Abstract
Similar to the disease affecting humans, osteoarthritis (OA) is a painful musculoskeletal condition affecting 20% of the adult canine population. Several solutions have been proposed, but the results achieved to date are far from being satisfactory. New approaches, such as intra‐articular delivery of cells (including mesenchymal stromal cells), have been proposed. Among the many sources, the adipose tissue is considered very promising. We evaluated the safety, feasibility, and efficacy of a single intra‐articular injection of autologous and micro‐fragmented adipose tissue (MFAT) in 130 dogs with spontaneous OA. MFAT was obtained using a minimally invasive technique in a closed system and injected in the intra‐ and/or peri‐articular space. Clinical outcomes were determined using orthopedic examination and owners’ scores for up to 6 months. In 78% of the dogs, improvement in the orthopedic score was registered 1 month after treatment and continued gradually up to 6 months when 88% of the dogs improved, 11% did not change, and 1% worsened compared with baseline. Considering the owners’ scores at 6 months, 92% of the dogs significantly improved, 6% improved only slightly, and 2% worsened compared with baseline. No local or systemic major adverse effects were recorded. The results of this study suggest that MFAT injection in dogs with OA is safe, feasible, and beneficial. The procedure is time sparing and cost‐effective. Post injection cytological investigation, together with the clinical evidence, suggests a long‐term pain control role of this treatment. The spontaneous OA dog model has a key role in developing successful treatments for translational medicine. stem cells translational medicine 2018;7:819–828
Significance Statement.
This study evaluates the safety, feasibility, and clinical efficacy of using autologous and micro‐fragmented adipose tissue for the treatment of spontaneous osteoarthritis in dogs. The procedure is simple, time sparing, cost‐effective, minimally invasive, one‐step, and eliminates the need for complex and time intensive cell culture processing. The lack of any complications and the long lasting successful results are of considerable importance for the use in human medicine.
Introduction
Osteoarthritis (OA) is a painful musculoskeletal condition, often secondary to structural abnormalities or ligament injury leading to articular instability and modifications of the normal cartilage matrix resulting in pain, joint stiffness, and muscular atrophy 1, 2. When surface erosion, bone sclerosis, and osteophyte production are severe enough to be clinically recognized, they are likely to be irreversible when treated with current therapies 3. OA is the most common cause of disability in the elderly population with an incidence of 10% in humans aged 60 years and older 4 and remains a huge concern to public health, in terms of both health‐related quality of life and the financial burden caused by the disease. A critical step toward understanding and mitigating the effects of this disease is translational research. Using animal models provides an extremely practical and clinically relevant way to study the natural history and response to treatment of OA, and the dog is probably the closest to a gold standard model for OA 5, 6. OA affects 20% of the adult canine population 7, with significant welfare implications. The disease affects dogs of all ages, sizes, and breeds 8, with large‐breeds developing more severe clinical signs.
A variety of treatments for the management of OA in dogs have been proposed (alternative therapies, functional food, intra‐articular agents, nutraceutical agents, pharmacological agents, physical therapies, surgical techniques, and weight control) with variable success rates 3, 9. Recently, new therapeutic approaches, such as the use of cellular therapies, including mesenchymal stromal cells (MSCs), have been introduced 10, 11, 12, 13, 14, 15 and adipose tissue is a very useful source of these naturally occurring regenerative cells because of its abundance and easy access. MSCs have been reported to have a perivascular origin and to be able to activate and influence the microenvironment by serving as “a site‐regulated drug store” 16. Through trophic, mitogenic, anti‐scarring, anti‐apoptotic, immunomodulatory 17, and antimicrobial actions, produced by a large amount of bioactive elements, growth factors and cytokines, these cells sense and signal changes in the microenvironment where they reside 17, 18, 19, 20.
The use of purified adipose‐derived MSCs has recently created a huge interest in the context of cartilage regeneration 21, 22 and both in vitro and in vivo studies demonstrated their anti‐inflammatory and regenerative properties 23. Nevertheless, enzymatic treatment and/or cell expansion have complex regulatory concerns related to good manufacturing practice (GMP) guidelines 24, 25, 26, 27, 28, 29 and the requirement for a biological license from the U.S. FDA. Hence, the availability of minimally manipulated autologous adipose tissue as a therapeutic option would have remarkable clinical relevance. For this reason, we employed a commercially available system (Lipogems®) that intraoperatively provides micro‐fragmented adipose tissue (MFAT) in a short time, without cell expansion, enzymatic treatment or other major manipulations 30, 31.
With this study, we evaluated the safety, feasibility, and clinical efficacy of autologous MFAT injections in dogs affected by spontaneous OA.
Materials and Methods
Study Design and Population
This is a multicenter prospective observational and independent study. One hundred and thirty client‐owned dogs with spontaneous OA were treated with autologous MFAT injections from 2014 to 2017 in seven private veterinary hospitals in Italy, Sweden, Israel, and U.K. Inclusion criteria for the participation in the study were OA diagnosed based on history, clinical signs, and radiographic evidence of arthritis in one or more joints. No age, sex, or weight limits were applied, but the dogs were required to be free from systemic diseases, have a normal complete blood count (CBC) and serum biochemical analysis and not enrolled in other clinical studies. Dogs with major comorbidities that could interfere or modify the results of the study, with radiographic evidences of joint mouse (intended as completely detached fragment of cartilage floating around the joint) and/or infected synovial fluid, or treated with systemic nonsteroidal anti‐inflammatory drugs (NSAIDs) or corticosteroids within 10 days and/or local intra‐articular medications 30 days before the treatment were excluded from the study. Participation in the study was interrupted in cases of any complication or lack of the owner to cooperate with procedures or restrictions. In case of death due to natural causes or owner request for euthanasia, dogs underwent autopsy and the treated joint/s underwent histological examinations. Each participating veterinary hospital followed guidelines established for Good Clinical Practice.
Pretreatment and Post‐Treatment Clinical Examination
To assess lameness and pain, detailed history from all dogs was obtained and gait was observed and recorded by video. The patients underwent general physical examination followed by orthopedic examination. Each joint was manipulated for pain, synovial effusion, crepitus, or altered range of motion with the intention to identify one or more affected joints. Because it is essential to have scoring methods that allow for reduced variability between various users and across multiple hospital settings 32, our follow‐up included both orthopedic examination at 2 weeks, 1, 3, and 6 months after treatment and monthly owner's pain assessments for up to 24 months with the Helsinki chronic pain index (HCPI) 33. To assess baseline severity of OA, disease progression and benefits of treatment, lameness scores were assigned using a modified numerical rating scale (NRS) ranging from 1 (clinically sound) to 4 (cannot be more lame) 34, 35. For the owner's pain assessments (HCPI), 11 items, divided between a simple descriptive scale for behavior and locomotion and the visual analog scale for pain, are scored 0–4. To better compare the HCPI with the lameness score, we multiplied the latter by 11 to obtain a customized score, the orthopedic score (OS), which was classified in normal (score = 11), mild (score = 22), moderate (score = 33), and severe (score = 44).
Radiographic Exam
Dogs underwent radiographic exams of the affected joints before treatment, 3 and 6 months after treatment or whenever a notable clinical change was reported. Anteroposterior, lateral, medial and, if necessary, oblique projections of the joints were used according to the anatomic region. Routine assessment parameters included joint capsular distention due to effusion, soft tissue thickening, intra‐articular mineralization, narrowed joint spaces, subchondral sclerosis, and osteophyte formation and progression 36. The dogs were graded according to I–IV numeric scale: I normal, II mild changes, III moderate changes, and IV severe radiographic changes.
Advanced Imaging Diagnostic
Computed tomography (CT) (Presto 4 slice, Hitachi, Japan and Somatom 16 slice, Siemens, Germany) provided improved accuracy for evaluation of articular abnormalities. Sagittal and dorsal reformatted images were used in conjunction with imaging in the transversal plane. Magnetic resonance imaging (MRI) (Vet‐MR Grande 0.25T, Esaote Spa, Genova, Italy) was used to assess additional bony, ligamentous, and soft‐tissue abnormalities since it is more sensitive in detecting early changes 37. T1‐weighted pre‐ and post‐contrast enhancement and T2‐weighted sequences were used in sagittal, dorsal, and transversal planes. Those methods were used for assessment of both extra‐ and intra‐articular structures although not routinely used in the initial evaluation of arthropathies, rather for resolving uncertain findings on conventional radiographs. Examples for such conditions are anterior cruciate ligament injuries, osteochondritis dissecans (OCD) and coronoid process fragmentation. Altogether, 68 patients underwent MRI and/or CT.
Synovial Fluid Examination
Each patient underwent a synovial fluid examination within 1 week before the treatment. Skin over the joint was prepared as for any sterile invasive procedure. The choice of needle diameter and length depended upon the joint involved and the size of the patient. Color, turbidity, viscosity, and quantity of synovial fluid were assessed. Cell count was done by CBC machine, whereas interpretation of fluid samples of low volume, was aided by methodical manual counting of cells on direct smears. Differential cell counts were done by MGG QUICK stain (Bio‐Optica Milano Spa, Milano, Italy) and microscopic examination 38. Protein concentration was measured by refractometer, paying attention to avoid small sample volumes in large amounts of EDTA (falsely high protein concentrations). The synovial fluid was classified in normal when cell count was <3,000 cells/μl (rare synoviocytes and lymphocytes); dogs were considered affected by a degenerative arthropathy when cell count was between 3,000 and 5,000 cells/μl (synoviocytes, macrophages, and lymphocytes). An inflammatory arthropathy was diagnosed when cell count was >5,000 cells/μl (mainly degenerated non‐granulocytes). In cases of suspected infectious arthropathy, a sample of synovial fluid was sent to the laboratory for bacterial culture.
Adipose Tissue Harvesting and Processing
The entire procedure was performed in a certified operating room. The harvesting procedure in humans has been extensively described 30, whereas in dogs, no previous experience has been published. Patients were sedated with medetomidine 1 μg/kg and metadone 0.2 mg/kg IM and then anesthetized by propofol 2–3 mg/kg IV ad effect until tracheal intubation was achieved. Anesthesia was maintained by isofluorane 1.2%. Each dog was placed in sternal recumbency. The procedure was performed making a single cutaneous hole, using an 18G needle, on the central line of the lumbar region above the fifth lumbar vertebra (Fig. 1). Through the cutaneous hole, a disposable 18G blunt cannula was inserted into the fat layer and the fat was infiltrated with a mixture of sterile saline 4 ml/kg b.wt. and adrenalin 0.05 mg/10 ml NaCl solution. The fat was then harvested by connecting a 16G blunt cannula to a Vaclock® (apex) 20‐ml syringe and then injected into the Lipogems® (apex) device (Lipogems International SpA, Milan, Italy), a disposable product that progressively reduces the size of the adipose tissue clusters while eliminating oily substances and blood residues with proinflammatory properties. The entire process, carried out in one surgical step, was performed in complete immersion in physiological solution minimizing any trauma to the cells. The resulting MFAT was collected in a 60‐cc syringe, positioned for decanting the excess saline solution and then transferred into several 10‐cc syringes to be injected in the patient. Randomly, every 10 patients, a sample of MFAT was sent to the laboratory for morphological and microbiological quality control.
Figure 1.
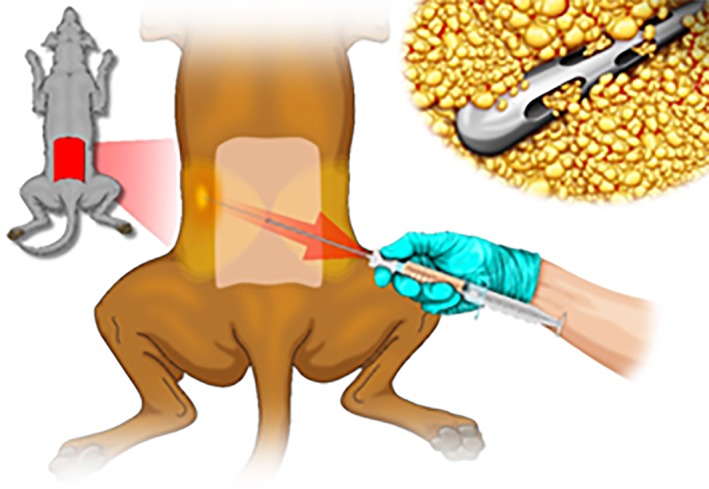
Operative scheme: lipoaspiration procedure in the dog.
MFAT Injection and Postoperative Care
In 90% of the patients, MFAT was injected in the intra‐articular space. In details, the needle was introduced into the joint, synovial fluid aspirated and MFAT injected through the same needle. The amount and site of injected material depended on patient's dimension, type of joint, type of arthropathy, and availability of material. Ideally, dogs >30 kg b.wt. had a 2 ml injection in or around each treated joint, dogs 15–30 kg had 1.5 ml, and dogs 1–14 kg had 1 ml. In cases of very small joints or massive osteophytes, MFAT was injected in the peri‐articular space (10% of the patients). The cutaneous hole was medicated with antibiotic cream and covered by plaster. The patient was sent home few hours after the procedure with Tramadol 2 mg/kg twice a day for 2 days. Owners were instructed to restrict the dog's activity for 5 days and encouraged to report to the personal veterinary surgeon any observed abnormality (pain, bleeding, infection, or others). HCPI survey compilation was explained to the owners together with the importance of client adherence to the follow‐up protocol. Finally, the possibility to forward films of the dog between the clinical controls was verified.
Histological Examinations
Histological examination was performed on the treated joints of two patients who died of natural causes not related to the primary articular disease 8 months after treatment and one patient who died 11 months after treatment. At necropsy, the articulations were taken and fixed in 4% phosphate buffered formalin. The bones were then routinely decalcified. Samples were taken after decalcification, embedded in paraffin and 4 μm sections were stained with hematoxylin–eosin (H&E), Masson trichrome and Alcian blue and examined microscopically. The expression of collagen I, II, III, and X was assessed by immunohistochemistry. Briefly, after dewaxing, slides were hydrated through a descending ethanol series and then rinsed in distilled water. Endogenous peroxidase was inactivated with 3% hydrogen peroxide in tris‐phosphate buffered saline (TBS) at room temperature. Antigen retrieval was achieved incubating the sections in a microwave oven with 10 mM citric acid solution (pH 6.0). Nonspecific reactivity was blocked with normal serum for 30 minutes. Slides were then incubated overnight with the following primary antibodies: rabbit polyclonal antibody to collagen I (Byorbit, Cambridge, U.K.; dilution 1:50); mouse monoclonal antibody to collagen II (Thermo Fisher, Waltham, MA, U.S., dilution 1:100) rabbit polyclonal antibody to collagen III (Byorbit, Cambridge, U.K.; dilution 1:50); rabbit polyclonal antibody to collagen X (Gene Tex, Irvine, CA; dilution 1:50). Sections were then respectively incubated with goat anti‐rabbit and goat anti‐mouse biotinylated antibody, followed by avidin‐biotin complex (ABCL‐2, Abcam, Cambridge, U.K.). Immunostaining was revealed with 3‐amino‐9ethyl‐carbazole and nuclei were counterstained with hematoxylin. Negative controls were performed by omitting the primary antibody.
MFAT morphology, for quality control purposes, was also evaluated. Thirteen samples were analyzed by means of light microscopy (LM), transmission electron microscopy (TEM), and scanning electron microscopy (SEM). Specifically, MFAT was fixed in formalin and embedded in paraffin for observation at light microscope. Five μm thick sections were stained with H&E and with Masson trichrome. For TEM, MFAT was fixed in glutaraldehyde, postfixed in osmium tetroxide and embedded in epoxy resin. Ultrathin sections were examined with a Philips EM 208 transmission electron microscope. For SEM, MFAT was fixed in 2.5% glutaraldehyde, dehydrated, coated with gold to a thickness of 15 nm and observed with a Philips XL30 scanning electron microscope.
Safety Assessment
To assess the safety of the procedure, serial blood samples were taken once a month, up to 6 months, for CBC and serum biochemical analysis.
Statistical Analysis
To guarantee standard operating procedures, all dogs were operated by the same surgeon (OZ). Clinical evaluations at any follow‐up time were performed in each center independently. Results are expressed as mean and standard deviation. Statistical analysis was performed using Graphpad Prism v7.04 software (GraphPad Software Inc., La Jolla, CA). One‐way analysis of variance (ANOVA) for repeated measures with Dunnett's post hoc test was used to analyze the data obtained at the different time points and for the comparison of data sets from each time point with the baseline values. Two‐way ANOVA for repeated measures was applied to test the influences of animal size and OA severity on owner's (HCPI) and orthopedic (OS) scores at the different time points. A p < .05 was considered statistically significant.
Ethics
For ethical reasons, the use of NSAIDs (mostly Robenacoxib 2 mg/kg per sid) during the study was allowed and limited to the shortest possible period. The owners of the dogs were thoroughly informed about the entire procedure and signed a formal agreement in acceptance of both anesthesia and therapy. They also accepted that their dogs would undergo post‐mortem examination of the joints.
Results
The 130 patients (49 breeds, 50.8% males, and 49.2% females) ranged in age from <1 to 13 years, with a mean age of six (SD 3.7). Considering the dog size, 10.8% of the dogs were small (<15 kg), 22.3% medium (15–25 kg), and 66.9% large (>25 kg). Three hundred forty‐six joints of eight types were treated with hips, elbows and stifles representing the majority (85%). On average, 35.6 ml (SD 10.5) of lipoaspirate and 7.3 ml (SD 4.3) of MFAT was obtained from each patient. Based on the pretreatment synovial fluid cytological examination, 10.0% of the dogs resulted normal (although presenting clinical signs of OA), 80.9% showed signs compatible with a degenerative arthropathy and 9.1% presented findings of an inflammatory non‐septic arthropathy. Regarding the severity of OA, detected by radiographic examination and/or advanced imaging diagnostic, 1.5% of the dogs were grade I, 30.0% grade II, 47.7% grade III, and the remaining 20.8% grade IV (Table 1).
Table 1.
Background data of the population
| Characteristic | Value |
|---|---|
| Gender | |
| M | 50.8% |
| F | 49.2% |
| Size | |
| Small | 10.8% |
| Medium | 22.3% |
| Large | 66.9% |
| Breeds | 49 |
| Age | |
| Mean | 6.0 yr.o. |
| SD | 3.7 |
| Total joints | 346 |
| Tarsus | 1.0% |
| Carpus | 5.0% |
| Metacarpus | 1.0% |
| Shoulder | 8.0% |
| Hip | 37.0% |
| Stifles | 21.0% |
| Paravertebral muscle | 2.0% |
| Elbow | 27.0% |
| Harvested fat | |
| Mean | 32.6 ml |
| SD | 10.6 ml |
| Obtained MFAT | |
| Mean | 7.3 ml |
| SD | 4.3 ml |
| Volumea of injected MFAT | |
| Tarsus | 0.5–2 ml |
| Carpus | 0.5–2 ml |
| Metacarpus | 0.3–1 ml |
| Shoulder | 0.5–2 ml |
| Hip | 0.5–3 ml |
| Stifles | 0.5–3 ml |
| Paravertebral muscle | 1–4 ml |
| Elbow | 0.5–2 ml |
| Synovial fluid | |
| NORMAL | 10.0% |
| DEG OA | 81.0% |
| INFL OA | 9.0% |
| Grade OA | |
| I | 1.5% |
| II | 30.0% |
| III | 47.7% |
| IV | 20.8% |
Minimum and maximum volume depending on the animal size.
Abbreviations: DEG, degenerative; F, female; INFL, inflammatory; M, male; MFAT, micro‐fragmented adipose tissue; OA, osteoarthritis; SD, standard deviation.
HCPI and OS scores both improved significantly at each time point with respect to baseline (p < .0001, Fig. 2). In 78% of the dogs, improvement in the OS was clear 1 month after MFAT injection and continued gradually up to 6 months. At 6 months, 88% of the dogs improved and 11% did not change compared to baseline. Only 1% worsened, presumably because of iatrogenic septic arthritis (Staphylococcus spp.) in the treated joint and treatment with antimicrobial drugs was successful. Considering the HCPI, based on the owner's knowledge of the dog's “normal” versus “abnormal” behaviors, gait and other typical activities 33, 39, at 6 months 63% of the dogs considerably improved (Δt6‐t0HCPI > 10), 29% significantly improved (Δt6‐t0HCPI ≥ 5 and ≤10), 6% improved only slightly (Δt6‐t0HCPI < 5), and 2% worsened compared to baseline evaluation (Fig. 2). Possible correlations between improvements in the clinical outcomes at 3 and 6 months and specific patient's categories such as sex, size, age, type of synovial fluid, severity of OA determined by imaging, owner's and orthopedic scores were also evaluated. Sex, age, and synovial fluid characteristics did not influence the outcomes. Conversely, OA severity influenced both OS and HCPI scores changes during time. In detail, dogs with OA grade III and IV showed more improvements compared to dogs with grade I and II (Δt6‐t0HCPI −14.2 vs. −9.4, p = .0001 and Δt6‐t0OS −14.0 vs. −10.4, p = .0092, Fig. 3).
Figure 2.
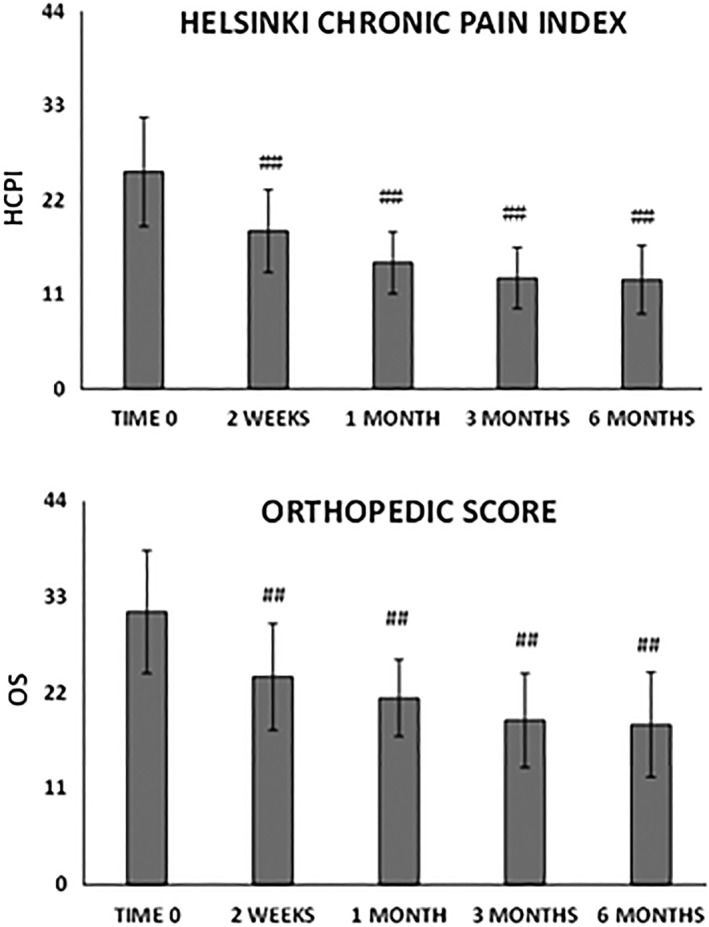
Trend of functional improvement from baseline to 6 months’ follow‐up. (Top): Helsinki chronic pain index. (Bottom): Orthopedic score. The severity of osteoarthritis is classified in normal (11 points), mild (22 points), moderate (33 points) and severe (44 points). Results are expressed as mean and standard deviation. A p < .01 was considered statistically significant (##).
Figure 3.
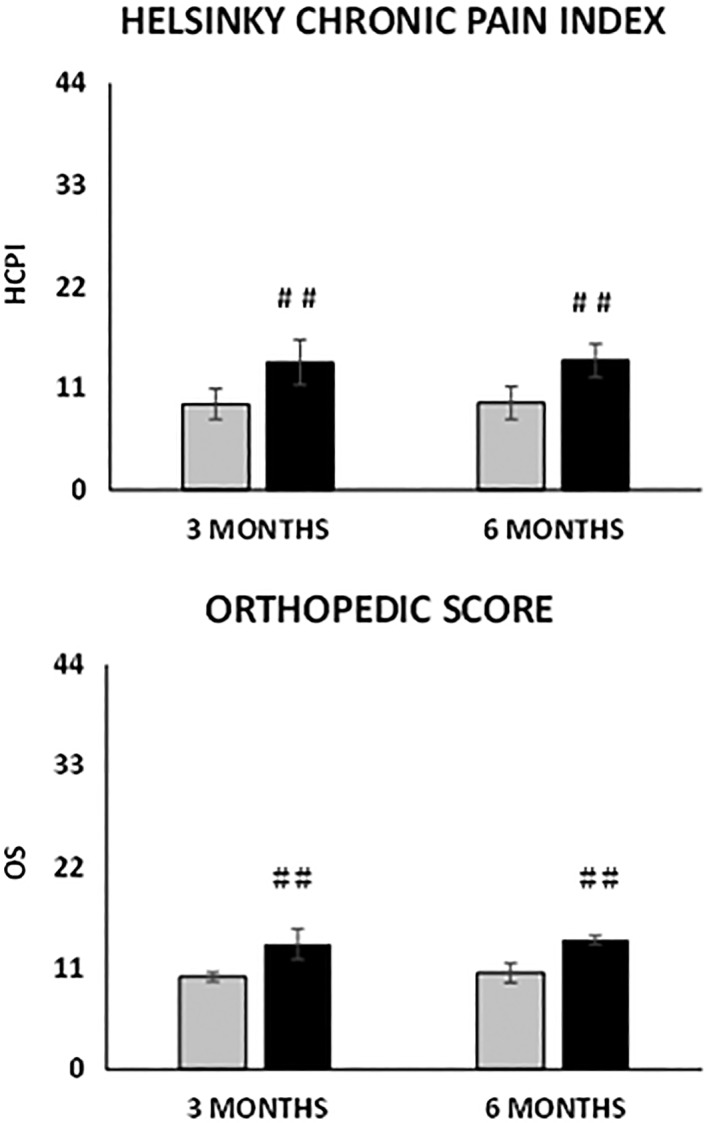
Change in the scores at 3 and 6 months’ follow‐up depending on the grade of radiographic osteoarthritis at baseline. (Top): Helsinki chronic pain index. (Bottom): Orthopedic score. Grey bars: OA grade I and II; black bars: OA grade III and IV. Results are expressed as mean and standard deviation. (##) p < .01 Δt3,6‐t0 grade III and IV versus I and II.
We observed the same trend for patients with an OS at baseline of moderate/severe that at 6 months showed a Δt6‐t0 HCPI of −14.5 versus the −8.3 of the patients starting from a normal/mild OS (p = 3.14E‐7) and a Δt6‐t0 OS of −15.4 versus the −6.9 (p = 2.18E‐10, Fig. 4).
Figure 4.
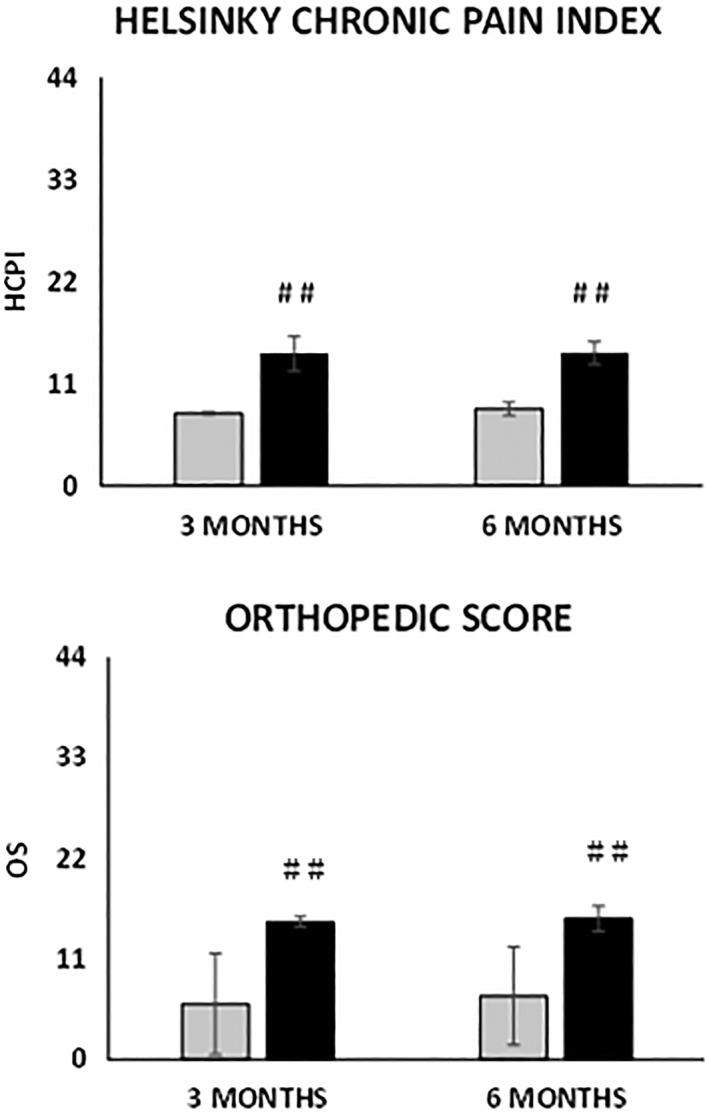
Change in the scores at 3 and 6 months’ follow‐up depending on the severity of osteoarthritis at baseline assessed by the orthopedic score. (Top): Helsinki chronic pain index. (Bottom): Orthopedic score. Grey bars: normal/mild; black bars: moderate/severe. Results are expressed as mean and standard deviation. (##) p < .01 Δtx‐t0 moderate/severe versus normal/mild.
The size of animal influenced the HCPI in relation to the time of observation, whereas OS values changings over time were independent from this parameter. Nevertheless, medium/large animals improved more compared to small animals (Δt6‐t0HCPI −13.3 vs. −9.3, p = .02 and Δt6‐t0OS −14.4 vs. −9.4, p = .03, Fig. 5). The improvements at each time point were confirmed in all different categories, in terms of animal size and gender.
Figure 5.
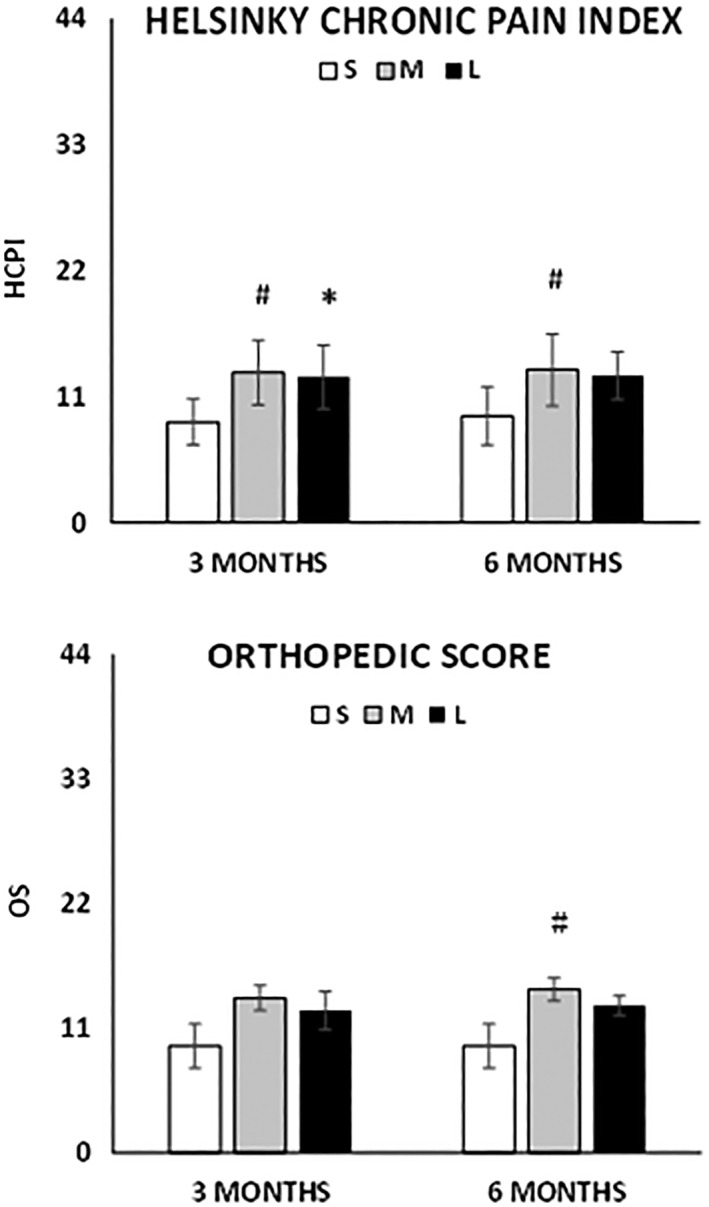
Change in the scores at 3 and 6 months’ follow‐up depending on the size of the animal. (Top): Helsinki chronic pain index. (Bottom): Orthopedic score. White bars (S): small size (<15 kg); grey bars (M): medium size (15–25 kg); black bars (L): large size (>25 kg). Results are expressed as mean and standard deviation. (#) p < .05 Δtx‐t0 medium versus small and (*) p < .05 Δtx‐t0 large versus small.
Post‐treatment radiographic assessment at 3 and 6 months showed variable increase in osteophyte dimension in adult dogs, in which hip, stifle, and shoulder joints were involved. However, in some cases osteophytes presented blunt extremity compared to pretreatment (Supporting information Fig. 1). In young dogs, mainly affected by OCD, the cartilage lesion became filled and covered by hyperdense material (Supporting information Fig. 2). In cases of stifle OA, post‐treatment MRI showed mildly decreased joint effusion, with bone marrow edema‐like lesions around the origin of the cranial cruciate ligament and a meniscus less clearly delineable, especially in the corpus/cranial horn transition area.
Clinical examinations at 1 month and every 3 months confirmed no local or systemic short‐ or long‐term major adverse effects. In addition, CBC and serum biochemical analysis did not show any significant abnormality.
The histological examination of a joint 11 months after treatment revealed an uneven articular surface. Various stages of degenerative lesions of the articular cartilage were observed and multiple foci of cell proliferation (chondrocyte clusters and/or disorientation of chondrocytes) were associated with these changes. Large eosinophilic polymorphic cells with cytoplasmic vacuoles were observed in multiple foci free within the articular cavity and lining the fibrous cartilage or the synovial membrane, suggesting the presence of the injected adipose tissue 11 months before (Supporting information Fig. 3).
Immunohistochemistry assessment showed that collagen expression varied significantly in the different areas. The widest area of immunostaining for type I collagen was observed in fibrocartilage and in fibrous‐like tissue; in these sites, collagen I was moderately but homogeneously expressed. In contrast, where hyaline cartilage was predominant, almost no staining for type I was detected. Collagen type II was abundantly expressed in preserved hyaline cartilage whereas collagen II immunostaining was mild and restricted to the lower regions of fibrocartilage. Immunohistochemistry demonstrated some immunopositivity for type III collagen in fibrocartilage while almost no staining for this type of collagen was seen in hyaline cartilage. Finally, immunoreactions to collagen X gave rise to a mild multifocally pericellular staining in fibrocartilage (Supporting information Fig. 4).
The morphological analysis of 13 samples of MFAT by means of LM and SEM revealed the adipocytes as empty structures due to the alcohol treatment that had removed the abundant lipid component and had preserved adipocyte membranes. A network of connective reticular tissue embedded by adipocytes and microvessels was observed (Fig. 6). At LM and TEM, different cell types were detected: empty adipocytes (Fig. 6A, 6B), red blood cells aligned within capillaries (Fig. 6E, 6G, 6H), long flat endothelial cells lined along the capillaries (Fig. 6G), large globular cells of possible stromal nature located in the interstices or close to vessel walls, and with an arrangement resembling the one described for pericytes (Fig. 6D, 6G, 6I). Neither medium nor large vessels were observed. No substantial differences were observed between samples taken from superficial or deep adipose tissue. Microbiological analysis confirmed the sterility of the injected MFAT.
Figure 6.
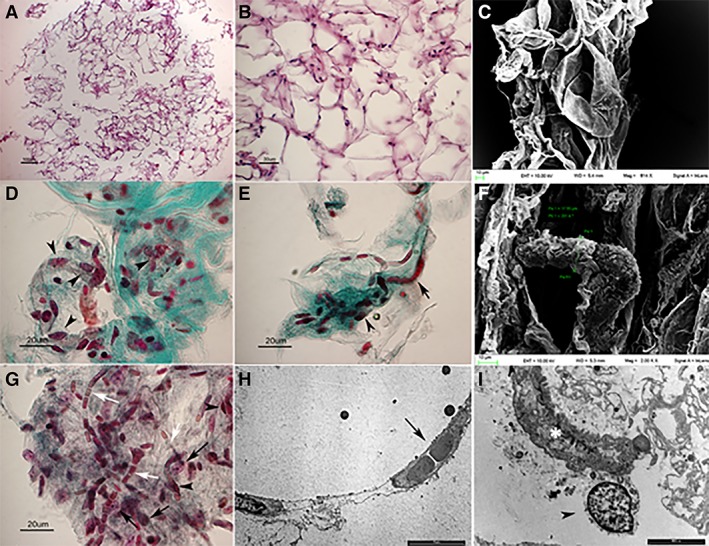
Micro‐fragmented adipose tissue obtained from canine lipoaspirate analyzed by light microscopy (LM), transmission electron microscopy (TEM) and scanning electron microscopy (SEM). (A): Lacunar spaces depict empty adipocytes surrounded by a network of reticular connective tissue (LM) (B): See A. High magnification. (C): Empty adipocytes surrounded by connective tissue (SEM). (D, E): Micro‐fragmented adipose tissue stromal vascular fraction: note the presence of micro‐vessels containing typically aligned red blood cells (arrow). Abundant stromal cells (arrowheads) may be detected within the connective fiber network (green). Masson trichrome staining. (F): A micro‐vessel embedded in connective tissue (SEM). (G): Micrograph showing red blood cells aligned within capillaries (white arrows), long and flat cells located along capillaries and referable to endothelial cells (black arrowheads); largest roundish cells located close to vessels’ wall, referable to pericytes (black arrows). Masson Trichrome staining (H): Electron micrograph (TEM) showing red blood cells typically aligned within a capillary (arrow). (I): A pericyte embracing a microvessel (TEM).
Discussion
This study assessed the safety, feasibility, and potential benefits of using autologous and micro‐fragmented adipose tissue in the treatment of OA in dogs. Animal models provide practical and clinically relevant ways to study both the natural history and response to treatment of OA and dog is probably the closest to a gold standard animal model for OA currently available. Indeed, similar to human medicine, OA is a painful musculoskeletal condition in dogs and once the degenerative process initiates, it becomes a vicious cycle and the released substances result in inflammation, pain, and further damage. A variety of treatments has been proposed for the management of OA, with variable success rates but none capable of long‐term resolution. A systematic review on randomized or semi‐randomized controlled clinical trials comparing intra‐articular corticosteroids with sham injection or no treatment in humans with knee OA revealed that the therapeutic effects in terms of pain, physical function and quality of life decrease over time and provided no evidence that an effect remains 6 months after a corticosteroid injection 40. A prospective, randomized, double‐blinded trial comparing outcomes in dogs with bilateral elbow OA treated with hyaluronic acid plus methylprednisolone (HA + S) or autologous conditioned plasma (ACP) showed improvements in activity, lameness and pain with HA + S and ACP suggesting that both treatments have beneficial effects up to 6 months 41. Similar results were observed in human medicine 42. In the long run, intra‐articular MSCs transplantation without scaffolds is a more attractive option for the treatment of OA. Through direct cell–cell interaction or the secretion of various factors, MSCs can initiate endogenous reparative activities in the osteoarthritic joint 13, 14, 16, 17, 18, 19, 20, 23, 43. Regarding the mechanism by which MFAT exerts its action within the joint, we hypothesize a strong analgesic, anti‐inflammatory, and trophic activity. Furthermore, the long‐lasting mesenchymal components may also exert a reparative action. This hypothesis is well supported by the various components of MFAT, as described by the histological examination 31, 44, 45.
The subjective nature of assessing pain in dogs makes it difficult to obtain useful, reliable, non‐biased, and repeatable data. Indeed, many outcome measures have been used, but there is no clear consensus on what is the most useful outcome and/or which is the best assessment method 46. In our study, we used both the lameness score and the HCPI because it is easy to use and validated 33. Our results demonstrated that MFAT injection significantly improved function and reduced pain and symptoms for at least 6 months with a trend of steady increase during time. Furthermore, we found that patients starting from a severe condition at baseline, mainly medium and large animals, showed greater improvements at 6 months compared to patients starting from a less severe condition, in line with a recent report investigating the therapeutic response in dogs with naturally occurring OA that revealed that dogs with a mild lameness are less prone to improve 47. Pretreatment and post‐treatment radiographic assessment indicated variable increase in osteophyte dimension and conformation at 3 and 6 months, in line with published data in which multiple injections of leukoreduced platelet rich plasma (PRP) were used in a canine model of cranial cruciate ligament and meniscal deficiency showing that radiographic OA significantly increased over time 48. However, in some cases osteophytes presented blunt extremity compared to pretreatment, which might be the result of improved range of movements. Notably, radiographic OA severity often did not correlate with the clinical severity of the joint, as previously described 49, 50, 51. Indeed, some post‐treatment stifle MRIs showed progressive damage of the ligaments although the clinical status improved (Supporting information Fig. 5). In a study on the radiographic progression of osteoarthritis of the stifle joint, secondary to cranial cruciate ligament deficiency, all OA features were characterized by significant changes over time, but osteophytosis had the greatest degree of change. In addition, 40% of contralateral joints showed progressive osteophytosis 36. These results justify the use of MFAT also in the contralateral joint, even though both radiographic and clinical signs were less extensive compared to the interested joint. Regarding the site of injection, we could not find any difference between intra‐ or peri‐articular injections in either small or large dogs.
In the treated joints of patients died of natural causes not related to the primary articular disease 8–11 months after treatment, morphological examination revealed the coexistence of severe changes of the articular cartilage together with wide areas covered by continuous cartilage. Cartilage was hyaline or fibrous or, more often, hybrid (hyaline and fibrous) as it showed a great deal of overlapping regions positive for collagen types I, II, and III. The presence of a hybrid cartilage covering the damaged areas of the articular surface supports the idea that MFAT may determine the formation of a tissue that certainly does not display the same mechanical properties of normal hyaline cartilage nor the same durability, but that partially restores joint function and minimizes pain. In our study, we observed a mild expression collagen X, a marker of chondrocyte hypertrophy usually expressed only in the growing skeleton, at the level of the growth plate. It has been demonstrated that MSCs retain a differentiation attitude that is analog to endochondral bone formation 52. In the long‐term, increased collagen X expression could become an adverse event. However, the very limited presence of the hypertrophic phenotype and the histological features observed in this study lead to exclude this occurrence at the time of the observation.
It is worthwhile to note that the reduced pain and functional impairment we observed in our study with MFAT injection is similar to those achieved in most of the PRP studies. However, no PRP studies with such a large number of dogs have been performed and multiple intra‐articular injections of PRP are needed rather than the single injection of MFAT. Regarding the long‐term results, most of the clinical trials report a follow up of 6 months, whereas in our study, owners continued to forward HCPI reports and films of their dogs showing the improvements were maintained up to 24 months (not shown).
Our conclusions are tempered by some limitations, such as the lack of a concurrent and randomized control group. Indeed, at least from the owners’ HCPI score side, the results could be attributed to a placebo effect. However, a number of randomized, controlled trials demonstrated variable efficacy of the major treatments (HA, PRP, or NSAIDS) in both dogs and humans 40, 41, 42, 43, 53, 54, 55, 56, 57, 58, 59, 60, 61. Furthermore, we have additional unpublished data regarding the use of bone marrow‐derived MSCS in dogs affected by spontaneous OA where, despite good clinical results, extended culture time, the need for specialized facilities, and high‐costs make it more limited compared to MFAT. Other limitations of our study include the lack of quantitative outcome measures, such as a pressure sensitive walkway to assess lameness and concentrations of selected cartilage biomarkers in the synovial fluid that are promising tools for objectively monitoring OA 62. However, these limits are compensated by the high number of enrolled patients and by the absence of concomitant treatments during the follow‐up period.
Finally, it is important to highlight that no side effects of either adipose tissue harvest or delivery were detected, except for iatrogenic septic arthritis in 1% of the cases, indicating that intra‐articular and/or peri‐articular injection of MFAT is a safe treatment.
Conclusion
The results of this study suggest that intra‐articular and/or peri‐articular injection of autologous, micro‐fragmented adipose tissue is a safe, feasible, and beneficial option for the treatment of OA in dogs. The procedure is simple, time sparing, cost‐effective, minimally invasive, one‐step, and eliminates the need for complex and time intensive cell culture processing. For at least 6 months, the results were very satisfactory and promising. The lack of any complications in the dog should be taken into account when considering this treatment in other species, including man. The study of spontaneous, naturally occurring OA in dogs is a model that provides a valuable role in developing successful, innovative treatment regimens for translational medicine facilitating the transfer of knowledge from the “bench” to the “bedside”.
Author Contributions
O.Z.: conception and design of the study, provision of study patients, data analysis and interpretation, manuscript writing and final approval of the manuscript; S.S., L.P., N.A., L.M., D.Z., and M.P.D.: collection of data; E.G., M.K., and D.M.L.: collection and assembly of data, data analysis and interpretation; L.F., L.P., L.L., G.A., and A.P.: data analysis and interpretation; D.S. and A.C.: provision of patients; M.A.: collection of data, data analysis and interpretation and final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
O.Z., G.A., L.P., and A.P. are scientific consultants of Lipogems International SpA. The other authors indicated no potential conflicts of interest.
Supporting information
Supporting Information Figure 1. Ventrodorsal projections of the hips in a 9‐year‐old German Shepherd. Osteophytes pretreatment (top), 10 (middle), and 23 months after (bottom). The femoral heads are severely deformed bilaterally and on the right side a hook‐shaped, on the left side a triangular osteophyte can be seen on the craniolateral acetabular margin. The sclerosis of the acetabular margins is progressing over time.
Supporting Information Figure 2. Humeral head OCD in 9 months old Golden Retriever, pretreatment (top) and 14 months after (bottom).
Supporting Information Figure 3. Histological examination of stifle joint 11 months after MFAT injection. Large eosinophilic polymorphic cells with cytoplasmic vacuoles were observed in multiple foci free within the articular cavity, lining the fibrous cartilage or the synovial membrane. Top) scale bar: 10×; bottom) scale bar: 40×.
Supporting Information Figure 4. Immunostaining for collagen type I, II, III, and X. (A–C): Moderate and homogeneous expression of collagen I by matrix fibers and cells of fibrocartilage and fibrous‐like tissue. (A) scale bar: 20×; (B) scale bar: 40×; (C): scale bar: 100×. (D): collagen II massive expression in normal hyaline cartilage. Scale bar, 20×. (E and F): Mild immunoreaction to collagen II in the lower areas of fibrocartilage. (E) scale bar: 40×; (F) scale bar: 100×. (G and H): Slight immunopositivity for type III collagen in fibrocartilage. (G) scale bar: 20X; (H) scale bar: 100×. (I): multifocal immunoreaction to collagen X in fibrocartilage. Scale bar: 100×.
Supporting Information Figure 5. Stifle MRI (STIR, fat suppression, dorsal plane, lateral to the left) pre (top), during (middle) and post (bottom) MFAT injection. The severe joint effusion (bright signal, arrow) is decreasing over time.
References
- 1. Martinez SA. Congenital conditions that lead to osteoarthritis in the dog. Vet Clin North Am Small Anim Pract 1997;27:735–758. [DOI] [PubMed] [Google Scholar]
- 2. Martinez SA, Coronado GS. Acquired conditions that lead to osteoarthritis in the dog. Vet Clin North Am Small Anim Pract 1997;27:759–775. [DOI] [PubMed] [Google Scholar]
- 3. Sanderson R, Beata C, Flipo R et al. Systematic review of the management of canine osteoarthritis. Vet Rec 2009;164:418–424. [DOI] [PubMed] [Google Scholar]
- 4. Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: A review of community burden and current use of primary health care. Ann Rheum Dis 2001;60:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gregory MH, Capito N, Kuroki K et al. A review of translational animal models for knee osteoarthritis. Arthritis 2012;2012:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pond M, Nuki G. Experimentally‐induced osteoarthritis in the dog. Ann Rheum Dis 1973;32:387–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnston SA. Osteoarthritis: Joint anatomy, physiology, and pathobiology. Vet Clin N Am Small Anim Pract 1997;27:699–723. [DOI] [PubMed] [Google Scholar]
- 8. Rychel JK. Diagnosis and treatment of osteoarthritis. Top Companion Anim Med 2010;25:20–25. [DOI] [PubMed] [Google Scholar]
- 9. Johnston SA, McLaughlin RM, Budsberg SC. Nonsurgical management of osteoarthritis in dogs. Vet Clin N Am Small Anim Pract 2008;38:1449–1470. [DOI] [PubMed] [Google Scholar]
- 10. De Siena R, Balducci L, Blasi A et al. Omentum‐derived stromal cells improve myocardial regeneration in pig post‐infarcted heart through a potent paracrine mechanism. Exp Cell Res 2010;316:1804–1815. [DOI] [PubMed] [Google Scholar]
- 11. Wh L, Fq S, Ln R et al. The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases. J Cell Mol Med 2015;19:511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Plock JA, Schnider JT, Zhang W et al. Adipose‐and bone marrow–derived mesenchymal stem cells prolong graft survival in vascularized composite allotransplantation. Transplantation 2015;99:1765–1773. [DOI] [PubMed] [Google Scholar]
- 13. Ude CC, Sulaiman SB, Min‐Hwei N et al. Cartilage regeneration by chondrogenic induced adult stem cells in osteoarthritic sheep model. PLoS One 2014;9:e98770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bajada S, Mazakova I, Richardson JB et al. Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med 2008;2:169–183. [DOI] [PubMed] [Google Scholar]
- 15. Muir P, Hans EC, Racette M et al. Autologous bone marrow‐derived mesenchymal stem cells modulate molecular markers of inflammation in dogs with cruciate ligament rupture. PLoS One 2016;11:e0159095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caplan AI, Correa D. The MSC: An injury drugstore. Cell Stem Cell 2011;9:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kean TJ, Lin P, Caplan AI et al. MSCs: Delivery routes and engraftment, cell‐targeting strategies, and immune modulation. Stem Cells Int 2013;2013:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076–1084. [DOI] [PubMed] [Google Scholar]
- 19. da Silva Meirelles L, Fontes AM, Covas DT et al. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 2009;20:419–427. [DOI] [PubMed] [Google Scholar]
- 20. English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol 2013;91:19–26. [DOI] [PubMed] [Google Scholar]
- 21. Black LL, Gaynor J, Gahring D et al. Effect of adipose‐derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: A randomized, double‐blinded, multicenter controlled trial. Vet Ther 2007;8:272. [PubMed] [Google Scholar]
- 22. Guercio A, Marco P, Casella S et al. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol Int 2012;36:189–194. [DOI] [PubMed] [Google Scholar]
- 23. Chamberlain G, Fox J, Ashton B et al. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007;25:2739–2749. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research Center for Devices and Radiological Health Office of Combination Products November 2017.
- 25. Ährlund‐Richter L, De Luca M, Marshak DR et al. Isolation and production of cells suitable for human therapy: Challenges ahead. Cell Stem Cell 2009;4:20–26. [DOI] [PubMed] [Google Scholar]
- 26. Arcidiacono JA, Blair JW, Benton KA. US Food and Drug Administration international collaborations for cellular therapy product regulation. Stem Cell Res Ther 2012;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lysaght T, Campbell AV. Regulating autologous adult stem cells: The FDA steps up. Cell Stem Cell 2011;9:393–396. [DOI] [PubMed] [Google Scholar]
- 28. Riis S, Zachar V, Boucher S et al. Critical steps in the isolation and expansion of adipose‐derived stem cells for translational therapy. Expert Rev Mol Med 2015;17:e11. [DOI] [PubMed] [Google Scholar]
- 29. Sensebé L, Bourin P, Tarte K. Good manufacturing practices production of mesenchymal stem/stromal cells. Hum Gene Ther 2010;22:19–26. [DOI] [PubMed] [Google Scholar]
- 30. Bianchi F, Maioli M, Leonardi E et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte‐like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 2013;22:2063–2077. [DOI] [PubMed] [Google Scholar]
- 31. Ceserani V, Ferri A, Berenzi A et al. Angiogenic and anti‐inflammatory properties of micro‐fragmented fat tissue and its derived mesenchymal stromal cells. Vasc Cell 2016;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sharkey M. The challenges of assessing osteoarthritis and postoperative pain in dogs. AAPS J 2013;15:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hielm‐Björkman AK, Rita H, Tulamo R‐M. Psychometric testing of the Helsinki chronic pain index by completion of a questionnaire in Finnish by owners of dogs with chronic signs of pain caused by osteoarthritis. Am J Vet Res 2009;70:727–734. [DOI] [PubMed] [Google Scholar]
- 34. Impellizeri JA, Tetrick MA, Muir P. Effect of weight reduction on clinical signs of lameness in dogs with hip osteoarthritis. J Am Vet Med Assoc 2000;216:1089–1091. [DOI] [PubMed] [Google Scholar]
- 35. Welsh E, Gettinby G, Nolan A. Comparison of a visual analogue scale and a numerical rating scale for assessment of lameness, using sheep as a model. Am J Vet Res 1993;54:976–983. [PubMed] [Google Scholar]
- 36. Innes J, Costello M, Barr F et al. Radiographic progression of osteoarthritis of the canine stifle joint: A prospective study. Vet Radiol Ultrasound 2004;45:143–148. [DOI] [PubMed] [Google Scholar]
- 37. D'ANJOU MA, Moreau M, Troncy E et al. Osteophytosis, subchondral bone sclerosis, joint effusion and soft tissue thickening in canine experimental stifle osteoarthritis: Comparison between 1.5 T magnetic resonance imaging and computed radiography. Vet Surg 2008;37:166–177. [DOI] [PubMed] [Google Scholar]
- 38. Raskin R, Meyer D. In: Edra ed. Citologia diagnostica del cane e del gatto, 2016. [Google Scholar]
- 39. Wiseman M, Nolan A, Reid J et al. Preliminary study on owner‐reported ehaviour changes associated with chronic pain in dogs. Vet Rec 2001;149:423–424. [DOI] [PubMed] [Google Scholar]
- 40. Jüni P, Hari R, Rutjes AW et al. Intra‐articular corticosteroid for knee osteoarthritis. Cochrane Libr 2015;10:1–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Franklin SP, Cook JL. Prospective trial of autologous conditioned plasma versus hyaluronan plus corticosteroid for elbow osteoarthritis in dogs. Can Vet J 2013;54:881. [PMC free article] [PubMed] [Google Scholar]
- 42. Kon E, Mandelbaum B, Buda R et al. Platelet‐rich plasma intra‐articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: From early degeneration to osteoarthritis. Arthrosc: J Arthrosc Relat Surg 2011;27:1490–1501. [DOI] [PubMed] [Google Scholar]
- 43. Qi Y, Feng G, Yan W. Mesenchymal stem cell‐based treatment for cartilage defects in osteoarthritis. Mol Biol Rep 2012;39:5683–5689. [DOI] [PubMed] [Google Scholar]
- 44. Bosetti M, Borrone A, Follenzi A et al. Human lipoaspirate as autologous injectable active scaffold for one‐step repair of cartilage defects. Cell Transplant 2016;25:1043–1056. [DOI] [PubMed] [Google Scholar]
- 45. Russo A, Condello V, Madonna V et al. Autologous and micro‐fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis. J Exp Orthopaed 2017;4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Belshaw Z, Asher L, Dean RS. Systematic review of outcome measures reported in clinical Canine osteoarthritis research. Vet Surg 2016;45:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gagnon A, Brown D, Moreau M et al. Therapeutic response analysis in dogs with naturally occurring osteoarthritis. Vet Anaesth Analg 2017;44:1373–1381. [DOI] [PubMed] [Google Scholar]
- 48. Cook JL, Smith PA, Bozynski CC et al. Multiple injections of leukoreduced platelet rich plasma reduce pain and functional impairment in a canine model of ACL and meniscal deficiency. J Orthop Res 2016;34:607–615. [DOI] [PubMed] [Google Scholar]
- 49. Morgan JP, Voss K, Damur DM et al. Correlation of radiographic changes after tibial tuberosity advancement in dogs with cranial cruciate‐deficient stifles with functional outcome. Vet Surg 2010;39:425–432. [DOI] [PubMed] [Google Scholar]
- 50. Boyd DJ, Miller CW, Etue SM et al. Radiographic and functional evaluation of dogs at least 1 year after tibial plateau leveling osteotomy. Can Vet J 2007;48:392. [PMC free article] [PubMed] [Google Scholar]
- 51. Gordon WJ, Conzemius MG, Riedesel E et al. The relationship between limb function and radiographic osteoarthrosis in dogs with stifle osteoarthrosis. Vet Surg 2003;32:451–454. [DOI] [PubMed] [Google Scholar]
- 52. Scotti C, Tonnarelli B, Papadimitropoulos A et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci 2010;107:7251–7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arrich J, Piribauer F, Mad P et al. Intra‐articular hyaluronic acid for the treatment of osteoarthritis of the knee: Systematic review and meta‐analysis. Can Med Assoc J 2005;172:1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bannuru RR, Vaysbrot EE, Sullivan MC et al. Relative efficacy of hyaluronic acid in comparison with NSAIDs for knee osteoarthritis: A systematic review and meta‐analysis Seminars in Arthritis and Rheumatism. New York: Elsevier, 2014:593–599. [DOI] [PubMed] [Google Scholar]
- 55. Görmeli G, Görmeli CA, Ataoglu B et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: A randomized, double‐blind, placebo‐controlled trial. Knee Surg Sports Traumatol Arthrosc 2017;25:958–965. [DOI] [PubMed] [Google Scholar]
- 56. Innes JF, Clayton J, Lascelles BDX. Review of the safety and efficacy of long‐term NSAID use in the treatment of canine osteoarthritis. Vet Rec 2010;166:226–230. [DOI] [PubMed] [Google Scholar]
- 57. Kon E, Mandelbaum B, Buda R et al. Platelet‐rich plasma intra‐articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: From early degeneration to osteoarthritis. Arthroscopy 2011;27:1490–1501. [DOI] [PubMed] [Google Scholar]
- 58. Lajeunesse D, Martel‐Pelletier J, Fernandes J et al. Treatment with licofelone prevents abnormal subchondral bone cell metabolism in experimental dog osteoarthritis. Ann Rheum Dis 2004;63:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lo GH, LaValley M, McAlindon T et al. Intra‐articular hyaluronic acid in treatment of knee osteoarthritis: A meta‐analysis. JAMA 2003;290:3115–3121. [DOI] [PubMed] [Google Scholar]
- 60. Raeissadat SA, Rayegani SM, Hassanabadi H et al. Knee osteoarthritis injection choices: Platelet‐rich plasma (PRP) versus hyaluronic acid (a one‐year randomized clinical trial). Clin Med Insights Arthritis Musculoskeletal Disord 2015;8:1–8. CMAMD. S17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang C‐T, Lin J, Chang C‐J et al. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee: A meta‐analysis of randomized controlled trials. J Bone Joint Surg Am 2004;86:538–545. [DOI] [PubMed] [Google Scholar]
- 62. Lindhorst E, Vail T, Guilak F et al. Longitudinal characterization of synovial fluid biomarkers in the canine meniscectomy model of osteoarthritis. J Orthop Res 2000;18:269–280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1. Ventrodorsal projections of the hips in a 9‐year‐old German Shepherd. Osteophytes pretreatment (top), 10 (middle), and 23 months after (bottom). The femoral heads are severely deformed bilaterally and on the right side a hook‐shaped, on the left side a triangular osteophyte can be seen on the craniolateral acetabular margin. The sclerosis of the acetabular margins is progressing over time.
Supporting Information Figure 2. Humeral head OCD in 9 months old Golden Retriever, pretreatment (top) and 14 months after (bottom).
Supporting Information Figure 3. Histological examination of stifle joint 11 months after MFAT injection. Large eosinophilic polymorphic cells with cytoplasmic vacuoles were observed in multiple foci free within the articular cavity, lining the fibrous cartilage or the synovial membrane. Top) scale bar: 10×; bottom) scale bar: 40×.
Supporting Information Figure 4. Immunostaining for collagen type I, II, III, and X. (A–C): Moderate and homogeneous expression of collagen I by matrix fibers and cells of fibrocartilage and fibrous‐like tissue. (A) scale bar: 20×; (B) scale bar: 40×; (C): scale bar: 100×. (D): collagen II massive expression in normal hyaline cartilage. Scale bar, 20×. (E and F): Mild immunoreaction to collagen II in the lower areas of fibrocartilage. (E) scale bar: 40×; (F) scale bar: 100×. (G and H): Slight immunopositivity for type III collagen in fibrocartilage. (G) scale bar: 20X; (H) scale bar: 100×. (I): multifocal immunoreaction to collagen X in fibrocartilage. Scale bar: 100×.
Supporting Information Figure 5. Stifle MRI (STIR, fat suppression, dorsal plane, lateral to the left) pre (top), during (middle) and post (bottom) MFAT injection. The severe joint effusion (bright signal, arrow) is decreasing over time.


