Abstract
Both vaccine and therapeutic approaches to malaria are based on conventional paradigms; whole organism or single antigen epitope-based vaccines administered with or without an adjuvant, and chemotherapeutics (anti-malaria drugs) that are toxic to the parasite. Two major problems that limit the effectiveness of these approaches are i) high levels of antigenic variation within parasite populations rendering vaccination efficacy against all variants difficult, and ii) the capacity of the parasite to quickly evolve resistance to drugs. We describe a new approach to both protection from and treatment of malaria parasites that involves the direct stimulation of the host innate immune response through the administration of a Toll-Like Receptor-2 (TLR2) agonist. The activity of PEG-Pam2Cys against the hepatocytic stages, erythrocytic stages and gametocytes of the rodent malaria parasite Plasmodium yoelii was investigated in laboratory mice. We show that administration of PEG-Pam2Cys, a soluble form of the TLR2 agonist S-[2,3-bis(palmitoyloxy)propyl] cysteine (Pam2Cys), significantly and dramatically reduces the numbers of malaria parasites that grow in the livers of mice following subsequent challenge with sporozoites. We also show that treatment can also clear parasites from the liver when administered subsequent to the establishment of infection. Finally, PEG-Pam2Cys can reduce the numbers of mosquitoes that are infected, and the intensity of their infection, following blood feeding on gametocytaemic mice. These results suggest that this compound could represent a novel liver stage anti-malarial that can be used both for the clearance of parasites following exposure and for the prevention of the establishment of infection.
Keywords: Malaria, Plasmodium, Pam2Cys, TLR-2
Graphical abstract
Highlights
-
•
TLR-2 agonist Pam2Cys reduces malaria parasite burden in the liver when administered prior to sporozoite challenge.
-
•
It also reduces malaria parasite burden in the liver when administered 24 h after sporozoite challenge.
-
•
It reduces the transmissibility of a malaria infection to mosquitoes.
1. Introduction
Clinically silent and typically low in number, the liver stages of the malaria parasite, Plasmodium spp., grow and develop in hepatocytes over the course of a number of days following the deposition of sporozoites into the skin of the host during an infected mosquito bite. Following their development in the liver, which involves the maturation of single nucleated invasive sporozoites into multi-nucleated schizonts containing thousands of merozoites capable of infecting erythrocytes, malaria parasites enter the blood stream where the symptomatic red-blood cell cycle is initiated. An intervention that effectively targets parasites in the liver has the capacity to prevent the onset of symptomatic malaria, and to break the transmission cycle of the parasite. There is currently only a single licensed drug available, primaquine, which can kill hypnozoites, the dormant form of malaria parasites in the liver. Its use, however, is associated with serious side effects in some groups of patients, including those with glucose-6-phosphate dehydrogenase (G6PD) deficiency in which it can cause severe hemolysis, and reports of parasite resistance to the drug are accumulating (Kristensen and Dragsted, 2014; Luzzatto and Seneca, 2014). New ways of attacking the malaria parasite in the liver are needed.
Liver stage malaria parasites cause no pathology but are recognized and targeted by the innate immune system, although the degree of protection is slight. The recognition of parasites in the liver is relatively poorly understood, but recent evidence suggests that it occurs through the activation of the cytosolic pattern recognition receptor melanoma differentiation-associated gene 5 (Mda5), which in turn triggers mitochondrial antiviral signaling protein (Mavs) stimulation to induce a type I interferon (IFN) response (Liehl et al., 2014). A role for IFNγ has also been shown, with parasite killing mostly mediated through the effector functions of liver natural killer T cells (NKT cells) (Miller et al., 2014). There is also evidence that sporozoites can activate the pattern recognition receptor Toll-like receptor-2 (TLR2) and that this can lead to suppression of the growth of hepatic stage parasites (Zheng et al., 2015). These findings thus suggest that harnessing and boosting the natural capacity of the innate immune system to kill hepatocytic malaria parasites could form the basis for a valuable intervention against the parasite.
The lipopeptide S-[2,3-bis(palmitoyloxy)propyl] cysteine (Pam2Cys), a synthetic analogue of the lipid component of macrophage activating lipopeptide-2 (MALP2), is a potent Toll-like Receptor 2 (TLR2) agonist, which has been shown to confer rapid protection against the influenza A virus (Tan et al., 2012; Chua et al., 2015) and secondary bacterial infections (Mifsud et al., 2016b). This protection is conferred through stimulation of the innate immune system, and specifically through the induction of macrophages and neutrophils to secrete inflammatory cytokines including interleukin-2 (IL-2), tumor necrosis factor alpha (TNF-α) and IFN-γ (Tan et al., 2012; Chua et al., 2015; Mifsud et al., 2016a, 2016b). Given the ability of PEG-Pam2Cys to invoke a response in this manner and the fact that liver stage malaria parasites are susceptible to innate immune responses involving the IFN-γ pathway, we hypothesized that PEG-Pam2Cys could work as both an immunotherapeutic to clear liver stage parasites, and also as an immunoprophylactic to prevent the establishment of parasites in the liver following sporozoite challenge. Here, we provide the results of experiments that show that administration of PEG-Pam2Cys several hours prior to challenge with sporozoites of the rodent malaria Plasmodium yoelii, significantly and dramatically reduces the numbers of parasites that are able to grow in the livers of mice. Furthermore, the administration of PEG-Pam2Cys 24 h after infection with sporozoites also provides significant protection against the development of parasites in the liver.
2. Materials and methods
2.1. Parasites and hosts
Laboratory animal experimentation was performed in accordance with the Japanese Humane Treatment and Management of Animals Law (Law No. 105 dated 19 October 1973 modified on 2 June 2006), and the Regulation on Animal Experimentation at Nagasaki University, Japan. The protocol was approved by the Institutional Animal Research Committee of Nagasaki University (permit: 1207261005–2).
The rodent malaria parasite Plasmodium yoelii yoelii, strain 17X1.1pp (hereafter referred to as P. yoelii) (Inoue et al., 2012) was used in all experiments. Eight-week old female BALB/c and CBA/n mice (SLC Inc., Shizuoka, Japan) were housed at 26 °C and fed on maintenance diet with 0.05% para-aminobenzoic acid (PABA)-supplemented water to assist parasite growth. Anopheles stephensi mosquitoes were housed in a temperature and humidity controlled insectary at 24 °C and 70% humidity, adult flies being maintained on 10% glucose solution supplemented with 0.05% PABA.
2.2. Synthesis of PEG-Pam2Cys
Pam2Cys is insoluble in physiological media and was therefore synthesized with a polyethylene glycol molecule attached to confer solubility for intravenous (i.v.) administration. Synthesis was performed in house using Fmoc-based chemistry as described previously (Tan et al., 2012). Fidelity of synthesis and the purification process was monitored by mass analysis (mass value found 1502.2 Da; expected mass 1502.1 Da) using an Agilent 1100 Series LC/MSD ion-trap mass spectrometer (Agilent, Palo Alto, CA, USA).
PEG-Pam2Cys was administered to mice at varying doses in 100 μL of PBS by the i.v. route via the tail vein. The lowest effective dose of Pam2Cys was used for each experiment, according to calibration in pilot studies.
2.3. Generation of sporozoites
Sporozoites were generated by allowing Anopheles stephensi mosquitoes to feed on mice inoculated i.v. with 1 × 106 P. yoelii blood stage parasites three days previously. Fifteen to 21 days post-feeding, mosquito salivary glands were dissected in sterile PBS (Sigma–Aldrich, Japan), crushed in a glass homogenizer, and sporozoite numbers determined using a haemocytometer.
2.4. Mosquito infection assay
In order to quantify the transmission blocking effect of PEG-Pam2Cys treatment, groups of 5 female BALB/c or CBA/n mice were inoculated with 1 × 106 Plasmodium yoelii yoelii infected red blood cells and treated 48 h later with PEG-Pam2Cys or a saline control. Twenty-four hours later, groups of 20–30 Anopheles stephensi mosquitoes were allowed to feed on each mouse, separately. The presence of oocysts on the midguts of gravid (and thus blood fed) mosquitoes was assessed by light microscopy 10–13 days later.
2.5. Challenge protocols
All challenge infections with P. yoelii sporozoites were performed by i.v. inoculation with sporozoites diluted in 100 μL of PBS. The number of sporozoites inoculated varied between experiments with 500–1500 used for evaluating the development of blood stage parasitaemia and 10,000–15,000 administered for measuring liver parasite burdens.
2.6. Quantitation of liver parasite burden using qPCR
Liver parasite burdens were assessed 42 h post-sporozoite inoculation using quantitative PCR (qPCR) measurement of parasite 18s rRNA gene copy number either directly on genomic DNA (gDNA) extracted from 0.5 cm3 sections of liver, or following reverse transcriptase PCR (RT-PCR) of total RNA extracted from whole mouse livers as previously described (Inoue et al., 2012).
2.7. Quantification of cytokines in blood by ELISA
ELISA was performed to quantify cytokines levels in the blood following PEG-Pam2Cys inoculation. Briefly, mice were inoculated i.v. with 7.5 nM PEG-Pam2Cys and 6 h later blood was collected and sera separated. Sera were then loaded onto ELISA plates (Ebiosciences Ready-SET-Go!® Kit) pre-coated with either anti-mouse IFN-γ, TNF-α, IL-6 or IL-10 capture antibodies. A standard containing either IFN-γ, TNF-α, IL-6 or IL-10 mouse recombinant protein was loaded onto the plate in a two-fold serial dilution series. The plates were analysed using the Multiskan™ Microplate Photometer (Thermo Scientific) and absorbance measurements uploaded onto Graphpad Prism (version 6.01) for estimation of cytokine levels in the serum. Absorbance measurements from the standards for each cytokine (at 450 nm) were used to generate a best fit of value curve from which concentrations of cytokines were extrapolated.
2.8. Quantification of liver NKT cells
To quantify liver NKT cells following inoculation with PEG-Pam2Cys, CBA/n mice were injected intravenously with 10 nM PEG-Pam2Cys or PBS, 72 h prior to liver perfusion into 5 ml RPMI (WAKO Japan) and homogenization with GentleMACS (Miltenyi Biotec, Germany) in 5 ml of RPMI supplemented with 10% FCS and 1% Penicillin. Homogenates were then filtered through a metal mesh into a 50 ml tube with RPMI followed by centrifugation at 1200 rpm for 10 min. Supernatants were discarded and cells were suspended in 25 ml of 33% Percoll (GE Healthcare UK ltd, Buckinghamshire, England) containing 2.5 ml of 5000U/5 ml Heparin (Mochida Pharmaceutical Co. Ltd, Tokyo, Japan) followed by centrifugation at 2200 rpm for 20 min. Red blood cells (RBCs) contained in the pellet were lysed with 3 ml of lysis buffer (0.83% Ammonium Chloride, Tris-HCl buffer pH 7.65) followed by washing with 50 ml HBSS. Cells were suspended in 500 μL RPMI and counted after staining with trypan blue. 50 μL of cell suspension was stained with PE-conjugated anti-mouse (eBioscience) and APC-conjugated anti-mouse DX5 (CD49b) antibodies (Biolegend Inc.) and were analysed by flow cytometry (BD FACSVerse, Nippon Becton Dickinson Company Ltd, Tokyo, Japan).
3. Results
PEG-Pam2Cys reduces the liver parasite burden when administered prior to sporozoite challenge and protects from the onset of blood-stage parasitaemia following mosquito-bite challenge.
In order to establish whether PEG-Pam2Cys could act as an immunoprophylactic, we tested its ability to protect against the establishment and growth of parasites in the liver of mice inoculated with sporozoites. Female BALB/c mice were inoculated intravenously with either 10 nM of PEG-Pam2Cys or PBS 6 h prior to challenge with 10,000 P. yoelii sporozoites. Liver parasite burden was quantified by qPCR 42 h after sporozoite challenge, a time when schizogony is advanced but prior to merosome release.
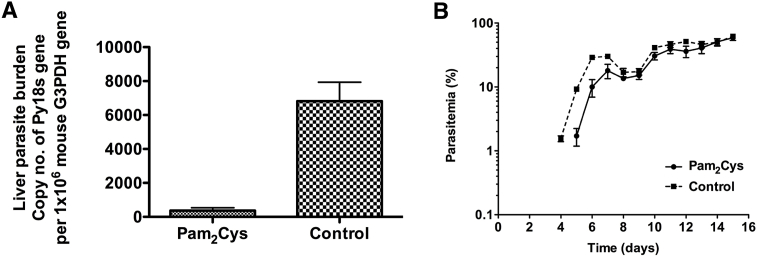
Mice that received PEG-Pam2Cys had significantly lower liver parasite burdens than those in the control group (Fig. 1A, P < 0.001, Student's two-tailed t-test, t = 5.394, df = 8, n = 5 mice per group). A further two groups of mice, one treated with PEG-Pam2Cys and the other with PBS, were similarly challenged and the course of blood stage parasitaemia monitored on days 4, 5 and 10 post-sporozoite challenge. The PEG-Pam2Cys treated group showed lower parasitaemia at all time points compared to the control group, but the growth rate of the parasites in the blood was not different (Fig. 1B), suggesting that the administration of PEG-Pam2Cys 6 h prior to sporozoite challenge reduces the numbers of parasites that are able to complete their development in the liver (or reduces the numbers of merozoites formed by a single hepatocytic schizont), but does not affect their growth in the blood.
Fig. 1.
Liver parasite burden of mice inoculated intravenously (iv) with 10,000 sporozoites of Plasmodium yoelii 17 × 1.1pp (Panel A), or exposed to the bites of 5 female Anopheles stephensi mosquitoes fed two weeks prior on mice infected with gametocytes of P. yoelii (Panel C). Livers were removed 42 hrs post-inoculation, homogenised in Isogen solution, total RNA extracted and converted to cDNA by reverse transcriptase PCR. Groups were treated 6 hrs prior to sporozoite inoculation with 10 nM inocula of PEG-Pam2Cys or saline control. Parasite liver burden was measured by qPCR quantification of P. yoelii 18s gene copy number with reference to the copy number of mouse g3pdh gene measured in the same sample. **P < 0.001, Student's two-tailed t-test, t = 5.394, df = 8. n = 5 mice per group (Panel A), and 3 mice in the Pam2Cys and 4 in the control group (Panel C). Panel B, Parasitaemia following challenge with 10,000 sporozoites of P. yoelii 17 × 1.1pp intravenously, following intravenous inoculation of 10 nM PEG-Pam2Cys or saline control 6 hrs earlier. n = 4 mice per group. Two-way ANOVA repeated measures mixed effects model; PEG-Pam2Cys accounts for 5.84% of the total variance (after adjusting for matching). F = 5.27. DFn = 1 DFd = 6, P = 0.0615. Data is representative of two repeat experiments. Panel C, percentage of mice that remained blood-stage parasite-free following the bites of three A. stephensi mosquitoes infected with P. yoelii sporozoites in groups inoculated 6-h prior to mosquito biting with 10 nM of PEG-Pam2Cys or saline control. Parasitaemia was recorded from day two post infection to day ten and the result is expressed as percentage of mice protected from developing parasitaemia. Log-rank (Mantel-Cox) test, p = 0.0004, df = 1, n = 10 mice per group. Panel E, survival of mice following intravenous inoculation of 15,000 sporozoites. Mice previously inoculated with PEG-Pam2Cys or saline control were inoculated with 15,000 sporozoites each, 6 h post-PEG-Pam2Cys or saline control inoculation. Survival of mice was monitored until day thirty post sporozoite inoculation. Log-rank (Mantel-Cox) test, p = 0.0027, df = 1, n = 5 mice per group.
The natural route of administration of malarial sporozoites is through the bites of infected mosquitoes. In the above experiment, we administered an atypically large number of sporozoites to facilitate the detection and quantitation of parasites in the liver by qPCR. In order to asses the anti-malarial prophylactic activity of PEG-Pam2Cys against the growth of malaria parasites transmitted via the natural route of infection, we performed an experiment in which PEG-Pam2Cys treated and untreated mice were exposed to the bites of five P. yoelii-infected mosquitoes. In the control group, the amount of parasite DNA in the liver at 42 h post-sporozoite challenge was low and variable, but present at detectable levels in each animal. In contrast, no malaria parasite DNA could be detected in the mice treated with PEG-Pam2Cys 6 h prior to sporozoite challenge (Fig. 1C).
We then investigated whether inoculation of PEG-Pam2Cys could protect mice from the onset of blood-stage parasitaemia following infected mosquito-bite challenge. Two groups of 10 mice, one of which was inoculated with PEG-Pam2Cys, and the other with saline solution, were exposed to the bites of three mosquitoes that had fed on P. yoelii infected blood 15 days previously. All mice in the saline group were parasitaemic four days after mosquito-bite challenge. In contrast, only two mice (20%) in the Pam2Cys treated group developed parasitaemia during ten days of monitoring, indicating a significant protective effect of Pam2Cys (log-rank (Mantel-Cox) test, p = 0.0004, d.f = 1, n = 10 mice per group, Fig. 1D).
Large sporozoite doses (>10,000) of P. yoelii 17X1.1pp lead to hyper-parasitaemia at the blood stage, resulting in host death in BALB/c mice. We tested the capacity of PEG-Pam2Cys to protect against this by inoculating two groups of mice with PEG-Pam2Cys or saline control and challenging with 15,000 sporozoites 6 h later. All mice in the saline control treated group succumbed to hyper-parasitaemia and died by day 9 post-sporozoite challenge. We found a significant protective effect of PEG-Pam2Cys treatment, with four out of five mice in this group surviving up to Day 30 post-sporozoite challenge, at which point parasitaemia had spontaneously resolved (log-rank (Mantel-Cox) test, p = 0.0027, d.f = 1, n = 5 mice per group, Fig. 1E).
3.1. PEG-Pam2Cys is an effective immunochemoprophylactic against malaria parasite growth in the liver at 5 nM, but has no effect at concentrations at or lower than 1 nM
To determine the lowest effective dose of PEG-Pam2Cys as an immunoprophylactic against liver stage malaria parasites, we inoculated groups of five mice with 5 nM, 1 nM, 0.1 nM and 0.01 nM of PEG-Pam2Cys or saline control.
Six hours after inoculation with PEG-Pam2Cys, mice were also challenged with 15,000 P. yoelii sporozoites and after a further 42 h, liver parasite burden was measured as above. We found that mice treated with 5 nM of PEG-Pam2Cys had significantly fewer parasites in the liver at 42 h post-infection than control mice, but that mice treated with 1 nM, 0.1 nM and 0.01 nM did not (Fig. 2A). Further groups of four mice treated with the same doses of PEG-Pam2Cys and control PBS were challenged with 500 sporozoites, and the growth of parasites in the blood followed for 16 days. We found no differences in parasite growth for mice treated with 1 nM, 0.1 nM, 0.01 nM and PBS, but mice treated with 5 nM of PEG-Pam2Cys displayed late onset and reduced parasitaemia during the first 8 days of infection, but no differences in parasite growth rates or peak parasitaemia (Fig. 2B).
Fig. 2.
Panel A: Liver parasite burden (LPB) of mice inoculated intravenously with 15,000 Plasmodium yoelii 17 × 1.1pp sporozoites 42 h post-inoculation measured from genomic DNA extracted from a 0.5 cm3 piece of liver tissue. Groups were treated 6 hrs prior to sporozoite inoculation with 5 nM, 1 nM, 0.1 nM, 0.01 nM PEG-Pam2Cys inocula or with saline as a negative control. LPB was measured by quantitative PCR of P. yoelii 18s gene copy number with reference to the copy number of mouse g3pdh gene measured in the same sample. Error bars indicate the standard error of the mean (SEM), n = 4 mice per group. *P value < 0.05, Student's two-tailed t-test (5 nM vs. 1 nM, t = 2.458; 5 nM vs. 0.1 nM, t = 3.190; 5 nM vs. Control, t = 3.678), but not significant for 5 nM vs. 0.01 nM, df = 6. Panel B: Parasitaemia of mice inoculated with 5 nM, 1 nM, 0.1 nM, 0.01 nM PEG-Pam2Cys or saline control 6 hrs prior to intravenous challenge with 500 sporozoites of P. yoelii 17X1.1pp. Error bars indicate the standard error of the mean (SEM), n = 4 mice per group.
3.2. PEG-Pam2Cys reduces liver parasite burden when administered after sporozoite infection
In order to determine whether PEG-Pam2Cys is capable of clearing parasites from the liver following their establishment and growth, we infected groups of four female BALB/c mice with 15,000 P. yoelii sporozoites and 24 h later inoculated 7.5 nM of PEG-Pam2Cys or PBS. The growth of parasites in the liver 42 h post sporozoite-challenge was then measured. There was a large, significant reduction in the amount of parasite DNA detected in the mice that received PEG-Pam2Cys, compared to those that received PBS alone (Fig. 3A, P value < 0.05, Student's two-tailed t-test, t = 5.72, df = 6). Further groups of five mice were treated as above, and blood stage parasitaemia was followed for 16 days. There was delayed onset, and lower parasitaemia during the first eight days of parasite growth, but no change in the rate of replication of parasites in the blood (Fig. 3B).
Fig. 3.
Panel A: Liver parasite burden (LPB) of mice inoculated intravenously with 15,000 Plasmodium yoelii 17 × 1.1pp sporozoites 42 h post-inoculation measured from genomic DNA extracted from a 0.5 cm3 piece of liver tissue. Mice were inoculated with sporozoites 24 hrs prior to intravenous administration of 7.5 nM PEG-Pam2Cys inoculation or with PBS (control). LPB was measured by quantitative PCR of P. yoelii 18s gene copy number with reference to the copy number of mouse g3pdh gene measured in the same sample. Error bars indicate the standard error of the mean (SEM), n = 4 mice per group. **P value < 0.05, Student's two-tailed t-test, t = 5.72, df = 6. Panel B: Parasitaemia of mice first inoculated intravenously with 500 sporozoites of P. yoelii 17X1.1pp intravenously then inoculated with 7.5 nM PEG-Pam2Cys 24 h later and the animals then monitored for 16 days. From Day 0 to Day 4 no parasitaemia was observed. Error bars indicate the standard error of the mean (SEM), n = 5 mice per group. On day 12 one mouse from the control group died.
3.3. PEG-Pam2Cys reduces the percentage of mosquitoes infected with malaria parasites, and the oocyst burden of those infected, following mosquito feeding on infected mice treated 24 h previously
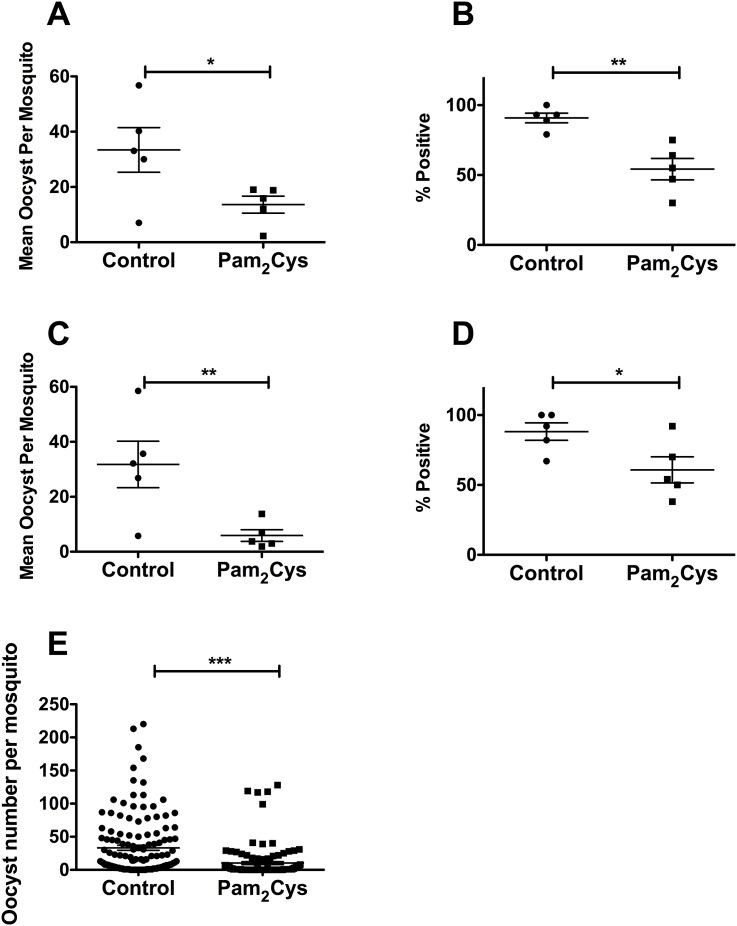
Gametocyte infectivity can be negatively affected by inflammatory cytokines (Naotunne et al., 1991), and we postulated that stimulation of TLR2 by PEG-Pam2Cys might reduce the capacity of blood stage infections to transmit to mosquitoes. To test this, Anopheles stephensi mosquitoes were allowed to feed on P. yoelii infected mice inoculated either with PEG-Pam2Cys or saline 24 h previously. Mosquitoes fed on mice inoculated with PEG-Pam2Cys were less likely to become infected and had lower oocyst burdens when they were infected (Fig. 4), indicating that TLR2 stimulation can reduce the infectivity of gametocytes to mosquitoes.
Fig. 4.
Percentage of Anopheles stephensi mosquitoes with at least one oocyst (A and C), and the mean number of oocysts per mosquito (B and D) for mosquitoes fed on gametocytaemic CBA/n (n = 5) (A and B) or BALB/c (n = 5) (C and D) mice. Mice were infected with 1 × 106Plasmodium yoelii yoelii parasitized red blood cells and treated with 10 nM PEG-Pam2Cys 24 hrs prior to mosquito feeding, which occurred on Day 3 post-parasite challenge. Groups of 20–30 mosquitoes were allowed to feed on individual mice. Oocysts were counted on dissected mosquito midguts 10 days post mosquito feeding. Data are representative of two independent experiments for each mouse genotype. Panel E shows the oocyst burden of individual mosquitoes fed on mice treated with PEG-Pam2Cys or saline solution in the two separate experiments represented in panels A–D (total of 261 mosquitoes fed on 10 individual mice). A, **P < 0.01, Student's one-tailed t-test, t = 4.369, df = 8 n = 5 mice per group; B, *P < 0.05, Student's one-tailed t-test, t = 2.292, df = 8 n = 5 mice per group; C, *P < 0.05, Student's one-tailed t-test, t = 2.440, df = 8 n = 5 mice per group; D, **P < 0.01, Student's one-tailed t-test, t = 2.965, df = 8 n = 5 mice per group; E, ***P < 0.0001, Student's one-tailed t-test, t = 5.086, df = 259. Error bars indicate the standard error of the means.
3.4. PEG-Pam2Cys inoculation during an ongoing blood stage malaria parasite infection can increase parasitaemia
Given the inhibitory effect of PEG-Pam2Cys treatment on the growth of liver stage parasites and the infectivity of gametocytes, we tested anti-schizontal activity on established blood stage infections in mice. Groups of mice were inoculated with PEG-Pam2Cys 6 h prior or three days after infection with 1 × 106 P. yoelii-infected RBCs, and parasitaemia was monitored daily by microscopy. No effect on blood stage parasite growth was observed in mice treated with PEG-Pam2Cys 6 h prior to parasite challenge compared to control mice, but mice inoculated with PEG-Pam2Cys on Day 3 post-infection experienced transient but significant increased parasitaemia on Days 4 and 5 (Supplementary Fig. 1).
3.5. PEG-Pam2Cys inoculation results in increased blood levels of IFN-γ, TNF-α and IL-6 but not IL-10 after 6 h
IFN-γ, TNF-α, IL-6, and IL-10 levels in blood were measured in groups of five female BALB/c mice 6 h following inoculation of 7.5 nM of PEG-Pam2Cys. Significantly elevated levels of IFN-γ, TNF-α and IL-6 were observed in mice treated with PEG-Pam2Cys compared to control animals, whereas IL-10 levels did not differ significantly between PEG-Pam2Cys and control groups (Fig. 5, TNF-α, P = <0.05, Student's two-tailed t-test, t = 3.230; IFN-γ, P = 0.0001, Student's two-tailed t-test, t = 7.346; IL6, P = 0.0002, Student's two-tailed t-test, t = 6.562, df = 8).
Fig. 5.
Blood concentration levels of the cytokines IFN-γ, TNF-α, IL-6 & IL-10 in mice inoculated with 7.5 nM PEG-Pam2Cys or saline solution (control) measured by ELISA. Error bars indicate the standard error of the mean (SEM), n = 5 mice per group. TNF-α *P value < 0.05, Student's two-tailed t-test, t = 3.230; IFN-γ ****P value 0.0001, Student's two-tailed t-test, t = 7.346; IL6 ***P value 0.0002, Student's two-tailed t-test, t = 6.562; IL10 values were not significant, df = 8.
3.6. PEG-Pam2Cys inoculation results in an increase in the numbers of natural killer (NK) T-cells in the livers of mice
IFN-γ has been shown to be involved in innate immune system mediated killing of liver stage parasites through the recruitment and activation of NKT cells (Miller et al., 2014). We investigated whether the increase in IFN-γ observed following treatment with PEG-Pam2Cys is associated with an increase in liver NKT cells. Groups of CBA/n mice (n = 8) were inoculated with 10 nM PEG-Pam2Cys and 72 h later, the numbers of NKT cells in liver homogenates were quantified by flow cytometry. Our results show that mice inoculated with PEG-Pam2Cys exhibited significantly higher numbers of NKT cells in their livers than those inoculated with saline (Fig. 6). There were no measurable changes in the numbers of CD4+ or CD8+ cells in the livers of mice inoculated with PEG-Pam2Cys (Fig. 6, Panels B and C).
Fig. 6.
Quantification of liver NKT cells in CBA mice (Panel A) inoculated intravenously with 10 nM of PEG-Pam2cys or saline control. Seventy-two hours later whole livers were perfused, homogenised, lymphocytes purified and NKT cells stained with appropriate antibodies, P = 0,0079, Student's two-tailed t-test, t = 3.679 df = 7, n = 8 mice per group, bars indicate the mean. Panels D and E show gating strategy for cells obtained from mice treated with PEG-Pam2Cys or PBS. Block Q2 contains double positive (DX5+, CD3+) NKT cells. Panels B and C, quantification of liver CD8+ (B) and CD4+ (C) cells in CBA mice. Panels F and G show gating strategy for cells obtained from mice treated with PEG-Pam2Cys or PBS respectively. Block Q1 contains PE-CD4 positive CD4 cells, block Q3 contains FITC-CD8 positive, CD8 cells.
4. Discussion
The control and prevention of malaria currently relies on anti-mosquito vector measures and the use of anti-malarial drugs. Whilst often highly effective, these methods are hampered by sub-optimal implementation, and by the emergence of drug resistant parasites (Cravo et al., 2006). Currently, there is renewed interest in malaria elimination, which aims to eradicate the disease entirely. Historically successful in moderate to low transmission areas, malaria eradication in the worst affected parts of the world (i.e. the tropics, especially sub-Saharan Africa) will be extremely difficult without the application of new therapeutics.
Here we have demonstrated a different approach to both protection from and treatment of malaria parasites that involves direct stimulation of the host innate immune response through the administration of a novel Toll-Like Receptor-2 (TLR2) agonist, PEG-Pam2Cys. Administration of PEG-Pam2Cys prior to inoculation of P. yoelii sporozoites either through artificial injection straight into the blood circulation, or through the bites of infected mosquitoes dramatically reduced the numbers of parasites that were present in the livers of mice 42 h later. Furthermore, administration of PEG-Pam2Cys 24 h after inoculation of sporozoites had a similar anti-parasitic effect. These results suggest that PEG-Pam2Cys could constitute a novel liver stage anti-malarial that can be used both for the clearance of parasites following exposure, and for the prevention of the establishment of infection as a novel immunochemoprophylactic.
Because the administration of PEG-Pam2Cys clears parasites even after 24 h of development in the liver, it is possible that it targets infected hepatocytes rather than preventing the establishment of infection at the sporozoites stage. PEG-Pam2Cys is a potent TLR-2 agonist (Jackson et al., 2004; Lau et al., 2006) that stimulates the production of inflammatory cytokines. We found elevated levels of IL-6, TNF-α and IFN-γ in the sera of mice following PEG-Pam2Cys inoculation. It is possible that increased levels of these cytokines, and especially of IFN-γ, are involved in liver stage parasite killing. IFN-γ has been shown to be involved in innate immune system mediated killing of liver stage parasites, through the recruitment and activation of NKT cells (Miller et al., 2014). We show that PEG-Pam2Cys inoculation increases the numbers of effector NKT cells in the liver, and it seems likely that this is mediated through elevation of IFN-γ. There is some evidence that TLRs are involved in the recognition of malaria parasites in the liver (Zheng et al., 2015), with recent work suggesting that recognition occurs through Plasmodium RNA mediated activation of Mda5, which in turn stimulates Mavs to up-regulate type I IFN production through interferon regulatory factors 3 and 7 (Ir3/Irf7) (Liehl et al., 2014). TLR-2 stimulation can provoke a Type I IFN response (Barbalat et al., 2009; Takeuchi and Akira, 2010) and it is therefore possible that the protection from liver stage parasites conferred by PEG-Pam2Cys is mediated in this manner. It is also possible that protection occurs through the IFN-γ pathway, as this is also known to be effective against hepatocytic malaria parasites (Miller et al., 2014; Liehl et al., 2015). Whatever the mechanism, the stimulation of TLR-2 by PEG-Pam2Cys results in innate immune system activation the end result of which is parasite clearance.
Evidence for the utility of an immunochemoprophylactic approach to the prevention of malaria parasite development in the liver has previously been provided by Gonzalez-Aseguinolaza et al., who showed that administration of the glycolipid α-galactosylceramide (α-GalCer) was a potent inhibitor of liver stage malaria parasite development when administered to mice 1–2 days prior to sporozoite challenge. The mechanism of this protection also appears to rely on activation of NKT cells and is IFN-γ dependent (Gonzalez-Aseguinolaza, G. et al., 2000).
Whilst our results suggest that PEG-Pam2Cys is effective at provoking an innate immune response that kills liver stage malaria parasites, further characterisation of its effects and side-effects are required before it can be proposed as an anti-malarial. Its prophylactic effect at the liver stage is dramatic, but we have only tested its ability to prevent parasite growth in the liver following inoculation 6 h prior to sporozoite challenge. How long it remains effective after inoculation remains to be tested. However, our previous findings have shown that innate immune responses induced by intranasal administration of PEG-Pam2Cys can protect against influenza virus challenge for up to 7 days (Tan et al., 2012; Chua et al., 2015; Mifsud et al., 2016b) and hints that a reasonable window of protection could also be achieved against liver parasites. Similarly, we have shown that PEG-Pam2Cys is effective at killing parasites in the liver 24 h after sporozoite inoculation but whether parasites can be killed later in their hepatocytic development is as yet untested. Of potential concern is that we found that PEG-Pam2Cys can exacerbate parasitaemia when administered during an ongoing blood stage infection. If it were to be used as a prophylactic, then there is the risk that any parasites escaping from the liver may lead to an abnormally high parasitaemia. However, we found that administration of PEG-Pam2Cys, whilst appearing to increase parasitaemia when inoculated during an ongoing blood stage infection, had no effect on blood stage parasite growth when administered 6 h prior to blood stage parasite inoculation, suggesting that this effect is short lived and may not exacerbate a break-out blood stage infection.
Inoculation of PEG-Pam2Cys during an on-going blood infection resulted in a decrease of transmitted infectivity to mosquitoes, a result consistent with previous work describing the anti-infectivity effect of pro-inflammatory cytokines (Naotunne et al., 1991). However, PEG-Pam2Cys inoculation during the blood stage infection was also shown to increase parasitaemia, making its use as a transmission-blocking agent problematic.
We have shown that PEG-Pam2Cys is a potent immunochemotherapeutic and immunochemoprophylactic against liver stage malaria parasites, and that it can inhibit the infectivity of gametocytes to mosquitoes. Because the TLR2 agonist stimulates a robust innate immune response that is not normally induced by the parasite, the compound should not be susceptible to the development of parasite resistance as are traditional drugs. We believe that the potential of PEG-Pam2Cys to protect against malaria, and indeed, other protozoan parasites warrants further investigation.
Funding
This work was supported by a JSPS Grant-in Aid for Scientific Research (16K21233) to RC. The funder played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report or in the decision to submit the article for publication.
Declarations of interest
None.
Acknowledgements
We are grateful to Professor Arnab Pain for discussion, and to Dr Taeko Moriyasu and Dr Risa Nakamura for technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2018.10.006.
Contributor Information
Brendon Chua, Email: bychua@unimelb.edu.au.
Richard Culleton, Email: richard@nagasaki-u.ac.jp.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Barbalat R., Lau L., Locksley R.M., Barton G.M. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat. Immunol. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua B.Y., Wong C.Y., Mifsud E.J., Edenborough K.M., Sekiya T., Tan A.C., Mercuri F., Rockman S., Chen W., Turner S.J., Doherty P.C., Kelso A., Brown L.E., Jackson D.C. Inactivated influenza vaccine that provides rapid, innate-immune-system-mediated protection and subsequent long-term adaptive immunity. mBio. 2015;6 doi: 10.1128/mBio.01024-15. e01024-01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravo P., Culleton R., Afonso A., Ferreira I., do Rosario V. Mechanisms of drug resistance in malaria: current and new challenges. Anti-Infect. Agents Med. Chem. 2006;5:63–73. [Google Scholar]
- Gonzalez-Aseguinolaza G., de Oliveira C., Tomaska M., Hong S., Bruna-Romero O., Nakayama T., Taniguchi M., Bendelac A., Van Kaer L., Koezuka Y., Tsuji M. α -galactosylceramide-activated Valpha 14 natural killer T cells mediate protection against murine malaria. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8461–8466. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Tang J., Miyakoda M., Kaneko O., Yui K., Culleton R. The species specificity of immunity generated by live whole organism immunisation with erythrocytic and pre-erythrocytic stages of rodent malaria parasites and implications for vaccine development. Int. J. Parasitol. 2012;42:859–870. doi: 10.1016/j.ijpara.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Jackson D.C., Lau Y.F., Le T., Suhrbier A., Deliyannis G., Cheers C., Smith C., Zeng W., Brown L.E. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15440–15445. doi: 10.1073/pnas.0406740101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen K.L., Dragsted U.B. Recurrent Plasmodium vivax malaria due to dose-dependent primaquine resistance: a case report. Scand. J. Infect. Dis. 2014;46:63–65. doi: 10.3109/00365548.2013.822093. [DOI] [PubMed] [Google Scholar]
- Lau Y.F., Deliyannis G., Zeng W., Mansell A., Jackson D.C., Brown L.E. Lipid-containing mimetics of natural triggers of innate immunity as CTL-inducing influenza vaccines. Int. Immunol. 2006;18:1801–1813. doi: 10.1093/intimm/dxl114. [DOI] [PubMed] [Google Scholar]
- Liehl P., Meireles P., Albuquerque I.S., Pinkevych M., Baptista F., Mota M.M., Davenport M.P., Prudencio M. Innate immunity induced by Plasmodium liver infection inhibits malaria reinfections. Infect. Immun. 2015;83:1172–1180. doi: 10.1128/IAI.02796-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liehl P., Zuzarte-Luis V., Chan J., Zillinger T., Baptista F., Carapau D., Konert M., Hanson K.K., Carret C., Lassnig C., Muller M., Kalinke U., Saeed M., Chora A.F., Golenbock D.T., Strobl B., Prudencio M., Coelho L.P., Kappe S.H., Superti-Furga G., Pichlmair A., Vigario A.M., Rice C.M., Fitzgerald K.A., Barchet W., Mota M.M. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat. Med. 2014;20:47–53. doi: 10.1038/nm.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L., Seneca E. G6PD deficiency: a classic example of pharmacogenetics with on-going clinical implications. Br. J. Haematol. 2014;164:469–480. doi: 10.1111/bjh.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifsud E.J., Tan A.C., Reading P.C., Jackson D.C. Mapping the pulmonary environment of animals protected from virulent H1N1 influenza infection using the TLR-2 agonist Pam(2)Cys. Immunol. Cell Biol. 2016;94:169–176. doi: 10.1038/icb.2015.81. [DOI] [PubMed] [Google Scholar]
- Mifsud E.J., Tan A.C., Short K.R., Brown L.E., Chua B.Y., Jackson D.C. Reducing the impact of influenza-associated secondary pneumococcal infections. Immunol. Cell Biol. 2016;94:101–108. doi: 10.1038/icb.2015.71. [DOI] [PubMed] [Google Scholar]
- Miller J.L., Sack B.K., Baldwin M., Vaughan A.M., Kappe S.H. Interferon-mediated innate immune responses against malaria parasite liver stages. Cell Rep. 2014;7:436–447. doi: 10.1016/j.celrep.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Naotunne T.S., Karunaweera N.D., Del Giudice G., Kularatne M.U., Grau G.E., Carter R., Mendis K.N. Cytokines kill malaria parasites during infection crisis: extracellular complementary factors are essential. J. Exp. Med. 1991;173:523–529. doi: 10.1084/jem.173.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tan A.C., Mifsud E.J., Zeng W., Edenborough K., McVernon J., Brown L.E., Jackson D.C. Intranasal administration of the TLR2 agonist Pam2Cys provides rapid protection against influenza in mice. Mol. Pharm. 2012;9:2710–2718. doi: 10.1021/mp300257x. [DOI] [PubMed] [Google Scholar]
- Zheng H., Tan Z., Zhou T., Zhu F., Ding Y., Liu T., Wu Y., Xu W. The TLR2 is activated by sporozoites and suppresses intrahepatic rodent malaria parasite development. Sci. Rep. 2015;5:18239. doi: 10.1038/srep18239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.