Abstract
Critical limb ischemia (CLI) is a devastating disease in patients undergoing hemodialysis (HD). Based on the unsatisfactory results of autologous mononuclear cell transplantation for patients with CLI undergoing HD, we conducted a phase II clinical trial to evaluate the safety and efficacy of granulocyte colony‐stimulating factor (G‐CSF)‐mobilized peripheral blood‐derived autologous purified CD34 positive (CD34+) cell transplantation for CLI in patients undergoing HD. Six patients with CLI (two with Rutherford category 4 and four with Rutherford category 5) were enrolled. As for primary endpoint, there were no major adverse events related to this therapy. As for efficacy, the amputation‐free survival rate was 100% at 1 year after cell therapy. Both rest pain scale and ulcer size were significantly improved as early as 4 weeks after therapy compared with baseline (p < .01), and three out of five ulcers completely healed within 12 weeks after cell transplantation. Clinical severity, including Fontaine scale and Rutherford category, significantly improved at 24 weeks after cell transplantation (p < .05), and further improved at 52 weeks (p < .01) compared with baseline. The improvement rate from CLI stage to non‐CLI stage was 83.3% at 52 weeks. Toe skin perfusion pressure and absolute claudication distance were also significantly improved. In conclusion, G‐CSF‐mobilized peripheral blood CD34+ cell transplantation was safe, feasible, and effective for patients with CLI undergoing HD. stem cells translational medicine 2018;7:774–782
Keywords: CD34 positive cells, Critical limb ischemia, Hemodialysis, Transplantation
Significance Statement.
Improvement of critical limb ischemia (CLI) is often very difficult. Because outcome in CLI patients, especially in hemodialysis (HD) patients, is very poor, effective treatment is urgently needed. CD34 positive cells have potential to vascular regeneration. However, the number of peripheral blood CD34 positive cells is severely decreased in HD patients with CLI due to uremic condition and inflammation. Mobilization by granulocyte colony‐stimulating factor (G‐CSF) significantly increased the number of CD34 positive cells in peripheral blood, and potential of CD34 positive cells was also confirmed in this study. Although this is a small study, regenerative therapy using autologous G‐CSF‐mobilized peripheral blood CD34 positive cell transplantation was highly effective in HD patients with CLI. This result may encourage novel cell‐based therapy for patients with CLI requiring HD.
Introduction
The prognosis of patients with critical limb ischemia (CLI) undergoing hemodialysis (HD) is poor 1, 2. In patients with CLI, revascularization therapy, including bypass surgery or endovascular therapy (EVT), is an essential treatment strategy. However, revascularization therapy still has significant limitations in patients with HD. Infrapopliteal arteries are the most frequent affected sites for CLI in patients undergoing HD, and these arteries usually show extensive vascular calcification 3. A recent report demonstrated that the restenosis rate is high after EVT for below‐knee arteries in patients with CLI undergoing HD, that is, 73% and 84% at 3 and 12 months, respectively 4. Moreover, outcomes after revascularization therapy are suboptimal. The amputation‐free survival (AFS) rates after EVT for infrapopliteal lesions in patients undergoing HD are 65.7% and 34.4% at 1 and 5 years, respectively 5. Another report demonstrated that the mortality rate is high after EVT, that is, 191 deaths among 547 patients (35%) after a median observation period of 557 days 6. In terms of outcomes after bypass surgery for below‐knee arteries in patients undergoing HD, the 1‐year AFS rates were 64% 7 and 60% 8, respectively.
In 1997, Asahara et al. isolated CD34 positive (CD34+) cells from the peripheral blood (PB) as endothelial progenitor cells (EPCs) 9 and confirmed the vasculogenic potential of these EPCs using a model of hind limb ischemia. Subsequently, several clinical trials of regenerative therapy for CLI using autologous mononuclear cells (MNCs) derived from bone marrow (BM) or PB were conducted 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22. Two types of MNCs were used, that is, whole MNCs containing several cell types and purified CD34+ cells after selection from MNCs. Transplantation of unselected peripheral blood mononuclear cells (PBMNCs) resulted in favorable outcomes in non‐HD patients with CLI (2‐year AFS 83%) 15; however, the outcomes in patients with CLI undergoing HD were disappointing (2‐year AFS 49%) 15. Transplantation with bone marrow mononuclear cells (BMMNCs) or PBMNCs 13, 15, 16, 17 did not provide advantages in AFS or overall survival rates for patients undergoing HD compared with those in revascularization therapy 5, 6, 7, 8.
In comparison with transplantation of whole PBMNCs, few attempts have been made to transplant purified CD34+ cells for CLI in patients undergoing HD, and the efficacy of treatment has not been confirmed 14, 22. Furthermore, these trials were not performed in a prespecified manner for patients with CLI undergoing HD. Human CD34+ cells express transcripts for, and secrete detectable amounts of vascular endothelial growth factor, hepatocyte growth factor, insulin‐like growth factor‐1, fibroblast growth factor 2, Flt‐3 ligand, and interleukin 8 23. Exosomes secreted from mobilized human CD34+ cells had significant vasculogenic paracrine activity in vitro and in vivo 24.
Therefore, this study conducted a prospective interventional phase II clinical trial using autologous granulocyte colony‐stimulating factor (G‐CSF)‐mobilized PB‐derived CD34+ cell transplantation focusing on HD patients with CLI.
Methods
Study Design and Criteria for Enrollment
The article was designed to prospectively evaluate the safety, feasibility, and efficacy of autologous G‐CSF‐mobilized CD34+ cells in patients with CLI requiring HD. The study protocol conformed to the Declaration of Helsinki and was approved by the institutional ethics committees (no. TGE00301‐024) and special committee for Class II regenerative medicine certified by the Ministry of Health, Labor, and Welfare in Japan (no. SKRM‐001). The article was registered to an official clinical trial registration site (UMIN no. 000015266).
Eligible subjects fulfilled all inclusion criteria as follows (a) atherosclerotic peripheral arterial disease (PAD) with greater than or equal to 70% luminal stenosis or obstruction in the leg arteries by digital subtraction angiography; (b) more than 3 months since the initiation of HD; (c) more than 6 months since the onset of lower limb ischemia; (d) CLI with a Rutherford category of 4–5; (e) failure of or no indication for transluminal angioplasty/stenting and bypass surgery; (f) men or women ages 20–80 years; and (g) provided written informed consent. The exclusion criteria were as follows: (a) Buerger's disease; (b) CLI of Rutherford category 6; (c) within 1 month after revascularization therapy (bypass surgery or EVT) or low‐density lipoprotein apheresis; (d) within 1 month after myocardial infarction, unstable angina pectoris, or stroke; (e) malignancy or history of malignancy within past 5 years; (f) diabetic retinopathy (new Fukuda Classification 25: B II—BV); (g) severely decreased cardiac function (left ventricular ejection fraction <25% on cardiac ultrasonography); (h) interstitial pneumonitis proved on chest computed tomography; (i) allergic reaction to G‐CSF or other reagents used in this study; (j) splenomegaly on computed tomography or ultrasonography; (k) at least one laboratory abnormality (white blood cell [WBC] ≤3000 per microliter or ≥15000 per microliter, hemoglobin concentration ≤8 g/dl, platelet count ≤104 per microliter, aspartate aminotransferase/alanine aminotransferase ≥100 IU/l, serum albumin ≤2 g/dl); (l) liver cirrhosis; (m) hematologic disease (leukemia, myeloproliferative or dysplastic disorder, and sickle cell anemia); and (n) pregnancy. After evaluation of the eligibility of each candidate for this cell‐based therapy by the case enrollment committee, appropriate case selection was confirmed at the independent case registration center in the Translational Research Informatics Center (TRI), Kobe, Japan.
Rules and Definitions
During the study period, the basic protocol treatment according to Trans‐Atlantic Inter‐Society Consensus II (medication, wound treatment) 26 was given to all patients. Anti‐platelet drugs and prostaglandin E1 or I2 analog were not allowed to be added during the study period. If these drugs had already been prescribed, the drug dose was not changed. Pain control was essentially performed using nonsteroidal anti‐inflammatories if needed.
Major cardiovascular events were defined as death due to coronary artery disease, nonfatal myocardial infarction, resuscitation after cardiac arrest, stroke, or EVT and/or bypass surgery involving the cerebral, coronary, aortic, or peripheral arteries. Major and minor amputations were distinguished by amputation sites proximal and distal to the ankle joint.
Treatment Procedures
Patients received subcutaneous administration of G‐CSF to mobilize EPCs from the BM. The dose of G‐CSF was 5 μg/kg per day for 5 days, and leukapheresis (COMTEC, Fresenius Kabi Japan Co., Tokyo, Japan) was performed to harvest PBMNCs on day 5. G‐CSF was scheduled to be cancelled when the WBC count was greater than or equal to 75,000 per microliter; however, the leukocyte count never exceeded 75,000 per microliter in any patient. The leukapheresis product was kept at a concentration of 2 × 108 cells per milliliter in autoplasma at 4°C–8°C overnight (≤18 hours) until the magnetic separation of CD34+ cells was started on day 6 using a CliniMACS Instrument (MiltenyBiotec, BergischGladbach, Germany), anti‐CD34 antibody‐labeled magnetic nanobeads, phosphate‐buffered saline/ethylenediaminetetraacetic acid buffer, and a tubing set (MiltenyiBiotec, BergischGladbach, Germany).
Cell transplantation was performed under general anesthesia immediately after CD34+ cell separation. All CD34+ cells dissolved in 10 ml physiological saline were administered intramuscularly into 40 sites (0.25 ml per site) of the leg with more severe ischemia in each patient. When the Rutherford severity was the same in bilateral legs, half of the cells were injected in each leg. The administration points consisted of 30 sites in the calf muscle, 6 sites in the sole muscle, and 4 sites in the intertie muscle, as described previously 14.
Endpoints
The primary endpoint was safety for 52 weeks after cell therapy. Safety was evaluated by adverse events, the severity of which was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
The secondary endpoint was efficacy, including the following parameters: (a) AFS; (b) rate of improvement from CLI stage to non‐CLI stage assessed by Fontaine stage and Rutherford category; (c) major and minor amputations; (d) death due to CLI; (e) all‐cause death; (f) cardiovascular event‐free survival; (g) changes in ulcer size, pain score, total walking distance, and pain‐free walking distance by the 6 minutes’ walking test, and physiological tests including ankle brachial pressure index (ABI), toe‐brachial pressure index (TBI; Form PWV/ABI: Omron Colin, San Antonio, TX, USA), skin perfusion pressure (SPP; S3000; Kaneka, Tokyo, Japan), and transcutaneous partial oxygen pressure (TcPO2; PO‐850; Sumitomo Electric System Solutions, Tokyo, Japan); and (h) efficiency of CliniMACS, including purity, viability, and recovery of CD34+ cells. If the number of transplanted CD34+ cells per limb was over 5 × 104 per kilogram, both safety and efficacy were evaluated. When the number of transplanted CD34+ cells per limb was below 5 × 104 per kilogram, only safety evaluation was performed, and the patients were excluded from efficacy evaluation.
Evaluation of Parameters
Rest pain was evaluated using the visual analog scale (VAS). Ulcers were evaluated by the maximum length and depth of the ulcer. Walking distance was evaluated using 6‐minute walking tests for determination of the absolute and initial claudication distance. Regarding microcirculatory impairment besides ABI, TBI, SPP, and TcPO2 were evaluated using Form PWV/ABI (Omron Colin), S3000 (Kaneka), and PO‐850 (Sumitomo Electric System Solutions) as previously described 14.
Data Management and Statistical Analysis
Data were managed at an independent data center of the TRI. Following data input, data cleaning, and logic check were performed to guarantee the data quality.
We presented categorical data as numbers (percentage) and continuous data as means (±SD). Wilcoxon rank sum tests were used for analysis of paired data (WBC count and CD34+ cell count between baseline and day 5 after G‐CSF treatment). Analysis of mean response over time was conducted using a mixed effects model for repeated measures. AFS and cardiovascular event‐free survival were calculated using the Kaplan–Meier method. All tests were two‐sided, and p values of less than .05 were considered significant. All analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patients
Six patients undergoing HD and seven legs with CLI were enrolled in the article from January 2015 to February 2016. All patients were men and five had diabetes mellitus. The cause of renal failure was diabetic kidney disease in four patients and nephrosclerosis in two patients. Although ischemic heart disease was frequently observed (83.3%), left ventricular ejection fraction was preserved in these patients (Table 1).
Table 1.
Baseline characteristics
| Characteristic | Value |
|---|---|
| Age (year) | 70.2 ± 8.0 |
| Male/female (n) | 6/0 |
| Underlying disease, n (%) | |
| Diabetic kidney disease | 4 (66.7) |
| Nephrosclerosis | 2 (33.3) |
| Hemodialysis duration (months) | 72.5 ± 40.4 |
| Comorbidity, n (%) | |
| Ischemic heart disease | 5 (83.3) |
| Stroke | 0 (0) |
| Hypertension | 6 (100) |
| Diabetes | 5 (83.3) |
| Dyslipidemia | 3 (50.0) |
| Smoking habit, n (%) | |
| No | 2 (33.3) |
| Ex | 4 (66.7) |
| Body mass index (kg/m2) | 22.9 ± 1.9 |
| Cardiac function | |
| LVEF (%) | 55.5 ± 7.3 |
| LVMI (g/m2) | 154.3 ± 38.7 |
| E/e’ | 16.9 ± 8.4 |
| Laboratory variables | |
| Blood urea nitrogen (mg/dl) | 41.3 ± 9.2 |
| Creatinine (mg/dl) | 8.5 ± 2.2 |
| Total protein (g/dl) | 6.6 ± 0.8 |
| Albumin (g/dl) | 3.7 ± 0.4 |
| Total cholesterol (mg/dl) | 145.2 ± 24.3 |
| Triglyceride (mg/dl) | 134.3 ± 45.9 |
| HDL cholesterol (mg/dl) | 54.0 ± 13.5 |
| LDL cholesterol (mg/dl) | 63.8 ± 21.1 |
| C‐reactive protein (mg/dl) | 0.75 ± 1.12 |
| Hemoglobin (g/dl) | 11.4 ± 1.7 |
| Hemoglobin A1c (%) | 6.9 ± 1.6 |
| Medication, n (%) | |
| Aspirin | 5 (83.3) |
| Clopidogrel | 4 (66.7) |
| Serotonin 5HT2 antagonist | 1 (16.7) |
| Cilostazol | 2 (33.3) |
| Prostanoid | 3 (50.0) |
| Statin | 4 (66.7) |
| ARB | 3 (50.0) |
Abbreviations: ARB, accumulative roll bonding; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index.
Two patients with Rutherford category 4 (Fontaine stage 3) and four patients with Rutherford category 5 (Fontaine stage 4) underwent cell therapy (Table 2). Cell therapy was performed in the right leg in cases 1–5. Case 6 had ulcer lesions in bilateral legs, and cell therapy was performed in bilateral legs. As shown in Table 2, VAS ranged from 2 to 7, and ulcer size ranged from 5 to 35 mm.
Table 2.
Information about CLI and cell transplantation
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 6 | |
|---|---|---|---|---|---|---|---|
| Parameters | Rt | Rt | Rt | Rt | Rt | Rt | Lt |
| Clinical severity | |||||||
| Fontaine stage | 3 | 4 | 4 | 4 | 3 | 4 | 4 |
| Rutherford category | 4 | 5 | 5 | 5 | 4 | 5 | 5 |
| Visual analog scale | 4 | 7 | 4 | 6 | 4 | 2 | 2 |
| Ulcer size in Rutherford 5 (mm) | 0 | 15 | 5 | 22 | 0 | 10 + 20 | 35 |
| 6 minutes’ walking distance (m) | |||||||
| Absolute claudication distance | 210 | 233 | 165 | 324 | 420 | 106 | |
| Initial claudication distance | 90 | 0 | 0 | 0 | 400 | 47 | |
| Diabetes | Yes | No | Yes | Yes | Yes | Yes | |
| Cell product | |||||||
| Apheresis product | |||||||
| Total MNC number (1010) | 1.9 | 2.8 | 2.1 | 2.4 | 3.5 | 3 | |
| CD34+ cell number (107) | 2 | 1.8 | 4.1 | 13.8 | 3.4 | 1 | |
| Cell product after magnetic sorting | |||||||
| Total cell number (106) | 35.1 | 13.8 | 37.1 | 100 | 32.8 | 21.3 | |
| CD34+ cell number (106) | 8.9 | 5.44 | 26.1 | 86.8 | 13.1 | 9.58 | |
| Purity (%) | 25.4 | 39.5 | 70.4 | 86.8 | 39.9 | 45 | |
| Viability (%) | 88.9 | 89.1 | 95.9 | 97.9 | 90.1 | 99.1 | |
| Cell transplantation | |||||||
| Transplanted cell number (105 per kilogram per limb) | 1.6 | 0.9 | 39 | 13.3 | 2.2 | 0.7 | 0.7 |
Abbreviation: MNC, mononuclear cell.
Outcomes of Mobilization, Harvesting, and Isolation of CD34+ Cells
Injection with G‐CSF (5 μg/kg) for 5 days significantly increased WBC count and CD34+ cell count in the PB from 5,700 ± 710 per microliter at baseline to 26,480 ± 4,614 per microliter and from 0.37 ± 0.14 per microliter at baseline to 2.80 ± 1.36 per microliter at day 5, respectively (Fig. 1). CD34+ cell count at baseline in patients with diabetes did not differ from that in a patient without diabetes. CD34+ cell count increased 7.6‐fold in total following G‐CSF administration. The CD34+ cell count in the PB increased 5.6 ± 2.6‐fold in diabetic patients and 9.9‐fold in nondiabetic patient following G‐CSF administration (not statistically significant).
Figure 1.
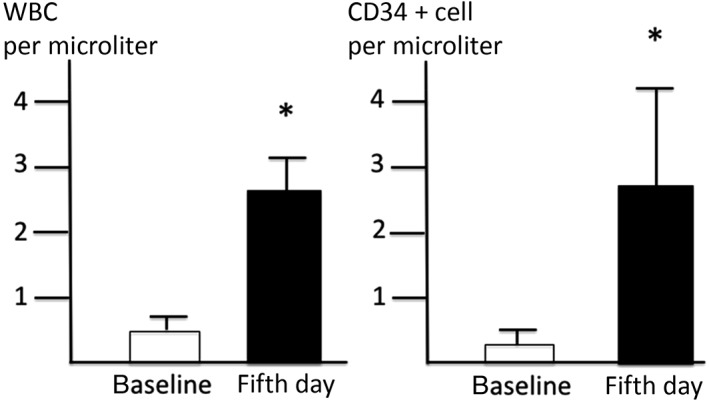
Change of WBC and CD34+ cell count before and after G‐CSF administration. Injection with G‐CSF (5 μg/kg) for 5 days significantly increased WBC count and CD34+ cell count in the peripheral blood. *p < .05 versus baseline data. Abbreviations: G‐CSF, granulocyte colony‐stimulating factor; WBC, white blood cell.
Cell products (apheresis products and magnetic sorting products) are shown in Table 2. The total MNC count and CD34+ cell counts obtained by apheresis on day 5 were 2.6 ± 0.24 × 1010 and 4.4 ± 2.0 × 107, respectively. The CD34+ cell count after magnetic sorting was 2.5 ± 3.1 × 107 with a purity of 51.2% ± 9.3% and viability of 93.5% ± 1.9%. Finally, magnetically sorted cells (8.4 ± 5.4 × 105 per kilogram) were transplanted into the ischemic limb. The transplanted cell number varied from 0.72 to 39 × 105/kg per limb. Cases 6 and 3 underwent minimum and maximum cell transplantation, respectively (Table 2).
Safety Evaluation
Adverse events during the 52‐week follow‐up after cell therapy are listed in Table 3. Angina pectoris, inguinal hernia, pneumonia, brain contusion, and skin ulcers due to worsening of arterial stenosis were serious adverse events for which in‐hospital treatment was necessary. However, none of these events were thought to directly relate to cell therapy. Nonserious adverse events, including constipation, colitis, neck pain, and fever due to G‐CSF injection, were found during the study period. Diabetic retinopathy was found in three patients at baseline, all of whom had the A3/A3 non‐proliferative stage according to the New Fukuda Classification. In these patients, case 6 showed mild vitreous hemorrhage in the left eye 5 months after cell transplantation. The hemorrhage subsided spontaneously without any treatment thereafter.
Table 3.
Adverse events during 52 weeks’ follow‐up period after cell transplantation
| Adverse event | Number of events |
|---|---|
| Serious adverse event | |
| Cardiovascular | |
| Angina | 1 |
| Arrhythmia (atrial fibrillation and atrial flutter) | 1 |
| Gastrointestinal | |
| Inguinal hernia | 1 |
| Infectious | |
| Pneumonia | |
| Central nervous system | |
| Brain contusion | 1 |
| Peripheral arterial | |
| Arterial stenosis | 1 |
| Skin | |
| Skin ulcer | 1 |
| Nonserious adverse event | |
| Ophthalmic | |
| Vitreous hemorrhage | 1 |
| Gastrointestinal | |
| Constipation | 1 |
| General | |
| Fever | 1 |
| Infectious | |
| Colitis | 1 |
| Musculoskeletal | |
| Neck pain | 1 |
| Cardiovascular | |
| Shunt vessel stenosis | 1 |
| Skin | |
| Contusion | 1 |
Efficacy Evaluation
All patients survived for 1 year without major or minor amputation. Thus, the 1‐year AFS rate was 100%. Cardiovascular event‐free survival was 83.3% at 24 weeks and 66.7% at 52 weeks (Fig. 2). The improvement rate from CLI stage to non‐CLI stage at 52 weeks after cell transplantation was 83.3% (five of six patients).
Figure 2.
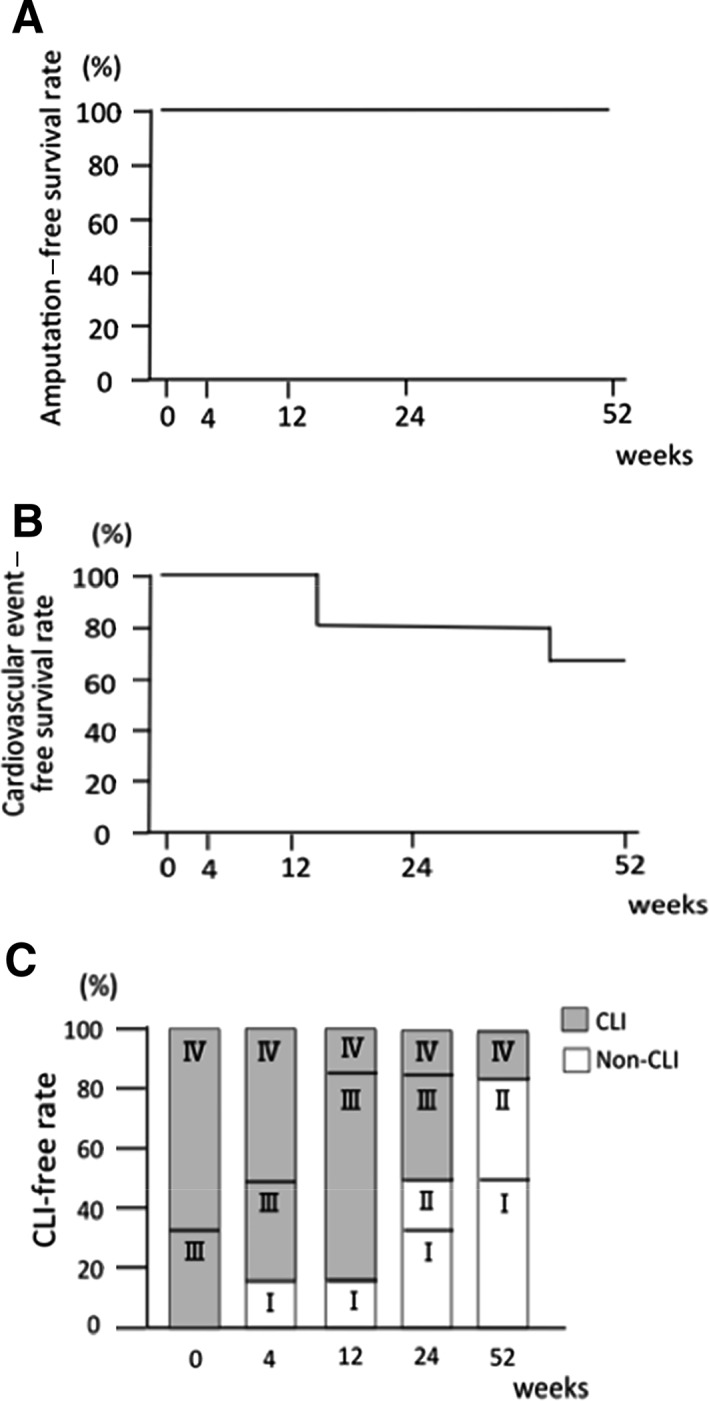
Amputation‐free survival, cardiovascular event‐free survival, and CLI‐free rate. (A): Amputation‐free survival at 1 year was 100%. (B): Cardiovascular event‐free survival rate was 66.7%. (C): Fontaine stage and CLI‐free rate. Grey bar indicates CLI, and open bar indicates non‐CLI. CLI‐free rate at 1 year was 83.3%. Abbreviation: CLI, critical limb ischemia
Both rest pain and intractable ulcers dramatically improved as early as 4 weeks after cell transplantation, and these effects continued with further improvement during the observation period (Fig. 3). Three out of five ulcers completely healed within 12 weeks after cell transplantation, and rest pain in two patients with Rutherford category 4 completely disappeared. As a result, the category of clinical severity significantly improved following cell therapy. Both Fontaine stage and Rutherford category significantly improved at 24 weeks after cell transplantation compared with those at baseline (p < .05), and further improvement was observed at 52 weeks after cell transplantation (p < .01; Fig. 3). Clinical severity did not improve only in one patient (case 6 receiving the minimum dose of CD34+ cells to bilateral legs). Ulcer size in case 6 once improved at 12 weeks after cell therapy (from 20 to 6 mm in the right leg and from 35 to 20 mm in the left leg). However, these ulcers did not heal at 52 weeks after cell therapy.
Figure 3.
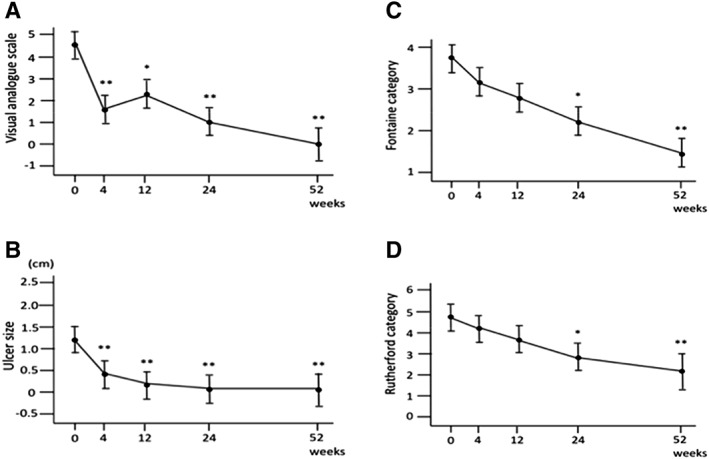
Change of pain score, ulcer size, and clinical severity. (A): Visual analog scale (VAS), (B): ulcer size, (C): Fontaine category, and (D): Rutherford category. VAS and ulcer size significantly improved as early as 4 weeks after cell transplantation, and these effects continued with further improvement during the observation period. Fontaine stage and Rutherford category significantly improved at 24 weeks from baseline, and further improvement was observed at 52 weeks after cell transplantation. *p < .05 and **p < .01 versus baseline data.
Improvement of clinical severity was accompanied by improvement in walking distance and microcirculation of ischemic limbs (Fig. 4). Absolute claudication distance significantly improved from 243.0 ± 51.4 m at baseline to 299.1 ± 51.9 m at 12 weeks (p < .05). Initial claudication distance also showed a tendency to improve from 89.5 ± 80.8 m at baseline to 304.1 ± 65.8 m at 24 weeks, although this change was not statistically significant. Mean dorsal SPP increased above 40 mmHg at 4 weeks (50.0 ± 7.0 mmHg), and remained above 40 mmHg until 52 weeks after cell transplantation. Toe SPP significantly increased from 27.3 ± 6.2 mmHg at baseline to 49.3 ± 6.2 mmHg at 52 weeks after cell transplantation (p = .024). TBI also increased from 0.51 ± 0.22 at baseline to 0.90 ± 0.25 at 52 weeks, although this change was not statistically significant (p = .25). ABI and TcPO2 did not show significant changes during the observational period.
Figure 4.
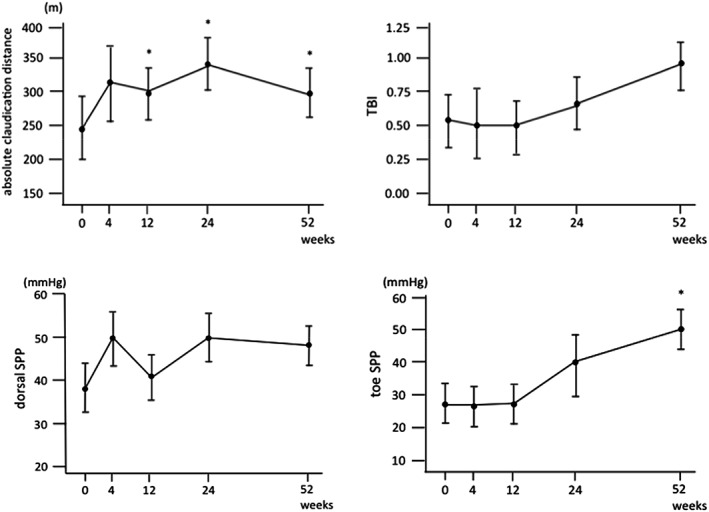
Change of absolute claudication distance, TBI, dorsal and toe SPP after cell transplantation. *p < .05 versus baseline data. Abbreviations: TBI, toe‐brachial index; SPP, skin perfusion pressure.
Whether the existence of diabetes affected the efficacy of this cell therapy was not clearly evaluated because of the small sample size of this study.
Discussion
Although this was a small study, we demonstrated that G‐CSF‐mobilized CD34+ cell therapy was safe and dramatically improved CLI in HD patients. To the best of our knowledge, this is the first report evaluating the effects of autologous CD34+ cell transplantation for patients with CLI undergoing HD as prespecified manner for HD patients. In consideration of the poor limb salvage rate after revascularization therapy and poor prognosis in patients with CLI undergoing HD, this result may encourage novel cell‐based therapy for patients with CLI requiring HD.
The number of CD34+ cells in the PB is usually significantly decreased in patients undergoing HD compared with that in patients not undergoing HD 27, 28 and is correlated with poor cardiovascular outcomes and all‐cause mortality in patients undergoing HD 29, 30. The chronic uremic milieu injures BM hematopoietic function in patients undergoing HD. Furthermore, several inflammatory cytokines suppress BM function to produce hematopoietic stem cells in patients with CLI 31. Therefore, patients with CLI undergoing HD may be considered a severely affected population with “BM failure” or “BM exhaustion.” Decreased nitric oxide (NO) production in patients with HD 32 may further decrease the mobilizing capacity of CD34+ cells to peripheral circulation, because NO is one of the strongest stimuli for CD34+ cell mobilization 33. However, G‐CSF administration augmented the mobilization of CD34+ cells, which have important functions in vascular repair, in patients undergoing HD. The potential of CD34+ cells for vasculogenesis in patients with CLI is reported to be weak compared with those in patients without CLI 31. However, autologous CD34+ cells in patients with CLI undergoing HD also showed significant potential for repairing severely ischemic intractable wounds. Diabetes did not influence on the efficacy of this cell therapy, and this cell therapy was significantly effective in diabetic patients with CLI undergoing HD as well.
PBMNC transplantation did not improve CLI in patients undergoing HD. Horie et al. 15 reported unsatisfactory results by transplantation of crude PBMNCs for patients with CLI undergoing HD. While their study provided good results in patients with PAD not undergoing HD, the 1‐year AFS rate after PBMNC transplantation was as high as 60% in patients with CLI undergoing HD. Furthermore, other reports of transplantation of BMMNCs 13 or PBMNCs 16 for patients with CLI undergoing HD have also shown poor clinical outcomes (overall survival and AFS). The 1‐year AFS rate was 52% following BMMNC transplantation and 46% following PBMNC transplantation. A pooled analysis of these two studies revealed no differences in overall survival rates and AFS rates between BMMNCs and PBMNCs 17. Unselected total MNCs contain several cell types, including inflammatory macrophages and fibroblasts, and worsened cardiac fibrosis has also been observed 34.
This study used a monoclonal antibody‐labeled magnetic sorting technique and found that the purity of CD34+ cells after the magnetic sorting in patients undergoing HD was not superior, but was instead inferior, to that in patients without HD in a previous report (92.7% ± 16.4%) 14. In our study, flow cytometry analysis of the cell population after magnetic sorting revealed significant amounts of cell aggregations, with platelets and/or cell debris with monocytes. We have previously reported that platelet/monocyte aggregation is enhanced in patients undergoing HD 35. This phenomenon may explain the decreased CD34+ cell purity in patients with CLI undergoing HD. Another possible reason for the low purity may be the dramatically reduced concentration of CD34+ cells in PBMNCs in patients undergoing HD.
Ulcer size and transplanted cell number may be an important factor predicting improvements in CLI 17. Ulcers measuring 35 and 20 mm in diameter in case 6 did not heal after cell transplantation of 0.72 × 105 CD34+ cells per kilogram for each limb, which was the lowest cell dose used in this study. In contrast, ulcers measuring 22 mm in case 4 and 15 mm in case 2 completely healed following cell transplantation of 13.3 × 105, and 0.95 × 105 CD34+ cells per kilogram per limb, respectively. These results suggested that more cells might be beneficial.
The small sample size and lack of control arm were major limitations in this study. However, this prospective interventional phase II study provided encouraging findings. Patients with CLI requiring HD showed improvement following autologous CD34+ cell transplantation. The AFS was 100%, and the CLI‐free ratio was 83.3% 1 year after cell therapy.
Conclusion
Autologous G‐CSF‐mobilized CD34+ cell transplantation cell was safe, feasible, and effective in patients with CLI undergoing HD. Long‐term observation and larger‐scale clinical studies are urgently needed to further confirm the potential benefits of this cell‐based therapy, particularly for patients with life‐threatening CLI who require HD.
Author Contributions
T.O.: conception and design, provision of study material or patients, collection and assembly of data, manuscript writing; Y.M., K.I., M.O., K.M., H.M., S. Hidaka: provision of study material or patients, and collection and assembly of data; S. Higashide and T.I.: data analyses as statistician; Y.F.: conception and design, technical advisor of cell isolation and transplantation, data analyses; A.K.: conception and design, technical advisor of cell isolation and transplantation, data analyses and interpretation, final approval of manuscript; M.F.: conception and design, data analyses and interpretation, final approval of manuscript; S.K.: principal investigator, conception and design, data interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
We deeply thank Dr. Takao Suzuki, the president of Tokushukai Medical Group, for his continuous and unifying support for this project. We thank Mr. Tsutomu Sato in Cell Processing Center for cell preparation using CliniMACS, medical engineers for cell separation, and rehabilitation staff for testing walking distance in our hospital. We thank Mr. Sizuhiro Yamada, Center for Clinical and Translational Science in our hospital for data assembly. We also thank the staffs of TRI for management, monitoring, assembly, fixation, and analysis of the study data.
References
- 1. Orimoto Y, Ohta T, Ishibashi H et al. The prognosis of patients on hemodialysis with foot lesion. J Vasc Surg 2013;58:1291–1299. [DOI] [PubMed] [Google Scholar]
- 2. Liu T, Liang KV, Rosenbaum A et al. Peripheral vascular disease severity impacts health outcomes and health‐related quality of life in maintenance hemodialysis patients in the HEMO study. Nephrol Dial Transplant 2012;27:2929–2936. [DOI] [PubMed] [Google Scholar]
- 3. Ohtake T, Oka M, Ikee R et al. Lower limbs’ arterial calcification on the prevalence and severity of PAD in patients on hemodialysis. J Vasc Surg 2011;53:676–683. [DOI] [PubMed] [Google Scholar]
- 4. Iida O, Soga Y, Kawasaki D et al. Angiographic restenosis and its clinical impact after infrapopliteal angioplasty. Eur J Vasc Endovasc Surg 2012;44:425–431. [DOI] [PubMed] [Google Scholar]
- 5. Nakano M, Hirano K, Yamauchi Y et al. Three‐year clinical outcome after inftapopliteal angioplasty for critical limb ischemia in hemodialysis patients with minor or major tissue loss. Catheter Cardiovasc Interv 2015;86:289–298. [DOI] [PubMed] [Google Scholar]
- 6. Suematsu N, Iida O, Takahara M et al. Prognostic factors in hemodialysis patients undergoing endovascular treatment for critical limb ischemia due to isolated below‐knee disease. Atheroscler Thromb 2015;22:404–414. [DOI] [PubMed] [Google Scholar]
- 7. Kodama A, Sugimoto M, Kuma S et al. Clinical outcomes after infrainguinal bypass grafting for critical limb ischemia in patients with dialysis‐dependent end‐stage renal failure. Eur J Vasc Endovasc Surg 2014;48:695–702. [DOI] [PubMed] [Google Scholar]
- 8. Kumada Y, Nogaki H, Ishii H et al. Clinical outcome after infrapopliteal bypass surgery in chronic hemodialysis patients with critical limb ischemia. J Vasc Surg 2015;61:400–404. [DOI] [PubMed] [Google Scholar]
- 9. Asahara T, Murohara T, Sullivan A et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967. [DOI] [PubMed] [Google Scholar]
- 10. Tateishi‐Yuyama E, Matsubara H, Murohara T et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone marrow cells: A pilot study and a randomized controlled trial. Lancet 2002;360:427–435. [DOI] [PubMed] [Google Scholar]
- 11. Huang P, Li S, Han M et al. Autologous transplantation of granulocyte colony‐stimulating factor‐mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care 2005;28:2155–2160. [DOI] [PubMed] [Google Scholar]
- 12. Kajiguchi M, Kondo T, Izawa H et al. Safety and efficacy of autologous progenitor cell transplantation for therapeutic angiogenesis in patients with critical limb ischemia. Circ J 2007;71:196–201. [DOI] [PubMed] [Google Scholar]
- 13. Matoba S, Tatsumi T, Murohara T et al. Long‐term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic angiogenesis by cell transplantation [TACT] trial). Am heart J 2008;156:1010–1018. [DOI] [PubMed] [Google Scholar]
- 14. Kawamoto A, Katayama M, Handa N et al. Intramuscular transplantation of G‐CSF‐mobilized CD34+ cells in patients with critical limb ischemia: A phase I/IIa, multicenter, single‐blinded, dose‐escalation clinical trial. Stem Cells 2009;27:2857–2864. [DOI] [PubMed] [Google Scholar]
- 15. Horie T, Onodera R, Akamatsu M et al. Long‐term clinical outcomes for patients with lower limb ischemia implanted with G‐CSF‐mobilized autologous peripheral blood mononuclear cells. Atherosclerosis 2010;208:461–466. [DOI] [PubMed] [Google Scholar]
- 16. Moriya J, Minamino T, Tateno K et al. Long‐term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circ Cardiovasc Intervent 2009;2:245–254. [DOI] [PubMed] [Google Scholar]
- 17. Onodera R, Teramukai S, Tanaka S et al. Bone marrow mononuclear cells versus G‐CSF‐mobilized peripheral blood mononuclear cells for treatment of lower limb ASO: Pooled analysis for long‐term prognosis. Bone Marrow Transplant 2011;46:278–284. [DOI] [PubMed] [Google Scholar]
- 18. Losordo DW, Kibbe MR, Mendelsohn F et al. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ Cardiovasc Interv 2012;5:821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dong Z, Chen B, Fu W et al. Transplantation of purified CD34+ cells in the treatment of critical limb ischemia. J Vasc Surg 2013;58:404–411. [DOI] [PubMed] [Google Scholar]
- 20. Gupta PK, Chullikana A, Parakh R et al. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med 2013;11:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujita Y, Kinoshita M, Furukawa Y et al. Phase II clinical trial of CD34+ cell therapy to explore endpoint selection and timing in patients with critical limb ischemia. Circ J 2014;78:490–501. [DOI] [PubMed] [Google Scholar]
- 22. Tanaka R, Masuda H, Kato S et al. Autologous G‐CSF‐mobilized peripheral blood CD34+ cell therapy for diabetic patients with chronic nonhealing ulcer. Cell Transplant 2014;23:167–179. [DOI] [PubMed] [Google Scholar]
- 23. Majka M, Janowska‐Wieczorek A, Ratajczak J et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood 2001;97:3075–3085. [DOI] [PubMed] [Google Scholar]
- 24. Sahoo S, Klychko E, Thorne T et al. Exosomes from human CD34+ stem cells mediate their proangiogenic paracrine activity. Circ Res 2011;109:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fukuda M. Clinical management of diabetic retinopathy. Nippon Ganka Gakkai Zasshi 1989;93:873–882. (Japanese). [PubMed] [Google Scholar]
- 26. Norgren L, Hiatt WR, Dormandy JA et al. Inter‐society consensus for the management of peripheral arterial disease. Int Angiol 2007;26:81–157. [PubMed] [Google Scholar]
- 27. Pala C, Altun I, Koker Y et al. The effect of diabetes mellitus and end‐stage renal disease on the number of CD34+ cells in the blood. Ann Hematol 2013;92:1189–1194. [DOI] [PubMed] [Google Scholar]
- 28. Jourde‐Chiche N, Dou L, Sabatier F et al. Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. J Thromb Haematol 2009;7:1576–1584. [DOI] [PubMed] [Google Scholar]
- 29. Maruyama S, Taguchi A, Iwashima S et al. Low circulating CD34+ cell count is associated with poor prognosis in chronic hemodialysis patients. Kidney Int 2008;74:1603–1609. [DOI] [PubMed] [Google Scholar]
- 30. Lee HJ, Kim W, Kim WS et al. Circulating endothelial progenitor cell levels predict cardiovascular events in end‐stage renal disease patients on maintenance hemodialysis. Nephron 2015;130:151–158. [DOI] [PubMed] [Google Scholar]
- 31. Teraa M, Sprengers RW, Westerweel PE et al. Bone marrow alterations and lower endothelial progenitor cell numbers in critical limb ischemia patients. PLoS One 2013;8:e55592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xiao S, Schmidt RJ, Baylis C. Plasma from ESRD patients inhibits nitric oxide synthase activity in cultured human and bovine endothelial cells. Acta Physiol Scand 2000;168:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thom SR, Bhopale VM, Velazquez OC et al. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Circ Physiol 2006;290:H1378–H1386. [DOI] [PubMed] [Google Scholar]
- 34. Kawamoto A, Iwasaki H, Kusano K et al. CD34‐positive cell exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation 2006;114:2163–2169. [DOI] [PubMed] [Google Scholar]
- 35. Kobayashi S, Miyamoto M, Kurumatani H et al. Increased leukocyte aggregations are associated with atherosclerosis in patients with hemodialysis. Hemodial Int 2009;13:286–292. [DOI] [PubMed] [Google Scholar]


