Abstract
Lymphocytes have always been among the prime targets in gene therapy, even more so since chimeric antigen receptor (CAR) T cells have reached the clinic. However, other gene therapeutic approaches hold great promise as well. The first part of this review provides an overview of current strategies in lymphocyte gene therapy. The second part highlights the importance of precise gene delivery into B and T cells as well as distinct subtypes of lymphocytes. This can be achieved with lentiviral vectors (LVs) pseudotyped with engineered glycoproteins recognizing lymphocyte surface markers as entry receptors. Different strategies for envelope glycoprotein engineering and selection of the targeting ligand are discussed. With a CD8-targeted LV that was recently used to achieve proof of principle for the in vivo reprogramming of CAR T cells, these vectors are becoming a key tool to genetically engineer lymphocytes directly in vivo.
Keywords: lymphocyte, gene therapy, cancer immunotherapy, entry targeting, lentiviral vector, in vivo gene transfer, CAR T cell
Main Text
Lymphocytes in Gene Therapy
Gene therapy looks back to a history of around 30 years. Since its early days, cells of the hematopoietic system, including lymphocytes, have been among the prime targets of research and clinical applications. In fact, the first clinical trial was performed in adenosine deaminase (ADA) deficiency-mediated severe combined immunodeficiency (ADA-SCID) patients by transferring an intact ADA gene copy into the patients’ T lymphocytes by an ex vivo gene delivery approach using γ-retroviral vectors.1 Although cure of this and other inherited immunodeficiencies in the end turned out to require gene delivery into hematopoietic stem cells (HSCs), B and T lymphocytes have remained in the focus. With two chimeric antigen receptor (CAR) T cell products having achieved marketing authorization, genetically modified T cells are major contributors to the success story of cancer immunotherapy.2 However, many other promising approaches for engineering of both, T and B cells, have been developed to date, which are summarized below as well.
T Lymphocytes
Equipping T cells with recombinant receptors recognizing antigens on diseased cells, be it CARs or T cell receptors (TCRs), represents one of the most innovative and successful strategies of T cell engineering for therapeutic purposes to date. Recombinant TCRs have been most extensively studied in the context of cancer, with the first TCR specific for the tumor-associated antigen MART-1 (melanoma-associated antigen recognized by T cells) applied clinically already in 2006.3 Today, many clinical trials have shown that TCR therapy can be beneficial for patients, with promising results obtained for melanoma, synovial cell sarcoma, and myeloma.4, 5, 6 Consequently, new studies are on the way involving both well-characterized and new lead TCRs with novel specificities for the treatment of various cancer types.7, 8
In comparison to response rates achieved with TCR T cell therapy, CAR T cells have been even more successful, as illustrated by the recent approval of the CD19-CAR T cell products Kymriah and Yescarta by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).9, 10 Efforts are being put into improving the CAR technology to increase safety and efficacy, reduce production costs, and make it applicable beyond hematological malignancies. Consequently, the number of clinical trials continues to increase exponentially.11
In addition, TCR and CAR therapies are now being expanded beyond cancer treatment. Regulatory T cells (Treg cells) representing the immunosuppressive arm of the T cell response have also been modified with CARs. For instance, an approach for the treatment of alloreactivity after organ transplantation used CAR Treg cells recognizing the human leukocyte antigen (HLA) A2.12 In autoimmunity, Tregs are equipped with CARs specific for self-antigens,13, 14 in some studies in combination with engineered expression of FOXP3.15 For treatment of antibody-mediated autoimmune disease, the autoantigen has been presented as an extracellular domain of the chimeric receptor, resulting in T cells redirected to anti-autoantigen B cell receptors that eliminated autoreactive B cells.16 Expanding this approach, it was recently shown that Treg cells expressing such an inverse CAR inhibit autoreactive B cells in a mouse model of hemophilia A.17
To increase T cell responses, cytokine or chemokine receptors as well as costimulatory receptors can be introduced. For instance, expression of CX3CR1 in T cells has improved T cell trafficking in preclinical tumor models.18 Likewise, genetic knockout of inhibitory receptors such as programmed death-1 (PD1) can improve T cell functions.19 Moreover, hybrid receptors have been introduced that combine the extracellular domain of an inhibitory receptor with the intracellular part of an activating receptor, thus converting the inhibitory signal into an activating one. Examples include a PD1 extracellular domain fused to a CD28 intracellular domain20 and an interleukin (IL)-4 receptor displaying the IL-7 receptor signaling domain.21, 22 Both approaches have resulted in superior antitumoral activities against large solid tumors established in mouse models over conventional CAR T cells used as controls. Besides, cytokine secretion can be engineered into CAR T cells in order to alter the immune environment within the tumor. Inducible release of IL-18 was recently shown to be superior over IL-12 with respect to safety and efficacy in a mouse model of melanoma.23
Finally, a lot of effort is currently put into gene therapy concepts targeting HIV-infected cells.24, 25 Here, T cells have been modified to express antisense transcripts in an attempt to confer protection from HIV infection.26 Similarly, the modification of T cells with membrane-anchored peptides inhibiting cell-virus fusion as well as TCRs recognizing viral antigens has been explored as a treatment option for HIV patients resistant to antiretroviral therapy.27, 28 Chronically or even latently infected cells are within special focus of ongoing anti-HIV strategies. Here, genome-modifying enzymes, such as transcription activator-like effector nucleases (TALENs) or CRISPR/Cas that precisely cleave the HIV provirus out of the cellular genome, as well as RNAi attacking viral RNA transcripts have appeared as promising novel tools.29 However, efficient delivery into resting T lymphocytes in vivo will be crucial for each of these strategies.
B Lymphocytes
As main players of the humoral immune response, B lymphocytes have been in the focus of genetic modification strategies as well. Best known for their ability to produce antigen-specific immunoglobulins, they can also act as antigen-presenting cells (APCs) to induce specific immune activation or immune tolerance. Given these essential functions, the genetic modification of B cells is of great interest for both, basic research and therapeutic applications. For instance, B lymphocytes were modified to express costimulatory molecules or pro-inflammatory cytokines for the improvement of their antigen-presenting function. Lee and colleagues30 could demonstrate enhanced antigen presentation by B cells that co-expressed the costimulatory ligands OX40L and 4-1BBL and the pro-inflammatory cytokine IL-12p40, which led to the induction of an antigen-specific T cell response in vitro. A similar approach was used to improve immune recognition of B cell malignancies. Introduction of CD80 and granulocyte-macrophage colony-stimulating factor (GM-CSF) into leukemic B cells ex vivo induced an anti-leukemic immune response.31 In autoimmune disease, engineering of antigen presentation by B cells can be used to induce immune tolerance, e.g., by delivering genes encoding antigen-immunoglobulin G (IgG) fusion proteins.32, 33
B cells and plasma cells in particular constitute nature’s protein-producing factories able to secrete large amounts of antibodies. This unique ability can be hijacked by modifying the cells to secrete a monoclonal antibody or another therapeutic protein of choice. Taking advantage of this strategy, B cells were engineered to secrete neutralizing antibodies or antibody derivatives for the treatment of HIV34, 35, 36 and hepatitis C virus (HCV).37 A very interesting recent study by Hung and colleagues38 achieved expression of human factor IX (FIX) and B cell-activating factor in human plasma cells by gene editing. Engineered human primary B cells secreting FIX have also been generated by lentiviral transduction.39 Transplantation of the modified cells into immunodeficient mice resulted in high-level expression of the therapeutic proteins in both studies, thus providing proof of concept for these novel therapeutic options to treat hemophilia.
Technical Challenges When Transducing Lymphocytes
Current approaches for lymphocyte engineering mainly rely on ex vivo gene transfer protocols. Following their isolation from either healthy donors or patients, the cells are activated and subsequently transduced by lentiviral vectors (LVs), the majority of which is pseudotyped with the glycoprotein G of vesicular stomatitis virus (VSV). The modified lymphocytes are then expanded and either used in functional in vitro assays or used for in vivo applications.
While ex vivo modification of B lymphocytes is possible and has been successfully applied as outlined above, it is not an easy task. Stable and efficient transduction of B cells by LVs is very difficult to achieve. Quiescent B cells are restrictive to transduction by conventional VSV G-pseudotyped LVs (VSV-LVs) due to a lack of low-density lipoprotein receptor (LDLR) expression,40 and, thus, they have to be activated prior to transduction. Efficient activation and culture of primary human B lymphocytes is tedious, as it involves carefully titrated activating stimuli in combination with cytokines followed by co-cultivation with feeder cells.41 Even under optimal activation and culture conditions, transduction efficiencies with VSV-LVs are notoriously low. The combination of all these difficulties may well serve as an explanation for the much lower number of clinical trials involving engineered B cells as compared to T cells.
In comparison, T lymphocyte manipulation by lentiviral transduction is easier to achieve. Still, the cells have to be activated prior to transduction with conventional VSV-LV, because, like B cells, they are otherwise not susceptible for transduction, again due to a lack of LDLR expression.40 Mainly due to the paramount success of CAR T cell therapy, the protocols for T cell isolation, activation, lentiviral transduction, and expansion have been extensively improved in recent years. Current state-of-the-art T cell activation relies on stimulation of the TCR activation pathway via CD3- and CD28-specific antibodies in combination with cytokines such as IL-7 and IL-15.42, 43
The need for activation of lymphocytes prior to transduction with conventional LVs harbors some disadvantages. First, it adds to the complexity of the overall procedure, involving additional steps before transduction of the cells can be carried out. This increases duration and costs of the manufacturing process. Second, the stimuli applied for activation in combination with the prolonged ex vivo culture likely change the cells, which can negatively impact on the quality of the final product. As a result, naive cells could differentiate into less preferential phenotypes that exhibit a higher degree of exhaustion, lower proliferative capacity, shorter in vivo persistence, and less functionality. This can have very important implications for therapeutic success. For instance, it has been shown that a central memory (CD45RO+/CD45RA+/CD62L+) or stem cell memory (CD45RO+/CD45RA−/CD62L+)44 phenotype is beneficial for T cell persistence and function in vivo.45 In this regard, a positive correlation of a CAR T cell central memory phenotype and a positive clinical response has been observed in several clinical studies,42, 46, 47 and, consequently, the infusion of purified central memory CAR T cells is now being investigated as well.48 Likewise, a central memory phenotype leads to functionally superior TCR-modified T cells.49 Minimal manipulation of lymphocytes during genetic modification is thus of tremendous clinical relevance.
Substantial progress toward transducing resting lymphocytes has been achieved by replacing the VSV G by envelopes of viruses that use a receptor expressed on quiescent cells for cell entry. Examples include the glycoproteins of measles virus (MV)50, 51, 52 or the envelope protein of Baboon endogenous retrovirus (BaEV),39, 53 which have been successfully used for the ex vivo modification of fully resting or minimally stimulated lymphocytes. The BaEV-pseudotyped LV was initially found to transduce mildly stimulated human HSCs at very high efficiency, thereby clearly outperforming VSV-LV.53 Subsequently, its clinical relevance was demonstrated by a study using the vector to deliver FIX into in vivo-differentiated human plasma B cells.39 Following transduction by BaEV-LVFIX in vitro, functional secretion of FIX by the transduced plasma cells was observed. Importantly, upon transplantation into immunodeficient hosts, function and long-term secretion of FIX was achieved, demonstrating engraftment of the transduced cells and the stability of gene transfer in vivo.
Transduction of quiescent cells of the hematopoietic system has also been achieved with particular receptor-targeted LVs (see below). Their advantage over the use of heterologous envelope proteins lies in the option to freely choose the entry receptor and, thus, control the cell type-specific gene transfer. Strategies for the required engineering process as well as the use of receptor-targeted LVs for ex vivo and in vivo applications are described in the following sections.
Strategies for LV Engineering
Transferring the gene of interest to the relevant cell type in a timely and spatially specific manner has been one of the most challenging tasks in gene delivery. Current applications in gene therapy have translated LVs into the clinic that mediate efficient gene delivery and, equally important, allow large-scale good manufacturing practice (GMP)-compliant production. That these LVs exert a broad tropism and deliver genes also into therapy-irrelevant cells have not been of concern so far, since gene delivery was performed ex vivo and the therapeutic genes were not harmful when delivered to therapy-irrelevant cells.
While this approach has been successful for the treatment of many fatal genetic diseases, it also has its limitations, especially when it comes to in vivo gene delivery. Accordingly, different strategies restricting gene expression to cell types of choice have been established, including, for example, cell type-specific promoters and microRNA (miRNA) target sequences.54 These approaches combine selective gene expression with the GMP-compatible VSV glycoprotein, and, therefore, may have straightforward access to the clinic. However, promoter targeting is not available for every therapeutically relevant cell type. This holds true also for lymphocytes and their subsets, despite an initial report on a CD4 gene-derived minimal promoter.55 In contrast to promoter targeting, entry-targeting strategies for LVs are readily available for any desired cell type. Importantly, entry targeting enables lymphocyte-restricted lentiviral gene transfer without the need for lymphocyte-specific promoters. For example, targeting ligands that bind to T cell surface markers with high affinity can be displayed on the LV particles. This way, gene delivery and subsequent transgene expression are restricted to the respective T cell subtype, even when controlled by strong and ubiquitous promoters.56, 57 Notably, this approach can simultaneously influence the biodistribution of vector particles by preventing their loss to therapy-irrelevant cells. Moreover, receptor targeting can be beneficial for cell physiology via cell surface receptor contact, thus, e.g., enabling the activation of lymphocytes.58
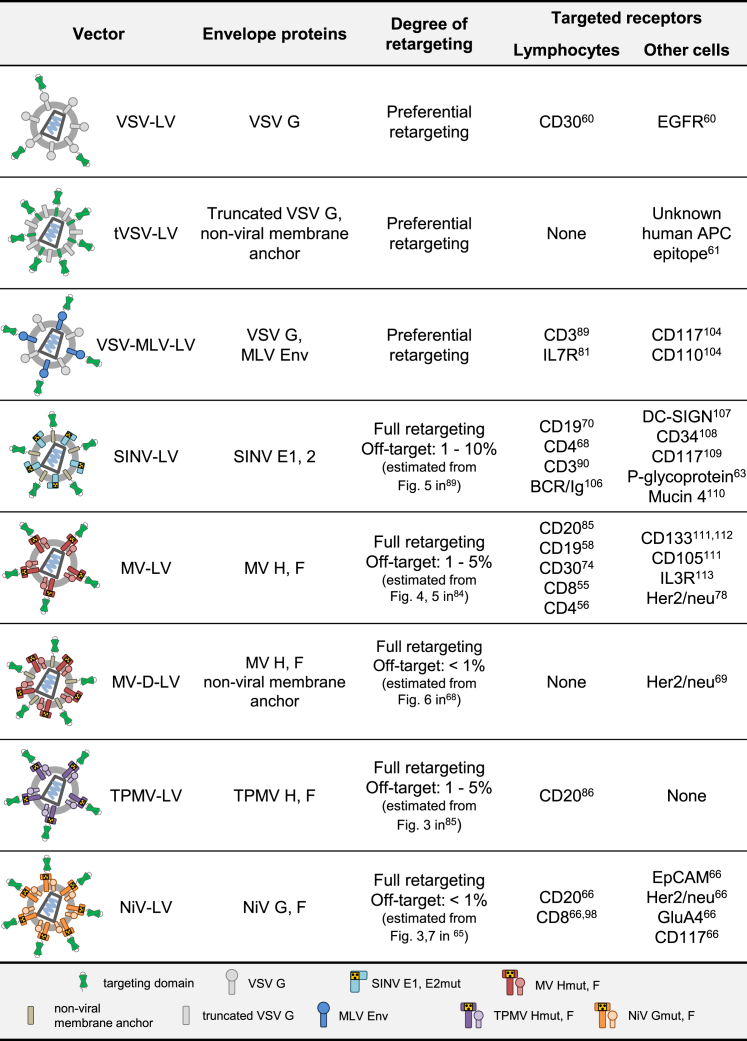
How does entry targeting work? There are essentially three components: First, a targeting ligand exhibiting high affinity for the cell surface receptor of choice has to be displayed on the LV particle surface. This by its own, if properly done, mediates binding of the particles to the cell type of interest, but not entry and transduction.59 Second, viral glycoproteins mediating cell entry must be present. If these are unmodified in their natural receptor recognition, cells expressing the natural entry receptor will be transduced as well. This results in the transduction of target receptor-negative but natural receptor-positive cells. For instance, co-display of unmodified glycoproteins like VSV G with a targeting ligand resulted in preferential transduction of target receptor-positive cells,60 an effect likely depending on the relative binding affinities for the target receptor and the natural entry receptor. Yet, these vectors require expression of the VSV G receptor LDLR for entry. Consequently, they are not only capable of transducing non-relevant LDLR+ cells but also incompatible with resting LDLR− lymphocytes (Figure 1). Thus, while this approach can be helpful for improving the transduction efficiency of rare cell types, it does not confer true cell type-specific gene delivery to these cells. The latter spares delivery to non-relevant cells and tissues and requires a third engineering step, namely the destruction of natural receptor binding. This task has not yet been achieved for any viral glycoprotein having receptor binding and membrane fusion combined in one polypeptide, as it is the case for VSV G or the retroviral envelope proteins. In this regard, an approach incorporating a truncated VSV G along with a membrane-anchored targeting ligand into LV particles61 (Figure 1) yielded promising first results, but it was later shown to mediate the transduction of non-target cells.62
Figure 1.
Overview of Lymphocyte-Retargeted LVs and the Respective Target Receptors
References refer to the initial description of the listed examples.55, 56, 58, 60, 61, 63, 65, 66, 68, 69, 70, 74, 78, 81, 84, 85, 86, 89, 90, 98, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113
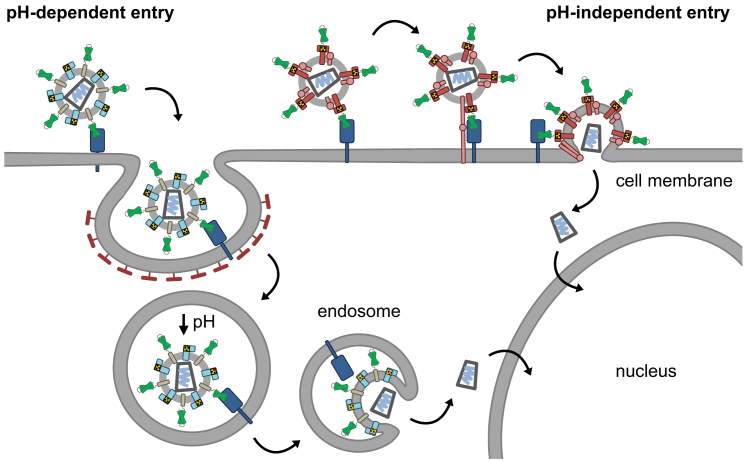
Destroying natural receptor binding while preserving membrane fusion activity has been accomplished for viral glycoprotein complexes in which the receptor attachment and membrane fusion functions are split on two polypeptides (Figure 1). In particular, this refers to the alphaviral glycoprotein complex E of Sindbis virus (SINV)63 and the glycoproteins of paramyxoviruses using proteins (not sugar residues) as natural entry receptor. The latter group encompasses MV,64 Tupaia paramyxovirus (TPMV),65 and Nipah virus (NiV).66 Notably, alphaviruses and paramyxoviruses use different entry modes (Figure 2). While both rely on membrane fusion, alphaviral glycoproteins, similar to VSV G, become fusogenic through the low pH encountered in endosomes. This trigger for conformational changes in the SINV E1 protein is essential for membrane fusion and, thus, cell entry.67 Accordingly, LV particles pseudotyped with these proteins exhibit the same entry mechanism. The paramyxovirus glycoproteins, in contrast, mediate cell entry directly at the cell membrane in a pH-independent manner. Here, receptor contact is the trigger for fusion activation.
Figure 2.
Cell Entry Routes of Receptor-Targeted LVs
Left: targeted (or untargeted) LVs pseudotyped with the glycoproteins of SINV or VSV exhibit a pH-dependent entry mechanism. Following receptor attachment, LV particles are taken up by the target cell via endocytosis. Endosomal acidification and the resulting pH drop lead to conformational changes in the glycoproteins and subsequent fusion with the endosomal membrane, enabling the capsid core containing the viral RNA to enter the cytoplasm and translocate to the nucleus. Right: in contrast, paramyxoviral receptor-targeted LVs use a pH-independent entry mechanism and enter the cell directly at the plasma membrane. By receptor binding, conformational changes result in activation of the fusion protein F, which mediates active fusion with the host cell membrane and release of the capsid core into the cytoplasm, from where it translocates to the nucleus.
This fundamental difference in entry has direct consequences for LV particles pseudotyped with these glycoproteins. VSV-LVs or LVs pseudotyped with targeted SINV glycoproteins require endocytosis of the targeted receptors for entry, and they are substantially reduced in the gene delivery efficiency when endocytosis is blocked. This was nicely illustrated when SINV-LV was targeted against the T lymphocyte marker CD4, which is endocytosed via the clathrin pathway.68 In sharp contrast, blocking of endocytosis in cells incubated with MV-LVs targeted to Her2/neu, a tumor surface antigen undergoing frequent endocytosis, resulted in substantially enhanced transduction, while LV particles pseudotyped with MV glycoproteins using the natural receptor CD46 were unaffected under these conditions.69 This shows that receptor-targeted LVs based on alphaviral and paramyxoviral glycoproteins have basically different demands on the properties of their target receptors. While for alphaviral LVs endocytosis of the target receptor is important, paramyxoviral LVs can cope equally well with target receptors undergoing no, occasional, or frequent endocytosis. In the latter case, transduction can be enhanced by blocking endocytosis, thereby prolonging the presence of the LV particles at the cell membrane.
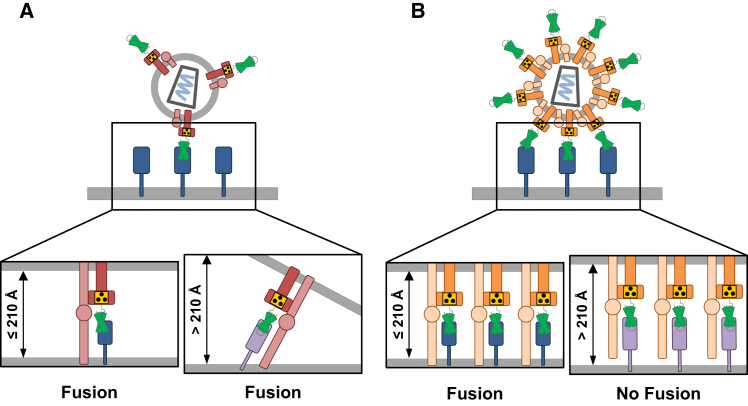
Although LVs pseudotyped with receptor-targeted NiV or MV glycoproteins rely on pH-independent entry at the cell membrane, they yet differ in their target receptor requirements. Interestingly, NiV-LVs appear to have a very precise requirement for a maximal distance of the target receptor binding site from the cell membrane, which may not be exceeded for efficient gene delivery to occur. While this distance hypothesis fits well with the architecture of the fusion protein in its fusion active state, it was surprising to observe this effect only for NiV-LVs, but not MV-LVs.66 Based on the very similar dimensions of their fusion proteins, both LV particle types should actually have similar distance requirements. Yet, MV-LV entry proved to be distance independent despite this similarity. What differs between these particles is glycoprotein density: NiV-LV particles contain approximately four times more glycoprotein than MV-LVs.66 It is, therefore, likely that NiV-LV particles form multiple receptor contacts, and, thus a more rigid complex with target cells than MV-LVs. The latter contact fewer receptors, and, therefore, they may still have some flexibility to compensate for a binding site that exceeds the maximal distance from the cell membrane (Figure 3). Selecting ligands that bind to membrane-proximal domains on the target receptor is thus an important aspect to be taken into account when designing novel NiV-LVs. Since these are usually not neutralized by human serum, they are preferable over MV-LVs when it comes to in vivo gene delivery.
Figure 3.
Working Model on the Different Consequences of Cell Entry upon Membrane-Distal Attachment of Receptor-Targeted NiV-LVs and MV-LVs
(A) MV-LV membrane fusion is independent of the distance of the targeting ligand’s binding site at the entry receptor from the host cell membrane. Because of the low glycoprotein density on the vector particle surface, MV-LVs bind to only a few target receptors. This leaves them more flexibility for positioning at the optimal distance to the cell surface that is required for the fusion peptide to become inserted into the cell membrane. Thus, gene delivery by MV-LVs is less influenced by the relative distance of the binding site on the target receptor from the membrane. (B) In contrast, NiV-LVs require target receptor binding close to the cell membrane for productive fusion. Due to the high glycoprotein density on the particle surface, NiV-LVs make multiple receptor contacts, which results in a rigid position of the particle-target receptor complex and a fixed distance from the target cell membrane. If this distance exceeds 210 Å, which is the distance covered by fusion-active F protein,66 insertion of the fusion peptide of the F protein into the cell membrane and subsequent fusion cannot take place.
Targeting Ligands
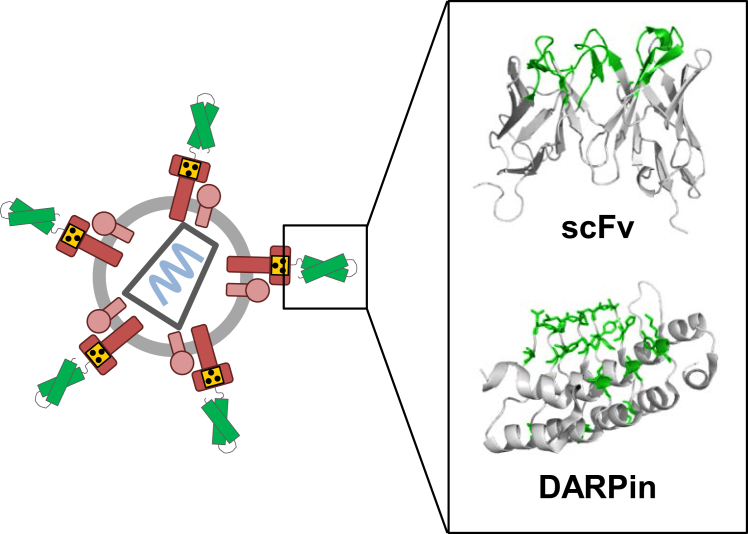
A critical component of receptor-targeted LVs is the targeting ligand, since it has to mediate highly affine and selective binding to the target receptor of choice. Most often, recombinant antibody molecules have been used for this purpose. In the context of the SINV E glycoproteins, complete Ig molecules have been incorporated into the LV envelope membrane.70 Alternatively, single-chain variable fragments (scFvs) are displayed on the glycoprotein either as genetic fusion or via bridging proteins71, 72 (Figure 4). The scFvs are composed of the antigen-binding domains derived from the heavy (VH) and the light (VL) chains of the IgG molecule and connected via an artificial linker domain.73 Accordingly, scFvs can be assembled at the genetic level from available or cloned sequences of monoclonal antibodies, or they are directly selected from phage display libraries.74
Figure 4.
Targeting Ligands
A schematic drawing of a receptor-targeted MV-LV (left) is shown. The engineered envelope proteins of MV are shown in red, the point mutations making H blind for its natural receptors are indicated by the blind symbol, and the targeting ligand is depicted in green. The inlet (right) shows the 3D structures of the two most frequently used targeting ligand scaffolds, scFv and the DARPin, in ribbon diagrams. The respective binding interfaces are highlighted in green. Contact residues of the DARPin are shown in stick representation in addition. 3D structures of scFv (PDB: 2GKI) and DARPin (PDB: 1MJ0) were generated using PyMol.
An inherent problem with scFvs is their tendency to aggregate by the formation of intermolecular VH-VL pairing. This instability varies from scFv to scFv and can be differentially pronounced when fused to the envelope glycoprotein and displayed on the surface of LV particles. In this setting, an unstable scFv can lead to aggregated vector particles resulting in low titers, or even the complete absence of the corresponding fusion protein due to a lack of cell surface expression in producer cells.75 The latter is an indispensable requirement for proper incorporation and surface display of the scFv on the LV particle. Cell surface expression upon transfection of the corresponding expression plasmid should, therefore, always be the first test when a new type of receptor-targeted LV is being generated.
From our experience, roughly half of the generated fusion proteins, consisting of MV hemagglutinin (H) and the candidate scFv, were properly expressed at the cell surface, and then gave rise to active receptor-targeted LVs. While the generation of an LV targeted with a new ligand is basically a trial-and-error type of process, Friedel et al.75 demonstrated that it is possible to convert a non-functional CD30-specific scFv into a scFv that mediates selective gene transfer into CD30-positive cells. This was achieved by introducing mutations in the framework regions of the scFv, while leaving the complementarity-determining regions (CDRs) untouched. The mutations had been identified by aligning the sequences of the VH and VL chains against sequences of antibodies of matching germlines. By systematically assessing the contribution of each amino acid mutation, the authors nicely demonstrated the positive correlation between physical properties of the scFv, such as thermostability and low tendency to aggregate, with the gene delivery activity of the corresponding CD30-LV. Although this approach can, in principle, be transferred to any scFv, it must be stressed that the molecular basis for the instability of scFvs is only partly understood. Such predictions from sequence analysis may, therefore, not always be successful.
Besides scFvs, other formats of recombinant antibodies, such as nanobodies, have been applied for LV receptor targeting.76 Since nanobodies are composed of a single antigen-binding domain, they are more stable than scFvs, and may, therefore, be better suited for LV targeting. However, there is by now more experience with another type of targeting ligand, which is the designed ankyrin repeat protein (DARPin)77 (Figure 4). Derived from cellular ankyrin proteins, these repeat proteins assemble as two or three α-helical repeat structures (33 amino acids each) and N- and C-terminal capping domains. Residues in the loop regions of each repeat form the binding surface. Notably, DARPins fold with rapid kinetics and are much more stable than scFvs. They were found to be compatible not only with the display on LV particles but also on the capsid of adeno-associated virus (AAV) vector particles, which do not tolerate VP2 capsid protein fusions with scFvs.78, 79
DARPins are selected from synthetic libraries covering more than 1012 variants. For selection, ribosomal display is applied, which is completely cell free and hence compatible with the screening of such large repertoires. Recently, the DARPin screening process and a novel DARPin library were adapted to the specific requirements of viral vectors.80 In this process, the extracellular part of the target receptor of choice is expressed in 293T cells and then provided as purified bait for selection. By choosing particular domains of the receptor, it is possible to control the exact binding site of the LV particle on the target receptor. Moreover, affinity maturation steps to reduce the off-rate of the DARPin receptor complex as well as counter-selections using related receptor proteins can be integrated. This way, the generation of LVs binding to such related receptors, and in consequence exhibiting reduced selectivity, can be prevented. An impressive example is the identification of a DARPin that can be used to target the glutamate receptor subunit 4 (GluA4), without recognition of the closely related GluA1–3 receptors.80
Besides synthetic binding domains, also natural ligands such as cytokines have been applied. These have initially been used to overcome the block in gene delivery in resting lymphocytes observed with γ-retroviral vectors or VSV-LV. Accordingly, cytokines such as IL-7 were displayed on the particle surface to activate lymphocytes. IL-7 is an important cytokine for lymphocyte development and T cell homeostasis. Its receptor (IL7R) is expressed on precursor cells of both lymphocyte lineages as well as adult T cells. To preferentially target lymphocytes and simultaneously apply an activating stimulus to the cells, Verhoeyen and coworkers81 fused the full-length human IL-7 to the murine leukemia virus (MLV) Env glycoprotein and co-incorporated this chimera along with VSV G into LV particles. The resulting vector transduced quiescent T lymphocytes without affecting their naive phenotypes, but it still induced minimal activation of the cells, as judged by CD25 and CD71 upregulation. Remarkably, IL7-MLV/VSV-LV retained the ability of IL-7 to promote T cell survival, as the cells could be cultured without the addition of cytokines after transduction. While this example nicely illustrates that surface engineering can influence the physiology of the targeted cell, these vector particles are not selective for T or B cells, because MLV Env and VSV G are still functional, and consequently, any cell expressing the natural receptors of MLV or VSV can be transduced. The vector particles are, thus, rather preferentially targeted to lymphocytes, which limits their application to ex vivo approaches. However, owing to their capability to transduce quiescent cells, they are a promising tool for the ex vivo modification of lymphocytes.
B Lymphocyte-Targeted LVs
For the generation of truly cell type-specific LVs, the targeted receptor should ideally be exclusively expressed on the target cell population. There are two candidates that make an excellent pick for B lymphocyte targeting due to their expression in cells of the B cell lineage, but not in any other cell type (Table 1). The first is the cell surface marker CD20, a protein expressed on B cells in all stages of development with the exception of early pro- and pre-B cells, plasma blasts, and plasma cells.82 The second candidate marker for B cell targeting is CD19, which is present on all cells of the B lymphocyte lineage except terminally differentiated plasma cells.83 Both proteins are thought to play a role in B cell activation.83, 84
Table 1.
Lymphocyte Receptors Targeted by LVs
| IL7R | CD30 | CD19 | CD20 | CD3 | CD4 | CD8 | ||
|---|---|---|---|---|---|---|---|---|
| Expressed on | B cells | yes | yesa | yes | yesb | – | – | – |
| T cells | yes | yesa | – | – | yes | yes | yes | |
| others | – | – | – | – | – | yesd | yese | |
| Targeted by display of | MLV/VSV-LV | IL-7c | – | – | scFv | – | – | |
| SINV-LV | – | – | – | mAB | mAB | – | – | |
| MV-LV | – | scFv | scFv | scFvc | – | DARPinc | scFv | |
| TPMV-LV | – | – | – | scFvc | – | – | – | |
| NiV-LV | – | – | – | scFv | – | – | scFv |
Expressed on activated lymphocytes only.
Not expressed on early pro-B cells and plasma cells.
Transduction of resting and/or minimally activated cells.
Monocyte-derived cells.
Dendritic cells and NK cell subsets.
The first truly B lymphocyte-specific LV was generated using SINV glycoproteins, and it was targeted to human B cells via CD20.70 To generate CD20-SINV-LV, a complete CD20-specific monoclonal antibody was co-incorporated into the lentiviral particles. Indeed, this vector was able to exclusively transduce activated human CD20+ B cells both in vitro and in vivo in a human peripheral blood mononuclear cell (PBMC) xenograft model. However, although the vector could readily transduce fully activated primary B cells, evidence on transduction of the resting B cells is so far lacking.
Another promising candidate for targeted gene delivery into human B cells was developed by Funke and colleagues85 using the modified MV glycoproteins for retargeting. Display of a CD20-specific scFv on the vector surface enabled selective long-term transduction of CD20+ cell lines and primary activated B cells in vitro. Most importantly, CD20-MV-LV also transduced unstimulated, fully resting primary human B cells. Engagement of CD20-MV-LV with the CD20 receptor induced minimal stimulation of the resting B cells, which resulted in upregulation of the activation marker CD71 on the cell surface and their transition from the G0 phase into the G1b phase of the cell cycle.58 To expand the panel of CD20-targeted LVs, TPMV-LVs were also retargeted using the same CD20-specific scFv.86 Remarkably, CD20-TPMV-LV transduced both activated and quiescent human primary B lymphocytes even more efficiently than CD20-MV-LV. Further improvement in the production of CD20-targeted LVs was achieved with the NiV glycoproteins, which resulted in an up-to-three log increase in titers compared to CD20-MV-LV.66 It will be interesting to test if these vector stocks allow the in vivo genetic modification of B cells upon systemic injection in humanized mouse models.
Important progress toward clinical translation of the CD20-targeted MV-LV was recently achieved by Wang and colleagues.87 In this interesting study, B cells were isolated from human CD20-transgenic BALB/c mice and used as target cells for genetic modification by CD20-MV-LV to induce tolerance in a mouse model of hemophilia B. Astonishingly, the authors could show that CD20-MV-LV successfully transduced CD20+ mouse B cells, but not CD20− cells, at similar efficiency as human primary B cells even without prior activation. To evaluate the clinical potential, CD20-transgenic mouse B cells were ex vivo transduced with CD20-MV-LV encoding human FIX, followed by adoptive transfer into recipient mice. Remarkably, treatment with FIX-secreting B cells protected the animals from FIX inhibitor formation following challenge with human FIX, indicating that the treatment had successfully induced tolerance.
To target B cells in all developmental stages, CD19-MV-LV was generated by displaying a CD19-specific scFv.58 CD19-MV-LV was shown to mediate efficient and exclusive transduction of CD19+ cells in mixed cultures even if only 1% of the total cell population was positive for CD19. Like CD20-MV-LV and CD20-TPMV-LV, CD19-MV-LV stably transduced both fully activated and resting primary human CD19+ B cells, albeit with slightly lower efficiency. Transduced quiescent B cells transiently upregulated the activation markers CD69 and CD71 and transitioned from G0 to G1b phase, indicating minimal activation. Although the exact mechanism still remains to be explored, crosslinking of the target receptor by LV particles displaying multiple scFv molecules resulting in signaling is the most likely scenario.88
T Lymphocyte-Targeted LVs
To achieve T cell-specific gene transfer, several cell surface receptors may be targeted (Table 1). An obvious candidate receptor for vector retargeting toward T lymphocytes is the T cell-exclusive marker CD3, which is part of the TCR-CD3 receptor complex expressed on all T cell subsets, but not on other cells. Indeed, fusion of an OKT3-derived scFv specific for the CD3ε chain to the MLV glycoprotein led to a preferential transduction of T lymphocytes in vitro.89 Moreover, the vector was able to efficiently transduce quiescent T cells, which was accompanied by upregulation of activation markers and entry into the G1b phase of the cell cycle. This indicates that CD3-MLV/VSV-LV partially retained the activating property of the parental antibody OKT3. Due to the presence of the wild-type VSV G in the vector particles, however, its use is rather restricted to in vitro applications.
Targeting of CD3 was also achieved by co-incorporation of a full-length, membrane-anchored OKT3 antibody along with the modified SINV glycoproteins into LV particles.90 The resulting CD3-SINV-LV mediated selective transduction of CD3+ Jurkat cells and activated primary human T lymphocytes in vitro, albeit at very low percentages. Transduction by CD3-SBV-LV decreased following treatment of the cells with ammonium chloride, confirming that CD3-SBV-LV cell entry is dependent on endocytosis of the targeted receptor. While proof of concept could be obtained in this study, gene transfer efficiency into primary human T cells with CD3-SBV-LV was very low, with only 5% of T cells transduced at day 4 post-transduction, which limits its use in therapeutic applications.
More promising data were generated with LVs equipped with modified paramyxovirus glycoproteins. To date, three highly specific and efficient LVs were described, two of which are targeted to CD8 on human cytotoxic T cells,56, 66 while the third recognizes human CD4 as entry receptor.57 The latter is especially interesting, as it is displaying a CD4-specific DARPin as targeting domain. As a result, CD4-MV-LV is exquisitely specific for human CD4+ T cells and can be produced at titers sufficient for in vivo applications.57 Moreover, it mediates efficient transduction of quiescent and minimally stimulated primary T lymphocytes. A very interesting observation was the induction of a temporary CD4 downregulation following transduction by CD4-MV-LV. As CD4 is linked to T cell activation pathways, its crosslinking with the vector particles could have triggered cell signaling and, thus, may explain the ability of CD4-MV-LV to transduce unstimulated cells.
The therapeutic potential of CD4-MV-LV was underlined by its ability to specifically modify CD4+ T cells with the HIV viral entry inhibitor maC46 in vitro, protecting them from infection with HIV. Furthermore, CD4-MV-LV was able to introduce an ErbB2-specific CAR selectively into over 90% of all CD4+ cells present in cultured human PBMCs. The generated CAR T cells were functionally active, as demonstrated by specific lysis of ErbB2+ tumor cells and secretion of the pro-inflammatory cytokines interferon γ (IFNγ) and tumor necrosis factor α (TNF-α) following antigen exposure.57
Highly encouraging results were also obtained with the CD8-targeted LV CD8-MV-LV, which is not only highly selective for CD8+ cytotoxic T cells present in human PBMCs but also, like CD4-MV-LV, transduces T lymphocytes that are minimally stimulated by IL-7.56 Additionally, TCR T cells generated with CD8-MV-LV lysed tumor cells more efficiently than conventionally generated CD8+ TCR T cells. Enhanced killing went along with a higher CD8 density on the transduced cells, a higher degree of activation, and elevated expression of cytolytic effector molecules. This could be attributed to the particular properties of the displayed scFv derived from the antibody OKT8, which has been shown to induce cytotoxic effector functions following CD8 binding.91 Exchanging the MV glycoproteins against those of NiV recently enabled the increase of vector titers to 108 TU/mL,66 an important step toward translating this promising vector type into the clinic.
One concern still to be addressed is the expression of CD4 and CD8 on other cell types. CD4 is also expressed on myeloid cells, such as dendritic cells (DCs), monocytes, and macrophages,92 while the homodimeric CD8αα isoform of CD8 is found on subsets of DCs93 and natural killer (NK) cells.94 However, since these cell types represent cell populations that are hard to transduce even when appropriately cultivated and activated ex vivo, transduction after systemic vector administration may be unlikely. Yet, the targeted vectors have to be carefully assessed for transduction of these and other receptor-positive cell types both ex vivo and in vivo.
In Vivo Gene Delivery
An exciting application for lymphocyte-targeted LVs is their systemic administration for in vivo gene delivery, which would make complex ex vivo manipulations of lymphocytes obsolete. Mouse models suitable for the evaluation of LVs targeted to markers on human lymphocytes must be immunodeficient and transplanted with human leukemic tumor cells, primary human PBMCs (PBMC-NOD-scid IL2rγnull [NSG] model), or with human CD34+ hematopoietic stem cells (CD34-NSG model).95 Mice subcutaneously transplanted with human T cell lines such as Jurkat cells can be a first step; however, these cells are much easier to transduce and not distributed in the circulation. A major advantage of the CD34-NSG model is the maturation and education of human thymocytes in the mouse environment, which leads to an induction of tolerance and prevents xenogeneic activation and associated adverse reactions like graft-versus-host disease (GvHD).95 Furthermore, the human T cells in these mice exhibit a resting state While they are thus less susceptible toward transduction by viral vectors, this better reflects the physiological situation. Alternatively, transgenic mice expressing the human target receptor on the relevant lymphocyte subpopulation can be used as, e.g., human CD20 on mouse B lymphocytes.87 Here, however, murine restriction factors can block transduction at post-entry steps, potentially reducing gene delivery efficiency with LVs.96
First attempts to achieve lymphocyte-specific gene transfer in vivo were carried out in PBMC- or Jurkat cell-transplanted mice. To investigate CD20-specific transduction, immunodeficient mice were transplanted by intravenous (i.v.) injection of human PBMCs, followed by almost immediate vector application 6 hr later.70 CD20-restricted transduction of the explanted human cells was observed 2 days later. While the data suggested vector selectivity, the stability of gene transfer was not investigated, and pseudo-transduction, i.e., transfer of GFP as protein, cannot be ruled out. The T cell-targeted CD3-SINV-LV was evaluated in a subcutaneous Jurkat cell xenograft mouse model.90 In this experiment, CD3-SINV-LV carrying luciferase and GFP as transgenes seemed to preferentially transduce CD3+ Jurkat cells, as judged by bioluminescence imaging several days post-injection. However, GFP expression in explanted cells was not evaluated, which limits conclusions about the selectivity of gene transfer.
Selectivity for lymphocytes was impressively demonstrated with paramyxoviral glycoprotein-based LVs delivering reporter and also therapeutic genes. CD4-MV-LV has been the first of these vectors tested in PBMC-NSG and CD34-NSG mice. Selective transduction of between 1% and 15% of human T cells was found after local intrasplenic or systemic intravenous injection in both mouse models.57 Notably, full activation of the circulating T cells in CD34-NSG mice by i.v. injection of the T cell-activating antibody OKT3, prior to systemic vector injection or minimal stimulation of T cells by injection of the homeostatic cytokine IL-7, did not change the outcome. Remarkably, CD4-restricted GFP expression was observed in both experiments, indicating that minimal stimulation of T cells by IL-7 administration is sufficient for gene delivery by CD4-MV-LV. This is especially important because IL-7 has been demonstrated to be safe and efficient in the stimulation of T cells in clinical applications.97
To evaluate the translational potential of CD4-MV-LV, the vector was used to deliver the therapeutically relevant FoxP3 gene to generate human regulatory T cells in vivo. NSG mice were intraperitoneally (i.p.) transplanted with unstimulated human PBMCs, followed by the administration of CD4-MV-LV delivering FoxP3 and a truncated nerve growth factor receptor (ΔNGFR) as reporter 2 days later. Up to 5% of ΔNGFR+/CD4+ cells could be detected 2 months after CD4-MV-LV was applied, showing that CD4-MV-LV can be used for sustained in vivo T cell reprogramming. Clearly, other applications of this powerful vector lie at hand: delivery of antiviral genes to HIV-infected CD4+ T cells is one example.
Another option of tremendous importance is the in vivo delivery of TCR or CAR genes by T lymphocyte-targeted LVs, possibly circumventing the tedious ex vivo manufacturing of TCR and CAR T cells. Proof of concept for the in vivo generation of functionally active CAR T cells by a single injection of a CD8-targeted LV was recently obtained by Pfeiffer and colleagues.98 In this study, CD8-NiV-LV delivered a CD19-CAR directly and selectively to human cytotoxic CD8+ T cells of PBMC-NSG and CD34-NSG mouse models. In both models, high numbers of CD8+ CAR T cells were present in spleen and blood, while CD8− cells remained devoid of CAR genes. The fraction of CAR+ cells encompassed up to 50% of the CD8+ T cells in some tissues of the PBMC-NSG mice and up to 14% in CD34-NSG mice. The overall expression level of the CAR in the fully humanized model was lower in comparison to the results obtained with PBMC-humanized mice, likely due to the minimal activation and lack of xenogeneic stimulation of the T cells in these animals. Most importantly, the generation of CAR T cells in this model was accompanied by the development of cytokine release syndrome (CRS)-like syndromes in some mice. Abnormal behavior, including apathy and neurological symptoms, went along with elevated inflammatory cytokines, including IL-6, IFNγ, and GM-CSF. Histological examination of various organs showed infiltration of CD8+ T cells, elimination of CD19+ cells from B lymphocyte-rich zones in spleen, as well as infiltration into the brain parenchyma, indicating that the observed syndrome is indeed reminiscent of CRS in human CAR T cell-treated patients.99 Taken together, this study clearly demonstrates the translational potential of T cell-targeted LVs in state-of-the-art immunotherapy.
Conclusions
Lymphocytes will remain in focus of gene therapy strategies as one of the most relevant cell types for many different types of diseases. The substantial progress made in LV surface engineering during the past years now allows the selective genetic engineering of defined subtypes of lymphocytes not only ex vivo, e.g., in cultivated human PBMCs but also in vivo upon systemic injection. Vector specificity was clearly demonstrated in many studies conducted by now.
The list of important target receptors on lymphocytes is by far not yet covered with the available vector types. Markers of less differentiated T cells could be an attractive target to achieve selective gene delivery into naive T cells. Besides, also NK cells that constitute the third group of lymphocytes are currently attracting more and more attention, especially since CAR-NK cells have shown promising clinical results.100 With NKp46, a first marker on these cells was recently targeted.80 Apart from immunotherapy, T cell-targeted LVs may be able to target the small reservoir of HIV latently infected cells.101 Receptor-targeted LVs recognizing this reservoir may have the potential to solve this delivery problem. In this case and potentially also others, however, target cells of interest may not be sufficiently defined by only a single cell surface marker but rather through the combination of two or more markers. It will be an important future task to develop the existing targeting strategies further to cope with this situation. Entering cells via binding to a primary attachment site followed by contacting the actual entry receptor, as it is exemplified by the cell entry of HIV, could be a natural template for this endeavor.
CAR T cell therapy is an attractive straightforward application for these vectors. This refers primarily to the in vivo delivery of CARs, a strategy that could substantially reduce the economic burden that the complex and cost-intensive ex vivo manipulation of T lymphocytes will cause. In this context, it is important to mention that a surface-engineered, fully synthetic nanoparticle targeted against murine CD3 has recently been shown to fulfill this task in a syngeneic mouse model.102 A transposase packaged along with the CAR gene into the interior of the particles mediated stable integration of the CAR gene into dividing T cells. Although this completely new type of gene delivery particle may still be far away from clinical applications, its development illustrates the different strategies followed and, thus, underlines the importance of this field. Besides in vivo CAR delivery, LVs targeted to subtypes of T cells may also improve ex vivo CAR gene delivery, since gene transfer into resting or minimally stimulated lymphocytes may reduce the complexity and cost of the current production process.
It will now be important to collect experience with these vectors in clinical applications. A first study with a surface-engineered LV targeted to DCs has recently been initiated.103 For in vivo delivery, large animal models will be important to be assessed next. This will show if the promising data seen in mouse models can indeed be translated to human patients. With the engineered NiV glycoproteins, production of receptor-targeted LVs has been substantially improved, now coming closer to titers obtained with VSV-LVs.66 Whether this will be sufficient to achieve GMP-compliant production for LVs with surface-engineered envelopes will have to be addressed in future studies toward the clinical translation of these promising new types of vector particles.
Author Contributions
C.J.B. conceived the structure of the article and the figures. A.M.F. prepared the figures and drafted parts of the manuscript comprising lymphocyte gene therapy and B and T cell-targeted as well as in vivo applications. C.J.B. revised the drafts and completed the manuscript. Both authors contributed to proofreading and finalization of text and figures.
Conflicts of Interest
C.J.B. is listed as co-inventor on patents about surface-engineered LVs that have been out-licensed.
Acknowledgments
We thank Dr. Jessica Hartmann for helpful advice with figure design. This work was supported by the LOEWE Center for Cell and Gene Therapy Frankfurt funded by Hessisches Ministerium für Wissenschaft und Kunst (llL5-518/17.004).
References
- 1.Ferrua F., Aiuti A. Twenty-Five Years of Gene Therapy for ADA-SCID: From Bubble Babies to an Approved Drug. Hum. Gene Ther. 2017;28:972–981. doi: 10.1089/hum.2017.175. [DOI] [PubMed] [Google Scholar]
- 2.June C.H., O’Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 3.Morgan R.A., Dudley M.E., Wunderlich J.R., Hughes M.S., Yang J.C., Sherry R.M., Royal R.E., Topalian S.L., Kammula U.S., Restifo N.P. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins P.F., Kassim S.H., Tran T.L.N., Crystal J.S., Morgan R.A., Feldman S.A., Yang J.C., Dudley M.E., Wunderlich J.R., Sherry R.M. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin. Cancer Res. 2015;21:1019–1027. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapoport A.P., Stadtmauer E.A., Binder-Scholl G.K., Goloubeva O., Vogl D.T., Lacey S.F., Badros A.Z., Garfall A., Weiss B., Finklestein J. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 2015;21:914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan R.A., Chinnasamy N., Abate-Daga D., Gros A., Robbins P.F., Zheng Z., Dudley M.E., Feldman S.A., Yang J.C., Sherry R.M. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karpanen T., Olweus J. T-cell receptor gene therapy--ready to go viral? Mol. Oncol. 2015;9:2019–2042. doi: 10.1016/j.molonc.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saudemont A., Jespers L., Clay T. Current Status of Gene Engineering Cell Therapeutics. Front. Immunol. 2018;9:153. doi: 10.3389/fimmu.2018.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartmann J., Schüßler-Lenz M., Bondanza A., Buchholz C.J. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol. Med. 2017;9:1183–1197. doi: 10.15252/emmm.201607485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald K.G., Hoeppli R.E., Huang Q., Gillies J., Luciani D.S., Orban P.C., Broady R., Levings M.K. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J. Clin. Invest. 2016;126:1413–1424. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skuljec J., Chmielewski M., Happle C., Habener A., Busse M., Abken H., Hansen G. Chimeric Antigen Receptor-Redirected Regulatory T Cells Suppress Experimental Allergic Airway Inflammation, a Model of Asthma. Front. Immunol. 2017;8:1125. doi: 10.3389/fimmu.2017.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon J., Schmidt A., Zhang A.-H., Königs C., Kim Y.C., Scott D.W. FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood. 2017;129:238–245. doi: 10.1182/blood-2016-07-727834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fransson M., Piras E., Burman J., Nilsson B., Essand M., Lu B., Harris R.A., Magnusson P.U., Brittebo E., Loskog A.S. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J. Neuroinflammation. 2012;9:112. doi: 10.1186/1742-2094-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellebrecht C.T., Bhoj V.G., Nace A., Choi E.J., Mao X., Cho M.J., Di Zenzo G., Lanzavecchia A., Seykora J.T., Cotsarelis G. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179–184. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang A.-H., Yoon J., Kim Y.C., Scott D.W. Targeting Antigen-Specific B Cells Using Antigen-Expressing Transduced Regulatory T Cells. J. Immunol. 2018;201:1434–1441. doi: 10.4049/jimmunol.1701800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqui I., Erreni M., van Brakel M., Debets R., Allavena P. Enhanced recruitment of genetically modified CX3CR1-positive human T cells into Fractalkine/CX3CL1 expressing tumors: importance of the chemokine gradient. J. Immunother. Cancer. 2016;4:21. doi: 10.1186/s40425-016-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Z., Shi L., Zhang W., Han J., Zhang S., Fu Z., Cai J. CRISPR knock out of programmed cell death protein 1 enhances anti-tumor activity of cytotoxic T lymphocytes. Oncotarget. 2017;9:5208–5215. doi: 10.18632/oncotarget.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X., Ranganathan R., Jiang S., Fang C., Sun J., Kim S., Newick K., Lo A., June C.H., Zhao Y., Moon E.K. A Chimeric Switch-Receptor Targeting PD1 Augments the Efficacy of Second-Generation CAR T Cells in Advanced Solid Tumors. Cancer Res. 2016;76:1578–1590. doi: 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leen A.M., Sukumaran S., Watanabe N., Mohammed S., Keirnan J., Yanagisawa R., Anurathapan U., Rendon D., Heslop H.E., Rooney C.M. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol. Ther. 2014;22:1211–1220. doi: 10.1038/mt.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammed S., Sukumaran S., Bajgain P., Watanabe N., Heslop H.E., Rooney C.M., Brenner M.K., Fisher W.E., Leen A.M., Vera J.F. Improving Chimeric Antigen Receptor-Modified T Cell Function by Reversing the Immunosuppressive Tumor Microenvironment of Pancreatic Cancer. Mol. Ther. 2017;25:249–258. doi: 10.1016/j.ymthe.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunert A., Chmielewski M., Wijers R., Berrevoets C., Abken H., Debets R. Intra-tumoral production of IL18, but not IL12, by TCR-engineered T cells is non-toxic and counteracts immune evasion of solid tumors. OncoImmunology. 2017;7:e1378842. doi: 10.1080/2162402X.2017.1378842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson C.W., Kiem H.-P. Cell and Gene Therapy for HIV Cure. Curr. Top. Microbiol. Immunol. 2018;417:211–248. doi: 10.1007/82_2017_71. [DOI] [PubMed] [Google Scholar]
- 25.Rogers G.L., Cannon P.M. Gene Therapy Approaches to Human Immunodeficiency Virus and Other Infectious Diseases. Hematol. Oncol. Clin. North Am. 2017;31:883–895. doi: 10.1016/j.hoc.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Levine B.L., Humeau L.M., Boyer J., MacGregor R.-R., Rebello T., Lu X., Binder G.K., Slepushkin V., Lemiale F., Mascola J.R. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc. Natl. Acad. Sci. USA. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egelhofer M., Brandenburg G., Martinius H., Schult-Dietrich P., Melikyan G., Kunert R., Baum C., Choi I., Alexandrov A., von Laer D. Inhibition of human immunodeficiency virus type 1 entry in cells expressing gp41-derived peptides. J. Virol. 2004;78:568–575. doi: 10.1128/JVI.78.2.568-575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perica K., Varela J.C., Oelke M., Schneck J. Adoptive T cell immunotherapy for cancer. Rambam Maimonides Med. J. 2015;6:e0004. doi: 10.5041/RMMJ.10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrera-Carrillo E., Berkhout B. Attacking HIV-1 RNA versus DNA by sequence-specific approaches: RNAi versus CRISPR-Cas. Biochem. Soc. Trans. 2016;44:1355–1365. doi: 10.1042/BST20160060. [DOI] [PubMed] [Google Scholar]
- 30.Lee J., Dollins C.M., Boczkowski D., Sullenger B.A., Nair S. Activated B cells modified by electroporation of multiple mRNAs encoding immune stimulatory molecules are comparable to mature dendritic cells in inducing in vitro antigen-specific T-cell responses. Immunology. 2008;125:229–240. doi: 10.1111/j.1365-2567.2008.02833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stripecke R., Cardoso A.A., Pepper K.A., Skelton D.C., Yu X.J., Mascarenhas L., Weinberg K.I., Nadler L.M., Kohn D.B. Lentiviral vectors for efficient delivery of CD80 and granulocyte-macrophage- colony-stimulating factor in human acute lymphoblastic leukemia and acute myeloid leukemia cells to induce antileukemic immune responses. Blood. 2000;96:1317–1326. [PubMed] [Google Scholar]
- 32.Melo M.E.F., Qian J., El-Amine M., Agarwal R.K., Soukhareva N., Kang Y., Scott D.W. Gene transfer of Ig-fusion proteins into B cells prevents and treats autoimmune diseases. J. Immunol. 2002;168:4788–4795. doi: 10.4049/jimmunol.168.9.4788. [DOI] [PubMed] [Google Scholar]
- 33.Wang X., Moghimi B., Zolotukhin I., Morel L.M., Cao O., Herzog R.W. Immune tolerance induction to factor IX through B cell gene transfer: TLR9 signaling delineates between tolerogenic and immunogenic B cells. Mol. Ther. 2014;22:1139–1150. doi: 10.1038/mt.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo X.M., Maarschalk E., O’Connell R.M., Wang P., Yang L., Baltimore D. Engineering human hematopoietic stem/progenitor cells to produce a broadly neutralizing anti-HIV antibody after in vitro maturation to human B lymphocytes. Blood. 2009;113:1422–1431. doi: 10.1182/blood-2008-09-177139. [DOI] [PubMed] [Google Scholar]
- 35.Hur E.M., Patel S.N., Shimizu S., Rao D.S., Gnanapragasam P.N.P., An D.S., Yang L., Baltimore D. Inhibitory effect of HIV-specific neutralizing IgA on mucosal transmission of HIV in humanized mice. Blood. 2012;120:4571–4582. doi: 10.1182/blood-2012-04-422303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joseph A., Zheng J.H., Chen K., Dutta M., Chen C., Stiegler G., Kunert R., Follenzi A., Goldstein H. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. J. Virol. 2010;84:6645–6653. doi: 10.1128/JVI.02339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fusil F., Calattini S., Amirache F., Mancip J., Costa C., Robbins J.B., Douam F., Lavillette D., Law M., Defrance T. A Lentiviral Vector Allowing Physiologically Regulated Membrane-anchored and Secreted Antibody Expression Depending on B-cell Maturation Status. Mol. Ther. 2015;23:1734–1747. doi: 10.1038/mt.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung K.L., Meitlis I., Hale M., Chen C.-Y., Singh S., Jackson S.W., Miao C.H., Khan I.F., Rawlings D.J., James R.G. Engineering Protein-Secreting Plasma Cells by Homology-Directed Repair in Primary Human B Cells. Mol. Ther. 2018;26:456–467. doi: 10.1016/j.ymthe.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy C., Fusil F., Amirache F., Costa C., Girard-Gagnepain A., Negre D., Bernadin O., Garaulet G., Rodriguez A., Nair N. Baboon envelope pseudotyped lentiviral vectors efficiently transduce human B cells and allow active factor IX B cell secretion in vivo in NOD/SCIDγc-/- mice. J. Thromb. Haemost. 2016;14:2478–2492. doi: 10.1111/jth.13520. [DOI] [PubMed] [Google Scholar]
- 40.Amirache F., Lévy C., Costa C., Mangeot P.-E., Torbett B.E., Wang C.X., Nègre D., Cosset F.L., Verhoeyen E. Mystery solved: VSV-G-LVs do not allow efficient gene transfer into unstimulated T cells, B cells, and HSCs because they lack the LDL receptor. Blood. 2014;123:1422–1424. doi: 10.1182/blood-2013-11-540641. [DOI] [PubMed] [Google Scholar]
- 41.Frecha C., Lévy C., Cosset F.-L., Verhoeyen E. Advances in the field of lentivector-based transduction of T and B lymphocytes for gene therapy. Mol. Ther. 2010;18:1748–1757. doi: 10.1038/mt.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Y., Zhang M., Ramos C.A., Durett A., Liu E., Dakhova O., Liu H., Creighton C.J., Gee A.P., Heslop H.E. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123:3750–3759. doi: 10.1182/blood-2014-01-552174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casucci M., Falcone L., Camisa B., Norelli M., Porcellini S., Stornaiuolo A., Ciceri F., Traversari C., Bordignon C., Bonini C., Bondanza A. Extracellular NGFR Spacers Allow Efficient Tracking and Enrichment of Fully Functional CAR-T Cells Co-Expressing a Suicide Gene. Front. Immunol. 2018;9:507. doi: 10.3389/fimmu.2018.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golubovskaya V., Wu L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers (Basel) 2016;8:36. doi: 10.3390/cancers8030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berger C., Jensen M.C., Lansdorp P.M., Gough M., Elliott C., Riddell S.R. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Louis C.U., Savoldo B., Dotti G., Pule M., Yvon E., Myers G.D., Rossig C., Russell H.V., Diouf O., Liu E. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fraietta J.A., Lacey S.F., Orlando E.J., Pruteanu-Malinici I., Gohil M., Lundh S., Boesteanu A.C., Wang Y., O’Connor R.S., Hwang W.T. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018;24:563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X., Popplewell L.L., Wagner J.R., Naranjo A., Blanchard M.S., Mott M.R., Norris A.P., Wong C.W., Urak R.Z., Chang W.C. Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood. 2016;127:2980–2990. doi: 10.1182/blood-2015-12-686725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu F., Zhang W., Shao H., Bo H., Shen H., Li J., Liu Y., Wang T., Ma W., Huang S. Human effector T cells derived from central memory cells rather than CD8(+)T cells modified by tumor-specific TCR gene transfer possess superior traits for adoptive immunotherapy. Cancer Lett. 2013;339:195–207. doi: 10.1016/j.canlet.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Frecha C., Costa C., Nègre D., Gauthier E., Russell S.J., Cosset F.-L., Verhoeyen E. Stable transduction of quiescent T cells without induction of cycle progression by a novel lentiviral vector pseudotyped with measles virus glycoproteins. Blood. 2008;112:4843–4852. doi: 10.1182/blood-2008-05-155945. [DOI] [PubMed] [Google Scholar]
- 51.Frecha C., Lévy C., Costa C., Nègre D., Amirache F., Buckland R., Russell S.J., Cosset F.L., Verhoeyen E. Measles virus glycoprotein-pseudotyped lentiviral vector-mediated gene transfer into quiescent lymphocytes requires binding to both SLAM and CD46 entry receptors. J. Virol. 2011;85:5975–5985. doi: 10.1128/JVI.00324-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Q., Schneider I.C., Gallet M., Kneissl S., Buchholz C.J. Resting lymphocyte transduction with measles virus glycoprotein pseudotyped lentiviral vectors relies on CD46 and SLAM. Virology. 2011;413:149–152. doi: 10.1016/j.virol.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 53.Girard-Gagnepain A., Amirache F., Costa C., Lévy C., Frecha C., Fusil F., Nègre D., Lavillette D., Cosset F.L., Verhoeyen E. Baboon envelope pseudotyped LVs outperform VSV-G-LVs for gene transfer into early-cytokine-stimulated and resting HSCs. Blood. 2014;124:1221–1231. doi: 10.1182/blood-2014-02-558163. [DOI] [PubMed] [Google Scholar]
- 54.Powell S.K., Rivera-Soto R., Gray S.J. Viral expression cassette elements to enhance transgene target specificity and expression in gene therapy. Discov. Med. 2015;19:49–57. [PMC free article] [PubMed] [Google Scholar]
- 55.Marodon G., Mouly E., Blair E.J., Frisen C., Lemoine F.M., Klatzmann D. Specific transgene expression in human and mouse CD4+ cells using lentiviral vectors with regulatory sequences from the CD4 gene. Blood. 2003;101:3416–3423. doi: 10.1182/blood-2002-02-0578. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Q., Schneider I.C., Edes I., Honegger A., Bach P., Schönfeld K., Schambach A., Wels W.S., Kneissl S., Uckert W., Buchholz C.J. T-cell receptor gene transfer exclusively to human CD8(+) cells enhances tumor cell killing. Blood. 2012;120:4334–4342. doi: 10.1182/blood-2012-02-412973. [DOI] [PubMed] [Google Scholar]
- 57.Zhou Q., Uhlig K.M., Muth A., Kimpel J., Lévy C., Münch R.C., Seifried J., Pfeiffer A., Trkola A., Coulibaly C. Exclusive Transduction of Human CD4+ T Cells upon Systemic Delivery of CD4-Targeted Lentiviral Vectors. J. Immunol. 2015;195:2493–2501. doi: 10.4049/jimmunol.1500956. [DOI] [PubMed] [Google Scholar]
- 58.Kneissl S., Zhou Q., Schwenkert M., Cosset F.-L., Verhoeyen E., Buchholz C.J. CD19 and CD20 targeted vectors induce minimal activation of resting B lymphocytes. PLoS ONE. 2013;8:e79047. doi: 10.1371/journal.pone.0079047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cosset F.L., Morling F.J., Takeuchi Y., Weiss R.A., Collins M.K., Russell S.J. Retroviral retargeting by envelopes expressing an N-terminal binding domain. J. Virol. 1995;69:6314–6322. doi: 10.1128/jvi.69.10.6314-6322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Höfig I., Barth S., Salomon M., Jagusch V., Atkinson M.J., Anastasov N., Thirion C. Systematic improvement of lentivirus transduction protocols by antibody fragments fused to VSV-G as envelope glycoprotein. Biomaterials. 2014;35:4204–4212. doi: 10.1016/j.biomaterials.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 61.Goyvaerts C., Dingemans J., De Groeve K., Heirman C., Van Gulck E., Vanham G., De Baetselier P., Thielemans K., Raes G., Breckpot K. Targeting of human antigen-presenting cell subsets. J. Virol. 2013;87:11304–11308. doi: 10.1128/JVI.01498-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goyvaerts C., De Vlaeminck Y., Escors D., Lienenklaus S., Keyaerts M., Raes G., Breckpot K. Antigen-presenting cell-targeted lentiviral vectors do not support the development of productive T-cell effector responses: implications for in vivo targeted vaccine delivery. Gene Ther. 2017;24:370–375. doi: 10.1038/gt.2017.30. [DOI] [PubMed] [Google Scholar]
- 63.Morizono K., Xie Y., Ringpis G.-E., Johnson M., Nassanian H., Lee B., Wu L., Chen I.S. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat. Med. 2005;11:346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- 64.Nakamura T., Peng K.-W., Harvey M., Greiner S., Lorimer I.A., James C.D., Russell S.J. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat. Biotechnol. 2005;23:209–214. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- 65.Springfeld C., von Messling V., Tidona C.A., Darai G., Cattaneo R. Envelope targeting: hemagglutinin attachment specificity rather than fusion protein cleavage-activation restricts Tupaia paramyxovirus tropism. J. Virol. 2005;79:10155–10163. doi: 10.1128/JVI.79.16.10155-10163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bender R.R., Muth A., Schneider I.C., Friedel T., Hartmann J., Plückthun A., Maisner A., Buchholz C.J. Receptor-Targeted Nipah Virus Glycoproteins Improve Cell-Type Selective Gene Delivery and Reveal a Preference for Membrane-Proximal Cell Attachment. PLoS Pathog. 2016;12:e1005641. doi: 10.1371/journal.ppat.1005641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li L., Jose J., Xiang Y., Kuhn R.J., Rossmann M.G. Structural changes of envelope proteins during alphavirus fusion. Nature. 2010;468:705–708. doi: 10.1038/nature09546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liang M., Morizono K., Pariente N., Kamata M., Lee B., Chen I.S. Targeted transduction via CD4 by a lentiviral vector uses a clathrin-mediated entry pathway. J. Virol. 2009;83:13026–13031. doi: 10.1128/JVI.01530-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rasbach A., Abel T., Münch R.C., Boller K., Schneider-Schaulies J., Buchholz C.J. The receptor attachment function of measles virus hemagglutinin can be replaced with an autonomous protein that binds Her2/neu while maintaining its fusion-helper function. J. Virol. 2013;87:6246–6256. doi: 10.1128/JVI.03298-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang L., Bailey L., Baltimore D., Wang P. Targeting lentiviral vectors to specific cell types in vivo. Proc. Natl. Acad. Sci. USA. 2006;103:11479–11484. doi: 10.1073/pnas.0604993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buchholz C.J., Friedel T., Büning H. Surface-Engineered Viral Vectors for Selective and Cell Type-Specific Gene Delivery. Trends Biotechnol. 2015;33:777–790. doi: 10.1016/j.tibtech.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 72.Lévy C., Verhoeyen E., Cosset F.-L. Surface engineering of lentiviral vectors for gene transfer into gene therapy target cells. Curr. Opin. Pharmacol. 2015;24:79–85. doi: 10.1016/j.coph.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Tiller K.E., Tessier P.M. Advances in Antibody Design. Annu. Rev. Biomed. Eng. 2015;17:191–216. doi: 10.1146/annurev-bioeng-071114-040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frenzel A., Schirrmann T., Hust M. Phage display-derived human antibodies in clinical development and therapy. MAbs. 2016;8:1177–1194. doi: 10.1080/19420862.2016.1212149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Friedel T., Hanisch L.J., Muth A., Honegger A., Abken H., Plückthun A., Buchholz C.J., Schneider I.C. Receptor-targeted lentiviral vectors are exceptionally sensitive toward the biophysical properties of the displayed single-chain Fv. Protein Eng. Des. Sel. 2015;28:93–106. doi: 10.1093/protein/gzv005. [DOI] [PubMed] [Google Scholar]
- 76.Goyvaerts C., De Groeve K., Dingemans J., Van Lint S., Robays L., Heirman C., Reiser J., Zhang X.Y., Thielemans K., De Baetselier P. Development of the Nanobody display technology to target lentiviral vectors to antigen-presenting cells. Gene Ther. 2012;19:1133–1140. doi: 10.1038/gt.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plückthun A. Designed ankyrin repeat proteins (DARPins): binding proteins for research, diagnostics, and therapy. Annu. Rev. Pharmacol. Toxicol. 2015;55:489–511. doi: 10.1146/annurev-pharmtox-010611-134654. [DOI] [PubMed] [Google Scholar]
- 78.Münch R.C., Mühlebach M.D., Schaser T., Kneissl S., Jost C., Plückthun A., Cichutek K., Buchholz C.J. DARPins: an efficient targeting domain for lentiviral vectors. Mol. Ther. 2011;19:686–693. doi: 10.1038/mt.2010.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Münch R.C., Janicki H., Völker I., Rasbach A., Hallek M., Büning H., Buchholz C.J. Displaying high-affinity ligands on adeno-associated viral vectors enables tumor cell-specific and safe gene transfer. Mol. Ther. 2013;21:109–118. doi: 10.1038/mt.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hartmann J., Münch R.C., Freiling R.-T., Schneider I.C., Dreier B., Samukange W., Koch J., Seeger M.A., Plückthun A., Buchholz C.J. A Library-Based Screening Strategy for the Identification of DARPins as Ligands for Receptor-Targeted AAV and Lentiviral Vectors. Mol. Ther. Methods Clin. Dev. 2018;10:128–143. doi: 10.1016/j.omtm.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verhoeyen E., Dardalhon V., Ducrey-Rundquist O., Trono D., Taylor N., Cosset F.-L. IL-7 surface-engineered lentiviral vectors promote survival and efficient gene transfer in resting primary T lymphocytes. Blood. 2003;101:2167–2174. doi: 10.1182/blood-2002-07-2224. [DOI] [PubMed] [Google Scholar]
- 82.Leandro M.J. B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies. Arthritis Res. Ther. 2013;15(Suppl 1):S3. doi: 10.1186/ar3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang K., Wei G., Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp. Hematol. Oncol. 2012;1:36. doi: 10.1186/2162-3619-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harwood N.E., Batista F.D. New insights into the early molecular events underlying B cell activation. Immunity. 2008;28:609–619. doi: 10.1016/j.immuni.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 85.Funke S., Maisner A., Mühlebach M.D., Koehl U., Grez M., Cattaneo R., Cichutek K., Buchholz C.J. Targeted cell entry of lentiviral vectors. Mol. Ther. 2008;16:1427–1436. doi: 10.1038/mt.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Enkirch T., Kneissl S., Hoyler B., Ungerechts G., Stremmel W., Buchholz C.J., Springfeld C. Targeted lentiviral vectors pseudotyped with the Tupaia paramyxovirus glycoproteins. Gene Ther. 2013;20:16–23. doi: 10.1038/gt.2011.209. [DOI] [PubMed] [Google Scholar]
- 87.Wang X., Herzog R.W., Byrne B.J., Kumar S.R.P., Zhou Q., Buchholz C.J., Biswas M. Immune Modulatory Cell Therapy for Hemophilia B Based on CD20-Targeted Lentiviral Gene Transfer to Primary B Cells. Mol. Ther. Methods Clin. Dev. 2017;5:76–82. doi: 10.1016/j.omtm.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buchholz C.J., Mühlebach M.D., Cichutek K. Lentiviral vectors with measles virus glycoproteins - dream team for gene transfer? Trends Biotechnol. 2009;27:259–265. doi: 10.1016/j.tibtech.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 89.Maurice M., Verhoeyen E., Salmon P., Trono D., Russell S.J., Cosset F.-L. Efficient gene transfer into human primary blood lymphocytes by surface-engineered lentiviral vectors that display a T cell-activating polypeptide. Blood. 2002;99:2342–2350. doi: 10.1182/blood.v99.7.2342. [DOI] [PubMed] [Google Scholar]
- 90.Yang H., Joo K.-I., Ziegler L., Wang P. Cell type-specific targeting with surface-engineered lentiviral vectors co-displaying OKT3 antibody and fusogenic molecule. Pharm. Res. 2009;26:1432–1445. doi: 10.1007/s11095-009-9853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou Q., Buchholz C.J. Cell type specific gene delivery by lentiviral vectors: New options in immunotherapy. OncoImmunology. 2013;2:e22566. doi: 10.4161/onci.22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jardine L., Barge D., Ames-Draycott A., Pagan S., Cookson S., Spickett G., Haniffa M., Collin M., Bigley V. Rapid detection of dendritic cell and monocyte disorders using CD4 as a lineage marker of the human peripheral blood antigen-presenting cell compartment. Front. Immunol. 2013;4:495. doi: 10.3389/fimmu.2013.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shortman K., Heath W.R. The CD8+ dendritic cell subset. Immunol. Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 94.Bonneville M., Lang F. CD8: from coreceptor to comodulator. Nat. Immunol. 2002;3:12–14. doi: 10.1038/ni0102-12. [DOI] [PubMed] [Google Scholar]
- 95.Walsh N.C., Kenney L.L., Jangalwe S., Aryee K.-E., Greiner D.L., Brehm M.A., Shultz L.D. Humanized Mouse Models of Clinical Disease. Annu. Rev. Pathol. 2017;12:187–215. doi: 10.1146/annurev-pathol-052016-100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gélinas J.-F., Gill D.R., Hyde S.C. Multiple Inhibitory Factors Act in the Late Phase of HIV-1 Replication: a Systematic Review of the Literature. Microbiol. Mol. Biol. Rev. 2018;82 doi: 10.1128/MMBR.00051-17. e00051-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mackall C.L., Fry T.J., Gress R.E. Harnessing the biology of IL-7 for therapeutic application. Nat. Rev. Immunol. 2011;11:330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pfeiffer A., Thalheimer F.B., Hartmann S., Frank A.M., Bender R.R., Danisch S., Costa C., Wels W.S., Modlich U., Stripecke R. In vivo generation of human CD19-CAR T cells results in B-cell depletion and signs of cytokine release syndrome. EMBO Mol. Med. 2018 doi: 10.15252/emmm.201809158. Published online September 17, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hay K.A., Hanafi L.-A., Li D., Gust J., Liles W.C., Wurfel M.M., López J.A., Chen J., Chung D., Harju-Baker S. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Glienke W., Esser R., Priesner C., Suerth J.D., Schambach A., Wels W.S., Grez M., Kloess S., Arseniev L., Koehl U. Advantages and applications of CAR-expressing natural killer cells. Front. Pharmacol. 2015;6:21. doi: 10.3389/fphar.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spragg C., De Silva Feelixge H., Jerome K.R. Cell and gene therapy strategies to eradicate HIV reservoirs. Curr. Opin. HIV AIDS. 2016;11:442–449. doi: 10.1097/COH.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith T.T., Stephan S.B., Moffett H.F., McKnight L.E., Ji W., Reiman D., Bonagofski E., Wohlfahrt M.E., Pillai S.P.S., Stephan M.T. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 2017;12:813–820. doi: 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pollack S.M., Lu H., Gnjatic S., Somaiah N., O’Malley R.B., Jones R.L., Hsu F.J., Ter Meulen J. First-in-Human Treatment With a Dendritic Cell-targeting Lentiviral Vector-expressing NY-ESO-1, LV305, Induces Deep, Durable Response in Refractory Metastatic Synovial Sarcoma Patient. J. Immunother. 2017;40:302–306. doi: 10.1097/CJI.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Verhoeyen E., Wiznerowicz M., Olivier D., Izac B., Trono D., Dubart-Kupperschmitt A., Cosset F.L. Novel lentiviral vectors displaying “early-acting cytokines” selectively promote survival and transduction of NOD/SCID repopulating human hematopoietic stem cells. Blood. 2005;106:3386–3395. doi: 10.1182/blood-2004-12-4736. [DOI] [PubMed] [Google Scholar]
- 105.Morizono K., Bristol G., Xie Y.M., Kung S.K., Chen I.S. Antibody-directed targeting of retroviral vectors via cell surface antigens. J. Virol. 2001;75:8016–8020. doi: 10.1128/JVI.75.17.8016-8020.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ziegler L., Yang L., Joo Ki., Yang H., Baltimore D., Wang P. Targeting lentiviral vectors to antigen-specific immunoglobulins. Hum. Gene Ther. 2008;19:861–872. doi: 10.1089/hum.2007.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang L., Yang H., Rideout K., Cho T., Joo K.I., Ziegler L., Elliot A., Walls A., Yu D., Baltimore D., Wang P. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat. Biotechnol. 2008;26:326–334. doi: 10.1038/nbt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liang M., Pariente N., Morizono K., Chen I.S. Targeted transduction of CD34+ hematopoietic progenitor cells in nonpurified human mobilized peripheral blood mononuclear cells. J. Gene Med. 2009;11:185–196. doi: 10.1002/jgm.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Froelich S., Ziegler L., Stroup K., Wang P. Targeted gene delivery to CD117-expressing cells in vivo with lentiviral vectors co-displaying stem cell factor and a fusogenic molecule. Biotechnol. Bioeng. 2009;104:206–215. doi: 10.1002/bit.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lafitte M., Rousseau B., Moranvillier I., Taillepierre M., Peuchant E., Guyonnet-Dupérat V., Bedel A., Dubus P., de Verneuil H., Moreau-Gaudry F., Dabernat S. In vivo gene transfer targeting in pancreatic adenocarcinoma with cell surface antigens. Mol. Cancer. 2012;11:81. doi: 10.1186/1476-4598-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anliker B., Abel T., Kneissl S., Hlavaty J., Caputi A., Brynza J., Schneider I.C., Münch R.C., Petznek H., Kontermann R.E. Specific gene transfer to neurons, endothelial cells and hematopoietic progenitors with lentiviral vectors. Nat. Methods. 2010;7:929–935. doi: 10.1038/nmeth.1514. [DOI] [PubMed] [Google Scholar]
- 112.Bayin N.S., Placantonakis D.G. Selective Targeting of CD133-Expressing Glioblastoma Stem Cells Using Lentiviral Vectors. Methods Mol. Biol. 2018;1741:91–101. doi: 10.1007/978-1-4939-7659-1_7. [DOI] [PubMed] [Google Scholar]
- 113.Ou W., Marino M.P., Suzuki A., Joshi B., Husain S.R., Maisner A., Galanis E., Puri R.K., Reiser J. Specific targeting of human interleukin (IL)-13 receptor α2-positive cells with lentiviral vectors displaying IL-13. Hum. Gene Ther. Methods. 2012;23:137–147. doi: 10.1089/hgtb.2012.054. [DOI] [PMC free article] [PubMed] [Google Scholar]