2-APB, 20 μM Ry, 10 μM Ru360, 0.5 μM rotenone or 10 μM AA and incubated for 6 h with 2.5 μM arsenite. Cells were then loaded (30 min) with Fluo-4-AM (A) or Rhod-2-AM (B) and processed to determine their respective fluorescence responses. In other experiments, RP-cells were treated as indicated above, supplemented with DHR (C) or MitoSOX red (D) and analyzed for their respective fluorescence responses. The results obtained using D- and RD-cells are shown in the insets. Results represent the means ± SD calculated from 4 to 7 separate experiments. *P < 0.05, **P < 0.01, as compared to untreated cells. (*)P < 0.05, (**)P < 0.01, as compared to cells treated with arsenite (one-way ANOVA followed by Dunnet's test).
These results are indicative of a pivotal role of the RyR in processes leading to mitochondrial Ca2+ accumulation in cells exposed to arsenite. This notion is also consistent with the outcome of experiments showing that arsenite slightly elevates the [Ca2+]c via 2-APB-sensitive and Ry- or Ru360- insensitive mechanisms in D-cells (Fig. 1A), which do not express the RyR. Under the same conditions, arsenite failed to increase the [Ca2+]m (Fig. 1B).
The results presented in this section indicate that arsenite elevates the [Ca2+]c and [Ca2+]m. It also appears that the fraction of the cation cleared by the mitochondria derives from the RyR and that mitochondrial Ca2+ uptake is mediated by a Ru360-sensitive mechanism. Some indication for the specificity of the effects of Ru360 is also provided by the absence of parallel effects on the [Ca2+]c.
3.2. Arsenite-induced mitoO2.- formation is Ca2+-dependent and requires mitochondrial Ca2+ accumulation
We recently demonstrated that the arsenite-induced Ca2+ mobilization from the IP3R and RyR is not affected by mitoO2.- released under the same conditions [28]. We therefore asked the question of whether the same is also true for events regulating mitochondrial Ca2+ uptake.
In order to address this question we used two different assays to measure ROS formation after a 6 h exposure of RP-cells to arsenite. We used DHR, a fluorescence probe allowing the detection of different radicals, including O2.-, generated in both intra- and extra-mitochondrial compartments [44], and MitoSOX red, a fluorochrome commonly employed for the detection of O2.- in the mitochondria of live cells [45].
Arsenite promoted significant fluorescence responses using either of the two probes (Supplementary Fig. 1C and D). These different approaches also produced identical outcomes, in line with the notion that arsenite promotes mitoO2.- formation, as we previously determined [24], [25], [26], [27]. Both responses were indeed abolished by the complex I inhibitor rotenone, as well as by AA, supplemented under conditions promoting high and low concentrations of the vitamin in the mitochondria and cytosol, respectively (Fig. 1C and D) [29], [30]. As indicated in Fig. 1B, these treatments however failed to affect the arsenite-induced increase in [Ca2+]m. Similar results were obtained by measuring the [Ca2+]c (Fig. 1A) [28].
We also performed other experiments in RD-cells exposed for 6 h to 2.5 μM arsenite. These cells do not express a functional respiratory chain [25], [26] and fail to respond to arsenite with increased mitoO2.- formation (inset to Fig. 1C and D), but nevertheless accumulate Ca2+ in their cytosol (Fig. 1A) and mitochondria (Fig. 1B). These responses were identical to those observed in RP-cells and equally modulated by 2-APB, Ry and Ru360.
These results indicate that, in cells exposed to arsenite, the processes leading to Ca2+ mobilization and mitochondrial Ca2+ accumulation are ROS-independent.
We next investigated the role of Ca2+ in events leading to the formation of mitoO2.- in cells exposed to arsenite. As illustrated in Fig. 1, the DHR- (C) and MitoSOX red (D) fluorescence responses detected in RP-cells exposed to the metalloid, besides being sensitive to rotenone or AA, were also suppressed by 2-APB, Ry or Ru360. The results illustrated in Table 1 indicate that the same treatments instead failed to affect the DHR-fluorescence response mediated by reagent H2O2, thereby providing evidence for the specificity of their protective effects.
Table 1.
Effect of various treatments on the DHR-fluorescence caused by H2O2.
| Treatment | DHR-fluorescence (arbitrary units) |
|---|---|
| Untreated RP-cells | 14.5 + 1.4 |
| H2O2 | 28.9 + 2.5* |
| + Rotenone | 29.6 + 2.2* |
| + AA | 27.4 + 2.4* |
| +2-APB | 30.1 + 3.3* |
| + Ry | 27.7 + 2.7* |
| + Ru360 | 29.0 + 2.6* |
RP-cells were treated for 15 min with 0 or 50 µM H2O2, in the absence or presence of rotenone (0.5 µM), 2-APB (50 µM), Ry (20 µM) or Ru360 (10 µM). Drugs were added to the cultures 5 min prior to H2O2. AA (10 µM) was given to the cells 15 min prior to H2O2. After treatments, the cells were analyzed for DHR-fluorescence. Results represent the means ± SD calculated from at least 3 separate experiments.
P < 0.001; as compared to untreated cells (one-way ANOVA followed by Dunnett's test).
These results are therefore consistent with the possibility that arsenite promotes mitoO2.- formation via a Ca2+-dependent mechanism, possibly regulated by the mitochondrial fraction of the cation. Consistent with this notion were the results from experiments using D-cells indicating the absence of mitoO2.- formation (inset to Fig. 1C and D) in cells in which arsenite slightly increased the [Ca2+]c, via a 2-APB-sensitive/Ry-insensitive mechanism, but failed to increase the [Ca2+]m (Fig. 1B).
We next used an alternative approach based on inhibition of mitochondrial Ca2+ efflux, an event expected to burst the arsenite-induced mitoO2.- formation. For this purpose, we used CGP-37157, an inhibitor of mitochondrial Na+/Ca2+ exchanger (mNCX) [46], and an exposure protocol (3 µM for 4 h) failing to produce detectable toxicity, as measured by either the trypan blue exclusion assay or the release of lactate dehydrogenase (not shown). An additional advantage of this exposure protocol is represented by the fact that, under these conditions, arsenite produces weak responses in terms of mitochondrial Ca2+ accumulation and mitoO2.- formation (Table 2). We also found that CGP-37157 alone caused a weak increase in [Ca2+]m in the absence of detectable effects on mitoO2.- formation. It was then interesting to observe that combined exposure to arsenite and the mNCX inhibitor synergistically increased the [Ca2+]m and mitoO2.- formation.
Table 2.
CGP-37157 enhances the Rhod 2- or MitoSOX-red-fluorescence responses mediated by arsenite.
| Treatment | Rhod 2-fluorescence (arbitrary units) | MitoSOX red-fluorescence (arbitrary units) |
|---|---|---|
| Untreated RP-cells | 11.5 ± 1.4 | 11.3 ± 1.1 |
| CGP-37157 | 16.9 ± 1.2 | 11.8 ± 1.3 |
| Arsenite | 20.4 ± 2.2 | 15.1 ± 1.5 |
| Arsenite + CGP-37157 | 36.4 ± 2.4* | 31.8 ± 2.6* |
RP-cells were exposed for 4 h to arsenite (2.5 µM) in the absence or presence of CGP-37157 (3 µM). After treatments, the cells were analyzed for Rhod 2- or MitoSOX red-fluorescence. Results represent the means ± SD calculated from 3 separate experiments.
P < 0.01, as compared to cells treated with arsenite (one-way ANOVA followed by Dunnett's test).
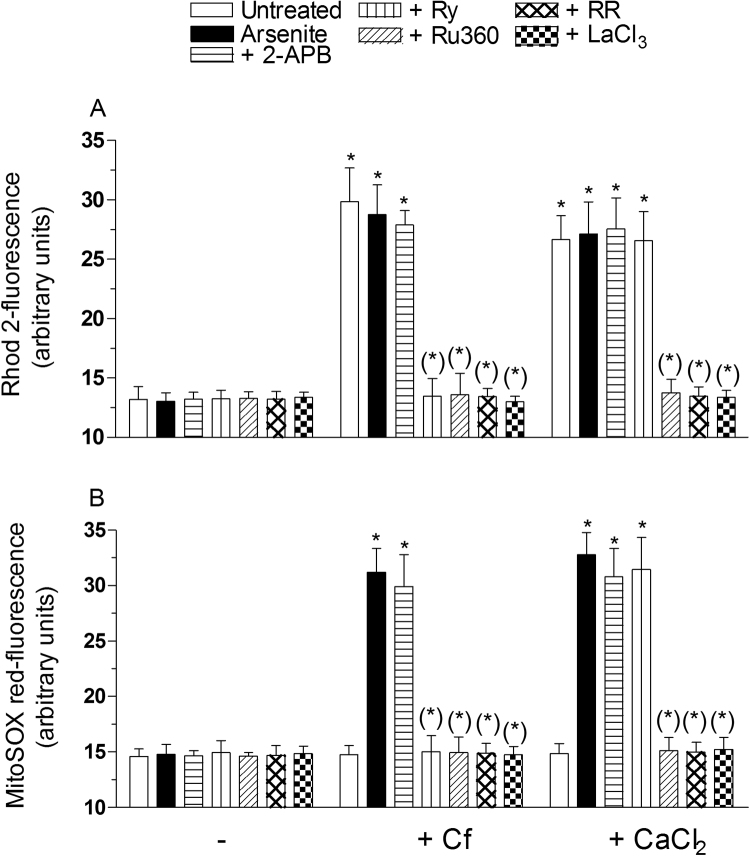
In order to provide a final, direct evidence linking the mitochondrial fraction of Ca2+ to mitoO2.- formation, we performed additional experiments using permeabilized RP-cells. In these experiments, the cells were first exposed to the appropriate fluorescence probe, subsequently permeabilized as detailed in the methods section, treated for 15 min with 2.5 μM arsenite, alone or with other additions, and finally analyzed for the [Ca2+]m (Fig. 2A) and mitoO2.- formation (Fig. 2B), as indicated above. Under these conditions, the metalloid failed to promote detectable effects, but nevertheless significantly enhanced mitoO2.- formation when the [Ca2+]m was increased via Cf supplementation. More specifically, we observed that Cf alone, or associated to arsenite, similarly increased the [Ca2+]m and that the enhanced mitoO2.- formation was only detected when the cells received both treatments. The increased [Ca2+]m and mitoO2.- formation were prevented by Ry, Ru360, RR (200 nM, a concentration specifically preventing mitochondrial Ca2+ uptake, [47]) and lanthanium ions (LaCl3,100 μM, which competitively inhibits Ca2+ uptake, [48]). 2-APB failed to produce detectable effects under the same conditions.
Fig. 2.
The role of mitochondrial Ca2+in events leading to mitoO2.-formation: studies in permealized cells RP-cells were incubated for 20 min with Rhod-2-AM (A), re-suspended in digitonin buffer and finally exposed for 10 min to 2.5 μM arsenite, either alone or associated with 10 mM Cf, or 30 μM CaCl2, in the absence or presence of 50 μM 2-APB, 20 μM Ry,10 μM Ru360, 200 nM RR or 100 μM LaCl3. After treatments, the cells were analyzed for Rhod-2-fluorescence. In other experiments (B), intact RP-cells were initially incubated for 20 min with MitoSOX red, processed and treated as indicated in (A), and finally analyzed to determine the resulting fluorescence responses. Results represent the means ± SD calculated from 3 separate experiments. *P < 0.01, as compared to untreated cells. (*)P < 0.01, as compared to cells treated with arsenite (one-way ANOVA followed by Dunnet's test).
We next performed experiments in which Cf was replaced with reagent Ca2+ (30 μM), and observed a significantly increased mitochondrial Ca2+ accumulation (Fig. 2A) associated with enhanced mitoO2.- formation (Fig. 2B). Furthermore, both responses were sensitive to Ru360, RR and LaCl3, but not to Ry, or 2-APB.
Collectively, the results presented in this section demonstrate that arsenite promotes mitochondrial Ca2+ accumulation via a mechanism insensitive to ROS. In addition, the experimental approach using both intact and permeabilized cells provided further indication for the specificity of the effects mediated by the different inhibitors and, most importantly, convincing experimental evidence causally linking the increased [Ca2+]m with mitoO2.- formation.
3.3. The increased mitochondrial Ca2+ concentrations are necessary to promote the indirect effects induced by arsenite on various biomolecules and on the Nrf2-dependent survival signaling
We previously determined that mitoO2.--derived H2O2 mediates both the toxic and adaptive responses triggered by arsenite [27]. We can therefore predict that 2-APB, Ry and Ru360, by preventing mitoO2.- formation (Fig. 1C and D), as previously observed with rotenone or AA [24], [26], [27], cause a parallel inhibition of the effects downstream to mitoO2.- formation.
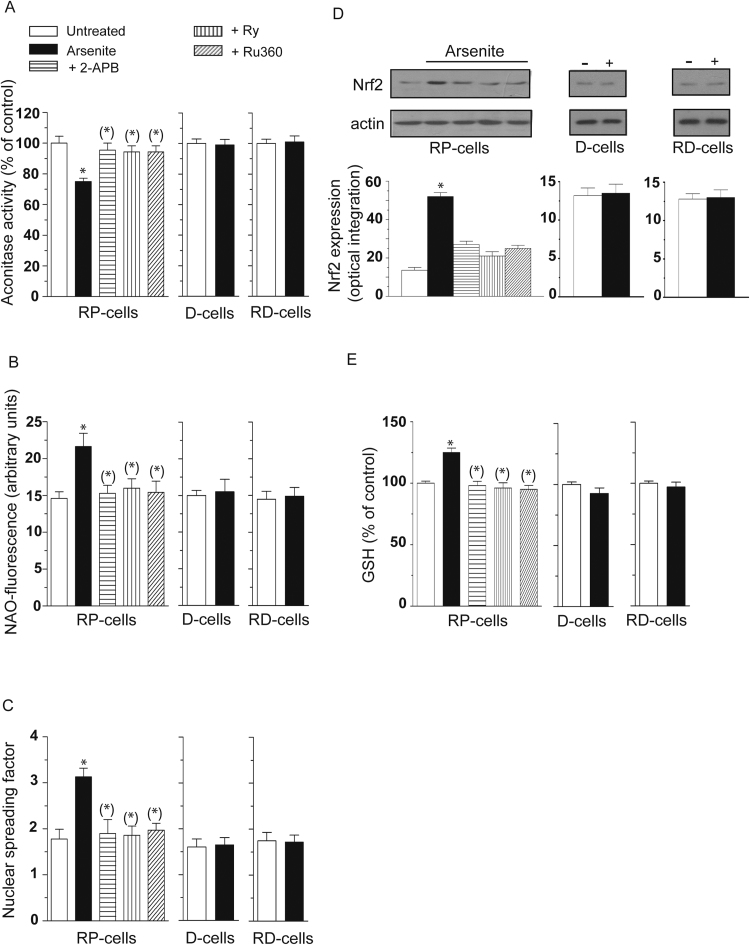
Indeed, as shown in Fig. 3, RP-cells exposed for 6 h to arsenite accumulated significant damage in their mitochondria in which we were able to detect decreased aconitase activity (A) and increased cardiolipin oxidation (B), using NAO as a fluorescent probe. Strand scission of genomic DNA, was also detected under the same conditions (C). In addition, we obtained evidence for increased Nrf2 expression (Fig. 3D) and GSH biosynthesis (Fig. 3E).
Fig. 3.
Mitochondrial Ca2+accumulation critically mediates the toxic effects induced by arsenite in mitochondrial and extramitochondrial compartments, as well as the parallel survival signaling. RP-, D- and RD-cells were exposed for 6 h to arsenite. RP cells also received the metalloid in the presence of 2-APB, Ry or Ru360. After treatments, the cells were analyzed for aconitase activity (A) NAO-fluorescence (B), DNA damage (C) and for their GSH content (E). In other experiments using identical conditions, except for the duration of arsenite exposure (4 h instead of 6 h), the cells were analyzed for Nrf2 expression (D). Blots shown in D (representative of three separate experiments) were re-probed for actin. Relative amounts of Nrf2 were quantified by densitometric analysis. Results represent the means ± SD calculated from 4 to 8 separate experiments. *P < 0.01, as compared to untreated cells. (*)P < 0.01, as compared to cells treated with arsenite (one-way ANOVA followed by Dunnet's test).
We found that all the above effects were prevented by 2-APB, Ry and Ru360. Arsenite instead failed to inhibit aconitase activity (A) and induce cardiolipin oxidation (B), DNA damage (C), Nrf2 expression (Fig. 3D) and GSH biosynthesis (Fig. 3E) in both D- and RD-cells, in which mitoO2.-formation did not take place (insets to Fig. 1C and D).
These results indicate that mitochondrial Ca2+ is critical for the arsenite-dependent induction of events associated with the formation of mitoO2.- and hence with the ensuing damage in diverse biomolecules and activation of the Nrf2/GSH survival responses.
3.4. Transient vs sustained increase in [Ca2+]c and [Ca2+]m
RP-cells were exposed for 6 h to 2.5 μM arsenite and incubated in fresh culture medium for additional 10 h prior to the assessment of the [Ca2+]c. The results illustrated in Fig. 4A indicate that the increase in [Ca2+]c detected after 6 h was transient, and indeed the cytosolic Ca2+ signal measured after recovery was identical to that measured in untreated cells. Different results were instead obtained by measuring the Ca2+ response mediated by a 16 h exposure to the metalloid. We found that the [Ca2+]c detected in these cells remained similar to that measured at the 6 h time-point.
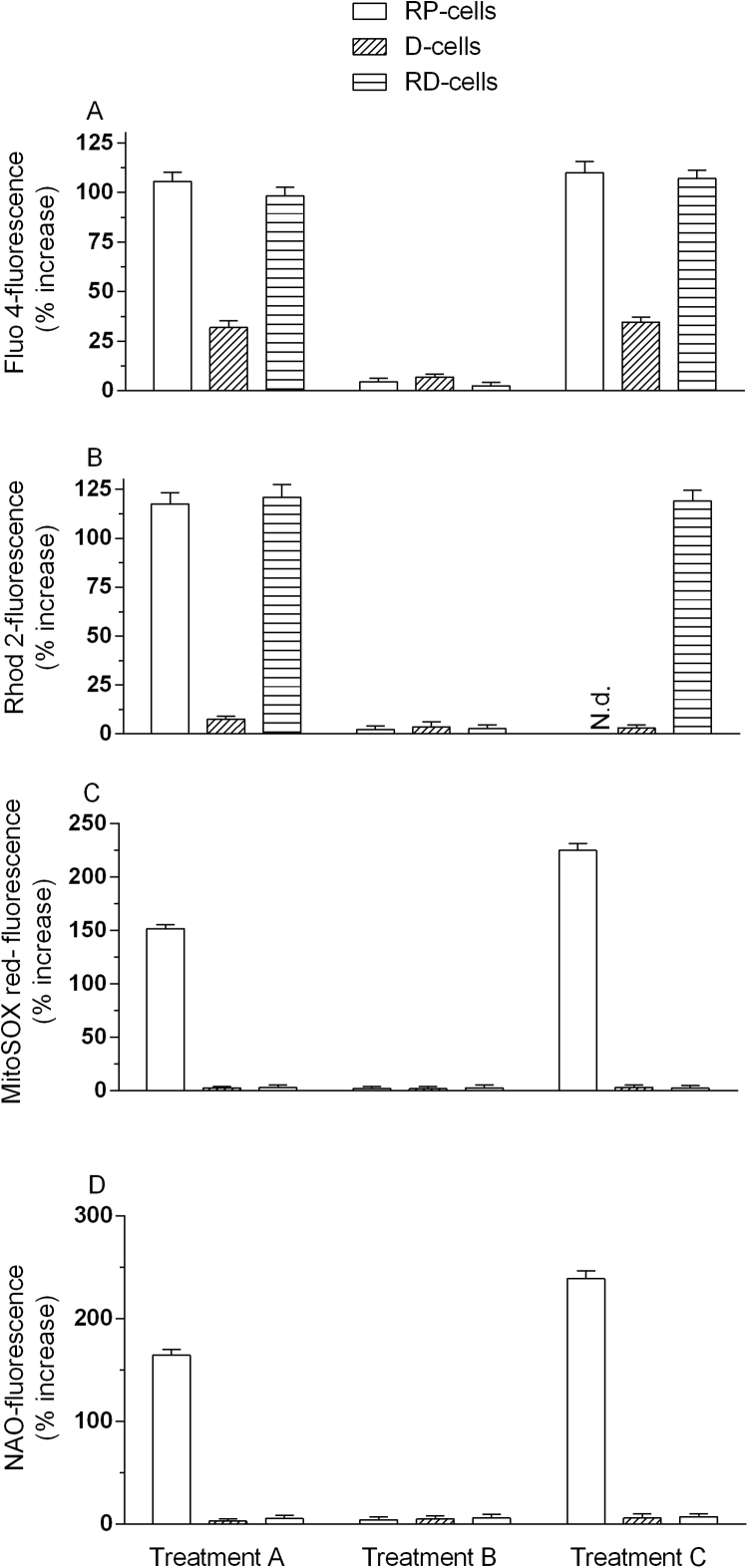
Fig. 4.
The effects of arsenite on the [Ca2+]cand [Ca2+]mare reversible: prolonged exposure to the metalloid is required to promote a sustained increase of the concentration of the cation in mitochondria, necessary for mitochondrial dysfunction and apoptosis. RP-, D- and RD-cells were treated for 6 h with arsenite (Treatment A). Cells received Treatment A and were then incubated for a further 10 h in fresh culture (Treatment B). Cells were treated for 16 h with arsenite (Treatment C). After treatments, the cells were analyzed for Fluo- 4- (A), Rhod-2- (B), MitoSOX (C) or NAO- (D) fluorescence. Results represent the means ± SD calculated from 3 to 6 separate experiments.
The results obtained with RD-cells were identical to those described above for RP-cells, whereas the small increase in [Ca2+]c detected in D-cells at 6 h disappeared after the post-treatment incubation and remained unchanged with the 16 h exposure regimen.
We subsequently found that the effects of the metalloid were also transient for the increased [Ca2+]m (Fig. 4B). We were instead unable to measure the [Ca2+]m in cells exposed for 16 h to arsenite, since under the same conditions a significant decline in mitochondrial membrane potential was also observed (Fig. 5A). Indeed Rhod 2, the probe employed in experiments measuring the [Ca2+]m, is taken up by the mitochondria as a function of their membrane potential [49].
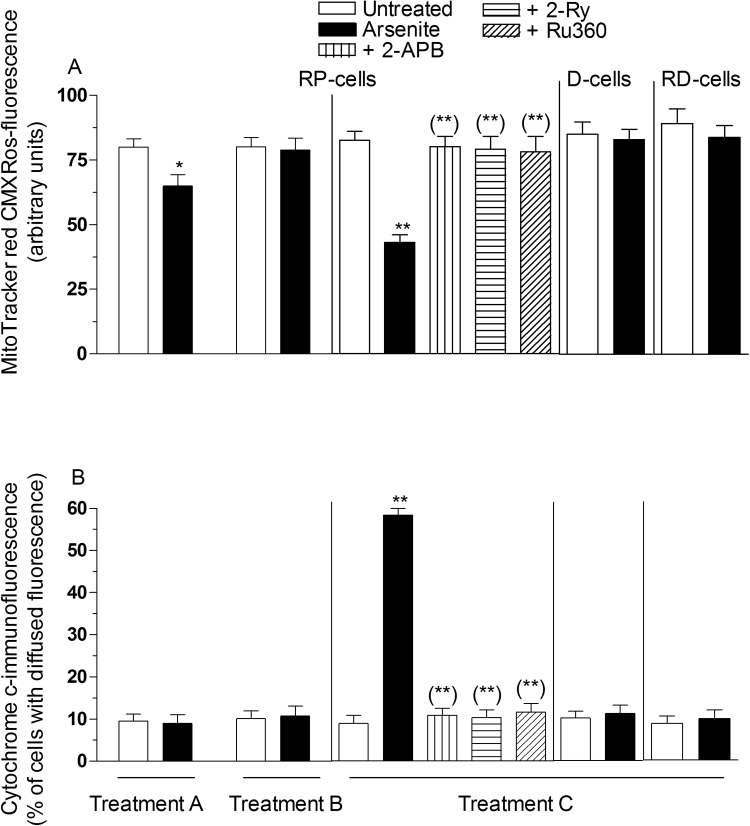
Fig. 5.
Impact of transientvssustained increase in the [Ca2+]mon mitochondrial dysfunction. Cells were treated as indicated in the legend to Fig. 4, in which a definition of Treatment A-C is also provided. RP-cells received Treatment C also in the presence of 2-APB, Ry or Ru360. After treatments, the cells were analyzed for MitoTracker red CMXRos-fluorescence (A) and cytochrome c localization (B), as detailed in the Methods section. Results represent the means ± SD calculated from 4 to 6 separate experiments. *P < 0.05, **P < 0.01, as compared to untreated cells. (**)P < 0.01, as compared to cells treated with arsenite (one-way ANOVA followed by Dunnet's test).
It is however important to note that RD-cells, while failing to produced ROS in response to arsenite (Fig. 1C), were also resistant to the effects on mitochondrial membrane potential (Fig. 5A). In addition, a 6 h exposure of RD-cells to arsenite caused an increased [Ca2+]m that returned to control levels after the 10 h post-incubation in fresh culture medium (Fig. 4B), as previously observed in RP-cells. RD-cells were therefore used to address the question of whether a sustained increase in [Ca2+]m is observed after the 16 h exposure to arsenite. The results illustrated in Fig. 4B indicate that this was indeed the case. As expected, there was no evidence of mitochondrial Ca2+ accumulation in D-cells receiving any of the above treatments (Fig. 4B).
We subsequently moved to experiments measuring mitoO2.- formation. As indicated in Fig. 4C, ROS formation in RP-cells exposed for 6 h to arsenite was suppressed by recovery in fresh culture medium, and significantly increased using the 16 h exposure paradigm (Fig. 4C). We also failed to detect increased mitoO2-.formation in D- or RD-cells receiving any of the above treatments (Fig. 4C).
We finally performed experiments measuring cardiolipin oxidation as an index of mitochondrial damage. As also previously indicated in Fig. 3B, a 6 h exposure to arsenite causes cardiolipin oxidation (Fig. 4D). This response was suppressed by recovery in fresh culture medium, and significantly increased using the 16 h exposure paradigm. In other words, the results obtained in these experiments were identical to those from experiments measuring mitoO2-.formation (Fig. 4C), which obviously makes sense since cardiolipin oxidation is mediated by mitoO2-.. As expected, there was no evidence of cardiolipin oxidation, in D- or RD-cells receiving any of the above treatments (Fig. 4D).
Collectively these results indicate that, under the conditions employed in the present study, the effects mediated by arsenite on the [Ca2+]c and [Ca2+]m are reversible upon post-treatment incubation of the cells in fresh culture medium. Evidence for mitoO2.- formation was therefore restricted to cells exposed to arsenite without recovery. The 16 h exposure protocol instead promoted a sustained increase in both [Ca2+]c and [Ca2+]m, associated with continuous and increasing mitoO2.- formation.
3.5. Sustained increase in [Ca2+]m is required to promote delayed toxic effects
As indicated above, and in our previous studies [24], [26], [27], inhibition of mitochondrial membrane potential is observed in RP-cells exposed for 16 h to arsenite (Fig. 5A). Furthermore, this event is associated with the mitochondrial loss of cytochrome c (Fig. 5B and Supplementary Fig. 1E), thereby confirming our previous findings providing evidence for the induction of MPT [24], [26], [27]. This notion is in keeping with the observation that CsA (0.5 μM) prevents both events (not shown). These same events were also sensitive to treatments preventing the mitochondrial accumulation of Ca2+. A longer exposure to arsenite (48 h) caused caspase 3 activation (Fig. 6A) and apoptotic DNA fragmentation (Fig. 6B). Both events were suppressed by CsA (not shown and [24], [26], [27]), 2-APB, Ry or Ru360.
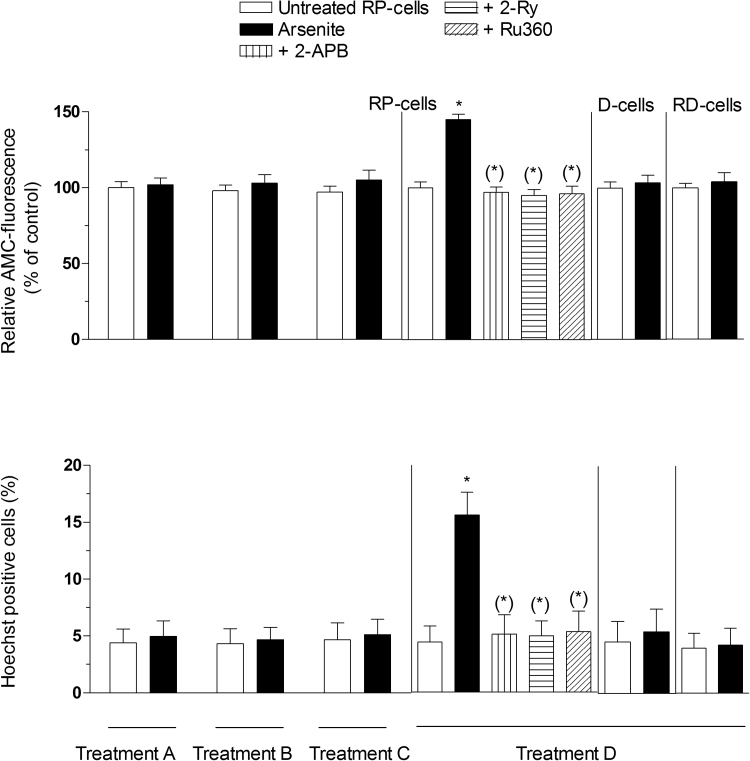
Fig. 6.
Sustained increase in the [Ca2+]mleads to apoptosis. Cells were treated as indicated in the legend to Fig. 4, in which a definition of Treatment A-C is also provided. Treatment D refers to cells exposed to the metalloid for 48 h. RP-cells received Treatment D also in the presence of 2-APB, Ry or Ru360. After treatments, the cells were analyzed for caspase-3 activity (A), or for apoptosis, by measuring chromatin fragmentation/condensation with the Hoechst assay (B). Results represent the means ± SD calculated from 5 separate experiments. *P < 0.01, as compared to untreated cells. (*)P < 0.01, as compared to cells treated with arsenite (one-way ANOVA followed by Dunnet's test).
We found no evidence for a decline in mitochondrial membrane potential (Fig. 5A), cytochrome c release (Fig. 5B), caspase 3 activation (Fig. 6A) and chromatin fragmentation (Fig. 6B) in RP-cells exposed for 6 h to arsenite, with or without recovery. As a final note, RD- and D-cells were resistant to mitochondrial dysfunction (Fig. 5A and B) and apoptosis (Fig. 6A and B) induced by prolonged arsenite exposure.
These results indicate that a sustained increase in [Ca2+]c and, more specifically in [Ca2+]m, is critical for the induction of the arsenite-dependent delayed events. The 6 h exposure to the metalloid instead promotes reversible changes in both the [Ca2+]c and [Ca2+]m. Exposure to arsenite followed by recovery in fresh culture medium was indeed associated with inhibition of mitoO2.- formation, mitochondrial dysfunction and delayed apoptosis.
4. Discussion
In a recent study, we investigated the effect of a low concentration of arsenite in a U937 cell line (RP-cells) and U937 cell variants with down-regulated RyR (D-cells) or characterized by a non-functional mitochondrial respiratory chain (RD-cells). These cells, along with use of specific inhibitors, allowed the identification of events leading to increased [Ca2+]c through the sequential involvement of the IP3R and RyR [28]. More specifically, we observed that arsenite promotes a weak stimulation of the IP3R and a significant amplification of the overall Ca2+ response through the RyR, an event dependent on direct effects of the metalloid on the intraluminal crosstalk between the IP3R and RyR.
Using the same approach, we now report that the effects of arsenite on the [Ca2+]c are associated with a significant increase in [Ca2+]m, likely mediated by the MCU, a channel characterized by a high selectivity and conductance for Ca2+, but a low apparent affinity for the cation [50], [51]. Although the involvement of MCU in the mitochondrial transport of the cation appears consistent with the outcome of various experimental studies (see below), it should nevertheless be mentioned that mitochondria can also uptake the cation by additional channels as the Ca2+/H+ exchanger Letm1, the mitochondrial RyR1 and transient receptor canonical 3 [52].
Our preliminary observation was that the fraction of Ca2+ mobilized by arsenite from the IP3R fails to promote a [Ca2+]c sufficiently high to allow transport of the cation in the mitochondria. A more correct interpretation of these findings should however be based on the well-established subcellular heterogeneity of the Ca2+ concentrations resulting from exposure to different stimuli in different cells [11], [12], [13]. The different subcellular concentrations of the cation in selected microdomains can therefore explain the specificity of the corresponding functional effects [11], [53], [54]. Likewise, the possibility of Ca2+ transport in mitochondria is restricted to conditions in which the concentration of the cation is particularly high in the close proximity of the MCU [51], [54]. These requirements therefore lead us to conclude that the Ca2+ released by the IP3R in response to arsenite fails to create local concentrations of the cation permissive for transport through the MCU.
We subsequently determined that the fraction of Ca2+ released by the RyR after IP3R stimulation induced by arsenite is instead efficiently cleared by the mitochondria. It therefore appears that the RyR-released Ca2+ promotes sufficient local concentrations of the cation in specialized microdomains created by the close association between mitochondria and the portion of the endoplasmic reticulum bearing the RyR channel. As a consequence, RyR-derived Ca2+ is readily transported by the MCU into mitochondria.
To summarize, the experimental results thus far discussed suggest that the direct effects of arsenite on the intraluminal IP3R/RyR crosstalk identified in our previous work [28] are functional to RyR-dependent Ca2+ release coupled with MCU-dependent mitochondrial Ca2+ accumulation.
Having previously demonstrated that mitoO2.-, and its dismutation product H2O2, produces hardly any effect on the arsenite-induced Ca2+ mobilization from the IP3R and the RyR [28], we investigated whether the same was also true for events leading to enhanced mitochondrial Ca2+ accumulation. Our approach, based on the use of treatments preventing ROS formation, or RD-cells, univocally demonstrated that mitochondrial Ca2+ accumulation is also ROS-independent.
We subsequently investigated whether Ca2+ is necessary for ROS formation and found that treatments preventing the increase in [Ca2+]c and [Ca2+]m produce parallel inhibitory responses in terms of mitoO2 formation in RP-cells. Furthermore, using RP-cells treated with Ry or D-cells, which do not express the RyR, we determined that the specific fraction of the cation involved in mitoO2.- formation is derived from the RyR. In other words, it appears that RyR-derived Ca2+ plays a critical role in the regulation of events associated with mitoO2.- formation. Since the fraction of Ca2+ taken up by the mitochondria also derives from the RyR, it would make sense to postulate its involvement in ROS formation. Our experiments performed in intact cells using Ru360 provided outcomes consistent with this conclusion. Indeed the MCU inhibitor, while failing to affect the [Ca2+]c, prevented the increase in [Ca2+]m and caused a parallel suppression of mitoO2-. formation.
Consistently, inhibition of Ca2+ efflux from mitochondria mediated by CGP-37157 was associated with a dramatic increase in the arsenite-induced mitoO2-. formation.
Inhibitor studies, however, while validated by parallel observations ruling out the possibility of non-specific effects on Ca2+ homeostasis and/or on ROS formation, require converging information from studies using different approaches.
We therefore tried to establish a more direct link between mitochondrial Ca2+ accumulation and mitoO2.- formation using permeabilized cells, which allow the use of treatments that cannot be employed in intact cells. We preliminarily demonstrated that, under these conditions, Cf (a RyR agonist) promotes mitochondrial Ca2+ accumulation. The observation that such event can be clearly observed in permeabilized cells, implies the existence of a close proximity between the RyR and mitochondria of RP-cells. The same conclusion was reached in studies using arsenite, which also promotes Ca2+ release from this channel and causes mitochondrial Ca2+ accumulation. Regardless of this consideration, however, an important observation we could make was that even a short-term exposure to arsenite promotes mitoO2.- formation in cells manipulated to induce an enforced mitochondrial Ca2+ accumulation. In addition, as also observed in intact cells, the increase in [Ca2+]m was necessary -but not sufficient- for ROS formation.
We also used a different approach in which reagent Ca2+ was directly delivered to the permeabilized cells to then obtain results consistent with those described above, in which Cf was used to promote mitochondrial Ca2+ accumulation. As a final note, a further proof of concept documenting the involvement of mitochondrial Ca2+ in ROS formation is provided by experiments using Ru360, low concentrations of RR or an excess concentration of lanthanium ions. These different treatments effectively prevented both the mitochondrial Ca2+ accumulation and ROS formation stimulated by arsenite in the presence of either Cf or reagent Ca2+.
Collectively, the above results provide solid experimental evidence supporting the notion that mitochondrial Ca2+ accumulation induced by arsenite is mediated by the MCU, ensues as a consequence of Ca2+ release form the RyR and critically mediates mitoO2.- formation.
O2.- formation in different subcellular compartments generates different local concentrations of H2O2. For this reason, and for the compartmentalized nature of the antioxidant defenses [3], [4], the indirect effects mediated by arsenite through the O2.-/H2O2 signaling may vary significantly with different sites of O2.- formation. It follows that the effects mediated by arsenite in the specific paradigm employed in this study are profoundly affected by the ability of the metalloid to uniquely produce mitoO2.- in U937 cells, which in turn appears to depend on mitochondrial Ca2+ accumulation consequent to the direct effects of the metalloid on the ER. Not surprisingly, prevention of mitochondrial Ca2+ accumulation was invariably associated with inhibition of the early effects mediated by arsenite through the intermediate formation of O2.- and H2O2 in mitochondrial (aconitase inhibition and cardiolipin oxidation) and extramitochondrial (strand scission of genomic DNA) compartments. Likewise, prevention of mitochondrial Ca2+ accumulation also abolished the cytoprotective signaling based on activation of the Nrf2 pathway leading to increased GSH biosynthesis.
Our previous work demonstrated that arsenite triggers two opposite events, both mediated by mitoO2.- and H2O2 [27]. On the one hand, the oxidant(s) promoted the formation of lesions on vicinal and distal biomolecules, as indicated above (6 h), culminating in mitochondrial dysfunction and MPT (16 h) and followed by a delayed (48 h) apoptotic response [24], [26], [27]. On the other hand, O2.- and H2O2 also promoted a survival signaling based on activation of Nrf2, enhanced γ-glutamilcysteine synthase catalytic heavy subunit expression and increased GSH biosynthesis [27]. This latter event reduced the rate/extent of mitoO2-. formation and, as a consequence, delayed and mitigated the extent of the above downstream deleterious effects.
We now provide details on the reasons why the above sequence of events requires the continuous presence of arsenite. Indeed, cells exposed for 6 h to the metalloid maintained intact and functional mitochondria, and remained viable when post-incubated in fresh culture medium since ROS formation ceased after the 10 h recovery, as a consequence of the inhibition of events causing deregulated Ca2+ homeostasis and increased mitochondrial Ca2+ accumulation. In addition, prevention of mitochondrial Ca2+ accumulation achieved with the use of various inhibitors, or D-cells, was invariably associated with the suppression of the toxic effects mediated by the metalloid. As previously noted, however, the increase in [Ca2+]m is necessary but not sufficient to promote cytotoxicity, since the effects detected in RD-cells were identical to those measured in RP-cells in terms of variations in [Ca2+]m, but different in terms of mitoO2.- formation and toxicity. Identical findings were obtained using RP-cells supplemented with agents preventing mitoO2.- formation.
We therefore conclude that mitochondrial Ca2+ accumulation represents the primum movens of the effects of arsenite in the specific cell line employed, in which the metalloid only generates mitoO2.-. The source of the cation appears to be the RyR and its significant accumulation in mitochondria, while failing per se to produce direct consequences, was nevertheless critical for mitoO2.- formation, the upstream species responsible for the cytoprotective and toxic effects mediated by the metalloid. These early events are however reversible and mitoO2.- formation ceases when the [Ca2+]m returns to normal levels. Sustained increase in [Ca2+]m is therefore required for continuous ROS formation, and for the onset of MPT [24], [26], [27], thereby making necessary a more prolonged exposure to arsenite in order to obtain evidence of toxicity.
Acknowledgements
This work was supported by Ministero dell’Università e della Ricerca Scientifica e Tecnologica, Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale, 2015, (Grant number: 2015MJBEM2-003. O.C.).
Acknowledgments
Author contribution
AG and OC conceived the research. AG, MF and LC performed the experiments. AG, MF, LC and OC analyzed the data. OC wrote the manuscript. AG, MF, LC and OC revised the manuscript. All authors approved the final version of the manuscript.
Conflict of interest statement
The authors have declared that they have no conflicts of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.10.015
Appendix A. Supplementary material
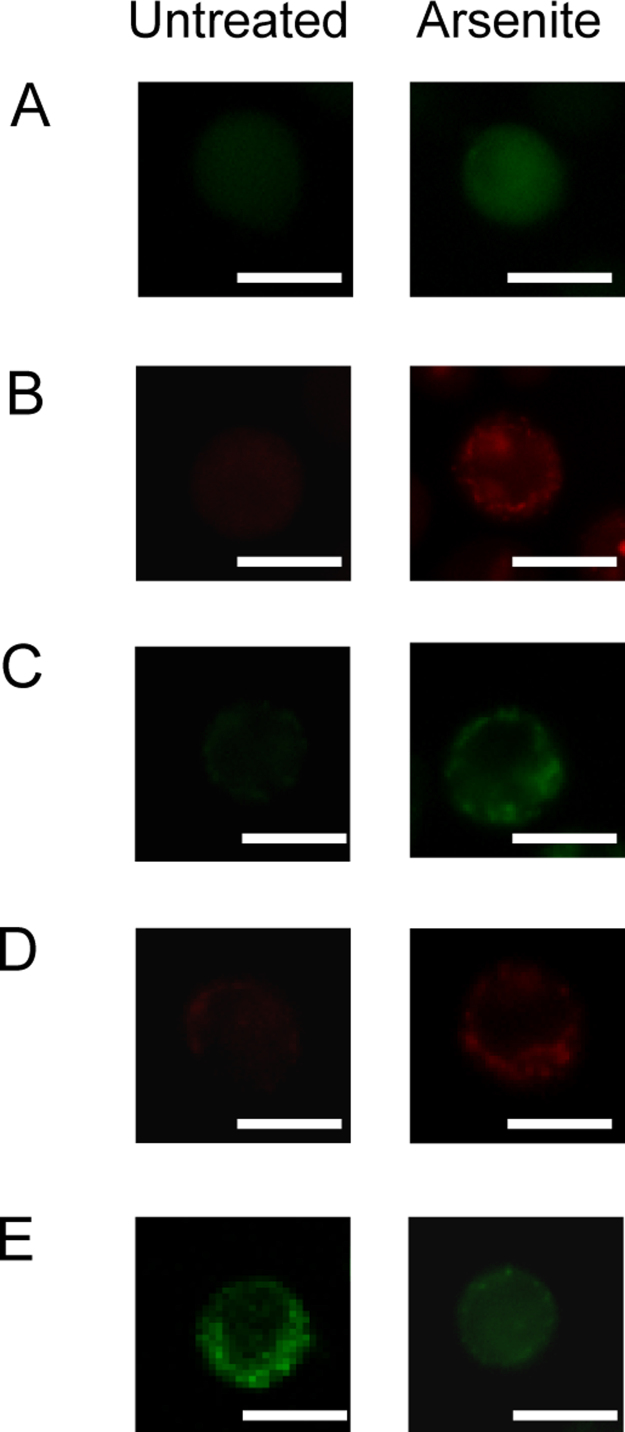
Fig. S1.
Representative micrographs of RP-cells incubated for 6 (A-D) or 16 (E) h, in the absence or presence of 2.5 µM arsenite. After treatments, the cultures were processed for the assessment of the fluorescence responses detected with Fluo 4 (A), Rhod 2 (B), DHR (C), MitoSOX red (D), as well as the cytochrome c immunofluorescence (E). Scale bars represent 20 µm.
References
- 1.Holmstrom K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell. Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 2.Kudryavtseva A.V., Krasnov G.S., Dmitriev A.A., Alekseev B.Y., Kardymon O.L., Sadritdinova A.F., Fedorova M.S., Pokrovsky A.V., Melnikova N.V., Kaprin A.D., Moskalev A.A., Snezhkina A.V. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget. 2016;7:44879–44905. doi: 10.18632/oncotarget.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 4.Handy D.E., Loscalzo J. Redox regulation of mitochondrial function. Antioxid. Redox Signal. 2012;16:1323–1367. doi: 10.1089/ars.2011.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertero E., Maack C. Calcium signaling and reactive oxygen species in mitochondria. Circ. Res. 2018;122:1460–1478. doi: 10.1161/CIRCRESAHA.118.310082. [DOI] [PubMed] [Google Scholar]
- 7.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulz E., Wenzel P., Munzel T., Daiber A. Mitochondrial redox signaling: interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 2014;20:308–324. doi: 10.1089/ars.2012.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chernorudskiy A.L., Zito E. Regulation of calcium homeostasis by ER redox: a close-up of the ER/mitochondria connection. J. Mol. Biol. 2017;429:620–632. doi: 10.1016/j.jmb.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Csordas G., Weaver D., Hajnoczky G. Endoplasmic reticular-mitochondrial contactology: structure and signaling functions. Trends Cell Biol. 2018;28:523–540. doi: 10.1016/j.tcb.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hidalgo C., Donoso P. Crosstalk between calcium and redox signaling: from molecular mechanisms to health implications. Antioxid. Redox Signal. 2008;10:1275–1312. doi: 10.1089/ars.2007.1886. [DOI] [PubMed] [Google Scholar]
- 13.Trebak M., Ginnan R., Singer H.A., Jourd'heuil D. Interplay between calcium and reactive oxygen/nitrogen species: an essential paradigm for vascular smooth muscle signaling. Antioxid. Redox Signal. 2010;12:657–674. doi: 10.1089/ars.2009.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guidarelli A., Sciorati C., Clementi E., Cantoni O. Peroxynitrite mobilizes calcium ions from ryanodine-sensitive stores, a process associated with the mitochondrial accumulation of the cation and the enforced formation of species mediating cleavage of genomic DNA. Free Radic. Biol. Med. 2006;41:154–164. doi: 10.1016/j.freeradbiomed.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Shakoor M.B., Nawaz R., Hussain F., Raza M., Ali S., Rizwan M., Oh S.E., Ahmad S. Human health implications, risk assessment and remediation of As-contaminated water: a critical review. Sci. Total Environ. 2017;601–602:756–769. doi: 10.1016/j.scitotenv.2017.05.223. [DOI] [PubMed] [Google Scholar]
- 16.Minatel B.C., Sage A.P., Anderson C., Hubaux R., Marshall E.A., Lam W.L., Martinez V.D. Environmental arsenic exposure: from genetic susceptibility to pathogenesis. Environ. Int. 2018;112:183–197. doi: 10.1016/j.envint.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Flora S.J. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011;51:257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Shen S., Li X.F., Cullen W.R., Weinfeld M., Le X.C. Arsenic binding to proteins. Chem. Rev. 2013;113:7769–7792. doi: 10.1021/cr300015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meissner G. The structural basis of ryanodine receptor ion channel function. J. Gen. Physiol. 2017;149:1065–1089. doi: 10.1085/jgp.201711878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen A., Cao E.H., Zhang T.C., Qin J.F. Arsenite-induced reactive oxygen species and the repression of alpha-tocopherol in the MGC-803 cells. Eur. J. Pharmacol. 2002;448:11–18. doi: 10.1016/s0014-2999(02)01901-5. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee C., Goswami R., Datta S., Rajagopal R., Mazumder S. Arsenic-induced alteration in intracellular calcium homeostasis induces head kidney macrophage apoptosis involving the activation of calpain-2 and ERK in Clarias batrachus. Toxicol. Appl. Pharmacol. 2011;256:44–51. doi: 10.1016/j.taap.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Suriyo T., Watcharasit P., Thiantanawat A., Satayavivad J. Arsenite promotes apoptosis and dysfunction in microvascular endothelial cells via an alteration of intracellular calcium homeostasis. Toxicol. Vitr. 2012;26:386–395. doi: 10.1016/j.tiv.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Pachauri V., Mehta A., Mishra D., Flora S.J. Arsenic induced neuronal apoptosis in guinea pigs is Ca2+ dependent and abrogated by chelation therapy: role of voltage gated calcium channels. Neurotoxicology. 2013;35:137–145. doi: 10.1016/j.neuro.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Guidarelli A., Carloni S., Balduini W., Fiorani M., Cantoni O. Mitochondrial ascorbic acid prevents mitochondrial O2.- formation, an event critical for U937 cell apoptosis induced by arsenite through both autophagic-dependent and independent mechanisms. Biofactors. 2016;42:190–200. doi: 10.1002/biof.1263. [DOI] [PubMed] [Google Scholar]
- 25.Guidarelli A., Fiorani M., Carloni S., Cerioni L., Balduini W., Cantoni O. The study of the mechanism of arsenite toxicity in respiration-deficient cells reveals that NADPH oxidase-derived superoxide promotes the same downstream events mediated by mitochondrial superoxide in respiration-proficient cells. Toxicol. Appl. Pharmacol. 2016;307:35–44. doi: 10.1016/j.taap.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Guidarelli A., Fiorani M., Cerioni L., Scotti M., Cantoni O. Arsenite induces DNA damage via mitochondrial ROS and induction of mitochondrial permeability transition. Biofactors. 2017;43:673–684. doi: 10.1002/biof.1375. [DOI] [PubMed] [Google Scholar]
- 27.Fiorani M., Guidarelli A., Capellacci V., Cerioni L., Crinelli R., Cantoni O. The dual role of mitochondrial superoxide in arsenite toxicity: signaling at the boundary between apoptotic commitment and cytoprotection. Toxicol. Appl. Pharmacol. 2018;345:26–35. doi: 10.1016/j.taap.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Guidarelli A.F., Cantoni M. O, Low concentrations of arsenite target the intraluminal inositol 1, 4, 5-trisphosphate receptor/ryanodine receptor crosstalk to significantly elevate intracellular Ca2+ J. Pharmacol. Exp. Ther. 2018;367:184–193. doi: 10.1124/jpet.118.250480. [DOI] [PubMed] [Google Scholar]
- 29.Fiorani M., Azzolini C., Cerioni L., Scotti M., Guidarelli A., Ciacci C., Cantoni O. The mitochondrial transporter of ascorbic acid functions with high affinity in the presence of low millimolar concentrations of sodium and in the absence of calcium and magnesium. Biochim. Biophys. Acta. 1848;2015:1393–1401. doi: 10.1016/j.bbamem.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Cantoni O., Guidarelli A., Fiorani M. Mitochondrial uptake and accumulation of vitamin C: what can we learn from cell cultures studies? Antioxid. Redox Signal. 2018;29:1502–1515. doi: 10.1089/ars.2017.7253. [DOI] [PubMed] [Google Scholar]
- 31.Azzolini C., Fiorani M., Cerioni L., Guidarelli A., Cantoni O. Sodium-dependent transport of ascorbic acid in U937 cell mitochondria. IUBMB Life. 2013;65:149–153. doi: 10.1002/iub.1124. [DOI] [PubMed] [Google Scholar]
- 32.Sugiyama T., Yamamoto-Hino M., Miyawaki A., Furuichi T., Mikoshiba K., Hasegawa M. Subtypes of inositol 1,4,5-trisphosphate receptor in human hematopoietic cell lines: dynamic aspects of their cell-type specific expression. FEBS Lett. 1994;349:191–196. doi: 10.1016/0014-5793(94)00662-8. [DOI] [PubMed] [Google Scholar]
- 33.Hosoi E., Nishizaki C., Gallagher K.L., Wyre H.W., Matsuo Y., Sei Y. Expression of the ryanodine receptor isoforms in immune cells. J. Immunol. 2001;167:4887–4894. doi: 10.4049/jimmunol.167.9.4887. [DOI] [PubMed] [Google Scholar]
- 34.Guidarelli A., Cerioni L., Fiorani M., Cantoni O. Differentiation-associated loss of ryanodine receptors: a strategy adopted by monocytes/macrophages to prevent the DNA single-strand breakage induced by peroxynitrite. J. Immunol. 2009;183:4449–4457. doi: 10.4049/jimmunol.0901260. [DOI] [PubMed] [Google Scholar]
- 35.Scotti M., Fiorani M., Guidarelli A., Cantoni O. Differentiation of promonocytic U937 cells to monocytes is associated with reduced mitochondrial transport of ascorbic acid. Oxid. Med. Cell. Longev. 2018;2018:4194502. doi: 10.1155/2018/4194502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiskum G., Craig S.W., Decker G.L., Lehninger A.L. The cytoskeleton of digitonin-treated rat hepatocytes. Proc. Natl. Acad. Sci. USA. 1980;77:3430–3434. doi: 10.1073/pnas.77.6.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardner P.R. Aconitase: sensitive target and measure of superoxide. Methods Enzymol. 2002;349:9–23. doi: 10.1016/s0076-6879(02)49317-2. [DOI] [PubMed] [Google Scholar]
- 38.Cantoni O., Guidarelli A. Indirect mechanisms of DNA strand scission by peroxynitrite. Methods Enzymol. 2008;440:111–120. doi: 10.1016/S0076-6879(07)00806-3. [DOI] [PubMed] [Google Scholar]
- 39.Guidarelli A., Cerioni L., Tommasini I., Fiorani M., Brune B., Cantoni O. Role of Bcl-2 in the arachidonate-mediated survival signaling preventing mitochondrial permeability transition-dependent U937 cell necrosis induced by peroxynitrite. Free Radic. Biol. Med. 2005;39:1638–1649. doi: 10.1016/j.freeradbiomed.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Cantoni O., Tommasini I., Cerioni L. The arachidonate-dependent survival signaling preventing toxicity in monocytes/macrophages exposed to peroxynitrite. Methods Enzymol. 2008;441:73–82. doi: 10.1016/S0076-6879(08)01205-6. [DOI] [PubMed] [Google Scholar]
- 41.Brundu S., Palma L., Picceri G.G., Ligi D., Orlandi C., Galluzzi L., Chiarantini L., Casabianca A., Schiavano G.F., Santi M., Mannello F., Green K., Smietana M., Magnani M., Fraternale A. Glutathione depletion is linked with Th2 polarization in mice with a retrovirus-induced immunodeficiency syndrome, murine AIDS: role of proglutathione molecules as immunotherapeutics. J. Virol. 2016;90:7118–7130. doi: 10.1128/JVI.00603-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maruyama T., Kanaji T., Nakade S., Kanno T., Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 43.Zazueta C., Sosa-Torres M.E., Correa F., Garza-Ortiz A. Inhibitory properties of ruthenium amine complexes on mitochondrial calcium uptake. J. Bioenerg. Biomembr. 1999;31:551–557. doi: 10.1023/a:1005464927366. [DOI] [PubMed] [Google Scholar]
- 44.Gomes A., Fernandes E., Lima J.L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods. 2005;65:45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Mukhopadhyay P., Rajesh M., Hasko G., Hawkins B.J., Madesh M., Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat. Protoc. 2007;2:2295–2301. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiesi M., Schwaller R., Eichenberger K. Structural dependency of the inhibitory action of benzodiazepines and related compounds on the mitochondrial Na+-Ca2+ exchanger. Biochem. Pharmacol. 1988;37:4399–4403. doi: 10.1016/0006-2952(88)90623-5. [DOI] [PubMed] [Google Scholar]
- 47.Carafoli E. Intracellular calcium homeostasis. Annu. Rev. Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- 48.Thomas C.E., Reed D.J. Effect of extracellular Ca++ omission on isolated hepatocytes. II. Loss of mitochondrial membrane potential and protection by inhibitors of uniport Ca++ transduction. J. Pharmacol. Exp. Ther. 1988;245:501–507. [PubMed] [Google Scholar]
- 49.Minta A., Kao J.P., Tsien R.Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J. Biol. Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- 50.Kirichok Y., Krapivinsky G., Clapham D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 51.Penna E., Espino J., De Stefani D., Rizzuto R. The MCU complex in cell death. Cell Calcium. 2018;69:73–80. doi: 10.1016/j.ceca.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 52.Mishra J., Jhun B.S., Hurst S., J O.U., Csordas G., Sheu S.S. The Mitochondrial Ca2+ uniporter: structure, function, and pharmacology. Handb. Exp. Pharmacol. 2017;240:129–156. doi: 10.1007/164_2017_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rizzuto R., Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 54.De Stefani D., Rizzuto R., Pozzan T. Enjoy the trip: calcium in mitochondria back and forth. Annu. Rev. Biochem. 2016;85:161–192. doi: 10.1146/annurev-biochem-060614-034216. [DOI] [PubMed] [Google Scholar]