Summary
Over the past decade, amniotic fluid‐derived stem cells have emerged as a novel experimental approach aimed at improving outcomes in children with congenital anomalies, including spina bifida, heart defects, and diaphragmatic hernia. Interest in these cells for the treatment of prenatally diagnosed diseases has arisen based on numerous studies demonstrating the relative ease of harvesting an abundant quantity of amniocytes from a small aliquot of fluid, the unique properties of amniocytes themselves, and the beneficial effects of amniotic fluid‐derived stem cells in experimental animal models. This report gives a brief overview of the rationale and current status of amniotic fluid stem cell‐based therapies, focusing on its relevance to birth defects affecting the fetus and neonate. The author proposes a roadmap for further study that would be required prior to clinical application of amniotic fluid stem cell technologies. stem cells translational medicine 2018;7:767–773
Significance Statement.
This article gives a pediatric surgeon‐scientist's perspective on the therapeutic potential of amniotic fluid‐derived stem cells in the management of a wide range of structural birth defects affecting the fetus and neonate. The characteristics of amniotic fluid‐derived stem cells are discussed in experimental animal models of congenital anomalies, including spina bifida, congenital heart disease, and congenital diaphragmatic hernia. Barriers to the clinical translation of amniotic fluid stem cells as a potential adjunct to surgical treatment in children are reviewed.
Introduction
Structural birth defects are the end products of aberrant organogenesis early in fetal life. Some of the more common prenatally diagnosed anomalies encountered by surgeons in the neonatal intensive care unit include congenital diaphragmatic hernia (CDH), abdominal wall defects, spinal bifida, and congenital heart disease. Thanks in part to the enhanced resolution of fetal ultrasound imaging, the vast majority of these anomalies are diagnosed during the second trimester of pregnancy, thereby allowing families the time to seek advanced perinatal care at a major pediatric referral center. However, despite advances in the medical and surgical care of these patients, these anomalies continue to inflict a major burden of pediatric disease and account for a significant proportion of infant mortality, morbidity, andhospitalization days worldwide. In the effort to further improve clinical outcomes in these young children, there has been increasing interest in the clinical application of various progenitor cell populations derived from amniotic fluid as a novel therapeutic adjunct to organ regeneration and surgical reconstruction in various pediatric disease processes 1, 2, 3, 4, 5.
Rationale for Amniotic Fluid‐Derived Stem Cells
The use of amniotic fluid stem cells represents a practical and logical choice for autologous cell‐based therapy in children with prenatally diagnosed congenital anomalies for a number of reasons. First, there is no need to wait until delivery for cell harvesting since amniocytes are easily accessible by needle aspiration (amniocentesis) of a small sample of amniotic fluid (e.g., 5 ml) 6. Because sampling amniotic fluid cells is already medically indicated as part of the diagnostic evaluation for many fetal anomalies to rule out aneuploidy, there is no added morbidity by procuring additional fluid for potential therapeutic benefit. After 15 weeks gestation, an amniocentesis is a safe procedure with a less than 1% rate of fetal loss when performed by experienced personnel under ultrasound guidance 7. By contrast, harvesting stem cells prenatally from placenta, chorionic villi, cord blood, liver, or skin is more technically challenging and associated with a higher risk of spontaneous abortion, infection, hemorrhage, and other morbidities 8. In fact, the safety of an amniocentesis has now enabled commercial banking of amniotic fluid in some developed countries.
Amniotic fluid‐derived stem cells genetically originate from the fetus itself, thereby allowing for autologous therapeutic applications to be performed without concern for immunologic rejection of donor cells upon delivery, either prenatally or in the early postnatal period 9, 10. Studies have shown that second‐trimester amniotic fluid stem cells can proliferate rapidly in culture compared to postnatal somatic cells under Good Manufacturing Practice conditions 6. Therefore, once the prenatal diagnosis of the anomaly is made during the second trimester, amniotic fluid stem cells can be easily expanded in vitro and ready for use as an autologous therapy in the perinatal period 11, 12. Given the relatively small size of the fetus, the importance of this issue should not be underestimated since a large number of cells may be required for therapeutic effect. In short, the use of amniotic fluid cell‐based approaches has substantial and clinically relevant advantages over other fetal and postnatal cell sources, including bone marrow and liver 13.
For many years, amniotic fluid was thought to be composed primarily of fetal urine with terminally differentiated epithelioid cells derived from the fetal skin and amnion. It is now well recognized that preterm amniotic fluid contains a heterogenous group of distinct progenitor cell populations, including CD34+ hematopoietic progenitors, c‐kit− mesenchymal stem/stromal cells (MSCs), and c‐kit+ multipotent stem cells 1. Each of these amniotic fluid cell types possesses its own unique tissue‐specific characteristics that lends itself to potential clinical translation based on differentiation capacity, anti‐inflammatory effects, and other trophic properties that are known to promote tissue repair and regeneration.
Amniotic Fluid‐Derived Mesenchymal Stem Cells
Amniotic fluid‐derived MSCs are a plastic‐adherent population of c‐kit− cells that fulfill the minimal criteria for MSCs (e.g., CD34−, CD45−, CD73+, CD90+, and CD105+) as originally described for cells derived from bone marrow 14. By definition, these cells have restricted differentiation potential and have been shown to differentiate into the osteogenic, adipogenic, and chondrogenic lineages in vitro 15, 16, 17, 18. However, increased plasticity of amniotic fluid‐derived MSCs (e.g., neural‐like cells) has also been demonstrated by some investigators, and others have found the expression of many of the same core pluripotency genes as undifferentiated embryonic cells, including OCT4 and SSEA4, albeit at lower levels in most cases 34. Pluripotent gene expression is usually variable due to their polyclonal nature and tends to decrease with gestational age and time in culture 19. Other investigators have similarly shown that unselected amniocytes have wide heterogeneity and express a range of genes and proteins specific to pluripotent, committed progenitors as well as fully differentiated cells 20, 21.
MSCs are perhaps best known for their potential immunomodulatory role in inflammatory conditions and disease processes 22. Although the mechanistic role of amniotic fluid‐derived MSCs in vivo remains poorly described, MSCs from amniotic fluid have been shown to inhibit T‐cell proliferation, to decrease memory T cells, to increase T regulator lymphocytes, and to polarize the Th2 subtype in association with increased IL‐10 and IL‐4 levels in coculture studies when compared to MSCs derived from bone marrow and placenta 23. It has been hypothesized that amniotic fluid‐derived MSCs may play a critically important paracrine role in the immune response at the maternal‐fetal interface through expression of the CD59 and HLA‐G, among others 9, 10.
c‐kit+ Amniotic Stem Cells
Shortly after MSCs derived from amniotic fluid were first reported, a specific subset of amniotic fluid stem cells expressing c‐kit (CD117), a tyrosine‐kinase receptor that specifically binds to the ligand, stem cell factor, was described by De Coppi et al. 24. These unique cells, which can be isolated using immunomagnetic beads during primary culture, constitute less than 1% of the total somatic cell population in amniotic fluid. c‐kit+ amniotic fluid cells express the same panel of MSCs phenotypic markers as c‐kit− MSCs derived from amniotic fluid but are clonal and capable of undergoing more than 250 population doublings, far exceeding the Hayflick limit as defined by the telomere length of the starting cell population. These cells have been associated with improved regeneration of damaged smooth muscle in rat cryoinjured bladders, among other tissues 25. Compared to MSCs derived from bone marrow or amniotic fluid, c‐kit+ amniotic fluid cells have much greater multipotent differentiation potential in vitro with clonal cell lines demonstrating differentiation into lineages within each of the three germ layers, including neurogenic (ectoderm), cardiogenic (mesoderm), and hepatogenic (endoderm) cell types 24, 26. Despite these findings, c‐kit+ amniotic fluid cells have not been fully embraced as having equivalent pluripotency to human embryonic stem cells or induced pluripotent stem cells (iPSCs). Nonetheless, a key potential advantage of c‐kit+ amniotic fluid cells is that they are not known to form teratomas when injected into immunodeficient mice and do not require xenogenic feeder layers to support their long‐term growth in culture. Similar to the situation with amniotic fluid‐derived MSCs, investigators have suggested that the immunoprivileged characteristics of c‐kit+ stem cells may enable their use in allogeneic cell therapy applications, particularly if the donor cells are ultimately delivered in the fetal environment 27, 28.
A major drawback of working with c‐kit+ amniotic fluid cells comes from their rarity. Unlike AF‐MSCs, which are abundant and easily obtained until term, c‐kit+ amniotic fluid cells cannot be reliably isolated from late second trimester and third trimester samples 29. These later time points are typically more clinically relevant since they correspond to the time at which most congenital anomalies are diagnosed by prenatal ultrasound. In general, c‐kit+ amniotic fluid cells isolation methods are also more laborious, time consuming, and costly. For these reasons, c‐kit+ amniotic fluid cells may be best suited as an “off‐the‐shelf” product amendable toward allogeneic cell transplantation applications.
Induced Pluripotent Stem Cells Derived from Amniocytes
The use of prenatal somatic cells from iPSCs represents another novel strategy to regenerate perinatal tissues that can be implanted to replace those that are severely damaged or missing. Over the past decade, substantial efforts have also been undertaken by numerous laboratories to reprogram early gestation amniocytes into pluripotent stem cells, enabling their use shortly at birth 30, 31, 32, 33. The embryonic origins, inherently primitive nature of certain populations (i.e., positive for OCT4, SSEA3, and SSEA4), and nascent epigenetic background of amniocytes may make them particularly well suited for rapid and highly efficient (e.g., 0.5%) genetic reprogramming for therapeutic use 31, 33. Indeed, investigators have suggested that the absence of age‐related epigenetic modifiers associated with alterations in DNA methylation and chromatin structure may help to explain why fewer than four Yamanaka reprogramming factors have been shown to be sufficient for successful reprogramming 32. More recently, various transgene‐free reprogramming methods, including exclusively chemical‐based techniques free from ectopic factors, have been reported 34, 35. iPSCs derived from amniotic fluid have also been shown to have immunoregulatory properties by impairng NK cell cytotoxicity, a mechanism that plays a role in allograft rejection 36.
Selected Congenital Anomalies Amenable to Amniotic Fluid Stem Cell Therapies
Neural Tube Defects
In myelomeningocele (MMC), one of the most severe types of neural tube defects, the natural sequelae in affected children include hydrocephalus, bowel and bladder incontinence, and distal lower motor dysfunction. Fetal surgical repair has now become the standard of care at some children's hospital in the U.S. based on a randomized trial demonstrating reduced hydrocephalus and modest gains in ambulation 37. Regenerative medicine‐based strategies, aimed at decreasing spinal cord gliosis within the defect during fetal repair, could represent a major additional advance in the quality of life for these children.
Several investigators have studied the role of amniotic fluid stem cell‐based therapies in animal models of fetal MMC (Fig. 1) 38, 39, 40, primarily as a means to provide tissue coverage in utero to protect the spinal cord from ongoing mechanical and chemical damage. For example, Fauza and colleagues have explored the intra‐amniotic delivery of high doses of amniotic fluid‐based stem cells (termed trans‐amniotic stem cell therapy, TRASCET). In this model, the cells have been shown to preferentially home to the neural tube defect to provide partial coverage of the spinal cord in syngeneic fetal rat models of MMC 39, 41. Another group of investigators has demonstrated the therapeutic concept of amniotic fluid‐based tissue engineering by repairing fetal rat neural tube defects using three‐dimensional tissue‐engineered skin from amniocytes. In this short‐term pilot study, the epidermal layer was generated from keratinocytes derived from human amniotic fluid iPSCs, whereas the dermis was created from human fibroblasts and collagen type I 40. Although promising, the long‐term effectiveness of these stem cell‐based strategies on functional spinal cord regeneration cannot be shown because postnatal survival is not feasible in any of the available rodent MMC models. Carefully conducted large animal studies using fetal lambs will likely be required in order to bring this technology to the clinical arena 42. Strategies such as the transplantation of amniotic fluid‐derived neural stem cells may offer additional benefits in terms of facilitating bona fide regeneration of neural derivatives. If amniotic fluid‐based stem cell therapies do eventually show evidence of enhanced neurologic function, one could easily envision a treatment paradigm in the future that would include deriving autologous stem cells following an amniocentesis once the diagnosis is made by ultrasound, typically between 16 and 20 weeks gestation. The amniotic fluid‐derived cells would be isolated and subsequently delivered to the MMC spinal cord injury later in gestation or at postnatal spinal cord closure.
Figure 1.
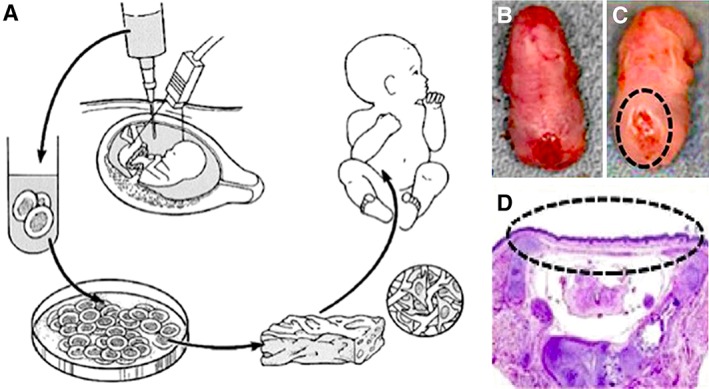
Tissue engineering from amniotic fluid‐derived stem cells for the treatment of prenatally diagnosed congenital anomalies. (A): Schematic diagram of autologous fetal stem cells obtained by amniocentesis during the second trimester, followed by ex vivo expansion of amniocytes in parallel with the remainder of gestation, and subsequent seeding of cells within biodegradable scaffolds in preparation for perinatal surgical implantation. Gross appearance of the lumbosacral neural tube defect in a fetal rat with myelomeningocele (MMC) (B) without treatment and (C) with treatment with amniotic fluid‐derived mesenchymal stem cells in utero. (D): Hematoxylin and eosin photomicrograph of a representative rat MMC defect at term after treatment with amniotic fluid‐derived mesenchymal stem cells in utero, showing rudimentary skin coverage (within dotted ellipse). Modified from 7, 31 with permission.
Congenital Heart Disease
Congenital heart defects are the most common major birth defects seen in children's hospitals worldwide, and those at the most severe end of the spectrum are diagnosed during the second trimester by fetal echocardiography. An alternative, regenerative medicine‐based approach to conventional therapies, including cardiac transplantation, would be to generate autologous replacement cardiomyomyocytes specific to each patient. c‐kit+ amniotic stem cells have been shown to differentiate into cardiomyocytes 26, 43. More recently, the differentiation of cells from autologous amniotic fluid iPSCs into functional cardiomyocytes by integration‐free reprogramming methods has also been demonstrated (Fig. 2) 44, 45. Amniotic fluid‐derived stem cells are likely to play an additional role as paracrine mediators of tissue regeneration based on experimental models of adult heart disease 28, 46. Although research in this area is quite preliminary, future work with the scale up and preconditioning of cardiomyocytes derived from amniotic fluid cells has enormous potential in helping to circumvent the scarcity of heart donors for infants born with severe heart failure because of hypoplastic left heart syndrome.
Figure 2.
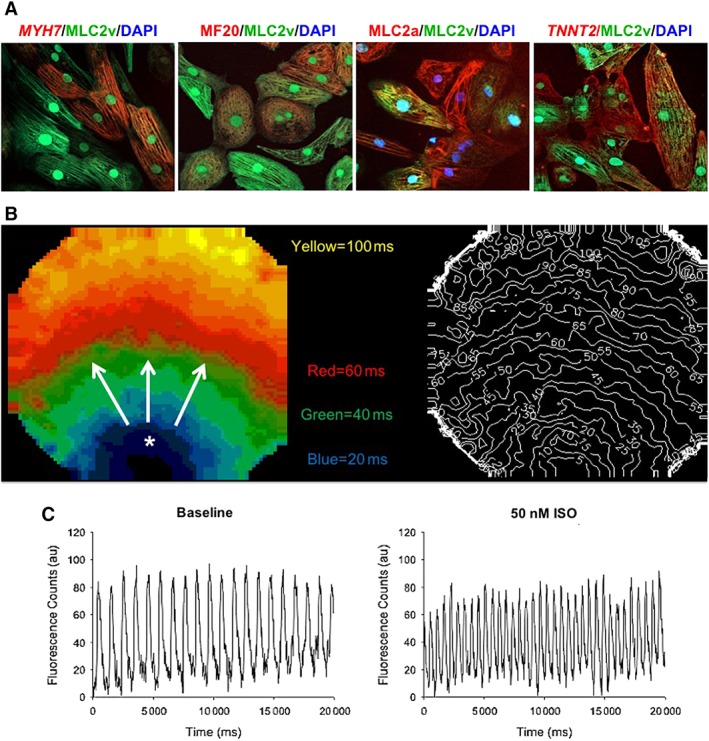
Generation of beating cardiomyocytes from pluripotent cells derived from human amniotic fluid. (A): Representative confocal microscopy images of cardiomyocyte subtypes exhibiting comparable mixed immunostaining patterns of sarcromeric proteins, including ventricle (MLC2v, FITC secondary, green) and other cardiac‐specific markers, including MYH7, MF20, MLC2a, and TTNT2 (magnification, ×40). (B): Representative pseudocolor activation map of intracellular calcium transient propagation in amniotic fluid cardiomyocyte monolayers showing typical radial spreading patterns after spontaneous activation. Isochrone lines (right) indicate differential activation times. (C): Representative calcium transient recordings after β‐adrenergic agonist (50 nM ISO) exposure of amniotic fluid cardiomyocytes demonstrating appropriate chronotropic responses compared to baseline. Modified from 35 with permission. Abbreviation: ISO, isoproterenol.
Surgical reconstruction of the heart valves and great vessels in children is currently performed using a prefabricated acellular prosthetic implant. Unfortunately, these implants typically fail to grow with the child into adulthood, resulting in the need for multiple operations to optimize heart function. By contrast, a cellularized, tissue‐engineered prosthetic would theoretically have the ability to grow and remodel over time, thereby avoiding serial revisional operations. Such an implant would also not mandate the need for long‐term anticoagulation. To date, the greatest experience with amniotic fluid based‐stem cell technologies has been with the fabrication of tissue‐engineered heart valves 47, 48. In one such study, Hoestrup and colleagues successfully created functional heart valves derived from human amniotic fluid cells 47. The cells were seeded onto synthetic heart valve leaflet scaffolds and conditioned within a pulse bioreactor to facilitate their maturation. Microscopic analyses revealed endothelialized tissue formation as well as biomechanical properties sufficient for implantation in vivo. Subsequent work in large animal models has demonstrated short‐term in vivo functionality with intact valvular integrity and absence of thrombus formation 48.
Respiratory Tract Anomalies
In CDH, the fetal diaphragm fails to correctly form, resulting in herniation of the abdominal viscera into the chest cavity. The diagnosis is made in the vast majority of cases by ultrasound at 18–20 weeks gestation. Although large holes in the diaphragm can be closed with a synthetic patch in the newborn period, recurrent herniation as the child grows is a well‐known complication following repair. Similar to the problems associated with prosthetic heart valves, it has been argued that a fully cellularized implant that has the ability to grow and remodel over time represents the ideal prosthesis for the CDH patient population. Fauza and colleagues have extensively explored this concept using sheep amniotic fluid‐derived fibroblasts resuspended in collagen hydrogels and seeded onto decellularized dermal scaffolds (Supporting Inforamtion Fig. S1) 49, 50. In these studies, the amniocytes were used to reconstruct a diaphragmatic defect in neonatal lambs, demonstrating lower rates of reherniation as well as improved modular and ultimate tensile strengths over time. Clinical trials using this treatment strategy are forthcoming.
In recent years, amniotic fluid stem cell‐based induction of lung growth has become another area of interest for the treatment of lung hypoplasia associated with CDH, giant omphalocele, and bronchopulmonary dysplasia. Given the known association of oligohydramnios with lung hypoplasia, it has been theorized that amniotic fluid‐derived stem cells may secrete growth factors that promote fetal growth vis‐à‐vis paracrine mediators and/or exosomes 51. Evidence for enhanced lung regeneration with amniotic fluid‐derived stem cells in experimental CDH models has been shown by numerous investigators 52, 53, 54. In an ex vivo fetal lung study 53, amniotic fluid MSCs augmented branching morphogenesis and lung epithelial maturation in the nitrofen rat model of CDH lung hypoplasia. Since nearly 30% of CDH neonates die secondary to overwhelming pulmonary hypoplasia and pulmonary hypertension, fetal augmentation of lung growth with amniotic fluid stem cell delivery might have a major clinical impact in severely affected fetuses.
Severe obstructive malformations of the trachea, including tracheal agenesis and atresia, are rare but well‐described entities in pediatric surgery. Methods of definitive tracheal reconstruction are currently limited by a paucity of available autologous tissue that can mimic the structural and mechanical properties of native airway cartilage. Chondrocytes derived from amniotic fluid‐derived MSCs could serve as a viable alternative for early tracheal repair soon after birth (Supporting Inforamtion Fig. S2). This therapeutic concept has been successfully demonstrated in an ovine model of tracheal repair 55. In this study, GFP‐labeled sheep amniocytes were seeded onto cylindrical polyglycolic acid‐based scaffolds and exposed to a chondrogenic medium containing transforming growth factor beta. The constructs were then used to reconstruct partial‐ or full‐circumferential tracheal defects in fetal lambs. Neonatal lambs were able to breathe at birth without major respiratory distress, and there was evidence of donor cell survival as revealed by GFP+ cells within fibrous cartilage. Of note, the successful implantation of a tissue‐engineered airway structure derived from autologous bone marrow MSCs has already reported in a child 56. However, to help bring this technology toward widespread clinical application, more research is clearly needed to evaluate the effect of novel biodegradable scaffolds with biomechanical properties that would enable long‐term tracheal growth and function.
Perinatal Gastrointestinal Disorders
Because of the known immunomodulatory properties of amniotic fluid stem cell populations with regards to decreasing T‐cell proliferation and polarizing the Th2 subtype in association with increased IL‐10 and IL‐4 levels, inflammatory disorders of the gastrointestinal tract in fetuses and neonates may be amenable to amniotic fluid cell‐based approaches. One such condition is gastroschisis, a congenital birth defect characterized by a failure of the abdominal wall to properly form at 10 weeks gestation. This subsequently leads to herniation of the abdominal viscera into the abdominal cavity with progressive chemical and mechanical damage to the small intestines while in utero. Similar to the case with MMC, TRASCET may have salutary, anti‐inflammatory effects on fetal gastroschisis intestines once the diagnosis is made in the fetus by ultrasound at 16–20 weeks gestation. TRASCET has been shown to improve intestinal histology at birth in a fetal rodent model of gastroschisis 57.
Although not considered to be a congenital anomaly per se, several investigators have also published promising data on amniotic fluid‐derived stem cells to abrogate the inflammatory response in necrotizing enterocolitis. Necrotizing enterocolitis is a potentially lethal disease (i.e., 40% mortality) that affects mostly premature infants and leads to progressive intestinal damage and necrosis 58, 59. Surgical removal of severely damaged intestines can be life saving, but current medical therapies, including antibiotics, are merely supportive and do little to halt the progressive inflammatory process in this disease. In the future, c‐kit+ donor cells or autologous amniotic fluid could be harvested in a sterile fashion at delivery for therapeutic purposes as described by others 18. One potential mechanism for the effectiveness of amniotic fluid stem cells may be by a reduction in intestinal permeability in neonatal rodent models 58.
Summary and Future Directions
The field of amniotic fluid stem cell therapy in the management of perinatal disease has enormous potential. Thus, it is not surprising that it has developed into one of the most furtile areas of translational research among fetal/pediatric scientists within the regenerative medicine community. Given the obvious clinical feasibility of amniotic fluid stem cell‐based therapies in those with prenatally diagnosed congenital anomalies, it is likely only a matter of time until some of the successful results that have already been demonstrated in preclinical animal models can be safely translated in humans.
Nevertheless, several limitations in this emerging field deserve special mention. First, as with most stem cell‐based therapies, we need to better define the optimal phenotype within a heterogeneous group of cells for each clinical application. Second, the biological basis for the paracrine function (e.g., growth factors, exosomes, etc.) of amniotic fluid stem cells remains to be elucidated. Third, determining the optimal gestational age for amniotic fluid stem cell isolation as well as dose/timing of therapy has made optimization for clinical use quite challenging. Fourth, the preparation of amniotic fluid stem cell for therapy may be variable and subject to large donor‐specific factors that have yet to be determined 60. Fifth, amniotic fluid cell‐based cultures often require the use of xenogeneic reagents that are either currently discouraged for human use or require close regulation by clinical safety boards, such as the Food and Drug Administration in the U.S. Finally, progress in amniotic fluid stem cell‐based therapy remains hampered by the relative rarity of each of these congenital anomalies, which often prohibits the ability to adequately study the efficacy of these novel treatments in a single center controlled trial. Overcoming these challenges will be essential if pediatric physicians and scientists are to bring these innovative and promising treatments to clinical fruition for the smallest and most vulnerable members of our society.
Disclosure of Potential Conflicts of Interest
The author indicated no potential conflicts of interest.
Supporting information
Figure S1. Repair of congenital diaphragmatic hernia (CDH) defects with a tissue‐engineered patch made from amniocytes. (A) Coronal MRI of a human fetus with a left CDH, showing herniation of liver (Lv) into the left chest relative to the right lung (R), left lung (L), and thymus (Th). (B) Gross appearance of diaphragmatic patch in an ovine model prior to implantation. (C) Intraoperative view showing adequate healing of a diaphragmatic patch several months after repair in a juvenile lamb. (D) Photomicrograph of amniocyte patch in a juvenile lamb illustrating collagen fiber alignment with Masson's trichrome staining (magnification, 10x). (E) Photomicrograph of amniocyte patch in a juvenile lamb showing positive smooth muscle actin (brown) staining (magnification, 10x). Modified from [41] with permission.
Figure S2. Tracheal reconstruction using a tissue‐engineered cartilaginous implant made from amniocytes. (A) Coronal MRI of a human fetus with tracheal atresia (yellow arrow). (B) Gross appearance of tracheal tube after in vitro chondrogenic differentation of amniotic fluid mesenchymal stem cells. (C) Gross appearance of trachea demonstrating mild stenosis at the implant site after two weeks in vivo. Modified from [11, 46] with permission.
Acknowledgments
I gratefully acknowledge Dr. Dario O. Fauza from Boston Children's Hospital, Harvard Medical School for providing selected images, as well as the National Institutes of Health (R01HD091323) for their financial support.
References
- 1. Cananzi M, Atala A, De Coppi P. Stem cells derived from amniotic fluid: New potentials in regenerative medicine. Reprod Biomed Online 2009;18(suppl 1):17–27. [DOI] [PubMed] [Google Scholar]
- 2. Klemmt PA, Vafaizadeh V, Groner B. The potential of amniotic fluid stem cells for cellular therapy and tissue engineering. Expert Opin Biol Ther 2011;11:1297–1314. [DOI] [PubMed] [Google Scholar]
- 3. Shaw SW, David AL, De Coppi P. Clinical applications of prenatal and postnatal therapy using stem cells retrieved from amniotic fluid. Curr Opin Obstet Gynecol 2011;23:109–116. [DOI] [PubMed] [Google Scholar]
- 4. Zhou Y, Besner G. Transplantation of amniotic fluid‐derived neural stem cells as a potential novel therapy for Hirschsprung's disease. J Pediatr Surg 2016;51:87–91. [DOI] [PubMed] [Google Scholar]
- 5. DeKoninck P, Toelen J, Zia S et al. Routine isolation and expansion late mid trimester amniotic fluid derived mesenchymal stem cells in a cohort of fetuses with congenital diaphragmatic hernia. Eur J Obstet Gynecol Reprod Biol 2014;178:157–162. [DOI] [PubMed] [Google Scholar]
- 6. Kunisaki SM, Armant M, Kao GS et al. Tissue engineering from human mesenchymal amniocytes: A prelude to clinical trials. J Pediatr Surg 2007;42:974–979. Discussion 979–980. [DOI] [PubMed] [Google Scholar]
- 7. Akolekar R, Beta J, Picciarelli G et al. Procedure‐related risk of miscarriage following amniocentesis and chorionic villus sampling: A systematic review and meta‐analysis. Ultrasound Obstet Gynecol 2015;45:16–26. [DOI] [PubMed] [Google Scholar]
- 8. Cadrin C, Golbus MS. Fetal tissue sampling—Indications, techniques, complications, and experience with sampling of fetal skin, liver, and muscle. West J Med 1993;159:269–272. [PMC free article] [PubMed] [Google Scholar]
- 9. Ge X, Wang IN, Toma I et al. Human amniotic mesenchymal stem cell‐derived induced pluripotent stem cells may generate a universal source of cardiac cells. Stem Cells Dev 2012;21:2798–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luo C, Jia W, Wang K et al. Human amniotic fluid stem cells suppress PBMC proliferation through IDO and IL‐10‐dependent pathways. Curr Stem Cell Res Ther 2014;9:36–45. [DOI] [PubMed] [Google Scholar]
- 11. Kaviani A, Guleserian K, Perry TE et al. Fetal tissue engineering from amniotic fluid. J Am Coll Surg 2003;196:592–597. [DOI] [PubMed] [Google Scholar]
- 12. Shaw SWS, Bollini S, Nader KA et al. Autologous transplantation of amniotic fluid‐derived mesenchymal stem cells into sheep fetuses. Cell Transplant 2016;25:615. [DOI] [PubMed] [Google Scholar]
- 13. Krupnick AS, Balsara KR, Kreisel D et al. Fetal liver as a source of autologous progenitor cells for perinatal tissue engineering. Tissue Eng 2004;10:723–735. [DOI] [PubMed] [Google Scholar]
- 14. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 15. In't Anker PS, Scherjon SA, Kleijburg‐van der Keur C et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003;102:1548–1549. [DOI] [PubMed] [Google Scholar]
- 16. Prusa AR, Marton E, Rosner M et al. Oct‐4‐expressing cells in human amniotic fluid: A new source for stem cell research? Hum Reprod 2003;18:1489–1493. [DOI] [PubMed] [Google Scholar]
- 17. Kunisaki SM, Jennings RW, Fauza DO. Fetal cartilage engineering from amniotic mesenchymal progenitor cells. Stem Cells Dev 2006;15:245–253. [DOI] [PubMed] [Google Scholar]
- 18. Moraghebi R, Kirkeby A, Chaves P et al. Term amniotic fluid: An unexploited reserve of mesenchymal stromal cells for reprogramming and potential cell therapy applications. Stem Cell Res Ther 2017;8:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miranda‐Sayago JM, Fernandez‐Arcas N, Benito C et al. Lifespan of human amniotic fluid‐derived multipotent mesenchymal stromal cells. Cytotherapy 2011;13:572–581. [DOI] [PubMed] [Google Scholar]
- 20. Roubelakis MG, Pappa KI, Bitsika V et al. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: Comparison to bone marrow mesenchymal stem cells. Stem Cells Dev 2007;16:931–952. [DOI] [PubMed] [Google Scholar]
- 21. Jezierski A, Gruslin A, Tremblay R et al. Probing stemness and neural commitment in human amniotic fluid cells. Stem Cell Rev 2010;6:199–214. [DOI] [PubMed] [Google Scholar]
- 22. Klein JD, Turner CG, Steigman SA et al. Amniotic mesenchymal stem cells enhance normal fetal wound healing. Stem Cells Dev 2011;20:969–976. [DOI] [PubMed] [Google Scholar]
- 23. Mareschi K, Castiglia S, Sanavio F et al. Immunoregulatory effects on T lymphocytes by human mesenchymal stromal cells isolated from bone marrow, amniotic fluid, and placenta. Exp Hematol 2016;44:138–150 e131. [DOI] [PubMed] [Google Scholar]
- 24. De Coppi P, Bartsch G Jr, Siddiqui MM et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 2007;25:100–106. [DOI] [PubMed] [Google Scholar]
- 25. De Coppi P, Callegari A, Chiavegato A et al. Amniotic fluid and bone marrow derived mesenchymal stem cells can be converted to smooth muscle cells in the cryo‐injured rat bladder and prevent compensatory hypertrophy of surviving smooth muscle cells. J Urol 2007;177:369–376. [DOI] [PubMed] [Google Scholar]
- 26. Guan X, Delo DM, Atala A et al. In vitro cardiomyogenic potential of human amniotic fluid stem cells. J Tissue Eng Regen Med 2011;5:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moorefield EC, McKee EE, Solchaga L et al. Cloned, CD117 selected human amniotic fluid stem cells are capable of modulating the immune response. PLoS One 2011;6:e26535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bollini S, Cheung KK, Riegler J et al. Amniotic fluid stem cells are cardioprotective following acute myocardial infarction. Stem Cells Dev 2011;20:1985–1994. [DOI] [PubMed] [Google Scholar]
- 29. Bai J, Wang Y, Liu L et al. Human amniotic fluid‐derived c‐kit(+) and c‐kit (−) stem cells: growth characteristics and some differentiation potential capacities comparison. Cytotechnology 2012;64:577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ye L, Chang JC, Lin C et al. Induced pluripotent stem cells offer new approach to therapy in thalassemia and sickle cell anemia and option in prenatal diagnosis in genetic diseases. Proc Natl Acad Sci USA 2009;106:9826–9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li C, Zhou J, Shi G et al. Pluripotency can be rapidly and efficiently induced in human amniotic fluid‐derived cells. Hum Mol Genet 2009;18:4340–4349. [DOI] [PubMed] [Google Scholar]
- 32. Galende E, Karakikes I, Edelmann L et al. Amniotic fluid cells are more efficiently reprogrammed to pluripotency than adult cells. Cell Reprogram 2010;12:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anchan RM, Quaas P, Gerami‐Naini B et al. Amniocytes can serve a dual function as a source of iPS cells and feeder layers. Hum Mol Genet 2011;20:962–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jiang G, Di Bernardo J, Maiden MM et al. Human transgene‐free amniotic‐fluid‐derived induced pluripotent stem cells for autologous cell therapy. Stem Cells Dev 2014;23:2613–2625. [DOI] [PubMed] [Google Scholar]
- 35. Moschidou D, Mukherjee S, Blundell MP et al. Human mid‐trimester amniotic fluid stem cells cultured under embryonic stem cell conditions with valproic acid acquire pluripotent characteristics. Stem Cells Dev 2013;22:444–458. [DOI] [PubMed] [Google Scholar]
- 36. Giuliani M, Oudrhiri N, Noman ZM et al. Human mesenchymal stem cells derived from induced pluripotent stem cells down‐regulate NK‐cell cytolytic machinery. Blood 2011;118:3254–3262. [DOI] [PubMed] [Google Scholar]
- 37. Adzick NS, Thom EA, Spong CY et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 2011;364:993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kunisaki SM. Congenital anomalies: treatment options based on amniotic fluid‐derived stem cells. Organogenesis 2012;8:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dionigi B, Ahmed A, Brazzo J 3rd et al. Partial or complete coverage of experimental spina bifida by simple intra‐amniotic injection of concentrated amniotic mesenchymal stem cells. J Pediatr Surg 2015;50:69–73. [DOI] [PubMed] [Google Scholar]
- 40. Kajiwara K, Tanemoto T, Wada S et al. Fetal therapy model of myelomeningocele with three‐dimensional skin using amniotic fluid cell‐derived induced pluripotent stem cells. Stem Cell Rep 2017;8:1701–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Turner CG, Pennington EC, Gray FL et al. Intra‐amniotic delivery of amniotic‐derived neural stem cells in a syngeneic model of spina bifida. Fetal Diagn Ther 2013;34:38–43. [DOI] [PubMed] [Google Scholar]
- 42. Meuli M, Meuli‐Simmen C, Hutchins GM et al. In utero surgery rescues neurological function at birth in sheep with spina bifida. Nat Med 1995;1:342–347. [DOI] [PubMed] [Google Scholar]
- 43. Connell JP, Ruano R, Jacot JG. Amniotic fluid‐derived stem cells demonstrate limited cardiac differentiation following small molecule‐based modulation of Wnt signaling pathway. Biomed Mater 2015;10:034103. [DOI] [PubMed] [Google Scholar]
- 44. Jiang G, Herron TJ, Di Bernardo J et al. Human cardiomyocytes prior to birth by integration‐free reprogramming of amniotic fluid cells. Stem Cells Translational Medicine 2016;5:1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Velasquez‐Mao AJ, Tsao CJM, Monroe MN et al. Differentiation of spontaneously contracting cardiomyocytes from non‐virally reprogrammed human amniotic fluid stem cells. PLoS One 2017;12:e0177824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yeh YC, Lee WY, Yu CL et al. Cardiac repair with injectable cell sheet fragments of human amniotic fluid stem cells in an immune‐suppressed rat model. Biomaterials 2010;31:6444–6453. [DOI] [PubMed] [Google Scholar]
- 47. Schmidt D, Achermann J, Odermatt B et al. Prenatally fabricated autologous human living heart valves based on amniotic fluid derived progenitor cells as single cell source. Circulation 2007;116:I64–I70. [DOI] [PubMed] [Google Scholar]
- 48. Weber B, Emmert MY, Behr L et al. Prenatally engineered autologous amniotic fluid stem cell‐based heart valves in the fetal circulation. Biomaterials 2012;33:4031–4043. [DOI] [PubMed] [Google Scholar]
- 49. Fuchs JR, Kaviani A, Oh JT et al. Diaphragmatic reconstruction with autologous tendon engineered from mesenchymal amniocytes. J Pediatr Surg 2004;39:834–838. Discussion 834–838. [DOI] [PubMed] [Google Scholar]
- 50. Kunisaki SM, Fuchs JR, Kaviani A et al. Diaphragmatic repair through fetal tissue engineering: A comparison between mesenchymal amniocyte‐ and myoblast‐based constructs. J Pediatr Surg 2006;41:34–39. discussion 34–39. [DOI] [PubMed] [Google Scholar]
- 51. Kotecha S. Lung growth: Implications for the newborn infant. Arch Dis Child Fetal Neonatal Ed 2000;82:F69–F74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pederiva F, Ghionzoli M, Pierro A et al. Amniotic fluid stem cells rescue both in vitro and in vivo growth, innervation, and motility in nitrofen‐exposed hypoplastic rat lungs through paracrine effects. Cell Transplant 2013;22:1683–1694. [DOI] [PubMed] [Google Scholar]
- 53. Di Bernardo J, Maiden MM, Hershenson MB et al. Amniotic fluid derived mesenchymal stromal cells augment fetal lung growth in a nitrofen explant model. J Pediatr Surg 2014;49:859–864. Discussion 864–855. [DOI] [PubMed] [Google Scholar]
- 54. DeKoninck P, Toelen J, Roubliova X et al. The use of human amniotic fluid stem cells as an adjunct to promote pulmonary development in a rabbit model for congenital diaphragmatic hernia. Prenat Diagn 2015;35:833–840. [DOI] [PubMed] [Google Scholar]
- 55. Kunisaki SM, Freedman DA, Fauza DO. Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J Pediatr Surg 2006;41:675–682. Discussion 675–682. [DOI] [PubMed] [Google Scholar]
- 56. Elliott MJ, De Coppi P, Speggiorin S et al. Stem‐cell‐based, tissue engineered tracheal replacement in a child: A 2‐year follow‐up study. Lancet 2012;380:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Feng C, Graham CD, Connors JP et al. Transamniotic stem cell therapy (TRASCET) mitigates bowel damage in a model of gastroschisis. J Pediatr Surg 2016;51:56–61. [DOI] [PubMed] [Google Scholar]
- 58. Zani A, Cananzi M, Fascetti‐Leon F et al. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX‐2 dependent mechanism. Gut 2014;63:300–309. [DOI] [PubMed] [Google Scholar]
- 59. McCulloh CJ, Olson JK, Zhou Y et al. Stem cells and necrotizing enterocolitis: A direct comparison of the efficacy of multiple types of stem cells. J Pediatr Surg 2017;52:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ekblad A, Qian H, Westgren M et al. Amniotic fluid: A source for clinical therapeutics in the newborn? Stem Cells Dev 2015;24:1405–1414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Repair of congenital diaphragmatic hernia (CDH) defects with a tissue‐engineered patch made from amniocytes. (A) Coronal MRI of a human fetus with a left CDH, showing herniation of liver (Lv) into the left chest relative to the right lung (R), left lung (L), and thymus (Th). (B) Gross appearance of diaphragmatic patch in an ovine model prior to implantation. (C) Intraoperative view showing adequate healing of a diaphragmatic patch several months after repair in a juvenile lamb. (D) Photomicrograph of amniocyte patch in a juvenile lamb illustrating collagen fiber alignment with Masson's trichrome staining (magnification, 10x). (E) Photomicrograph of amniocyte patch in a juvenile lamb showing positive smooth muscle actin (brown) staining (magnification, 10x). Modified from [41] with permission.
Figure S2. Tracheal reconstruction using a tissue‐engineered cartilaginous implant made from amniocytes. (A) Coronal MRI of a human fetus with tracheal atresia (yellow arrow). (B) Gross appearance of tracheal tube after in vitro chondrogenic differentation of amniotic fluid mesenchymal stem cells. (C) Gross appearance of trachea demonstrating mild stenosis at the implant site after two weeks in vivo. Modified from [11, 46] with permission.


