Introduction
Merkel cell carcinoma (MCC) is a rare, aggressive neuroendocrine cutaneous tumor with a high mortality rate.1 Evidence supports 2 molecular subclasses of MCC: virus-positive MCC associated with oncogenic Merkel cell polyomavirus (MCPyV), and virus-negative MCC (VN-MCC) associated with UV signature mutations.1, 2, 3 Typically, MCC presents as a pink papule or nodule on sun-exposed skin of white, elderly individuals.1 MCC most frequently presents in the head and neck area, followed by the upper extremities.1 MCC commonly metastasizes to regional lymph nodes and skin, with potential for distant metastases to skin, lung, liver, central nervous system, and bone.3 Patients may have a high burden of satellite and in-transit cutaneous metastases, which may be histologically identical to primary MCC tumors.1, 3
In approximately 1% to 2% of patients with MCC, a second MCC tumor will arise that is clinically compatible with a new primary MCC.1, 4 In such cases, molecular analyses may be useful in distinguishing a second primary MCC tumor from a distant cutaneous metastasis.4, 5, 6, 7, 8
Some MCC tumors have concurrent squamous cell carcinoma (SCC), either in situ or invasive, which has been proposed to favor primary MCC over a cutaneous metastasis.1 Concurrent SCC/MCC is a distinct phenomenon from MCC “mixed tumors” with intratumoral squamous differentiation (multiple foci of squamous differentiation dispersed throughout the tumor, rather than a discrete squamous lesion).1 MCC associated with SCC is usually MCPyV-negative, with rare exceptions.9 Although both SCC and MCC may arise in association with photodamage, it is unknown if concurrent SCC/MCC represent biologically related neoplasms.
We present a case of an elderly man found to have MCC of the left forehead. A second focus of MCC was subsequently identified on the left zygoma, in association with a previously diagnosed invasive SCC. Single nucleotide polymorphism (SNP) array analysis confirmed clonal relationship between the foci of MCC, supporting a metastatic process, and demonstrated the SCC to be unrelated. Our findings suggest that concurrent SCC and MCC can represent unrelated collision tumors. Our case also indicates that nonmelanoma skin cancers (NMSCs) in the lymphatic draining area of MCC tumors should be scrutinized for potential involvement by MCC.
Case report
An 84-year-old man presented to the Department of Dermatology at the University of Puerto Rico with a new lesion of concern. He had a history of multiple NMSCs, including SCC of the left zygoma that was diagnosed by biopsy 6 months before the current visit (this tumor was not excised at the time because the patient was temporarily lost to follow-up). At the current visit, we identified a new, ill-defined erythematous scaly plaque on the left upper forehead (Fig 1), that upon biopsy showed MCC with characteristic CK20 expression and negative immunohistochemistry for MCPyV large T antigen. There was no associated SCC or squamous differentiation. Wide local excision found residual tumor with a tumor thickness of 2.2 mm and 6 to 8 mitoses per high-power field. Margins were negative. Sentinel lymph node biopsy was not pursued, in part because of patient comorbidities.
Fig 1.
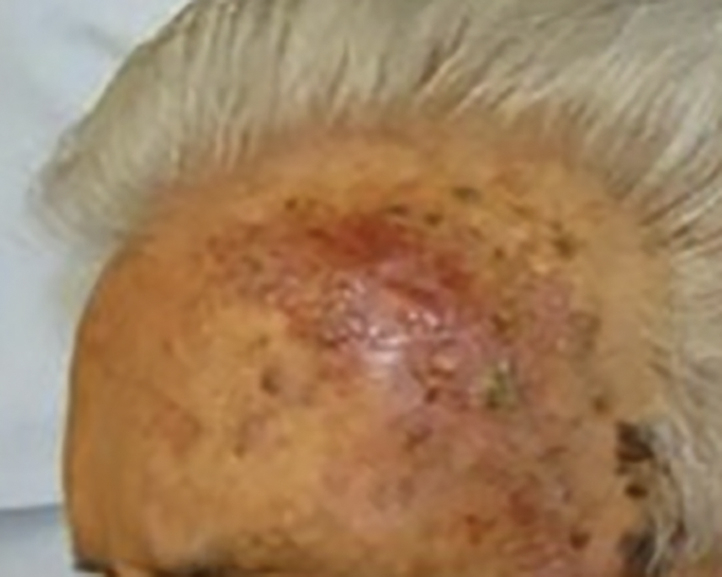
Clinical appearance of MCC on the left forehead, presenting as erythematous plaque.
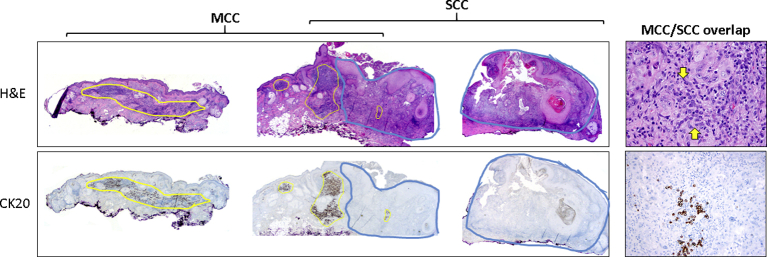
Three months after the initial diagnosis of MCC, the previously biopsied SCC on the left zygomatic area (Fig 2) was removed by Mohs micrographic surgery (MMS). Microscopic examination during the first stage of MMS found a second population of small blue cells in addition to the SCC. Four MMS stages were necessary to obtain negative margins. Permanent sections of the debulking specimen confirmed the diagnosis of SCC and MCC, presenting as adjacent tumors with focal intermingling (Fig 3). CK20 immunohistochemistry was positive, and MCPyV large T antigen immunohistochemistry was negative in this focus of MCC.
Fig 2.
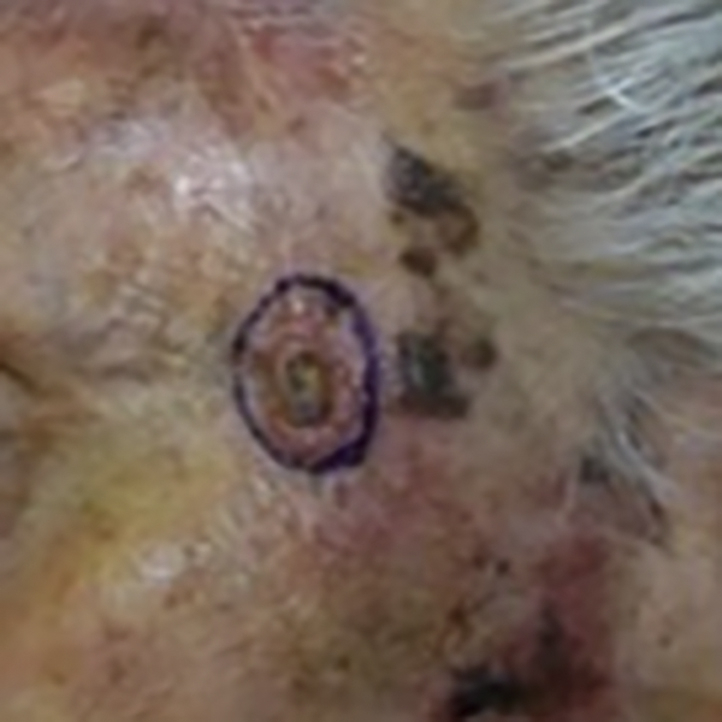
Clinical lesion on left zygoma with biopsy-proven SCC, later found to have concurrent MCC upon excision.
Fig 3.
Microscopic appearance of lesion on left zygoma. Breadloafed sections (original magnification x20, left) display MCC (yellow circles) highlighted by cytokeratin 20 (CK20) immunohistochemistry, adjacent to and intermingled with SCC (blue circles). Higher magnification (original magnification x400, top right) of the area with both components shows SCC with a subtle population of small round cells (area flanked by yellow arrows) that are challenging to identify because of intermingled lymphocytes. Cytokeratin-20 immunohistochemistry (lower right) confirms the presence of MCC.
At the time the left zygoma lesion was excised, a punch biopsy from a new erythematous plaque on the left preauricular area was also diagnosed as MCC, consistent with a local cutaneous metastasis. Staging positron emission tomography/computed tomography was negative. The left preauricular MCC was treated with radiotherapy.
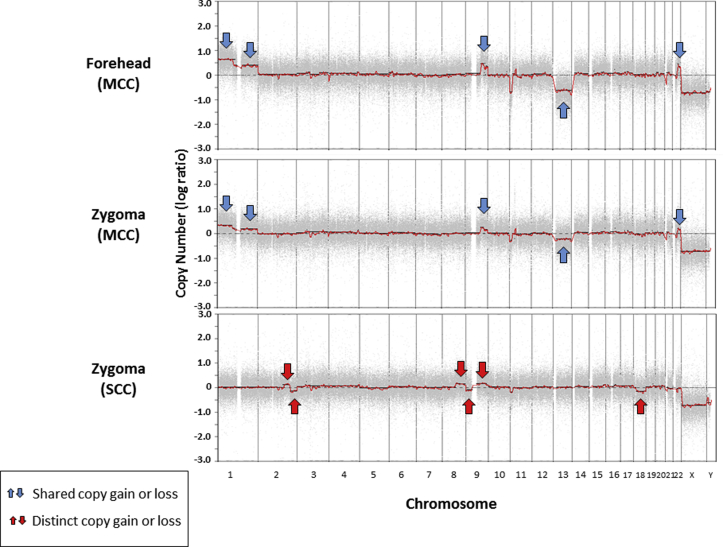
The presence of concurrent SCC with the left zygoma lesion was considered unusual for metastatic MCC and raised the possibility of a second metachronous primary tumor. SNP array analysis found identical chromosomal copy number aberrations between the two MCC tumors (Fig 4). The concurrent SCC on the left zygoma was separately analyzed and demonstrated a distinct pattern of chromosomal copy number aberrations and loss of heterozygosity (Fig 4).
Fig 4.
Chromosomal copy number change by SNP array shows identical gains and losses in the MCC tumors from the forehead and zygoma (blue arrows), and a distinct pattern of gains and losses in the SCC from the zygoma (red arrows).
Discussion
We present a case of multifocal VN-MCC on the face of an elderly man. Several months after the initial diagnosis of MCC on the forehead, additional sites of MCC were identified in the lymphatic draining area, including a focus associated with an SCC. The forehead tumor was clinically considered the primary tumor, in part based on larger size and the absence of MCC in the original biopsy from the zygoma. However, we acknowledge the possibility that the zygoma MCC may be the primary tumor, which was not detected in the initial biopsy because of sampling.
Multiple primary MCC tumors may be challenging to distinguish from cutaneous metastases.1 Microscopic findings are usually not helpful for this distinction, although the presence of concurrent SCC has been proposed to favor primary MCC.1 Different MCPyV status between 2 tumors would support them to be unrelated (metachronous primaries); however, in practice, this may not be a useful test, as patients with multiple primary MCC tumors tend to consistently have either MCPyV-driven or photo damage–associated tumors.4 Molecular studies can distinguish clonally related MCC metastases from independent primary tumors.4, 5, 6, 7, 8 In our case, SNP array found clonal identity between 2 foci of MCC. By contrast, the associated SCC had a distinct, nonoverlapping set of chromosomal copy number changes, compatible with an unrelated neoplasm. Therefore, in our case, concurrent SCC/MCC appears to represent a collision phenomenon rather than divergent differentiation within a single clonal malignancy. We predict this observation would not be applicable to true MCC mixed tumors (ie, tumors displaying diffuse intratumoral squamous metaplasia, rather than a concurrent squamous neoplasm).10, 11
Our case raises several important observations. We build upon previous observations that chromosomal copy number analysis is useful for demonstrating MCC clonality.4, 8 Our findings suggest that, at least in the current case, SCC and MCC may coexist as a result of collision rather than divergent differentiation of a single neoplasm. We propose that concurrent SCC is not a reliable feature for favoring a primary MCC, as collision is possible in a patient with high NMSC burden. Finally, NMSCs in the lymphatic draining area of a previously diagnosed MCC require careful scrutiny for involvement by MCC that may be easily overlooked, especially when obscured by inflammation and reparative changes.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Harms P.W. Update on Merkel cell carcinoma. Clin Lab Med. 2017;37(3):485–501. doi: 10.1016/j.cll.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Feng H., Shuda M., Chang Y., Moore P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schadendorf D., Lebbe C., Zur Hausen A. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53–69. doi: 10.1016/j.ejca.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Harms K.L., Lazo de la Vega L., Hovelson D.H. Molecular profiling of multiple primary Merkel cell carcinoma to distinguish genetically distinct tumors from clonally related metastases. JAMA Dermatol. 2017;153(6):505–512. doi: 10.1001/jamadermatol.2017.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagy J., Feher L.Z., Sonkodi I., Lesznyak J., Ivanyi B., Puskas L.G. A second field metachronous Merkel cell carcinoma of the lip and the palatine tonsil confirmed by microarray-based comparative genomic hybridisation. Virchows Arch. 2005;446(3):278–286. doi: 10.1007/s00428-004-1176-0. [DOI] [PubMed] [Google Scholar]
- 6.Schrama D., Thiemann A., Houben R., Kahler K.C., Becker J.C., Hauschild A. Distinction of 2 different primary Merkel cell carcinomas in 1 patient by Merkel cell polyomavirus genome analysis. Arch Dermatol. 2010;146(6):687–689. doi: 10.1001/archdermatol.2010.121. [DOI] [PubMed] [Google Scholar]
- 7.Ahronowitz I.Z., Daud A.I., Leong S.P. An isolated Merkel cell carcinoma metastasis at a distant cutaneous site presenting as a second 'primary' tumor. J Cutan Pathol. 2011;38(10):801–807. doi: 10.1111/j.1600-0560.2011.01757.x. [DOI] [PubMed] [Google Scholar]
- 8.Eluri M., Feneran A., Bordeaux J.S. Multiple Merkel cell carcinomas: late metastasis or multiple primary tumors? A molecular study. JAAD Case Rep. 2017;3(2):131–134. doi: 10.1016/j.jdcr.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou T.C., Tsai K.B., Wu C.Y., Hong C.H., Lee C.H. Presence of the Merkel cell polyomavirus in Merkel cell carcinoma combined with squamous cell carcinoma in a patient with chronic arsenism. Clin Exp Dermatol. 2016;41(8):902–905. doi: 10.1111/ced.12954. [DOI] [PubMed] [Google Scholar]
- 10.Pulitzer M.P., Brannon A.R., Berger M.F. Cutaneous squamous and neuroendocrine carcinoma: genetically and immunohistochemically different from Merkel cell carcinoma. Mod Pathol. 2015;28(8):1023–1032. doi: 10.1038/modpathol.2015.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter M.D., Gaston D., Huang W.Y. Genetic profiles of different subsets of Merkel cell carcinoma show links between combined and pure MCPyV-negative tumors. Hum Pathol. 2018;71:117–125. doi: 10.1016/j.humpath.2017.10.014. [DOI] [PubMed] [Google Scholar]