Abstract
Background
lncRNA GAS5 acts as a tumor-suppressor gene in various types of malignancies, but its involvement in esophageal cancer has not been well studied.
Material/Methods
A total of 112 patients with esophageal cancer and 55 volunteers with normal physiological conditions were included in this study. Tumor tissues and adjacent healthy tissues were collected from esophageal cancer patients and blood was extracted from patients and controls. Expression of GAS5 in those tissues was detected by qRT-PCR. All patients were followed up for 5 years and diagnostic and prognostic values of serum GAS5 for esophageal cancer were investigated by ROC curve analysis and survival curve analysis, respectively. Effects of GAS5 expression on cell proliferation and migration were investigated by CCK-8 assay and Transwell cell migration assay, respectively. Effects of GAS5 overexpression on expression of PI3K/AKT/mTOR-related proteins were explored by Western blot analysis.
Results
GAS5 expression level was lower in tumor tissues than in adjacent healthy tissues. Serum level of GAS5 was lower in cancer patients than in healthy controls, and serum level of GAS5 was decreased with increase in stage of primary tumor (T stage). GAS5 overexpression inhibited tumor cell proliferation and migration, while treatment with PI3K activator reduced the inhibitory effects. GAS5 overexpression decreased the expression level of PI3K and phosphorylation levels of Akt and mTOR in esophageal cancer cells, while PI3K activator treatment showed no significant effects on GAS5 expression.
Conclusions
GAS5 was downregulated in esophageal cancer patients compared to healthy controls, and GAS5 overexpression suppressed proliferation and migration of esophageal cancer cells by inactivating the PI3K/AKT/mTOR pathway.
MeSH Keywords: Cell Migration Inhibition, Cell Proliferation, Esophageal Neoplasms
Background
Esophageal cancer is a malignancy that develops from the esophagus, and now is the 8th most common type of cancer and the 6th leading cause of cancer-related deaths [1]. More than 80% of new cases of esophageal cancer were reported in China [2]. In spite of the development of multimodality therapies, such as chemotherapy, surgery, and radiation therapy, treatment outcomes and prognosis of patients with esophageal cancer are still poor, possibly due to its extremely aggressive nature [3]. The 5-year survival rate is usually below 10% and is especially poor for esophageal cancer patients with tumor metastasis [2]. Therefore, early diagnosis and treatment is critical for the survival of patients with esophageal cancer.
Genetic factors play pivotal roles in the development of esophageal cancer [4]. Long non-coding RNAs (lncRNAs) are a group of RNA transcripts composed of more than 200 nucleotides and having no protein-coding ability [5]. Studies in the last several decades have shown that lncRNAs play pivotal roles in almost all critical physiological as well as pathological processes, such as the pathogenesis of different types of malignant tumors, including esophageal cancer [6,7]. lncRNA GAS5 has been proved to play a role as a tumor-suppressor gene in the development of several malignancies, such as cervical cancer [8] and gastric cancer [9]. Downregulation of GAS5 in tumor tissues promotes the growth and metastasis of tumors [8,9]. The involvement of GAS5 in esophageal cancer has not been well studied. Therefore, we investigated the functionality of lncRNA GAS5 in esophageal cancer. We found that GAS5 overexpression suppresses proliferation and migration of esophageal cancer cell by inactivating the PI3K/AKT/mTOR pathway. Our study provides useful information and references for the diagnosis and treatment of esophageal cancer.
Material and Methods
Subjects
A total of 112 patients with esophageal cancer diagnosed via pathological examination and imaging methods were enrolled at the First Affiliated Hospital of Zhengzhou University from May 2010 to May 2012. Those patients included 60 males and 52 females, with an age range of 30–77 years and a mean age of 52±6.6 years. Inclusion criteria were: (1) patients pathologically diagnosed as esophageal cancer and (2) patients signed informed consent. Exclusion criteria were: (1) patients with other malignancies and (2) patients with severe coagulation dysfunction. At the same time, a total of 55 healthy people with similar age and sex distributions were included to serve as control group. This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University. Primary tumor staging was performed according to the following criteria: Tis, high-grade dysplasia (n=18); T1, tumor invaded muscularis mucosae, submucosa or lamina propria (n=22); T2, tumor invaded muscularis propria (n=24); T3, tumor invaded adventitia (n=23); and T4, tumor invaded adjacent structures (n=25).
Specimen collection
Blood (20 ml) was extracted from each participant on the day of admission. Blood was kept on the bench at room temperature for 2 h, followed by centrifugation at 1875 rpm to obtain serum. All patients received surgical resection of the primary tumor, and tumor tissues and adjacent healthy tissues within 5 cm around tumors were collected during the operation. All tissues were stored in liquid nitrogen before use.
Cell lines and cell culture
Homo sapiens esophageal cancer cell lines EC9706 and KYSE510 were provided by the American Type Culture Collection (Manassas, VA, USA). Cells of those 2 cell lines were cultured in RPMI-1640 medium (GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen, Waltham, MA, USA) in an incubator (37°C, 5% CO2). FBS was not added in case of GAS5 expression assay with PI3K activator SC79 (10 μM) treatment (including control cells).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Tumor tissues and adjacent healthy tissues were ground in liquid nitrogen, followed by addition of TRIzol® reagent (Thermo Fisher Scientific, Inc.) to extract total RNA. TRIzol® reagent was also directly mixed with in vitro cultured cells to extract total RNA. cDNA was synthesized using the SuperScript IV reverse transcriptase kit (Thermo Fisher Scientific, Inc.) with total RNA as template. Reverse transcription conditions were: 5 min at 25°C, 30 min at 50°C, and 5 min at 80°C. The PCR reaction system was prepared using SYBR® Green Real-Time PCR Master Mix (Thermo Fisher Scientific, Inc.). PCR reactions were performed on the CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Sequences of primers used in PCR reactions were: 5′-CCATGGATGACTTGCTTGGG-3′ (forward) and 5′-TGCATGCTTGCTTGTTGTGG-3′ (reverse) for human lncRNA GAS5; 5′-CCCACTCCTCCACCTTTGAC-3′ (forward) and 5′-ATGAGGTCCACCACCCTGTT-3′ (reverse) for human GAPDH. PCR reaction conditions were: 95°C for 25 s, followed by 40 cycles of 95°C for 12 s and 50°C for 40 s. Data were processed using 2−ΔΔCt method, and relative expression level of lncRNA GAS5 was normalized to endogenous control GAPDH.
Construction of GAS5 expression vector and transfection
GAS5 cDNA with EcoRI cutting site on both ends were amplified by PCR using the following primers: 5′-GAATTCCTTTTCGAGGTAGGAGTC-3′ (forward) reverse: 5′-GAATTCAGACACTGTTTTAATCTTC-3′ (reverse). The lncRNA-GAS5 expression vector was established by inserting an EcoRI-EcoRI fragment containing full-length lncRNA-GAS5 cDNA into pIRSE2-EGFP (Clontech, Palo Alto, CA, USA). Cells of EC9706 and KYSE510 cell lines were cultured overnight to reach 80~90% confluence, and lipofectamine 2000 (11668-019, Invitrogen, Carlsbad, USA) was used to transfect 10 nM vector into 5×106 cells. Transfection with empty pIRSE2-EGFP vector was used as a negative control. Expression of GAS5 before Western blot analysis and before and after proliferation and migration assay was checked to make sure the upregulation rate was above 150%.
Cell proliferation assay
Cells were collected during logarithmic growth phase and cell suspension with a density of 4×104 cells/ml was prepared. We added 100 μL cell suspension containing 4×103 cells into each well of 96-well plates. Cells were cultured at 37ºC with 5% CO2, and 10 μL CCK-8 solution was added into each well at 24, 48, 72, and 96 h later. Cells were cultured at 37°C for another 4 h, and a Fisherbrand™ accuSkan™ GO UV/Vis Microplate Spectrophotometer was used to measure OD values at 450 nm.
Transwell migration assays
Cell migration ability of each cell line was measured by Transwell cell migration assay (polyester inserts, BD Biosciences, Franklin Lakes, NJ, USA). Briefly, ~4×103 cells in 100 μl serum-free PMI-1640 was added into the upper chamber, and the lower chamber was filled with RPMI-1640 medium containing 20% FBS. Cells were cultured for 24 h and membranes were collected and stained with 0.5% crystal violet at room temperature for 20 min. After staining, cells on the membrane were counted under a light microscope (Olympus Corporation, Tokyo, Japan).
Western blot analysis
RIPA solution (Thermo Fisher Scientific, Inc.) was mixed with in vitro cultured cells to extract total protein. Protein concentration was measured by BCA method. We performed 10% SDS-PAGE gel electrophoresis with 20 μg protein per lane, followed by gel transfer to PVDF membranes (Thermo Fisher Scientific). After washing with TBST buffer 3 times, 15 min each time, membranes were incubated with primary antibodies, including rabbit anti-PI3K (1: 2000, ab182651, Abcam); anti-AKT1 antibody (1: 2000, ab28422, Abcam), anti-p-AKT1 (S473, 1: 1000, ab8932, Abcam), anti-mTOR (1: 2000, ab2732, Abcam), anti-p-mTOR (phospho S2448, 1: 1000, ab109268, Abcam), and anti-GAPDH polyclonal antibody (1: 2000, ab181602, Abcam) overnight at 4°C. Membranes were washed with TBST buffer 3 times, 15 min each time, followed by incubation with goat anti-rabbit IgG-horseradish peroxidase secondary antibody (1: 1,000, MBS435036, MyBioSource) at room temperature for 2 h. After washing with TBST buffer 3 times, 15 min each time, chemiluminescence method was used to detect signals, and the relative expression level of each protein was normalized to endogenous control GAPDH using Image J 1.48 software (NIH, Bethesda, MD, USA).
Statistical analysis
GraphPad Prism 7 was used for all statistical analyses. Comparisons of measurement between 2 groups and among multiple groups were performed by t test and one-way analysis of variance followed by LSD test, respectively. Count data were compared by chi-square test. p<0.05 indicated a statistically significant difference.
Results
Expression of lncRNA GAS5 in tumor tissues and adjacent healthy tissues of esophageal cancer patients
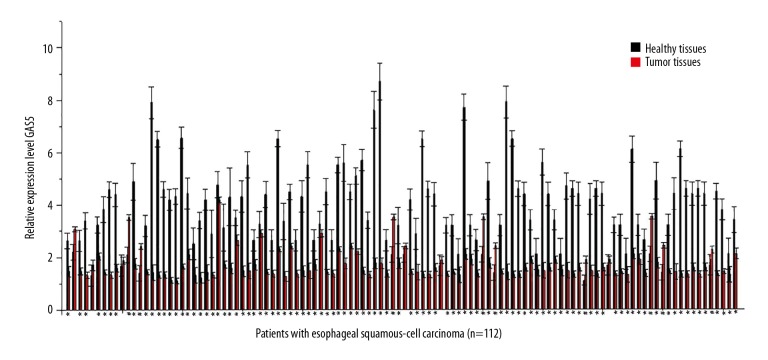
Expression of lncRNA GAS5 in tumor tissues and adjacent healthy tissues of 112 esophageal cancer patients was detected by qRT-PCR. As shown in Figure 1, significantly lower expression level of lncRNA GAS5 in tumor tissues than in adjacent healthy tissues was found in 98 out of 112 patients, accounting for 87.5%. In contrast, significantly higher expression level of lncRNA GAS5 in tumor tissues than in adjacent healthy tissues was found in 9 out of 112 patients, only accounting for 8%. No significant difference was found in 5 cases, accounting for 4.5%. Those data suggest that downregulation of lncRNA GAS5 is very likely to be involved in the pathogenesis of esophageal cancer.
Figure 1.
Expression of lncRNA GAS5 in tumor tissues and adjacent healthy tissues of esophageal cancer patients. This figure basically shows that GAS5 is downregulated in most patients with esophageal cancer. Measurement was performed in triplicate. * Compared with adjacent healthy tissues, p<0.05; # compared with tumor tissues, p<0.05.
Expression of lncRNA GAS5 in serum of healthy people and esophageal cancer patients with different stages of primary tumor
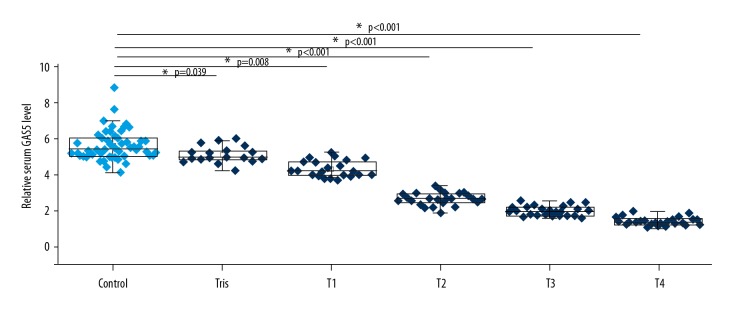
Expression of lncRNA GAS5 in serum of the 55 healthy people and 112 esophageal cancer patients was also detected by qRT-PCR. As shown in Figure 2, serum levels of lncRNA GAS5 were significantly lower in esophageal cancer patients than in healthy controls (p<0.05). In addition, serum level of lncRNA GAS5 decreased with the increase of primary tumor stage.
Figure 2.
Expression of lncRNA GAS5 in serum of healthy people and esophageal cancer patients with different stages of primary tumors. Serum levels of lncRNA GAS5 were significantly lower in esophageal cancer patients than in healthy controls, and serum levels of lncRNA GAS5 decreased with the increase of primary tumor stage. Measurement was performed in triplicate. * p<0.05.
Diagnostic and prognostic values of serum lncRNA GAS5 for esophageal cancer
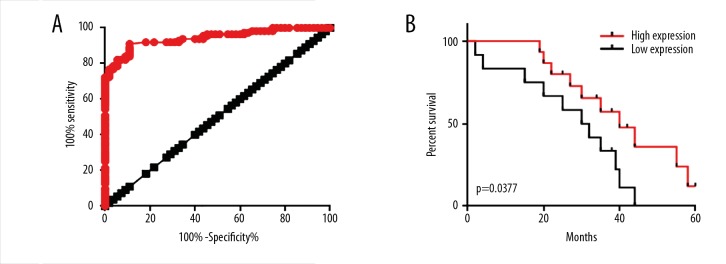
The diagnostic value of serum lncRNA GAS5 for esophageal cancer was analyzed by ROC curve analysis using serum levels of 112 esophageal cancer patients and 55 healthy controls. As shown in Figure 3A, the area under the curve (AUC) was 0.9435 with a 95% confidence interval of 0.9110 to 0.9760 (p<0.0001). These data suggest that serum lncRNA GAS5 may serve as a promising diagnostic biomarker for esophageal cancer. Patients in different stages of esophageal cancer were divided into a high expression group (n=56) and a low expression group (n=56) according to the median serum level of lncRNA GAS5. After discharge, patients were followed up for 5 years (until May 2017) or until their deaths, and patients who failed to complete follow-up or died of other diseases of accidents were not included in this study. Kaplan-Meier method was used to plot survival curves with default parameters based on the survival conditions collected during follow-up. Survival curves were compared by log rank t test. Results showed that the overall survival of patients with high serum levels of lncRNA GAS5 was significantly better than that of patients with low serum levels of lncRNA GAS5 (p<0.05, Figure 3B) only in stage 4 (p=0.00377) and no significant differences were found in other stages. These data suggest that serum lncRNA GAS5 may serve as a promising prognostic biomarker only for advanced esophageal cancer.
Figure 3.
Diagnostic and prognostic values of serum lncRNA GAS5 for esophageal cancer. (A) ROC curve analysis of the diagnostic value for esophageal cancer. Red dots represent diagnostic curve and black squares represent line of identity. (B) Comparison of survival curves of stage 4 patients with high and low serum levels of lncRNA GAS5.
Effects of lncRNA GAS5 overexpression on PI3K/AKT/mTOR-related proteins in esophageal cancer cells
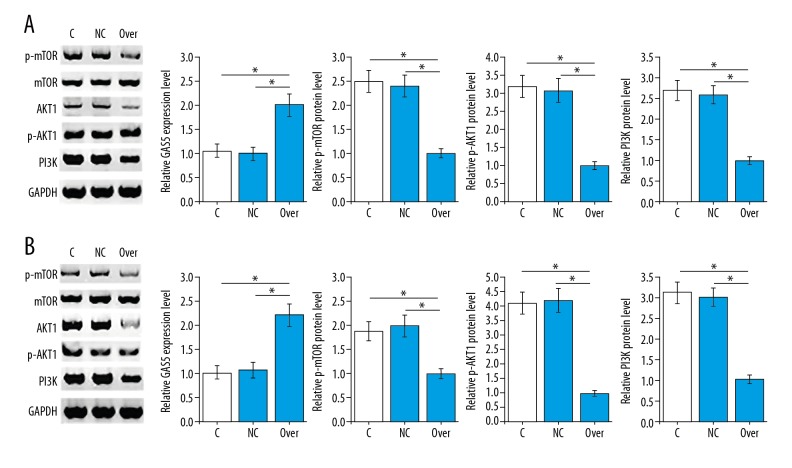
It is known that lncRNA GAS5 may achieve its biological functions through interactions with the PI3K/AKT/mTOR pathway [10]. Therefore, effects of GAS5 overexpression on PI3K/AKT/mTOR-related proteins in esophageal cancer cells were explored in this study. As shown in Figure 4, GAS5 overexpression showed no significant effects on expression level of Akt and mTOR (p>0.05), while GAS5 overexpression significantly decreased the expression level of PI3K and phosphorylation levels of Akt and mTOR in EC9706 (p<0.05, Figure 4A) and KYSE510 (p<0.05, Figure 4B) cell lines.
Figure 4.
Effects of lncRNA GAS5 overexpression on PI3K/AKT/mTOR-related proteins in esophageal cancer cells. The figure shows the effects of lncRNA GAS5 overexpression on PI3K/AKT/mTOR-related proteins in EC9706 (A) and KYSE510 (B) cell lines. Gray-scale of each protein was normalized to that of GAPDH endogenous control. This assay was performed in triplicate. * p<0.05.
Effects of lncRNA GAS5 overexpression on proliferation and migration of esophageal cancer cells
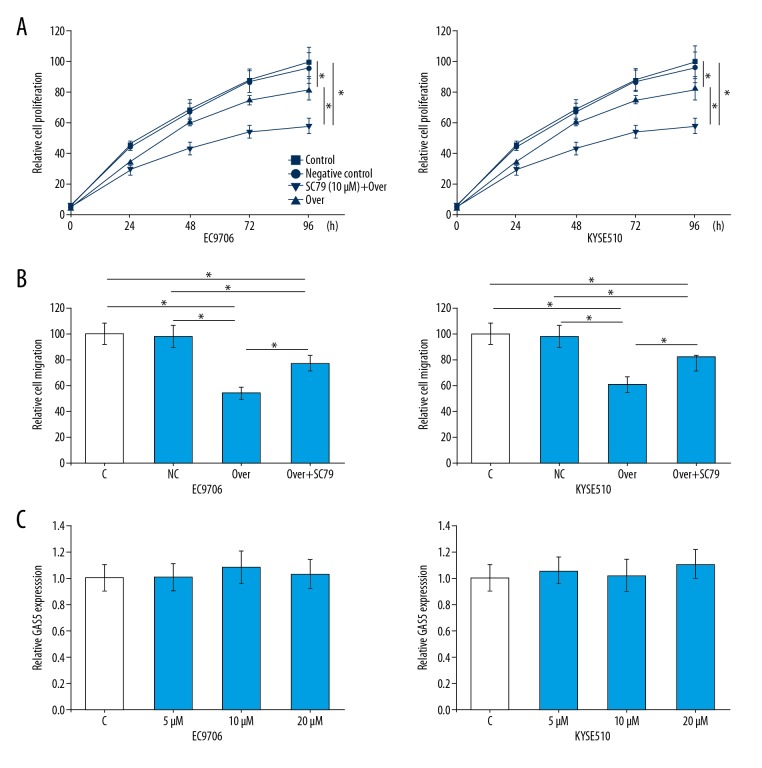
Effects of lncRNA GAS5 overexpression on proliferation and migration of cells of the 2 esophageal cancer cell lines were explored by CCK-8 cell proliferation assay and Transwell cell migration assay, respectively. As shown in Figure 5, lncRNA GAS5 overexpression significantly inhibited the proliferation (Figure 5A) and migration (Figure 5B) of cells of the 2 esophageal cancer cell lines (p<0.05), while treatment with PI3K activator SC79 (10 μM, Sigma-Aldrich) for 1 h significantly increased the phosphorylation level of Akt (data not shown) and reduced the inhibitory effects of GAS5 overexpression on cell proliferation and migration (p<0.05). In addition, treatment with SC79 at 3 different concentrations (5 μM, 10 μM, and 20 μM) showed no significant effects on the expression of lncRNA GAS5 in cells of the 2 esophageal cancer cell lines (Figure 5C).
Figure 5.
Effects of lncRNA GAS5 overexpression on proliferation and migration of esophageal cancer cells. (A) Effects of lncRNA GAS5 overexpression on proliferation of esophageal cancer cells. (B) Effects of lncRNA GAS5 overexpression on migration of esophageal cancer cells. (C) Effects of SC79 on lncRNA GAS5 expression in esophageal cancer cells. This assay was performed in triplicate. * p<0.05; Control (C) – control cells without any transfection; Negative control (NC) – cells transfected with empty vector; Over – transfection with GAS5 expression vector; SC79 – treatment with SC79.
Discussion
lncRNA GAS5 generally plays a role as tumor suppressor gene in development of various types of cancers. In the study of pancreatic cancer, Lu et al. reported that lncRNA GAS5 was significantly downregulated in tumor tissues and cancer cells compared with normal tissues and normal pancreatic cells [11]. In another study, downregulation of GAS5 was also observed in bladder cancer, and the reduced expression level of GAS5 was closely correlated with the accelerated growth of tumors [12]. It has also been reported that the development of esophageal cancer is also accompanied by decreased expression level of GAS5 [13], and inhibition of GAS5 may serve as a possible target for the treatment of this disease [14]. In the present study, significantly lower expression levels of GAS5 in tumor tissues compared to adjacent healthy tissues were found in most patients with esophageal cancer. In addition, serum levels of GAS5 were also significantly lower in esophageal cancer patients than in healthy controls, and serum levels of GAS5 were further decreased with the increase of primary tumor stage. Those data further confirmed that downregulation of GAS5 is very likely involved in the pathogenesis of esophageal cancer.
Survival of esophageal cancer patients is still challenged by poor treatment outcomes and prognosis due to the fact that most patients are diagnosed at advanced stages that are inappropriate for surgical resection [15]. In addition, due to its extremely aggressive nature, the postoperative recurrence rate of esophageal cancer is high, which in turn leads to poor survival [16]. Therefore, early diagnosis and treatment of esophageal cancer is critical. Development of human diseases is usually accompanied by changes in certain substances in blood, and the detection of changes in these substances may provide references for treatment of diseases [17]. In the present study, results of ROC curve analysis confirmed that serum lncRNA GAS5 can be used to effectively distinguish esophageal cancer patients from healthy controls. In addition, patients in stage 4 with lower serum levels of lncRNA GAS5 showed shorter survival times compared with patients with higher serum levels of lncRNA GAS5. These data suggest that lncRNA GAS5 may serve as a promising biomarker for esophageal cancer. However, downregulation of GAS5 was observed in multiple types of cancers [8–11], which affects the specificity of the application of GAS5 in the diagnosis and prediction of prognosis of patients with esophageal cancer. Therefore, multiple biomarkers should be combined to increase the specificity of the diagnosis of esophageal cancer.
It has been well established that lncRNA GAS5 is involved in the regulation of the proliferation and migration of cancer cells [18,19]. In the present study, lncRNA GAS5 overexpression significantly reduced the proliferation and migration of cells of 2 esophageal cancer cell lines. It has been reported that lncRNA GAS5 may achieve its biological functions through interactions with the PI3K/AKT/mTOR pathway [10], which is a critical signaling in nearly all critical aspects of tumor growth and metastasis [20]. Upregulation of AS5 inactivates the PI3K/AKT/mTOR pathway, while low expression levels of GAS5 activate the PI3K/AKT/mTOR pathway to promote cancer development [10]. In the present study, lncRNA GAS5 overexpression significantly activated the PI3K/AKT/mTOR pathway in esophageal cancer cells, while PI3K activator treatment showed no significant effects on GAS5 expression. In addition, PI3K activator treatment significantly reduced the inhibitory effects of lncRNA GAS5 overexpression on cell proliferation and migration. These data suggest that lncRNA GAS5 inhibits esophageal cancer by reducing the proliferation and migration of cancer cells through inactivation of the PI3K/AKT/mTOR pathway. However, the mechanism of action of GAS5 in regulating the PI3K/AKT/mTOR pathway is still unknown. GAS5 may directly target this pathway or might indirectly interact with this pathway through other signaling molecules. Therefore, more studies are needed.
Conclusions
GAS5 expression was lower in esophageal cancer patients than in healthy controls, and serum GAS5 is a promising diagnostic and prognostic biomarker for esophageal cancer. GAS5 overexpression inhibited tumor cell proliferation and migration, while treatment with PI3K activator reduced this inhibitory effect. GAS5 overexpression decreased the expression level of PI3K and phosphorylation levels of Akt and mTOR in esophageal cancer cells, while PI3K activator treatment showed no significant effects on GAS5 expression. Therefore, we conclude that GAS5 overexpression may suppress the proliferation and migration of esophageal cancer cell by inactivating the PI3K/AKT/mTOR pathway.
Footnotes
Source of support: National Natural Science Foundation of China (Grant No. 81702343)
References
- 1.Arnal MJD, Arenas ÁF, Arbeloa ÁL. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21(26):7933–43. doi: 10.3748/wjg.v21.i26.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng H, Zheng R, Zhang S, et al. Esophageal cancer statistics in China, 2011: Estimates based on 177 cancer registries. Thorac Cancer. 2016;7(2):232–37. doi: 10.1111/1759-7714.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ida S, Watanabe M, Yoshida N, et al. Sarcopenia is a predictor of postoperative respiratory complications in patients with esophageal cancer. Ann Surg Oncol. 2015;22(13):4432–37. doi: 10.1245/s10434-015-4559-3. [DOI] [PubMed] [Google Scholar]
- 4.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Seminars in radiation oncology. Elsevier. 2007;17(1):2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15(Suppl 1):R17–29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 6.Gutschner T, Diederichs S. The hallmarks of cancer: A long non-coding RNA point of view. RNA Biol. 2012;9(6):703–19. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abraham JM, Meltzer SJ. Long noncoding RNAs in the pathogenesis of Barrett’s esophagus and esophageal carcinoma. Gastroenterology. 2017;153(1):27–34. doi: 10.1053/j.gastro.2017.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao S, Liu W, Li F, et al. Decreased expression of lncRNA GAS5 predicts a poor prognosis in cervical cancer. Int J Clin Exp Pathol. 2014;7(10):6776–83. [PMC free article] [PubMed] [Google Scholar]
- 9.Sun M, Jin F, Xia R, et al. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer. 2014;14(1):319. doi: 10.1186/1471-2407-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue D, Zhou C, Lu H, et al. LncRNA GAS5 inhibits proliferation and progression of prostate cancer by targeting miR-103 through AKT/mTOR signaling pathway. Tumor Biol. 2016;37(12):16187–97. doi: 10.1007/s13277-016-5429-8. [DOI] [PubMed] [Google Scholar]
- 11.Lu X, Fang Y, Wang Z, et al. Downregulation of gas5 increases pancreatic cancer cell proliferation by regulating CDK6. Cell Tissue Res. 2013;354(3):891–96. doi: 10.1007/s00441-013-1711-x. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Wang W, Jiang J, et al. Downregulation of GAS5 promotes bladder cancer cell proliferation, partly by regulating CDK6. PloS One. 2013;8(9):e73991. doi: 10.1371/journal.pone.0073991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He J, Liu D, Li Y, et al. Decreased expression of lncRNA GAS5 predicts poor survival and aggressive phenotype in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2017;10(2):1603–10. [Google Scholar]
- 14.Wang K, Li J, Xiong G, et al. Negative regulation of lncRNA GAS5 by miR-196a inhibits esophageal squamous cell carcinoma growth. Biochem Biophys Res Commun. 2018;495(1):1151–57. doi: 10.1016/j.bbrc.2017.11.119. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 16.Markar S, Gronnier C, Duhamel A, et al. The impact of severe anastomotic leak on long-term survival and cancer recurrence after surgical resection for esophageal malignancy. Ann Surg. 2015;262(6):972–80. doi: 10.1097/SLA.0000000000001011. [DOI] [PubMed] [Google Scholar]
- 17.Davis JL, Moutinho V, Jr, Panageas KS, et al. A peripheral blood biomarker estimates probability of survival: The neutrophil – lymphocyte ratio in noncancer patients. Biomark Med. 2016;10(9):953–57. doi: 10.2217/bmm-2016-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Huang H, Li Y, et al. Decreased expression of long non-coding RNA GAS5 promotes cell proliferation, migration and invasion, and indicates a poor prognosis in ovarian cancer. Oncol Rep. 2016;36(6):3241–50. doi: 10.3892/or.2016.5200. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Guo Y, Song Y, et al. Long noncoding RNA GAS5 inhibits malignant proliferation and chemotherapy resistance to doxorubicin in bladder transitional cell carcinoma. Cancer Chemother Pharmacol. 2017;79(1):49–55. doi: 10.1007/s00280-016-3194-4. [DOI] [PubMed] [Google Scholar]
- 20.Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4:64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]