Abstract
Background
Methotrexate (MTX) is an effective drug for the treatment of adult malignancies, but toxicity remains a significant problem. Toxic reactions may occur when patients use high-dose MTX (HD-MTX), but the correlation between its toxicity and concentration in adults is controversial. The purpose of this study was to examine the relationship between MTX concentration and renal function, as well as to assess toxic reactions to MTX in Chinese adults with acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL).
Material/Methods
This retrospective study enrolled 97 patients who had been diagnosed with ALL or NHL, and who were treated at the Hemopathology Department of Shanghai Changhai Hospital from January 2015 to June 2016.
Results
Forty-one (27.5%) episodes of elimination delay were observed. We found negative correlations between creatinine clearance rate before MTX infusion and the plasma concentrations of MTX at 36 h after MTX infusion (P=0.005). The serum creatinine at 48 h and plasma concentrations of MTX at 48 h and72 h were significantly and positively correlated (both p=0.000). High blood concentration of MTX was positively associated with nephrotoxicity > grade 1 (P<0.01). Infection > grade 1 was more likely to occur if a patient had high MTX levels at 36 h, 48 h, and 72 h (P<0.01).
Conclusions
Our results show that renal function is associated with MTX concentration, and high MTX concentration can predict the occurrence of renal toxicity and infection related to MTX.
MeSH Keywords: Creatinine, Drug-Related Side Effects and Adverse Reactions, Methotrexate
Background
Methotrexate (MTX) is an antimetabolite that disturbs the metabolism of folic acid. MTX is widely used in the treatment of various neoplastic diseases [1,2]. Doses of 500 mg/m2 or higher are defined as high-dose methotrexate (HD-MTX). Higher doses are often more effective than lower doses, but can cause significant toxicity, which includes nephrotoxicity, hepatotoxicity, gastrointestinal mucositis, bone marrow suppression, and neurotoxicity [3–5]. It is reported that the incidence rate of reversible chemical hepatitis is up to 60% and 25% have hyperbilirubinemia [6]. Oral mucositis is a dose-limiting of HD-MTX; grade IV mucositis is related to infections, extension of chemotherapy regimens, and even death [7]. In contrast to other antineoplastic drugs, the cytotoxic effects of MTX can be partially antagonized by leucovorin (LV), which allows use of high-dose intravenous MTX up to 33.6 g/m2 [8,9]. Despite appropriate supportive care measures during administration of HD-MTX, acute kidney injury (AKI) develops in 2–12% of patients [10], so renal toxicity is a particular concern [11]. For instance, the probability of renal injury in patients with lymphoma who receive HD-MTX therapy is 9.1%, and the probability of renal damage due to sarcomas is only 1.5% [12,13].
MTX concentrations >1 μmol/L at approximately 48 h or >0.1 μmol/L at 72 h are defined as MTX elimination delay [14,15], and delayed MTX elimination after administration of HD-MTX remains an important problem. Therefore, serum MTX concentration monitoring is still a standard approach for identifying patients at high risk of developing toxicity. Unfortunately, there is little research on MTX concentration and renal function, and the relationship between MTX concentration and the toxicity due to MTX is controversial in Chinese adults with ALL and NHL. Monitoring of plasma concentrations of MTX is difficult to perform in some resource-constrained settings. Therefore, we performed the present retrospective analysis to explore the association between MTX concentration and its toxicities in patients with hematological malignancy, as well as to investigate the association between renal function and MTX elimination, which be useful in clinical practice to prevent severe adverse events.
Material and Methods
Materials
We enrolled 97 people ≥18 years old who were diagnosed with ALL or NHL in our hospital from January 2015 to June 2016, after informed consent was obtained. The study was approved by the Medical Ethics Committee of Changhai Hospital of the Second Military Medical University. A total of 149 courses of MTX were administered, and the median age of included patients was 40.87 years. The study patients had to have a leukocyte count (WBC) ≥4.0×109/L, absolute neutrophil count (ANC) ≥2.0×109/L, and blood platelet count (PLT) ≥100×109/L, with normal liver and kidney function. During HD-MTX chemotherapy, they were all in full remission.
MTX administration and measurements of plasma MTX concentration
First 10% of HD-MTX was given over the first 30 min and 90% was given over the next 23.5 h. After 36 h of MTX infusion, it is recommend use leucovorin (LV) for rescue, the rescue dosage depending on the concentration of MTX until the plasma concentration of MTX reaches <0.1 μmol/L, and a fluorescent polarization immunoassay is used to measure the MTX concentrations at 24, 36, 48, 72, and 96 h. Patients who have MTX elimination delay should be continuously monitored for MTX concentrations until they reach <0.1 μmol/L.
Toxicity evaluation
HD-MTX-related toxicity was assessed. According to the World Health Organization adverse effect grading system, the group that had an adverse reaction (AE) was compared with those without an AE. The incidence of diarrhea, renal toxicity, hepatotoxicity, and infection after HD-MTX therapy were compared between the 1–3 g/m2 group and the 3–5 g/m2 group, and the between the groups with and without elimination delay.
CF rescuing regimen
We defined the severity based on the MTX concentration, and MTX concentration between 1 μmol/L and 10 μmol/L was defined as low severity in adults, MTX concentration between 10 μmol/L and 100 μmol/L was defined as moderate severity adults, and MTX concentration greater than 100 μmol/L was defined as high severity in adults. At 36 h after MTX administration, LV rescue was started with either 15 mg/m2 for the low-severity adults or 30 mg/m2 for the moderate- and high-severity adults. LV was stopped when the plasma MTX concentration was <0.1 μmol/L. For patients with metabolic delay, the rescue dose was be increased depending on the MTX concentration.
Assessment of renal function
The following formula was used to calculate the creatinine clearance rate (CCr): Ccr (ml/min)=[(140-age) ×weight (kg)]/[0.818×Cr (umol/L)] (for women, the calculation used 0.85)
Adverse events (AEs)
In summary, we assessed the adverse events (AEs) grade ≥1, and hematological toxicity and CNS toxicity were excluded in our study because almost all (>99%) patients appeared to have neutropenia and we failed to find any CNS toxicity in our study.
Statistics analysis
The continuous variables are expressed as means and standard deviation (SD) or median. The comparisons of patients’ demographic features and creatinine metabolism parameters between normal and delayed MTX elimination groups were performed by Mann-Whitney U test. Categorical variables are expressed as numbers and percentages. Spearman correlation coefficient was used to estimate the correlation between 2 variables. Spearman’s rank correlation analysis was used to calculate the creatinine metabolism parameters and serum MTX concentrations. We compared differences between patients with an adverse event (AE) versus those without an AE by Wilcoxon’s rank sum test.
All date assessments were double-tailed and p≤0.05 was considered statistically significant. SPSS 19.0 statistical software was used for statistical analysis.
Results
A total of 149 courses of MTX infusion were enrolled in this study. For dosages, we defined 1–3 g MTX/m2 as the low-dose group and 3–5 g/m2 as the high-dose group. There were 89 courses of 1–3 g MTX/m2 and 60 courses of 3–5 g MTX/m2. Forty-one episodes of elimination delay were observed, accounting for 27.5% of total courses.
There were no statistically significant differences in body height, body weight, age, body surface area (BSA), Cr concentrations before MTX infusion, Cr concentrations at 48 h, CCr before MTX infusion, and CCr at 48 h between group B and group A. Cr concentrations before MTX infusion and after 48-h MTX infusion were significantly higher in the delayed elimination group compared with the normal group (P=0.003 and P=0.000, respectively).
The CCr at 48 h was significantly higher in the normal elimination group compared with the delayed elimination group (P=0.001). There was no significant difference in the rest of the parameters between the 2 groups (Table 1).
Table 1.
Comparison of patients, characteristics by methotrexate (MTX) dose and by MTX elimination status.
| Characteristic | Overall N=149 | MTX dose* | P value | MTX elimination# | P value | ||
|---|---|---|---|---|---|---|---|
| Group A N=89 | Group B N=60 | Normal N= 108 | Delayed N=41 | ||||
| Body height (cm) | 167.6±8.2 | 167.20±8.74 | 168.17±7.35 | 0.396 | 166.50±8.06 | 170.46±7.93 | 0.006 |
| Body weight (kg) | 62.6±14.9 | 62.68±16.04 | 62.43±13.09 | 0.949 | 60.81±15.04 | 67.26±13.54 | 0.005 |
| Age (years) | 40.9±15.8 | 42.55±17.04 | 38.37±13.59 | 0.108 | 45.00±16.10 | 39.30±15.51 | 0.071 |
| BSA (m2) | 1.8±1.2 | 1.79±0.23 | 1.79±0.19 | 0.804 | 1.76±0.21 | 1.86±0.19 | 0.002 |
| Cr (μmol/L), 0 h | 57.7±30.4 | 55.73±19.04 | 60.62±41.99 | 0.845 | 52.37±17.78 | 71.66±47.94 | 0.003 |
| Cr (μmol/L), 48 h | 69.5±55.5 | 66.79±44.47 | 73.59±73.59 | 0.550 | 53.95±18.21 | 111.55±90.76 | 0.000 |
| CCr (mL/min), 0 h | 135.7±49.6 | 136.73±50.87 | 140.06±47.36 | 0.386 | 139.60±43.54 | 125.55±15.51 | 0.083 |
| CCr (mL/min), 48 h | 127.0±50.3 | 125.80±51.84 | 129.06±48.41 | 0.405 | 138.20±45.26 | 96.73±51.42 | 0.000 |
Comparison of patients, characteristics by methotrexate (MTX) dose and by MTX elimination status; Data are presented as mean ± standard deviation. BSA – body surface area; CCr – creatinine clearance rate; MTX – methotrexate; Cr – serum creatinine concentration;
Group A=1–3 g/m2 MTX, group B=3–5 g/m2 MTX.
Delayed elimination of MTX was indicated by plasma MTX concentrations ≥1.0 μmol/L at 48 hours or ≥0.1 μmol/L at 72 hours.
We found that MTX concentration in the high-dose group is more likely to be associated with MTX metabolic delay (Table 2).
Table 2.
Summary of high plasma MTX concentrations at 48 and 72 hr after MTX dosing.
| Group | No. of courses | MTX concentration at 48 hr | MTX concentration at 72 hr | ||
|---|---|---|---|---|---|
| Mean ±SD | Median (Min, Max) | Mean ±SD | Median (Min, Max) | ||
| Low dosage | 89 | 0.85±0.24 | 0.15 (0.00, 16.50) | 0.27±0.91 | 0.03 (0.00, 5.40) |
| High dosage | 60 | 2.5±0.72 | 0.32 (0.00, 29.42) | 0.92±0.35 | 0.08 (0.00,16.97) |
After comparing the demographic features and some potential characteristics that may be correlated with MTX elimination, including height, weight, age, body surface area (BSA), and Cr and CCr levels, we found no difference between the low-dose group and the high-dose group in terms of patient demographic characteristics. However, patients with MTX clearance delay showed higher serum creatinine concentrations at 0 h and 48 h, and a more severe CCr decrease at 48 h. There was also a significant difference between the normal elimination and delayed elimination according to height, weight, and BSA. The relationship between plasma MTX concentration and toxicities induced by MTX, including renal toxicity, hepatotoxicity, stomatitis, and infection, was statistically analyzed to find which toxicity is associated with blood-drug concentration of MTX (Table 3).
Table 3.
The correlation between the plasma MTX concentration and MTX-related toxicities that include nephrotoxicity, hepatic toxicity, stomatitis, and infection.
| Adverse event (number of patients) | P value | ||
|---|---|---|---|
| Without AE (N=131) | With AE (N=18) | ||
| Hepatic toxicity > grade 1 | |||
| 24 h | 41.94 (6.98, 337.60) | 31.99 (12.02, 128.90) | 0.276 |
| 36 h | 4.24 (0.10, 46.68) | 2.76 (0.22, 9.66) | 0.804 |
| 48 h | 1.62 (0.00, 29.42) | 0.80 (0.03, 4.45) | 0.271 |
| 72 h | 0.59 (0.00, 16.97) | 0.17 (0.00, 0.73) | 0.199 |
| 96 h | 0.28 (0, 6.60) | 0.05 (0.00, 0.39) | 0.921 |
| Adverse event (number of patients) | P value | ||
| Without AE (N=137) | With AE (N=12) | ||
| Nephrotoxicity > grade 1 | |||
| 24 h | 45.91 (7.11, 337.60) | 30.18 (6.98, 139.20) | 0.070 |
| 36 h | 4.13 (0.11, 32.76) | 3.93 (0.10, 46.68) | 0.001 |
| 48 h | 1.32 (0.00, 19.94) | 1.93 (0.00, 29.42) | 0.000 |
| 72 h | 0.39 (0.00, 6.32) | 0.83 (0.00, 16.97) | 0.014 |
| 96 h | 0.19 (0.00, 3.89) | 0.38 (0.00, 6.60) | 0.139 |
| Adverse event (number of patients) | P value | ||
| Without AE (N=124) | With AE (N=25) | ||
| Oral mucositis > grade 1 | |||
| 24 h | 41.96 (6.98, 337.60) | 34.69 (10.30, 99.75) | 0.541 |
| 36 h | 4.11 (0.15, 32.76) | 3.84 (0.10, 46.68) | 0.247 |
| 48 h | 1.46 (0.00, 19.94) | 1.84 (0.00, 29.42) | 0.623 |
| 72 h | 0.48 (0.00, 8.50) | 0.81 (0.00, 16.97) | 0.627 |
| 96 h | 0.24 (0.00, 5.40) | 0.33 (0.00, 6.60) | 0.755 |
| Adverse event (number of patients) | P value | ||
| Without AE (N=100) | With AE (N=49) | ||
| Infection > grade 1 | |||
| 24 h | 45.91 (7.11, 337.60) | 30.18 (6.98, 139.20) | 0.070 |
| 36 h | 4.13 (0.11, 32.76) | 3.93 (0.10, 46.68) | 0.010 |
| 48 h | 1.32 (0.00, 19.94) | 1.93 (0.00, 29.42) | 0.000 |
| 72 h | 0.39 (0.00, 6.32) | 0.83 (0.00, 16.97) | 0.014 |
| 96 h | 0.19 (0.00, 3.89) | 0.38 (0.00, 6.60) | 0.139 |
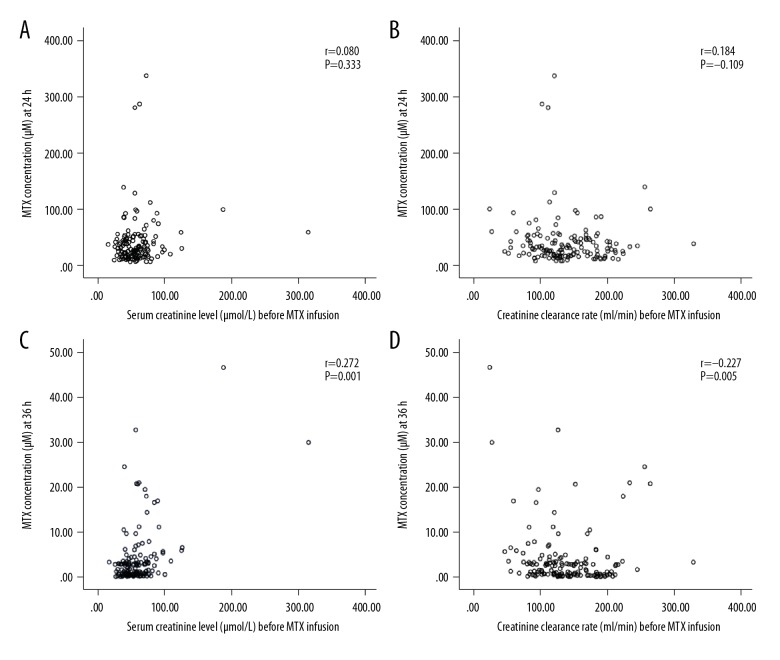
We found that nephrotoxicity > grade 1 and MTX concentration were significantly correlated. Infection > grade 1 was more likely to occur if a patient had high MTX levels at 36 h, 48 h, and 72 h, but hepatic toxicity and oral mucositis had no correlations with MTX levels. As show in Figure 1, there was no significant correlation between MTX concentrations at 24 h and Cr concentrations or CCr before MTX infusion (Figure 1A, 1B) (P=0.333 and P=0.184, respectively). The MTX concentrations at 36 h and Cr concentrations before MTX infusion were significantly and positively correlated (Figure 1C) (P=0.001) and the CCr level before MTX infusion and the plasma MTX concentration at 36 h were negatively correlated (Figure 1D) (P=0.005).
Figure 1.
(A) MTX concentrations at 24 h and Cr concentrations. (B) MTX concentrations at 24 h and CCr before MTX infusion. (C) MTX concentrations at 36 h and Cr concentrations before MTX infusion. (D) CCr before MTX infusion and plasma MTX concentration at 36 h.
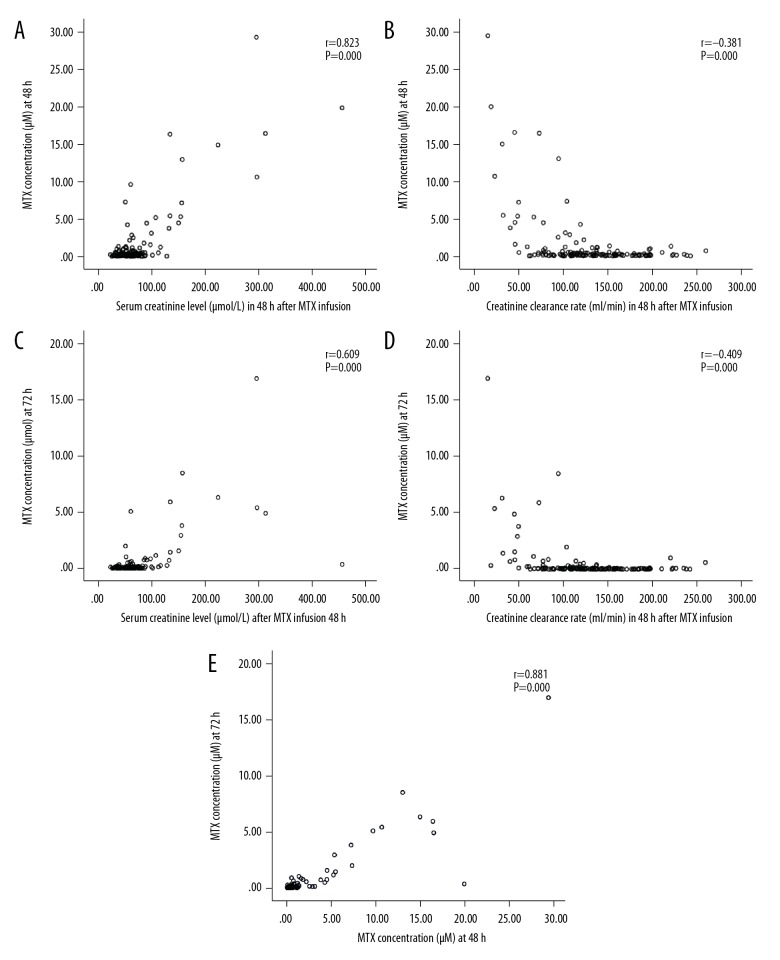
The results indicate that renal function before MTX infusion can be used to calculate the MTX concentrations at 36 h, meaning that MTX concentrations at 36 h can reflect the MTX metabolism status. The correlation between Cr at 48 h and concentration of MTX was significantly and positively correlated (P=0.000 and P=0.000, respectively) (Figure 2A, 2B). There was a significant negative correlation between the CCr level at 48 h and the plasma MTX concentrations at 48 h and 72 h (P=0.000 and P=0.000, respectively) (Figure 2C, 2D). MTX concentrations at 48 h and 72 h were also a significantly and positively correlated (Figure 2E).
Figure 2.
(A) Cr at 48 h and MTX concentrations at 48 h. (B) Cr at 48 h and MTX concentrations at 72 h. (C) CCr at 48 h and the plasma MTX concentration at 48 h. (D) CCr at 48 h and the plasma MTX concentration at 72 h. (E) MTX concentrations at 48 h and 72 h.
Discussion
HD-MTX treatment of adult malignant tumors is not evidence-based, and the treatment plan mainly relies on the doctor’s experience. Much previous research has focused on assessing the relationship between the polymorphisms of MTX and MTX-related toxicities, but it is still difficult to use the patient’s genotype to predict MTX toxicity and to correctly adjust the dosage [16,17].
We found that kidney function is good predictor of MTX toxicity because the kidneys are the main pathway for MTX elimination, and approximately 70–90% of MTX is excreted unchanged from urine [18]. The relationship between serum MTX concentration and creatinine clearance is controversial. Hempel et al. [19] found that MTX concentration was negatively associated with CCr level, which agrees with the present findings. However, Evans et al. [20] and Joannon et al. [21] reported that MTX concentrations have no relationship with CCr, but these studies were performed in children, not adults.
Our study shows a positive correlation between kidney function and MTX concentration in adults, and the Cr and CCr levels at 48 h after MTX infusion are positively correlated with MTX concentration. Our results revealed that MTX concentration and nephrotoxicity were significantly and high plasma MTX levels can induced nephrotoxicity. Using serum creatinine and CCr levels, clinicians can evaluate the MTX elimination status before the MTX concentration report is available and could help to enhance alkalization and hydration as early as possible. Our results also suggest that the blood concentration of MTX can predict whether MTX will induce infection, so clinicians could prophylactically administer antibiotics in advance based on MTX concentration.
Acute kidney injury (AKI) develops in 2–12% of patients [22]. Our results suggest that the incidence rate of nephrotoxicity was 8.05% in our study (12/149 courses). It is unrealistic to monitor blood drug concentration of MTX, but regular measurement of renal function is feasible.
This study has certain limitations. First of all, it is limited by the relatively small number of patients included. Secondly, we did not consider the effect of homogeneity of disease severity on the results and this may have influenced the results. Thirdly, we did not consider hydration, alkalinization, or the frequency of chemotherapy. Therefore, our findings require replication in a study involving a larger cohort of patients and considering the possible factors what we described above. The predictors mentioned above may sometimes be more useful than regular measurement of MTX concentrations, which is difficult. Clearly, further studies are needed to confirm our results, but we believe our results are reliable and of clinical value.
Conclusions
Our results show that renal function is associated with MTX concentrations and the occurrence of renal toxicity and infection can be predicted when MTX concentration is high during HD-MTX in Chinese adult with ALL or NHL.
Acknowledgements
We thank the Pharmacy Department of Changhai Hospital for support and encouragement.
Footnotes
Source of support: 1. Shanghai Municipal Commission of Health and Family Flanning-Construction of clinical pharmacy service system (2016ZB0303). 2. Scientific Research Project of Hospital Pharmacy of Shanghai Pharmaceutical Association (2017-YY-02-13). 3. Military Construction of The National Key Clinical Specialist – Clinical Pharmacy
References
- 1.Mantadakis E, Cole PD, Kamen BA. High-dose methotrexate in acute lymphoblastic leukemia: Where is the evidence for its continued use? Pharmacotherapy. 2005;25(2):748–55. doi: 10.1592/phco.25.5.748.63584. [DOI] [PubMed] [Google Scholar]
- 2.Abramson JS, Hellmann M, Barnes JA, et al. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer. 2010;116(18):4283–90. doi: 10.1002/cncr.25278. [DOI] [PubMed] [Google Scholar]
- 3.Sterba J, Valík D, Bajciová V, et al. High-dose methotrexate and/or leucovorin rescue for the treatment of children with lymphoblastic malignancies: Do we really know why, when and how? Neoplasma. 2005;52(6):456–63. [PubMed] [Google Scholar]
- 4.Schmiegelow K. Advances in individual prediction of methotrexate toxicity: A review. Br J Haematol. 2009;146(5):489–503. doi: 10.1111/j.1365-2141.2009.07765.x. [DOI] [PubMed] [Google Scholar]
- 5.Relling MV, Fairclough D, Ayers D, et al. Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol. 1994;12(8):1667–72. doi: 10.1200/JCO.1994.12.8.1667. [DOI] [PubMed] [Google Scholar]
- 6.Ackland SP, Schilsky RL. High-dose methotrexate: A critical reappraisal. J Clin Oncol. 1987;5:2017–31. doi: 10.1200/JCO.1987.5.12.2017. [DOI] [PubMed] [Google Scholar]
- 7.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–84. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 8.Evans WE, Crom WR, Abromowitch M, et al. Clinical pharmacodynamics of high-dose methotrexate in acute lymphocytic leukemia. Identification of a relation between concentration and effect. N Engl J Med. 1986;314(8):471–77. doi: 10.1056/NEJM198602203140803. [DOI] [PubMed] [Google Scholar]
- 9.Cohen IJ. Defining the appropriate dosage of folinic acid after high-dose methotrexate for childhood acute lymphatic leukemia that will prevent neurotoxicity without rescuing malignant cells in the central nervous system. J Pediatr Hematol Oncol. 2004;26(3):156–63. doi: 10.1097/00043426-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Widemann BC, Balis FM, Kim A, et al. Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: Clinical and pharmacologic factors affecting outcome. J Clin Oncol. 2010;28(25):3979–86. doi: 10.1200/JCO.2009.25.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takami M, Kuniyoshi Y, Oomukai T, et al. Severe complications after high-dose methotrexate treatment. Acta Oncol. 1995;34(5):611–12. doi: 10.3109/02841869509094036. [DOI] [PubMed] [Google Scholar]
- 12.Perazella MA, Moeckel GW. Nephrotoxicity from chemotherapeutic agents: Clinical manifestations, pathobiology, and prevention/therapy. Semin Nephrol. 2010;30:570–81. doi: 10.1016/j.semnephrol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 13.May J, Carson KR, Butler S, et al. High incidence of methotrexate associated renal toxicity in patients with lymphoma: A retrospective analysis. Leuk Lymphoma. 2014;55:1345–49. doi: 10.3109/10428194.2013.840780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoller RG, Hande KR, Jacobs SA, et al. Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med. 1977;297(12):630–34. doi: 10.1056/NEJM197709222971203. [DOI] [PubMed] [Google Scholar]
- 15.Kodidela S, Chandra PS, Dubashi B. Pharmacogenetics of methotrexate in acute lymphoblastic leukemia: Why still at the bench level. Eur J Clin Pharmacol. 2014;70(3):253–60. doi: 10.1007/s00228-013-1623-4. [DOI] [PubMed] [Google Scholar]
- 16.Kishi S, Cheng C, French D, et al. Ancestry and pharmacogenetics of anti-leukemic dug toxicity. Blood. 2007;109(10):4151–57. doi: 10.1182/blood-2006-10-054528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmiegelow K. Advances in individual prediction of methotrexate toxicity: A review. Br J Haematol. 2009;146(5):489–503. doi: 10.1111/j.1365-2141.2009.07765.x. [DOI] [PubMed] [Google Scholar]
- 18.Adamson PC, Balis FM, Berg W, Blaney SM. General principles of chemotherapy. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 5th edition. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2005. pp. 290–365. [Google Scholar]
- 19.Hempel L, Misselwitz J, Fleck C, et al. Influence of high-dose methotrexate therapy (HD-MTX) on glomerular and tubular kidney function. Med Pediatr Oncol. 2003;40(6):348–54. doi: 10.1002/mpo.10293. [DOI] [PubMed] [Google Scholar]
- 20.Evans WE, Crom WR, Stewart CF, et al. Methotrexate systemic clearance influences probability of relapse in children with standard-risk acute lymphocytic leukaemia. Lancet. 1984;1(8373):359–62. doi: 10.1016/s0140-6736(84)90411-2. [DOI] [PubMed] [Google Scholar]
- 21.Joannon P, Oviedo I, Campbell M, et al. High-dose methotrexate therapy of childhood acute lymphoblastic leukemia: Lack of relation between serum methotrexate concentration and creatinine clearance. Pediatr Blood Cancer. 2004;43(1):17–22. doi: 10.1002/pbc.20032. [DOI] [PubMed] [Google Scholar]
- 22.Howard SC, McCormick J, Pui CH, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21(12):1471–82. doi: 10.1634/theoncologist.2015-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]