Abstract
Primary cilia (PC) are antenna‐like organelles that protrude from most mammalian cells. They are essential for the regulation of several signaling pathways such as Hedgehog and WNT. It is therefore not surprising that a dysfunction of PC is frequently associated with pathologies. Originally, PC were found to be involved in a variety of diseases commonly referred to as ciliopathies including cystic kidney diseases. Evidence is accumulating that PC play also an important role in cancer formation and regulation, which is the focus of this review.
Keywords: cancer, Hedgehog, primary cilia
Subject Categories: Cancer, Signal Transduction
Glossary
- ADAM
A disintegrin and metalloproteinase
- ADAM
A disintegrin and metalloproteinase
- APC
Adenomatosis polyposis coli
- Arl13b
ADP ribosylation factor‐like 13b
- BCC
Basal cell carcinoma
- BOC
Brother of CDO
- CBF‐1
C‐promoter‐binding factor‐1
- ccRCC
Clear cell renal cell carcinoma
- CCRK
Cell cycle‐related kinase
- CDK2
Cyclin‐dependent kinase 2
- Cdo
Cell adhesion molecule down‐regulated by oncogenes
- CK1
Casein kinase 1
- CP110
Centriolar coiled‐coil protein of 110 kDa
- CPC
Choroid plexus carcinoma
- CP
Choroid plexus
- CROCC
Ciliary rootlet Coiled‐coil
- Dll
Delta‐like ligand
- Dvl
Disheveled
- Dync2h1
Cytoplasmic dynein 2 heavy chain 1
- EGF
Epidermal growth factor
- EMT
Epithelial–mesenchymal transition
- ENU
Ethylnitrosourea
- Erk
Extracellular signal‐regulated kinase
- EVC‐1
Ellis‐van Creveld syndrome protein‐1
- FGF
Fibroblast growth factor
- Gas‐1
Growth arrest‐specific‐1
- GBMC
Glioblastoma cells
- GBM
Glioblastoma
- Gli
Glioma‐associated oncogene
- GNP
Granule neuron progenitors
- GPR161
G‐protein‐coupled receptor 161
- GSK3
Glycogen synthase kinase 3
- GTP
Guanosine triphosphate
- HDAC2
Histone deacetylase 2
- Hh
Hedgehog
- HIF
Hypoxia‐inducible factor
- HMLE
Human mammary epithelial cell line
- IFT88
Intraflagellar transport protein of 88 kDa
- IGF
Insulin growth factor
- INPP5E
Inositol‐polyphosphate‐5‐phosphatase
- Intu
Inturned
- IPMN
Intraductal papillary‐mucinous neoplasia
- Kif3a
Kinesin family member 3a
- Kif7
Kinesin family member 7
- KO
Knockout
- LEF
Lymphoid‐enhancing factor
- LPAR1
Lysophosphatidic acid receptor 1
- LRP
Lipoprotein receptor‐related protein
- MAPK
Mitogen‐activated protein kinase
- MEF
Murine embryonic fibroblast
- Nek2
NIMA‐related kinase 2
- NEK8
Never in mitosis gene A‐related kinase 8
- NICD
Notch intracellular domain
- OFD1
Oral‐facial‐digital syndrome protein 1
- OSE
Ovarian surface epithelium cells
- PCP
Planar cell polarity
- PC
Primary cilia
- PD‐1
Programmed cell death protein 1
- PDAC
Pancreatic ductal adenocarcinoma
- PDGFR
Platelet‐derived growth factor receptor
- PI(4‐5)P2
Phosphoinositol‐4,5‐diphosphate
- PI3 kinase
Phosphoinositide 3‐kinase
- PIN
Prostatic intraepithelial neoplasia
- PIP
Phosphoinositol 4‐phosphate
- PKA
Protein kinase A
- PlK1
Polo‐like kinase 1
- Plk4
Polo‐like kinase 4
- Ptch
Patched
- PTM
Post‐translational modifications
- Rbl1
Retinoblastoma‐like 1
- ROR2
Receptor tyrosine kinase‐like orphan receptor
- RTK
Receptor tyrosine kinase
- RYK
Receptor‐like tyrosine kinase
- SCC
Squamous cell carcinoma
- SMAD
small worm phenotype/mothers against decapentaplegic
- Smo
Smoothened
- SPEN
nuclear protein split ends
- SuFu
Suppressor of fused
- TACE
TNFalpha‐converting enzyme
- TCF
T‐cell factor
- TGF‐beta
Transforming growth factor‐beta
- Trp53
Transformation‐related protein 53
- VHL
von Hippel–Lindau
- Wnt
Wingless integration site
- ZEB‐1
Zinc finger E‐box‐binding homeobox
Introduction
Most of the differentiated cells in humans display a hair‐like organelle protruding from their surface, which is referred to as the cilium (from the Latin word for eyelashes). Two kinds of cilia have been described: motile cilia and primary cilia. The principal function of the former was easy to understand by mere observation: Motile cilia beat in a regular and coordinated fashion and, by doing so, put fluids in motion around them, like the mucus that is pushed upwards from the bronchi to the trachea 1, 2. The date of the first description of primary cilia is somewhat controversial, but has been at least as early as 1898 3. Already at that time, a sensory function for PC was suggested. Nevertheless, despite finer description by electron microscopy 4, 5 they were considered for a long time as anomalous, rudimentary structures without any function. This just changed in the 1990s 6, when PC were associated with the onset of human diseases 7, 8. Nowadays, PC are commonly considered as crucial sensors, antenna or rheostat of the cell by providing the ability to fine‐tune cell signaling and thus key cellular processes. Malfunctioning cilia can therefore be the cause of a diverse set of diseases called ciliopathies. These syndromes include rare inborn disorders like Joubert syndrome, Bardet–Biedl syndrome, and Meckel syndrome, but also more common pathologies like polycystic kidney diseases (PKDs). Ciliopathies have been extensively reviewed elsewhere 9, 10. In this review, focus will be set on the role of primary cilia in tumorigenesis.
Two aspects of cilia biology are important when considering their role in cancer: the coordination of cilia formation (ciliogenesis) during cell cycle, and the regulation of cancer‐linked signaling pathways. We will first discuss the former and subsequently the role of PC in modulating signaling pathways.
The protruding part of the primary cilium is composed of an axoneme made of nine doublets of post‐translationally modified microtubules ensheathed by a ciliary membrane, which is continuous with the plasma membrane but of different composition in lipids and proteins 11. The axoneme is rooted by the basal body, which is anchored at the membrane (Fig 1). Because the basal body is derived from the mother centriole of the centrosome, which has to assemble the mitotic spindle, the primary cilium can only be formed in the G0 or G1 phase of the cell cycle and is usually resorbed during the G1/S or G2/M phases 12, 13, although some cells retain primary cilia in S/G2/M phases (reviewed in ref. 13). Based on this concept, a model emerged that primary cilia develop only in quiescent or differentiated cells: The highest the mitotic index, the least ciliated are the cells in an unsynchronized population. In support of this idea, cell cycle‐dependent actors tightly regulate assembly and disassembly of the primary cilium. First, CP110 (centriolar coiled‐coil protein of 110 kDa), a protein that caps the distal end of the mother centriole to prevent ciliary formation, is tightly regulated at both the transcriptional and translational levels during the cell cycle and is phosphorylated by CDK2 (cyclin‐dependent kinase 2) 14. Moreover, the mitotic kinases, Aurora A 12, 15, Plk1 (Polo‐like kinase) 16 and Nek2 (NIMA‐related Kinase) 17, were shown to block the assembly of the primary cilium. As cancer cells have an elevated proliferation compared to normal cells and several types of cancer cells were reported to be unciliated 18, the general consensus was that cancer cells are unlikely to display cilia. However, the improvement of reagents and techniques for the detection of PC showed that PC can be also present in cancer cells 19. Today, a growing body of work demonstrates that transformed cells are capable of retaining the ability to form cilia even in culture without serum starvation 19, 20.
Figure 1. Basic architecture of primary cilia.
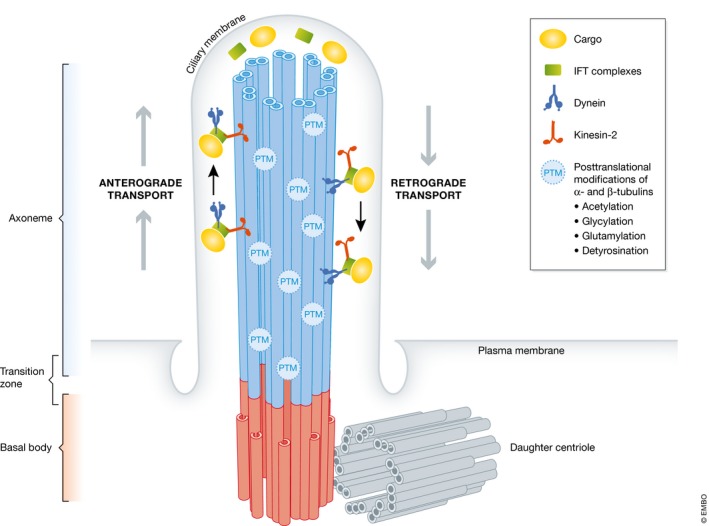
The scaffold of primary cilia is composed of nine doublets of microtubules arranged in a circle, the so‐called axoneme. The microtubules consist of polymerized alpha‐ and beta‐tubulin dimers and are stabilized by post‐translational modifications (PTM) such as acetylation, glycylation, and glutamylation 130. The axoneme is anchored in the cell via the basal body that consists of gamma‐tubulin and originates from the mother centriole (the associated daughter centriole is shown in gray). In the absence of PC, mother and daughter centrioles form the centrosome. Cargo proteins are transported along the axoneme by intraflagellar transport proteins, a process that is driven by motor proteins such as kinesin‐2 toward the ciliary tip (anterograde) and dynein toward the base (retrograde).
Two landmark studies published in 2009 nicely demonstrate that the presence of primary cilium can both promote and suppress tumorigenesis in medulloblastoma and basal cell carcinoma (BCC) 21, 22. Interestingly, these two cancers have a dysregulation of the Hedgehog pathway in common, a major signaling pathway regulated through the primary cilium.
Ciliary modulation of signaling pathways
Primary cilia are crucial regulators of cell signaling. The receptors and downstream effectors of many signaling proteins are enriched and sequestered in the cilium, and this compartmentalization allows for fine temporal and spatial regulation of pathway activation as well as downstream signal propagation 23. This chapter will provide a brief overview on the signaling pathways that are thought to be regulated by the primary cilium and that have been linked to cancer.
Hedgehog (Hh) signaling
In vertebrates, the best‐studied cilia‐linked pathway is the Hedgehog (Hh) pathway, which plays fundamental roles during development and in adult tissue homeostasis. Indeed, this pathway is aberrantly activated in several cancers (reviewed in ref. 24). The first description of a formal link between the primary cilium and Hedgehog signaling was established during a screen aimed at analyzing embryonic mutations induced by ethylnitrosourea (ENU) in mice. The authors 8 showed that mutations in IFT72 and IFT88, two proteins of the intraflagellar transport machinery, perturb hedgehog signaling. After this first description, numerous studies investigated how PC and Hh signaling are connected. A striking result of this body of work is that all the key components of the Hh pathway are enriched in the cilium 25, 26, 27, 28 and that their localization changes dynamically in response to the activation of the pathway. The regulation of this pathway is extremely sophisticated comprising the two receptors Patched (which binds the hedgehog ligands) and Smoothened (which is responsive to specific molecules including oxysterols and purmorphamine), several co‐receptors (such as Cdo, BOC, Gas‐1), kinases (including GSK3b, PKA, CK1), as well as activators and repressors (including GPR161, SuFu, EVC1, and EVC2). For recent reviews on Hedgehog signaling and its actors, see 29, 30.
Briefly, in its “Off‐state”, Patched, the receptor of Hh ligands accumulates in the ciliary membrane (Fig 2). It is thought to repress the entry of Smoothened (Smo) in the cilium and its subsequent activation. The transcription factors of the pathway (Gli1, Gli2 and Gli3) are transported to the tip of the cilium in a complex with SuFu and Kif7 26, 31, 32. Unlike Gli1, which is a strict activator and functions as part of a positive feedback loop (33), the Gli2 and Gli3 transcription factors both have the potency to be activator or repressor of their target genes. They are potential activators (GliA) in their full‐length form 34, but binding of the Glis to SuFu in the Off‐state prevents their entry into the nucleus. Moreover, the full‐length Glis are phosphorylated by a kinase complex consisting of protein kinase A (PKA), glycogen synthase kinase 3 (GSK3), and casein kinase 1 (CK1a), which leads to their partial proteolysis into a shorter repressor form (GliR) with help of the G‐protein‐coupled receptor GPR161 35. Upon binding of Sonic Hedgehog to Patched, the receptor is removed from the cilium as well as GPR161, de‐repressing Smo, which can in turn enter the cilium and get modified to activate downstream signaling. The Gli transcription factors accumulate at the tip of the cilium at higher levels than in the Off‐state and, along the way, detach from SuFu, which allows GliA to enter the nucleus to activate their target genes.
Figure 2. Hedgehog signaling in PC .
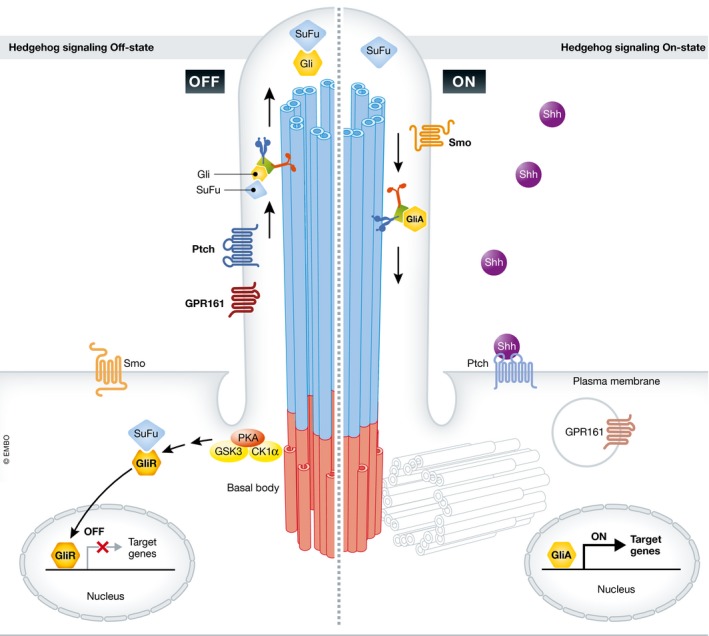
In the absence of Hh ligands such as Sonic Hh (Shh), the negative regulators Ptch and GPR161 are present on the ciliary membrane. In this state (OFF), suppressor of fused (SuFu) forms a complex with the Gli transcription factors at the tip of the cilium. Upon phosphorylation by the kinases PKA, glycogen synthase kinase 3 (GSK3) and casein kinase 1α (CK1α) 1 at the basal body, Gli is processed in a repressor form (GliR) and migrates into the nucleus, where it represses the transcription of target genes. Ligand binding to Ptch (ON) induces its translocation away from the cilium and promotes the entry of the activating receptor Smo. This process allows the migration of active Gli (GliA) into the nucleus where transcription of Hh target genes is activated. Whether SuFu dissociates from Gli or migrates associated with GliA into the nucleus remains controversial.
The movements of some actors of this pathway within the cilium are at least partly supported by the intraflagellar transport machinery along the axoneme for their removal from the cilium 23, 36, 37, which explains that mutations on proteins from the IFT lead to impaired Hh signaling. It is worth noting that the regulation of the composition of the ciliary membrane is also a major regulator of signaling. Indeed, two recent studies underlined the role of phosphoinositide metabolism in regulating Hh signaling in the cilium 38, 39. They described that the ciliary membrane is enriched in phosphoinositol 4‐phosphate (PIP) and that this enrichment is maintained by the inositol phosphatase INPP5E. When INPP5E is nonfunctional, the main phosphoinositol in the ciliary membrane becomes the diphosphate PI(4‐5)P2, which disrupts Hh signaling, notably, because it leads to the accumulation of the repressor GPR161.
Finally, Gli transcription factors can be also activated independently of Hh ligands or Smo through the so‐called non‐canonical Hh signaling. Nevertheless, this pathway appears to be rather cilia‐independent and thus is beyond the frame of this review. For reviews on this topic see 40, 41.
Wnt signaling
The Wnt signaling pathway is initiated by binding of Wnt ligands, which are lipid‐modified secreted ligands to the Frizzled family of receptors that can have different co‐receptors, such as low‐density lipoprotein receptor‐related protein 5 (LRP5) and 6 (LRP6) (Fig 3). Nineteen Wnt ligands and ten Frizzled receptors forming homo‐ and heteromeric complexes create a rather complex signaling network (Reviewed in ref. 42). Moreover, Wnt ligands can also bind non‐Frizzled receptors, such as the receptor tyrosine kinases ROR2 and RYK 43. The key feature of the canonical Wnt signaling pathway is the stabilization in the cytoplasm and subsequent translocation of the protein beta‐catenin into the nucleus, where it activates transcription factors of the T‐cell factor/lymphoid‐enhancing factor (TCF/LEF) family. In its Off‐state, beta‐catenin is degraded in the cytoplasm by a protein complex that includes Axin, adenomatosis polyposis coli (APC), glycogen synthase kinase 3 (GSK3), and casein kinase 1α (CK1α) 44. Upon binding of Wnt ligands to Frizzled, LRP is phosphorylated and recruits the protein disheveled (Dvl), which becomes activated. Activated Dvl interacts with the destruction complex, thus preventing beta‐catenin degradation and favoring its translocation into the nucleus. Wnt can also signal through the non‐canonical, PCP (planar cell polarity) pathway, which is modulated through Dvl and independent of beta‐catenin. This complex pathway is a major player for the establishment of cell polarity in the plane of the epithelium. Due to its capacity to regulate both the canonical and non‐canonical Wnt signaling, Dvl was suggested to participate in the molecular switching between the two pathways 45.
Figure 3. Wnt signaling in PC .

(A) In the absence of Wnt ligand binding to the Frizzled‐LRP5/6 receptor complex, beta‐catenin is part of protein complex, including Axin, adenomatosis polyposis coli (APC), glycogen synthase kinase 3 (GSK3), and casein kinase 1α (CK1α) that leads to its destruction. (B) Ligand binding to Frizzled‐LRP5/6 activates disheveled that destabilizes the destruction complex thus releasing active beta‐catenin that can then migrate to the nucleus. There it binds to the transcription factors T‐cell factor/lymphoid enhancer‐binding factor (TCF/LEF) to switch on the transcription of Wnt target genes.
Because of the similarities of the Wnt pathways to Hedgehog, it was tempting to imagine a role of the primary cilium in the regulation of the Wnt pathways, especially since many actors of the pathways are present at the cilium or basal bodies. These include vangl‐2, GSK3 beta, APC and inversin that have been described to target Dvl for degradation 45, 46, 47, 48. However, the role of the primary cilium in regulating Wnt pathways remains controversial. A striking example of this controversy lies in the result of two studies that used knockdown approaches for genes that are essential for ciliogenesis. Corbit et al 49 observed an increased susceptibility to Wnt ligand in mouse embryos as well as murine embryonic fibroblasts (MEFs) deficient for the ciliary genes Kif3a, Ift88 or Ofd1, whereas another study observed no altered response to Wnt ligands in embryos or MEFs deficient for Kif3a, Ift88, or Dync2h1 28. These results, among others (reviewed in refs. 43, 50, 51), are difficult to reconcile especially since zebrafish mutants of IFT88 and ciliopathic mouse models fail to show phenotypes indicative of disrupted canonical or PCP (e.g., convergent extension phenotypes) Wnt signaling 28, 52. To conclude, the role of the primary cilium in Wnt signaling is still rather unsettled.
Notch signaling
The Notch signaling system consists of three Delta‐like (Dll1, Dll3, and Dll4), two Jagged ligands (Jagged1, Jagged2), and four receptors (Notch 1–4). Ligand–receptor binding induces two proteolytic cleavages first by ADAM/TACE followed by gamma‐secretase releasing the Notch intracellular domain (NICD) into the cytoplasm. NICD binds to the transcriptional repressor C‐promoter‐binding factor‐1 (CBF1) to form a complex called CSL that can then activate the key transcription factors Hes and Hey. Components of the Notch pathway were found in the PC/basal body, but the impact of PC on Notch signaling appears to be tissue specific 53, 54. A recent article describes that PC deficiency in corneal epithelial cells leads to an increased proliferation, but decreased Notch signaling 55. This is in line with the observation that PC‐deficient keratinocytes in vitro and in the embryonic epidermis of mice have defects in Notch signaling 53. Other reports, however, described active Notch signaling in the absence of PC in murine pancreas, a retinal pigment epithelial cell line as well as zebrafish tissue 54, 56, 57. The way Notch signaling is regulated by PC appears therefore to be cell type specific.
Receptor tyrosine kinases
Primary cilia were also reported to coordinate receptor tyrosine kinases (RTKs), including several receptors for growth factors, such as fibroblast growth factor (FGF), epidermal growth factor (EGF), insulin‐like growth factor (IGF), though probably the best‐studied RTKs are platelet‐derived growth factor receptor (PDGFR) and transforming growth factor‐beta (TGF‐beta) receptors 58. Binding to their cognate ligands induces phosphorylation of tyrosine in the cytoplasmic tail of the receptor that allows recruitment of adaptor molecules and the subsequent initiation of signaling cascades, such as mitogen‐activated protein kinases (MAPK) and PI‐3/Akt kinase. PDGFR consist of two isoforms, i.e., alpha and beta, which can form homo‐ and heterodimers. PDGFRalpha, but not the beta isoform, was shown to be localized to PC in quiescent cells 59. Studies on ciliary mutants revealed that PDGFRalpha localizes to the plasma membrane in the absence of PC, where it is over‐activated upon ligand binding 60. Notably, aberrant PDGFRalpha signaling has been associated with pathologies such as gastrointestinal stromal tumors 61.
TGF‐beta is a classical pleiotropic cytokine that is well illustrated by its dual role during tumorigenesis, as it can have a tumor suppressive function in normal and early‐stage tumor cells, but acts rather as a tumor promoter in more advanced disease stages 62. Different isoforms for TGF‐beta ligand and receptors are known that contribute to the complexity of this ligand/receptor system. Binding of the ligand to TGF‐beta type 2 receptor leads to the recruitment and phosphorylation of TGF‐beta type 1 receptor. Activated TGF‐beta type 1 receptor can trigger different signaling cascades by phosphorylating and activating signal transducers such as the MAPK ERK1/2 or PI‐3/Akt kinase as well as SMAD transcription factors, the main component of the canonical TGF‐beta/Smad pathway. More specifically, TGF‐beta‐initiated signaling leads to the phosphorylation of a SMAD2/3 complex that can then associate with SMAD4. This trimeric complex translocates to the nucleus to induce gene expression.
TGF‐beta receptors have been shown to localize to PC and that their effector signaling components ERK1/2 and SMAD3 accumulate at the ciliary base upon TGF‐beta‐induced activation 63, 64. PC‐mediated signaling of TGF‐beta was shown to be regulated by endocytosis 63 and ceramide 65.
To conclude, the PC is a hub that tunes a large panel of signaling pathways. The main and best understood pathway in terms of its relation with the PC is Hh signaling, and the way how the PC delegates the other signaling pathways still requires further investigation especially regarding tissue and cell specificity. In the following section, we will discuss how the PC regulation of signaling pathways can modulate carcinogenesis. Nevertheless, many studies linking primary cilia and cancer are largely observational and do not elaborate on the mechanisms of cancer regulation by PC.
PC in brain cancers
Medulloblastoma
It is well established that Hh signaling and PC play an important role in the development of the brain. Several reports investigated their implication in medulloblastoma, the most common pediatric brain cancer, and glioblastoma, an aggressive primary brain tumor in adults. The work of Han et al 21 focused on the role of PC in medulloblastoma. The authors were intrigued by reports that the proliferation of cerebellar neuron precursors (GNPs) is PC and Hh dependent 66, 67 and that GNPs can be at the origin of medulloblastoma. For this, they generated mice with constitutive expression of an active form of Smo, i.e., SmoM2, in GNPs using a specific cre‐transgenic mouse strain. These mice have normal PC and develop medulloblastoma. This strain was crossed with mice carrying conditional knockout (KO) alleles (flx/flx) for genes essential for cilia formation, i.e., the kinesin family member 3A (Kif3a) 68 and intraflagellar transport protein 88 (Ift88) 69. Depletion of Kif3a or Ift88 and the resulting removal of PC in GNPs completely blocked medulloblastoma formation. This concurs with a study using mutant mice that harbor only one Ptch allele which develop spontaneous medulloblastoma 70. GNP‐specific deletion of Kif3a abrogated tumor development in this mouse strain as well 70. To further explore the link between PC expression and tumorigenesis, Han el al 21 generated a mouse strain that allows expression of a constitutively active form of the Hh effector Gli2 in GNPs. Surprisingly, ectopic expression of the active form of Gli2 in GNPs did not result in tumor formation in those mice. Tumors were only detectable after deletion of primary cilia by crossing with Kif3aflx/flx mice. Thus, PC appear to have a dual function in medulloblastoma formation in mice by either promoting or blocking tumorigenesis, depending on the nature of the oncogenic initiating event. The authors proposed a model in which PC regulate the activity of both Smo and Gli3, the main repressor of the pathway. In fact, PC‐deficient GNP cells that express ectopically active Gli2 display decreased Gli3 repressor expression levels. Notably, similar conclusions have been made by Wong et al 22, who studied the role of PC and Hh in basal cell carcinoma (see below). To translate their observations made in mice to human pathology, Han and colleagues correlated the gene expression profile with PC presence in biopsies of 24 medulloblastoma patients and detected PC mainly in tumors with active Hh or Wnt signaling, suggesting that PC also regulate Wnt signaling in medulloblastoma. A more extensive analysis of biopsies is required to confirm whether high Hh signaling is only present in tumors displaying PC and whether PC could serve as a biomarker for medulloblastoma. In fact, one analysis of 14 medulloblastoma biopsies identified PC on 12 of the tested samples, but no correlation with either tumor subtype or signaling pathway 71. The latter study also described that PC were not detectable on CD15+ cancer stem cells.
Several mechanisms have been recently described to regulate Sonic Hedgehog (Shh)‐driven medulloblastoma. One study identifies the inositol phosphatase INPP5E as an important regulator for dampening cilia‐associated phosphoinositide PI3‐kinase/serine/threonine kinase AKT signaling 72. The authors show that INPP5E localizes to PC in tumor cells and that its absence leads to an activation of the PI 3‐kinase/Akt pathway and in turn to a destabilization of PC. The findings obtained in mice and ex vivo cultures were supported by the analysis of biopsies correlating low INPP5E transcript levels with an increased overall survival in neuroblastoma patients.
Two other cilia based tumor regulators have been recently identified for Shh‐dependent medulloblastoma 73, 74. First, the GTPase ADP ribosylation factor‐like 13b (ARL13b) is specifically expressed in PC and a widely used marker for PC. Bay et al discovered that deletion of ARL13b in Ptch‐deficient mice controlled medulloblastoma formation. ARL13b knockdown in human and mouse medulloblastoma cell lines reduced Shh signaling and proliferation 73. In addition, they reported that postnatal depletion of ARL13b has no overt phenotype in mice, though they observed a few cysts in the kidney. This led the authors to propose ARL13b as a suitable drug target for medulloblastoma.
Second, the G‐protein‐coupled receptor Gpr161 has been described as a negative regulator of the Hh pathway 35. Shimada and colleagues found that conditional depletion of Gpr161 in GNPs or neural stem cells induces their hyperproliferation in a PC‐dependent manner leading to spontaneous formation of cerebellar tumors in mice 74. Gene expression profiling of those tumors revealed a Shh medulloblastoma subtype signature. Moreover, they reported that low Gpr161 expression correlates with poor prognosis in Shh medulloblastoma patients supporting the concept that Gpr161 is a tumor suppressor.
Glioblastoma
Two reports describe the presence of PC in glioblastoma specimens using light and electron microscopy. Sarkisian and colleagues detected PC in up to 25% of cells in 23 human glioblastoma biopsies tested 75. PC were also detected in different regions of the tumor microenvironment. Moreover, the presence of PC correlated with expression of the proliferation marker Ki‐67 and the transcription factor ZEB1 that is linked to tumor initiation and invasiveness. In the same study, recently derived primary tumor cell lines were also shown to display PC, which is in contradiction with the work from Moser and colleagues. In fact, the latter report describes perturbated ciliogenesis in cultured glioblastoma cell lines (GBMC) 76. This finding was confirmed in a later study by the same authors, where they describe a disruption of ciliogenesis at early tumor stages in the majority of seven patient samples tested 77. It is tempting to speculate that the rudimentary cilia still display some signaling activity or even promote different signaling pathways than intact PC. An analysis of a larger cohort of tissues should reconcile the reports on PC expression in glioblastoma and clarify whether subsets with distinct ciliogenesis ability exist.
Two other studies describe mechanisms, which dampen ciliogenesis and thus promote tumor cell growth in glioblastoma cells. First, Yang et al 78 described the cell cycle‐related kinase (CCRK) as an inhibitor of ciliogenesis which in turn promotes proliferation of glioblastoma cells. It should be interesting to study how the CCRK expression status correlates with PC presence in tumor tissues. Second, Loskutov et al 79, identified an accumulation of lysophosphatidic acid receptor 1 (LPAR1) in primary cilia of human astrocytes. Astrocytes are one of the cell types that can be on the origin of GBM. Loskutov et al could show that loss of PC redistributes LPAR1 to the plasma membrane, where it binds to effector molecules and thus initiates proliferation. Importantly, a LPAR antagonist was found to block growth of GBM patient‐derived xenografts in vivo.
Choroid plexus tumor
Choroid plexus (CP) tumor is a rare type of pediatric brain tumor. Li et al 80 generated a mouse model with constitutive active Notch1 in CP epithelium that induced tumor development. These tumors displayed elevated Hh signaling activity, and while normal CP epithelial cells have been described to exhibit multiple PC 81, CP tumor cells in this study were found to have only one primary cilium. Importantly, an analysis of human specimens showed that in normal CP epithelium, only multiciliated cells were detectable, whereas malignant CP carcinomas (CPC) and about half of the tested benign CP papillomas (CPP) displayed only PC. Primary cell cultures revealed that epithelial cells from Notch‐induced CP tumors, expressing only one PC but not normal multiciliated epithelial cells, were responsive to Shh agonists. In addition, human CP tumors displayed a signature of activated Notch and Hh signaling. As progenitors of normal CP epithelial cells have also only one PC with active Hh signaling, the authors propose a model that aberrant Notch activation in progenitor cells blocks differentiation and promotes tumorigenesis. In support of the model that Notch preserves PC‐mediated Hh signaling is the work of Stasiulewicz el al 82 showing that Notch regulates Hh activity by controlling the localization of Hh components in the cilium.
PC in skin cancer
Basal cell carcinoma
Basal cell carcinoma (BCC) is the most common skin cancer and frequently caused by either mutational inactivation of Ptch or activation of Smo in epidermal keratinocytes 83. Concurring with the established link between PC and Hh signaling, expression of PC has been found in biopsies of BCC patients and skin lesions in mice constitutively expressing active Smo 22. A more recent study reported abundant ciliated keratinocytes in BCC specimens in comparison with healthy skin and biopsies of squamous cell carcinoma (SCC), another form of skin cancer 20. To explore the role of PC and Hh in BCC, Wong and colleagues used an approach similar to the one described by Han el al in their study on medulloblastoma (see above). They generated mouse strains expressing a constitutively active form of either Smo or Gli2 in keratinocytes and found that active Smo but not Gli2 induced tumor formation. Depletion of PC in keratinocytes abolished tumor development in the mouse model with constitutively active Smo, but induced tumorigenesis in mice with constitutive expression of active Gli2. Thus, as in medulloblastoma, PC appear to have a dual function in BCC formation by either promoting or blocking tumorigenesis in mice depending on the nature of the oncogenic initiating event.
Primary cilia formation and Hh signaling in BCC were found to be regulated by inturned (INTU), a cilia and planar polarity effector protein 20. INTU expression was found to be elevated in biopsies of BCC, as compared to those of SCC patients and healthy donors and correlated with the presence of PC. This together with the correlation of INTU and Hh responsive genes suggests an association between ciliogenesis, Hh signaling activity and INTU expression in BCC. Next, Yang el al found that Intu depletion in keratinocytes blocks PC formation and Hh signaling and importantly protects mice from SmoM2‐induced BCC. Constitutively, active Gli2, however, was capable to trigger Hh activity in Intu‐deficient MEFs, suggesting that INTU acts upstream of Gli transcription factors.
Melanoma
One study by Kim et al 84 analyzed the expression during melanoma development in the biopsies of 62 patients. Interestingly, PC expression was prominent in melanocytic nevi and nearly completely absent in the further advanced stages, i.e., melanoma in situ, invasive melanoma, and metastatic melanoma. The authors concluded that PC down‐regulation is a promoter and potential biomarker of melanoma development. Another report proposed that polo‐like kinase 4 (PLK4), a regulator of centrosome duplication, regulates PC expression in the skin 85. PLK4 over‐expression in mice induces tissue hyperplasia in skin, which was associated with compromised melanocyte differentiation as well as centriole amplification and PC down‐regulation in the epidermis. Notably, transcript levels of PLK4 were found to be elevated in half of the 48 tested gastric cancers when compared to corresponding non‐cancerous tissues 86.
A recent study further explored the mechanism responsible for the down‐regulation of PC during melanoma development 87. This study identified the methyltransferase EZH2 as a silencer of ciliary genes. In fact, EZH2 is part of the polycomb repressive complex 2 (PRC2) known for its ability to suppress gene expression via histone methylation. Notably, transcriptome analysis showed a significant upregulation of EZH2 levels in malignant melanoma compared to benign nevi and, moreover, inverse correlation to PC expression during melanoma development. Mouse models confirmed that EZH2 drives melanoma formation but is not sufficient to induce tumor formation. Finally, EZH2‐driven suppression of ciliary genes in human melanoma cells resulted in the disassembly of PC that in turn promoted Wnt signaling.
Taken together, while PC are present in BCC they appear to be down‐regulated in SCC and melanoma supporting the concept that the presence of PC is tumor‐type specific.
PC in epithelial cancers
Breast cancers
Several studies investigated the role of PC in epithelial tumors, such as breast cancer. Different classifications for breast cancer exist; among them, one is related to the expression status of hormone receptors such as estrogen, progesterone and epidermal growth factor. Breast cancer cells not expressing those hormone receptors are termed triple‐negative. One report describes decreased presence of PC in human breast cancer cell lines and tissues 88. In this study, PC were detected in fibroblasts of normal breast tissue and nearly exclusively in stromal fibroblasts of breast cancer biopsies. This corresponds with the report of Nobutani and colleagues that PC are hardly detectable in epithelial cells of breast cancer 89. Menzl el al 90 reported a down‐regulation of PC during breast cancer progression in both the epithelial and stromal compartments. The same group investigated the polyoma middle T mouse breast cancer model and detected that inhibition of cilia formation in mammary epithelial cells increases Hh signaling and tumorigenesis as well as tumor grade 91.
Mammary and mammary cancer stem cells (or tumor‐initiating cells) depend on Hh signaling as well as mechanisms related to epithelial–mesenchymal transition (EMT). Guen and coworkers discovered that PC can link both processes 92. They identified PC in murine mammary glands in the basal epithelial stem cell compartment, but hardly in luminal epithelial cells. The presence of PC correlated with the EMT associated transcription factor Slug. In agreement with other reports, PC were also found in stromal cells of mammary glands 89, 93. In addition, PC were detected in a stem cell‐like subset of a human mammary epithelial (HMLE) cell line. EMT induction by either ectopic expression of EMT‐related transcription factors or deletion of E‐cadherin significantly increased the number of PC and activation of Hh signaling. Similar observations were made for HMLE cells transformed by expression of a constitutively active form of H‐RAS. Finally, PC were found to be required for the maintenance of mammary stem cells as well as tumor‐initiating cells. The authors emphasize that those findings were made on basal mammary stem cells and the transformed HMLER cells that form tumors of the basally derived claudin‐low subtype and might be different for other breast cancer subtypes that originate from luminal breast epithelium. In fact, this model would reconcile the results of Guen and colleagues with the reports on decreased PC expression on breast cancer cells 88, 89, 90 and that PC act as tumor suppressor in polyomavirus middle T‐induced mammary tumors that originate from the lumen 91.
One analysis of human breast cancer cell lines reported that the nuclear protein split ends (SPEN) regulates cilia formation and migration of PC expressing breast cancer cells 94. Elevated transcript levels of SPEN were found to be indicative of a better prognosis in triple‐negative breast cancer. The hormone‐independent function of SPEN complements to the original finding that SPEN acts as a tumor suppressor in breast cancer by acting as a transcriptional co‐repressor of estrogen receptor alpha 95, 96.
Pancreatic cancers
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal cancers, since patients are often diagnosed when tumors are already at advanced stages. Several reports describe PC expression in pancreatic cancer. Bailey et al 97 studied mice, in which pancreatic tumor cell lines were orthotopically implanted and described that over‐expression of Shh promotes tumor formation. Primary cilia were detected in normal pancreatic ductal epithelium, but in the tumors, only stromal fibroblasts and not epithelial cells displayed PC. The authors proposed a model, in which Shh secreted by the pancreatic epithelium triggers PC formation in stromal fibroblasts. Seeley el al 98 investigated oncogenic Kras‐driven mouse models for pancreatic cancer displaying a down‐regulation of PC during the transition from normal pancreas tissue to precursor lesions. Tissue‐specific knockdown of PC in this mouse model was found in another study to promote tumor formation by activating the mevalonate pathway 99. The work of Seeley et al also described the absence of PC in precursor lesions of patients and concluded that the down‐regulation of PC is an early event in pancreatic carcinogenesis. Emoto and coworkers detected PC on cancer cells in 25 of 100 analyzed biopsies of PDAC patients 100. Patients with PC‐positive cancer had an increased number of lymph node metastasis and poorer overall survival, and the authors proposed that PC expression could be an independent prognostic indicator. The presence of PC was also reported on stromal cells, though this was not quantified. A subsequent report described PC presence in paraffin‐embedded pancreas taken from healthy subjects and patients at different disease stages including precursor lesions in intraductal papillary‐mucinous neoplasia (IPMN) and different adenocarcinoma stages 101. This study displayed a gradual down‐regulation of PC numbers in epithelial cells during tumor progression from healthy tissue to PDAC that was paralleled by an increase in PC numbers in stromal cells. In line of the reported restriction of active Hh signaling in the stromal compartment of pancreatic cancer tissues, the authors promote a model that PC regulate Hh signaling in the stromal tumor environment, which is in agreement with observations made by Bailey et al 97. In this context, we would like to refer to an excellent review that was published during preparation of this manuscript describing in detail the role of PC in the crosstalk between cancer cells and their microenvironment 102.
One study explored the mechanism inducing the down‐regulation of ciliogenesis during pancreatic carcinogenesis and identified histone deacetylase 2 (HDAC2) as a regulator of PC formation 103. Kobayashi el al analyzed PDAC cell lines and found that down‐regulation of HDAC2 decreases the expression of the mitotic kinase Aurora A that has been shown to promote disassembly of PC.
Renal cancers
About 2–3% of all malignancies are kidney cancers among which clear cell renal cell carcinomas (ccRCC) are the most frequent. Nearly all ccRCC patients harbor inactivating mutations in the von Hippel–Lindau (VHL) tumor suppressor gene that is involved in controlling hypoxia‐inducible factor‐α (HIFα) and thus oxygen levels, but was also shown to stabilize microtubules and thus PC 103. In fact, ccRCC tissues were reported to have significantly lower numbers of PC though no correlation between PC and either cell proliferation or VHL inactivation was found 105, 106. Dere and colleagues analyzed RCC cell lines and described that VHL‐knockdown regulates beta‐catenin activity that in turn increases levels of the cell cycle regulator Aurora kinase A, thus leading to a down‐regulation of ciliogenesis 107. Ding el al found that VHL‐knockdown upregulates another cell cycle regulator, i.e., NEK8 (never in mitosis gene A‐related kinase 8) via HIFα that also leads to PC disassembly 107. Nevertheless, specific deletion of VHL in renal epithelial cell in mice does not cause ccRCC, suggesting that additional mutations are required 109. In fact, triple‐knockout mice for VHL, Trp53, and Rbl1 develop ccRCC resembling the human disease 110. These mice, however, developed tumors during aging, suggesting that they acquired additional mutations. Strikingly, exome sequencing showed that each of the tested mouse tumors exhibited mutations in at least one ciliary gene. Moreover, 40% of 488 human ccRCC samples analyzed displayed mutations in primary cilium related genes supporting the importance of PC inactivation in at least part of ccRCC.
Ovarian cancer
A critical role of Aurora kinase A was also described for cancer ovarian surface epithelium (OSE) cells by Egeberg el al 111. The authors found a decreased frequency of PC in cancer OSE compared to normal OSE cells that correlated with decreased Hh signaling and PDGRFalpha expression as well as centrosomal location of Aurora kinase A.
Gastrointestinal cancer
Primary cilia were shown to dampen Wnt signaling (Lancaster et al, see signaling section) that plays a crucial role in colon homeostasis and carcinogenesis 112. In fact, Lancaster et al proposed a model in which the decreased presence of PC promotes WNT signaling during colon carcinogenesis. We found that decreased numbers of PC in the colon do not modulate colon homeostasis, but correlate with increased susceptibility to chemically induced colon carcinogenesis in mice 113. However, further analysis is required to validate this correlation and to decipher the signaling pathways involved in this model. One study on tissue samples from small bowel and colorectal adenocarcinoma patients reported low frequency of PC in the tumors, as well as a correlation between overall survival and frequency of PC 114. Using the same cohort of samples a follow‐up study revealed a correlation between frequency of PC and the immune suppressor programmed cell death protein 1 (PD‐1) 115. The relevance of these observations needs to be validated by the analysis of additional patient cohorts.
Prostate cancer
Another cancer where PC have been associated with WNT signaling is prostate cancer. One report described PC at different stages during prostate cancer formation, i.e., normal prostate, prostatic intraepithelial neoplasia (PIN) and invasive prostate cancer displaying a decrease in the percentage of ciliated cells in prostatic neoplasia compared to normal tissue 116. Notably, unciliated cells had higher levels of nuclear beta‐catenin thus increased WNT signaling. Primary cilia expression on stromal cells was found to be unaltered.
PC in other cancer types
The group of Dynlacht explored the role of PC and Hh signaling during myoblast differentiation and progression of rhabdomyosarcoma, an aggressive pediatric cancer that develops from undifferentiated myoblasts 117. They describe a gradual loss of PC expression during myoblast differentiation that is paralleled by decreased Hh signaling. In fact, PC‐mediated Hh signaling is required for expression of proteins that regulate myoblast differentiation. Most of the analyzed tumor tissues had no detectable PC and the authors promoted a model, in which PC absence or deregulation promotes proliferation at the expanse of differentiation in myoblasts and thus rhabdomyosarcoma formation.
A distinct relation between PC expression and Hh signaling was described by Ho et al in neoplastic chondrocytes 118. Primary cilia were significantly decreased in neoplastic chondrocytes from human chondrosarcomas compared to normal chondrocytes, but the remaining PC in human chondrosarcomas explants attenuated Hh activity. This was supported by the finding that ablation of PC in chondrocytes promoted Gli2‐induced tumorigenesis in mice. Surprisingly, reduced expression of the intraflagellar transport protein Ift88 led to a reduction in PC on chondrocytes and increased Hh signaling, which was sufficient to induce cartilage tumors.
General considerations and remarks
Nowadays, PC are recognized as important regulators in tumorigenesis, but their role in the context of each organ and also cell type is variable (Fig 4). This is illustrated by several studies that report either higher or lower numbers of cilia in tumor tissue compared to controls. Moreover, PC can function as both tumor promoters and suppressors. This has to be taken into account when investigating the role of PC during tumorigenesis but underlines the potential of PC to serve as a biomarker.
Figure 4. Overview on reported functions of PC in different cancer types.
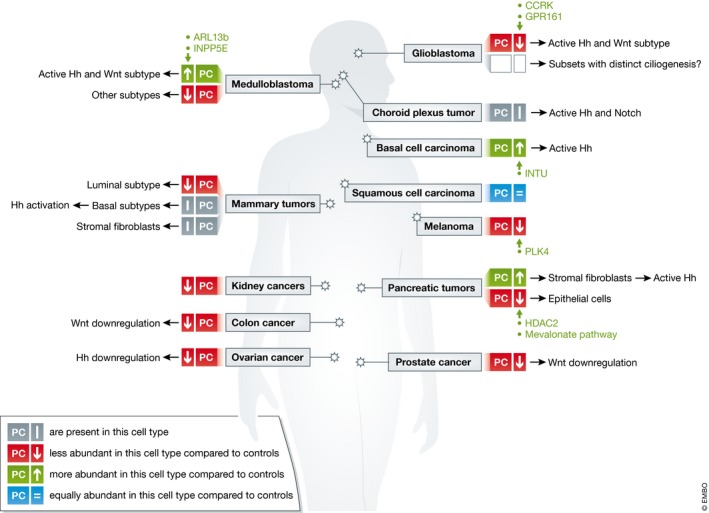
Of note, this schema is a simplification for the sake of clearness; more details are found in the manuscript. ARL13b: ADP ribosylation factor like GTPase 13B; CCRK: cell cycle‐related kinase; Gpr161: G‐protein‐coupled receptor 161; HDAC2: histone deacetylase 2; INPP5E: inositol‐polyphosphate‐5‐phosphatase E; Intu: inturned planar cell polarity protein; PLK4: Polo‐like kinase 4.
A crucial point is obviously the technique for the identification of PC. Most studies use rather well‐established antibodies for post‐translational modifications, such as glutamylation and acetylation 119 or for ARL13b to identify PC on cells or tissue sections. Ideally, these stainings are performed in combination with an antibody to identify basal bodies, such as anti‐gamma‐tubulin. The proposed role for ARL13b to act as a tumor suppressor raises the possibility that ARL13b expression in PC could be altered during tumor progression, and in fact, most authors now use two different markers for PC to validate their findings. One study used an antibody for ciliary rootlet coiled‐coil (CROCC) for the identification of PC displaying an increase in PC numbers in lung, colon, and pancreatic adenocarcinoma compared to the respective normal tissue and unaltered expression in stomach, prostate, and breast cancer 120. The findings obtained using CROCC for the identification of PC are somewhat different from the PC expression patterns in the respective tumor tissues described above. It remains to be clarified whether CROCC is labeling the same PC identified by anti‐ARL13b and post‐translational modifications of tubulin. Nevertheless, the possibility that PC could have different molecular composition during tumorigenesis is intriguing. One such possibility is an altered pattern of post‐translational modifications (PTM) of tubulin in the cilium 121. For example, tubulin glycylation was previously shown to control PC length in vitro 122. In addition, it has been reported that PC length can be altered in some human tumor tissues 116, 123. Future studies will reveal whether PTM patterns in PC could serve as a biomarker and how altered length could modulate the function of PC during tumorigenesis.
Primary cilia have been also associated with the development of novel therapeutic approaches. One study proposes the restoration of PC in tumor cells as a therapeutic strategy 96. Indeed, several compounds, including a panel of glucocorticoids, were found to be able to re‐establish PC expression in cancer cell lines and attenuate their proliferation. A distinct approach is suggested in a recent study describing that PC can mediate resistance of cancer cell lines to kinase inhibitors 124. The resistance to kinase inhibitors in tumor cells was associated with increased number and length of primary cilia and elevated Hh signaling. This was linked to decreased levels of tubulin‐glutamylation of PC. Moreover, drug resistance could be overcome by pharmacological or genetic targeting of PC. Future work will reveal whether either of the described mechanisms can be recapitulated in tumor models in vivo.
Looking at the extensive list of studies linking PC, Hh signaling, and tumorigenesis, it is not surprising that this pathway became an interesting cancer drug target. Smo inhibitors are FDA‐approved for treatment of advanced BCC and tested in clinical trials in medulloblastoma patients 125, 126. The success of those therapeutics was, however, limited due to the development of drug resistance. One study demonstrated that the presence of PC on epithelial cancer cell lines does not predict their responsiveness to a SMO agonist or antagonist 127. A recent report described that drug resistance against Smo inhibitors is associated with frequent mutations in the ciliogenesis gene oral‐facial‐digital syndrome type I (OFD1) 128. Zhao et al could demonstrate that loss of PC renders medulloblastoma cells resistant to Smo inhibitors allowing a basal level of Hh activity and tumor cell growth. Notably, datasets of Smo inhibitor‐resistant BCC tumors displayed a significantly elevated incidence of mutations of ciliary genes. Thus, while active Smo expressing PC were shown to trigger maximal Hh signaling and thus tumorigenesis in BCC, tumor cells can become resistant to Smo inhibitors by down‐regulating PC formation.
Emerging data suggest that the poor outcome of patients as well as the recurrence of CRC is due to the resistance to current clinical therapies of a small population of abnormal cells called cancer stem cells (CSC) or tumor‐initiating cells (TIA) 129. It has been suggested that these cells are entirely responsible for the development of the tumor and represent the only cell population able to sustain tumor growth and progression. Only few studies have addressed so far, whether PC can regulate CSC 71, 92, but this possibility is fascinating and will certainly attract the attention in the next years.
Taken together, the way how PC regulate carcinogenesis differs between tumor types and within tumor subtypes. Possibly, this notion will also hold for tumor stages, e.g., tumor initiation and metastasis. Though the importance of PC in cancer is clearly emerging, there is still a long way to go to decipher the mixed signals they transmit (see also Box 1).
Box 1:In need of answers† .
-
Can the presence of primary cilia serve as a biomarker for diagnosis or prognosis during carcinogenesis?
To address this question the analysis of large cohorts of patient samples is required. Notably, these samples should comprise different tumor subtypes.
-
Do different types of primary cilia, either with different molecular composition and/or length exert different functions during tumorigenesis, e.g. trigger different signaling pathways?
The thorough characterization of cilia in tumor cells, as well as the generation of novel tools for the characterization of primary cilia should provide new insights to address this question.
-
Can the primary cilium be a therapeutic target in oncology?
As crucial modulators of different signaling pathways, primary cilia are a potential therapeutic target to either enhance or reduce specific signaling depending on the cellular context. Given the complexity of the signaling pathways, however, a better understanding of ciliary signaling is required that would allow to target primary cilia in specific tumor subtypes.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
MH has been supported by Fondation ARC pour la Recherche sur le Cancer (n° SL220120605303), Institut National Du Cancer (grants PLBIO15‐057, 16‐161, 16‐143, 17‐183), and SIRIC Montpellier cancer grant INCA_INSERM_DGOS_12553. We are grateful to Drs. B. Delaval, V. Pinet, B. Pradet‐Balade, and P. Seksik for their careful reading of the manuscript.
EMBO Reports (2018) 19: e46589
See the Glossary for abbreviations used in this article.
Note
†Correction added on 30 October 2018 after first online publication: The content of Box 1 has been corrected.
Contributor Information
Thibaut Eguether, Email: thibaut.eguether@upmc.fr.
Michael Hahne, Email: michael.hahne@igmm.cnrs.fr.
References
- 1. Purkinje JE, Valentin GG (1834) Entdeckung continuierlicher durch Wimperhaare erzeugter Flimmerbewegungen. Arch Anat Physiol 1: 391–400 [Google Scholar]
- 2. Satir P (1980) Structural basis of ciliary movement. Environ Health Perspect 35: 77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zimmerman KW (1898) Beitrage zur kenntnis einiger drusen und epithelien. Arch Mikrosk Anat 52: 552–706 [Google Scholar]
- 4. Barnes BG (1961) Ciliated secretory cells in the pars distalis of the mouse hypophysis. J Ultrastruct Res 5: 453–467 [DOI] [PubMed] [Google Scholar]
- 5. Sorokin S (1962) Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol 15: 363–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fawcett DW (1981) The cell. Philadelphia, PA: Saunders; [Google Scholar]
- 7. Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG (2000) Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 151: 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV (2003) Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426: 83–87 [DOI] [PubMed] [Google Scholar]
- 9. Goetz SC, Anderson KV (2010) The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11: 331–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hildebrandt F, Benzing T, Katsanis N (2011) Ciliopathies. N Engl J Med 364: 1533–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rohatgi R, Snell WJ (2010) The ciliary membrane. Curr Opin Cell Biol 22: 541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Plotnikova OV, Pugacheva EN, Golemis EA (2009) Primary cilia and the cell cycle. Methods Cell Biol 94: 137–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ke Y‐N, Yang W‐X (2014) Primary cilium: an elaborate structure that blocks cell division? Gene 547: 175–185 [DOI] [PubMed] [Google Scholar]
- 14. Tsang WY, Dynlacht BD (2013) CP110 and its network of partners coordinately regulate cilia assembly. Cilia 2: 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inoko A, Matsuyama M, Goto H, Ohmuro‐Matsuyama Y, Hayashi Y, Enomoto M, Ibi M, Urano T, Yonemura S, Kiyono T et al (2012) Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. J Cell Biol 197: 391–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang G, Chen Q, Zhang X, Zhang B, Zhuo X, Liu J, Jiang Q, Zhang C (2013) PCM1 recruits Plk1 to the pericentriolar matrix to promote primary cilia disassembly before mitotic entry. J Cell Sci 126: 1355–1365 [DOI] [PubMed] [Google Scholar]
- 17. Kim S, Lee K, Choi J‐H, Ringstad N, Dynlacht BD (2015) Nek2 activation of Kif24 ensures cilium disassembly during the cell cycle. Nat Commun 6: 8087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wheatley DN (1995) Primary cilia in normal and pathological tissues. Pathobiology 63: 222–238 [DOI] [PubMed] [Google Scholar]
- 19. Kowal TJ, Falk MM (2015) Primary cilia found on HeLa and other cancer cells. Cell Biol Int 39: 1341–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang N, Leung EL‐H, Liu C, Li L, Eguether T, Jun Yao X‐J, Jones EC, Norris DA, Liu A, Clark RA et al (2017) INTU is essential for oncogenic Hh signaling through regulating primary cilia formation in basal cell carcinoma. Oncogene 36: 4997–5005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han Y‐G, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez‐Buylla A (2009) Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med 15: 1062–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong SY, Seol AD, So P‐L, Ermilov AN, Bichakjian CK, Epstein EH, Dlugosz AA, Reiter JF (2009) Primary cilia can both mediate and suppress Hedgehog pathway–dependent tumorigenesis. Nat Med 15: 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eguether T, Cordelieres FP, Pazour GJ (2018) Intraflagellar transport is deeply integrated in hedgehog signaling. Mol Biol Cell 29: 1178–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hassounah NB, Bunch TA, McDermott KM (2012) Molecular pathways: the role of primary cilia in cancer progression and therapeutics with a focus on hedgehog signaling. Clin Cancer Res 18: 2429–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DYR, Reiter JF (2005) Vertebrate Smoothened functions at the primary cilium. Nature 437: 1018–1021 [DOI] [PubMed] [Google Scholar]
- 26. Haycraft CJ, Banizs B, Aydin‐Son Y, Zhang Q, Michaud EJ, Yoder BK (2005) Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 1: e53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rohatgi R, Scott MP (2007) Patching the gaps in Hedgehog signalling. Nat Cell Biol 9: 1005–1009 [DOI] [PubMed] [Google Scholar]
- 28. Ocbina PJR, Tuson M, Anderson KV (2009) Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS One 4: e6839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pak E, Segal RA (2016) Hedgehog signal transduction: key players, oncogenic drivers, and cancer therapy. Dev Cell 38: 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu F, Zhang Y, Sun B, McMahon AP, Wang Y (2017) Hedgehog signaling: from basic biology to cancer therapy. Cell Chem Biol 24: 252–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tukachinsky H, Lopez LV, Salic A (2010) A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu‐Gli protein complexes. J Cell Biol 191: 415–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He M, Subramanian R, Bangs F, Omelchenko T, Liem KF, Kapoor TM, Anderson KV (2014) The kinesin‐4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nat Cell Biol 16: 663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hui C‐C, Angers S (2011) Gli proteins in development and disease. Annu Rev Cell Dev Biol 27: 513–537 [DOI] [PubMed] [Google Scholar]
- 34. Cohen MM (2010) Hedgehog signaling update. Am J Med Genet A 152A: 1875–1914 [DOI] [PubMed] [Google Scholar]
- 35. Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK (2013) The ciliary G‐protein‐coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell 152: 210–223 [DOI] [PubMed] [Google Scholar]
- 36. Keady BT, Samtani R, Tobita K, Tsuchya M, San Agustin JT, Follit JA, Jonassen JA, Subramanian R, Lo CW, Pazour GJ (2012) IFT25 links the signal‐dependent movement of Hedgehog components to intraflagellar transport. Dev Cell 22: 940–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eguether T, San Agustin JT, Keady BT, Jonassen JA, Liang Y, Francis R, Tobita K, Johnson CA, Abdelhamed ZA, Lo CW et al (2014) IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment. Dev Cell 31: 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chávez M, Ena S, Van Sande J, de Kerchove d'Exaerde A, Schurmans S, Schiffmann SN (2015) Modulation of ciliary phosphoinositide content regulates trafficking and sonic hedgehog signaling output. Dev Cell 34: 338–350 [DOI] [PubMed] [Google Scholar]
- 39. Garcia‐Gonzalo FR, Phua SC, Roberson EC, Garcia G, Abedin M, Schurmans S, Inoue T, Reiter JF (2015) Phosphoinositides regulate ciliary protein trafficking to modulate hedgehog signaling. Dev Cell 34: 400–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gu D, Xie J (2015) Non‐canonical hh signaling in cancer‐current understanding and future directions. Cancers (Basel) 7: 1684–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gonnissen A, Isebaert S, Haustermans K (2015) Targeting the Hedgehog signaling pathway in cancer: beyond Smoothened. Oncotarget 6: 13899–13913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nusse R (2003) Wnts and Hedgehogs: lipid‐modified proteins and similarities in signaling mechanisms at the cell surface. Development 130: 5297–5305 [DOI] [PubMed] [Google Scholar]
- 43. Daulat AM, Borg J‐P (2017) Wnt/Planar cell polarity signaling: new opportunities for cancer treatment. Trends Cancer 3: 113–125 [DOI] [PubMed] [Google Scholar]
- 44. Salic A, Lee E, Mayer L, Kirschner MW (2000) Control of beta‐catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol Cell 5: 523–532 [DOI] [PubMed] [Google Scholar]
- 45. Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Krönig C, Schermer B, Benzing T, Cabello OA, Jenny A et al (2005) Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 37: 537–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morgan D, Eley L, Sayer J, Strachan T, Yates LM, Craighead AS, Goodship JA (2002) Expression analyses and interaction with the anaphase promoting complex protein Apc2 suggest a role for inversin in primary cilia and involvement in the cell cycle. Hum Mol Genet 11: 3345–3350 [DOI] [PubMed] [Google Scholar]
- 47. Ross AJ, May‐Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S et al (2005) Disruption of Bardet‐Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet 37: 1135–1140 [DOI] [PubMed] [Google Scholar]
- 48. Veland IR, Awan A, Pedersen LB, Yoder BK, Christensen ST (2009) Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol 111: 39–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen M‐H, Chuang P‐T, Reiter JF (2008) Kif3a constrains beta‐catenin‐dependent Wnt signalling through dual ciliary and non‐ciliary mechanisms. Nat Cell Biol 10: 70–76 [DOI] [PubMed] [Google Scholar]
- 50. Wallingford JB, Mitchell B (2011) Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev 25: 201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Elliott KH, Brugmann SA (2018) Sending mixed signals: Cilia‐dependent signaling during development and disease. Dev Biol 10.1016/j.ydbio.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang P, Schier AF (2009) Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development 136: 3089‐3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E (2011) A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell 145: 1129–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leitch CC, Lodh S, Prieto‐Echagüe V, Badano JL, Zaghloul NA (2014) Basal body proteins regulate Notch signaling through endosomal trafficking. J Cell Sci 127: 2407–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grisanti L, Revenkova E, Gordon RE, Iomini C (2016) Primary cilia maintain corneal epithelial homeostasis by regulation of the Notch signaling pathway. Development 143: 2160–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cervantes S, Lau J, Cano DA, Borromeo‐Austin C, Hebrok M (2010) Primary cilia regulate Gli/Hedgehog activation in pancreas. Proc Natl Acad Sci USA 107: 10109–10114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu YP, Tsai I‐C, Morleo M, Oh EC, Leitch CC, Massa F, Lee B‐H, Parker DS, Finley D, Zaghloul NA et al (2014) Ciliopathy proteins regulate paracrine signaling by modulating proteasomal degradation of mediators. J Clin Invest 124: 2059–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Christensen ST, Morthorst SK, Mogensen JB, Pedersen LB (2017) Primary cilia and coordination of receptor tyrosine kinase (RTK) and transforming growth factor β (TGF‐β) signaling. Cold Spring Harb Perspect Biol 9: a028167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST (2005) PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol 15: 1861–1866 [DOI] [PubMed] [Google Scholar]
- 60. Schmid FM, Schou KB, Vilhelm MJ, Holm MS, Breslin L, Farinelli P, Larsen LA, Andersen JS, Pedersen LB, Christensen ST (2018) IFT20 modulates ciliary PDGFRα signaling by regulating the stability of Cbl E3 ubiquitin ligases. J Cell Biol 217: 151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Corless CL, Barnett CM, Heinrich MC (2011) Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer 11: 865–878 [DOI] [PubMed] [Google Scholar]
- 62. Colak S, Ten Dijke P (2017) Targeting TGF‐β signaling in cancer. Trends Cancer 3: 56–71 [DOI] [PubMed] [Google Scholar]
- 63. Clement CA, Ajbro KD, Koefoed K, Vestergaard ML, Veland IR, Henriques de Jesus MPR, Pedersen LB, Benmerah A, Andersen CY, Larsen LA et al (2013) TGF‐β signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep 3: 1806–1814 [DOI] [PubMed] [Google Scholar]
- 64. Labour M‐N, Riffault M, Christensen ST, Hoey DA (2016) TGFβ1 ‐ induced recruitment of human bone mesenchymal stem cells is mediated by the primary cilium in a SMAD3‐dependent manner. Sci Rep 6: 35542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gencer S, Oleinik N, Kim J, Panneer Selvam S, De Palma R, Dany M, Nganga R, Thomas RJ, Senkal CE, Howe PH et al (2017) TGF‐β receptor I/II trafficking and signaling at primary cilia are inhibited by ceramide to attenuate cell migration and tumor metastasis. Sci Signal 10: eaam7464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Spassky N, Han Y‐G, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia‐Verdugo JM, Alvarez‐Buylla A (2008) Primary cilia are required for cerebellar development and Shh‐dependent expansion of progenitor pool. Dev Biol 317: 246–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wechsler‐Reya RJ, Scott MP (1999) Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 22: 103–114 [DOI] [PubMed] [Google Scholar]
- 68. Marszalek JR, Ruiz‐Lozano P, Roberts E, Chien KR, Goldstein LS (1999) Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin‐II. Proc Natl Acad Sci USA 96: 5043–5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK (2007) Intraflagellar transport is essential for endochondral bone formation. Development 134: 307–316 [DOI] [PubMed] [Google Scholar]
- 70. Barakat MT, Humke EW, Scott MP (2013) Kif3a is necessary for initiation and maintenance of medulloblastoma. Carcinogenesis 34: 1382–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gate D, Danielpour M, Bannykh S, Town T (2015) Characterization of cancer stem cells and primary cilia in medulloblastoma. CNS Neurol Disord Drug Targets 14: 600–611 [DOI] [PubMed] [Google Scholar]
- 72. Conduit SE, Ramaswamy V, Remke M, Watkins DN, Wainwright BJ, Taylor MD, Mitchell CA, Dyson JM (2017) A compartmentalized phosphoinositide signaling axis at cilia is regulated by INPP5E to maintain cilia and promote Sonic Hedgehog medulloblastoma. Oncogene 36: 5969–5984 [DOI] [PubMed] [Google Scholar]
- 73. Bay SN, Long AB, Caspary T (2018) Disruption of the ciliary GTPase Arl13b suppresses Sonic hedgehog overactivation and inhibits medulloblastoma formation. Proc Natl Acad Sci USA 115: 1570–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shimada IS, Hwang S‐H, Somatilaka BN, Wang X, Skowron P, Kim J, Kim M, Shelton JM, Rajaram V, Xuan Z et al (2018) Basal suppression of the sonic hedgehog pathway by the G‐Protein‐Coupled receptor Gpr161 restricts medulloblastoma pathogenesis. Cell Rep 22: 1169–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sarkisian MR, Siebzehnrubl D, Hoang‐Minh L, Deleyrolle L, Silver DJ, Siebzehnrubl FA, Guadiana SM, Srivinasan G, Semple‐Rowland S, Harrison JK et al (2014) Detection of primary cilia in human glioblastoma. J Neurooncol 117: 15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Moser JJ, Fritzler MJ, Rattner JB (2009) Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer 9: 448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Moser JJ, Fritzler MJ, Rattner JB (2014) Ultrastructural characterization of primary cilia in pathologically characterized human glioblastoma multiforme (GBM) tumors. BMC Clin Pathol 14: 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yang Y, Roine N, Mäkelä TP (2013) CCRK depletion inhibits glioblastoma cell proliferation in a cilium‐dependent manner. EMBO Rep 14: 741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Loskutov YV, Griffin CL, Marinak KM, Bobko A, Margaryan NV, Geldenhuys WJ, Sarkaria JN, Pugacheva EN (2018) LPA signaling is regulated through the primary cilium: a novel target in glioblastoma. Oncogene 37: 1457–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li L, Grausam KB, Wang J, Lun MP, Ohli J, Lidov HGW, Calicchio ML, Zeng E, Salisbury JL, Wechsler‐Reya RJ et al (2016) Sonic Hedgehog promotes proliferation of Notch‐dependent monociliated choroid plexus tumour cells. Nat Cell Biol 18: 418–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Narita K, Takeda S (2015) Cilia in the choroid plexus: their roles in hydrocephalus and beyond. Front Cell Neurosci 9: 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stasiulewicz M, Gray SD, Mastromina I, Silva JC, Björklund M, Seymour PA, Booth D, Thompson C, Green RJ, Hall EA et al (2015) A conserved role for Notch signaling in priming the cellular response to Shh through ciliary localisation of the key Shh transducer Smo. Development 142: 2291–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A et al (1998) Activating Smoothened mutations in sporadic basal‐cell carcinoma. Nature 391: 90–92 [DOI] [PubMed] [Google Scholar]
- 84. Kim J, Dabiri S, Seeley ES (2011) Primary cilium depletion typifies cutaneous melanoma in situ and malignant melanoma. PLoS One 6: e27410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Coelho PA, Bury L, Shahbazi MN, Liakath‐Ali K, Tate PH, Wormald S, Hindley CJ, Huch M, Archer J, Skarnes WC et al (2015) Over‐expression of Plk4 induces centrosome amplification, loss of primary cilia and associated tissue hyperplasia in the mouse. Open Biol 5: 150209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shinmura K, Kurabe N, Goto M, Yamada H, Natsume H, Konno H, Sugimura H (2014) PLK4 overexpression and its effect on centrosome regulation and chromosome stability in human gastric cancer. Mol Biol Rep 41: 6635–6644 [DOI] [PubMed] [Google Scholar]
- 87. Zingg D, Debbache J, Peña‐Hernández R, Antunes AT, Schaefer SM, Cheng PF, Zimmerli D, Haeusel J, Calçada RR, Tuncer E et al (2018) EZH2‐mediated primary cilium deconstruction drives metastatic melanoma formation. Cancer Cell 34: 69–84.e14 [DOI] [PubMed] [Google Scholar]
- 88. Yuan K, Frolova N, Xie Y, Wang D, Cook L, Kwon Y‐J, Steg AD, Serra R, Frost AR (2010) Primary cilia are decreased in breast cancer: analysis of a collection of human breast cancer cell lines and tissues. J Histochem Cytochem 58: 857–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nobutani K, Shimono Y, Yoshida M, Mizutani K, Minami A, Kono S, Mukohara T, Yamasaki T, Itoh T, Takao S et al (2014) Absence of primary cilia in cell cycle‐arrested human breast cancer cells. Genes Cells 19: 141–152 [DOI] [PubMed] [Google Scholar]
- 90. Menzl I, Lebeau L, Pandey R, Hassounah NB, Li FW, Nagle R, Weihs K, McDermott KM (2014) Loss of primary cilia occurs early in breast cancer development. Cilia 3: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hassounah NB, Nunez M, Fordyce C, Roe D, Nagle R, Bunch T, McDermott KM (2017) Inhibition of ciliogenesis promotes hedgehog signaling, tumorigenesis, and metastasis in breast cancer. Mol Cancer Res 15: 1421–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Guen VJ, Chavarria TE, Kröger C, Ye X, Weinberg RA, Lees JA (2017) EMT programs promote basal mammary stem cell and tumor‐initiating cell stemness by inducing primary ciliogenesis and Hedgehog signaling. Proc Natl Acad Sci USA 114: E10532–E10539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. McDermott KM, Liu BY, Tlsty TD, Pazour GJ (2010) Primary cilia regulate branching morphogenesis during mammary gland development. Curr Biol 20: 731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Légaré S, Chabot C, Basik M (2017) SPEN, a new player in primary cilia formation and cell migration in breast cancer. Breast Cancer Res 19: 104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Légaré S, Cavallone L, Mamo A, Chabot C, Sirois I, Magliocco A, Klimowicz A, Tonin PN, Buchanan M, Keilty D et al (2015) The estrogen receptor cofactor SPEN functions as a tumor suppressor and candidate biomarker of drug responsiveness in hormone‐dependent breast cancers. Cancer Res 75: 4351–4363 [DOI] [PubMed] [Google Scholar]
- 96. Khan NA, Willemarck N, Talebi A, Marchand A, Binda MM, Dehairs J, Rueda‐Rincon N, Daniels VW, Bagadi M, Thimiri Govinda Raj DB et al (2016) Identification of drugs that restore primary cilium expression in cancer cells. Oncotarget 7: 9975–9992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bailey JM, Mohr AM, Hollingsworth MA (2009) Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene 28: 3513–3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Seeley ES, Carrière C, Goetze T, Longnecker DS, Korc M (2009) Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res 69: 422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Deng Y‐Z, Cai Z, Shi S, Jiang H, Shang Y‐R, Ma N, Wang J‐J, Guan D‐X, Chen T‐W, Rong Y‐F et al (2017) Cilia loss sensitizes cells to transformation by activating the mevalonate pathway. J Exp Med 215: 177–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Emoto K, Masugi Y, Yamazaki K, Effendi K, Tsujikawa H, Tanabe M, Kitagawa Y, Sakamoto M (2014) Presence of primary cilia in cancer cells correlates with prognosis of pancreatic ductal adenocarcinoma. Hum Pathol 45: 817–825 [DOI] [PubMed] [Google Scholar]
- 101. Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, Scales SJ, de Sauvage FJ (2009) Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci USA 106: 4254–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liu H, Kiseleva AA, Golemis EA (2018) Ciliary signalling in cancer. Nat Rev Cancer 18: 511–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kobayashi T, Nakazono K, Tokuda M, Mashima Y, Dynlacht BD, Itoh H (2017) HDAC2 promotes loss of primary cilia in pancreatic ductal adenocarcinoma. EMBO Rep 18: 334–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W (2003) Regulation of microtubule stability by the von Hippel‐Lindau tumour suppressor protein pVHL. Nat Cell Biol 5: 64–70 [DOI] [PubMed] [Google Scholar]
- 105. Schraml P, Frew IJ, Thoma CR, Boysen G, Struckmann K, Krek W, Moch H (2009) Sporadic clear cell renal cell carcinoma but not the papillary type is characterized by severely reduced frequency of primary cilia. Mod Pathol 22: 31–36 [DOI] [PubMed] [Google Scholar]
- 106. Basten SG, Willekers S, Vermaat JS, Slaats GG, Voest EE, van Diest PJ, Giles RH (2013) Reduced cilia frequencies in human renal cell carcinomas versus neighboring parenchymal tissue. Cilia 2: 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dere R, Perkins AL, Bawa‐Khalfe T, Jonasch D, Walker CL (2015) β‐catenin links von Hippel‐Lindau to aurora kinase A and loss of primary cilia in renal cell carcinoma. J Am Soc Nephrol 26: 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ding X‐F, Zhou J, Hu Q‐Y, Liu S‐C, Chen G (2015) The tumor suppressor pVHL down‐regulates never‐in‐mitosis A‐related kinase 8 via hypoxia‐inducible factors to maintain cilia in human renal cancer cells. J Biol Chem 290: 1389–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Frew IJ, Moch H (2015) A clearer view of the molecular complexity of clear cell renal cell carcinoma. Annu Rev Pathol 10: 263–289 [DOI] [PubMed] [Google Scholar]
- 110. Harlander S, Schönenberger D, Toussaint NC, Prummer M, Catalano A, Brandt L, Moch H, Wild PJ, Frew IJ (2017) Combined Vhl, Trp53 and Rb1 mutation causes clear cell renal cell carcinoma in mice. Nat Med 23: 869–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Egeberg DL, Lethan M, Manguso R, Schneider L, Awan A, Jørgensen TS, Byskov AG, Pedersen LB, Christensen ST (2012) Primary cilia and aberrant cell signaling in epithelial ovarian cancer. Cilia 1: 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Medema JP, Vermeulen L (2011) Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature 474: 318–326 [DOI] [PubMed] [Google Scholar]
- 113. Rocha C, Papon L, Cacheux W, Marques Sousa P, Lascano V, Tort O, Giordano T, Vacher S, Lemmers B, Mariani P et al (2014) Tubulin glycylases are required for primary cilia, control of cell proliferation and tumor development in colon. EMBO J 33: 2247–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dvorak J, Hadzi Nikolov D, Dusek L, Filipova A, Richter I, Buka D, Ryska A, Mokry J, Filip S, Melichar B et al (2016) Prognostic significance of the frequency of primary cilia in cells of small bowel and colorectal adenocarcinoma. J BUON 21: 1233–1241 [PubMed] [Google Scholar]
- 115. Dvorak J, Hadzi Nikolov D, Dusek L, Filipova A, Richter I, Buka D, Ryska A, Mokry J, Filip S, Melichar B et al (2017) Association of the combined parameters including the frequency of primary cilia, CD8+ tumor infiltrating lymphocytes and PD‐1 expression with the outcome in intestinal cancer. J BUON 22: 1477–1487 [PubMed] [Google Scholar]
- 116. Hassounah NB, Nagle R, Saboda K, Roe DJ, Dalkin BL, McDermott KM (2013) Primary cilia are lost in preinvasive and invasive prostate cancer. PLoS One 8: e68521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Fu W, Asp P, Canter B, Dynlacht BD (2014) Primary cilia control hedgehog signaling during muscle differentiation and are deregulated in rhabdomyosarcoma. Proc Natl Acad Sci USA 111: 9151–9156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ho L, Ali SA, Al‐Jazrawe M, Kandel R, Wunder JS, Alman BA (2013) Primary cilia attenuate hedgehog signalling in neoplastic chondrocytes. Oncogene 32: 5388–5396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Magiera MM, Janke C (2013) Investigating tubulin posttranslational modifications with specific antibodies. Methods Cell Biol 115: 247–267 [DOI] [PubMed] [Google Scholar]
- 120. Yasar B, Linton K, Slater C, Byers R (2017) Primary cilia are increased in number and demonstrate structural abnormalities in human cancer. J Clin Pathol 70: 571–574 [DOI] [PubMed] [Google Scholar]
- 121. Janke C (2014) The tubulin code: molecular components, readout mechanisms, and functions. J Cell Biol 206: 461–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gadadhar S, Dadi H, Bodakuntla S, Schnitzler A, Bièche I, Rusconi F, Janke C (2017) Tubulin glycylation controls primary cilia length. J Cell Biol 216: 2701–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lee J, Yi S, Kang YE, Chang JY, Kim JT, Sul HJ, Kim JO, Kim JM, Kim J, Porcelli AM et al (2016) Defective ciliogenesis in thyroid hürthle cell tumors is associated with increased autophagy. Oncotarget 7: 79117–79130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jenks AD, Vyse S, Wong JP, Kostaras E, Keller D, Burgoyne T, Shoemark A, Tsalikis A, de la Roche M, Michaelis M et al (2018) Primary cilia mediate diverse kinase inhibitor resistance mechanisms in cancer. Cell Rep 23: 3042–3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bakshi A, Chaudhary SC, Rana M, Elmets CA, Athar M (2017) Basal cell carcinoma pathogenesis and therapy involving hedgehog signaling and beyond. Mol Carcinog 56: 2543–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bautista F, Fioravantti V, de Rojas T, Carceller F, Madero L, Lassaletta A, Moreno L (2017) Medulloblastoma in children and adolescents: a systematic review of contemporary phase I and II clinical trials and biology update. Cancer Med 6: 2606–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Spann AL, Yuan K, Goliwas KF, Steg AD, Kaushik DD, Kwon Y‐J, Frost AR (2015) The presence of primary cilia in cancer cells does not predict responsiveness to modulation of smoothened activity. Int J Oncol 47: 269–279 [DOI] [PubMed] [Google Scholar]
- 128. Zhao X, Pak E, Ornell KJ, Pazyra‐Murphy MF, MacKenzie EL, Chadwick EJ, Ponomaryov T, Kelleher JF, Segal RA (2017) A transposon screen identifies loss of primary cilia as a mechanism of resistance to SMO inhibitors. Cancer Discov 7: 1436–1449 [DOI] [PubMed] [Google Scholar]
- 129. Colak S, Medema JP (2014) Cancer stem cells–important players in tumor therapy resistance. FEBS J 281: 4779–4791 [DOI] [PubMed] [Google Scholar]
- 130. Janke C, Bulinski JC (2011) Post‐translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol 12: 773–786 [DOI] [PubMed] [Google Scholar]


