Passaris et al. show that the well-studied spv virulence operon of Salmonella Typhimurium displays a bimodal expression pattern. Using quantitative single-cell fluorescence microscopy, they demonstrate that this expression pattern originates in the bimodal expression...
Keywords: bimodality, Salmonella typhimurium, spv operon, spvA
Abstract
The well-studied spv operon of Salmonella typhimurium is important for causing full virulence in mice and both the regulation and function of the Spv proteins have been characterized extensively over the past several decades. Using quantitative single-cell fluorescence microscopy, we demonstrate the spv regulon to display a bimodal expression pattern that originates in the bimodal expression of the SpvR activator. The spv expression pattern is influenced by growth conditions and the specific S. typhimurium strain used, but does not require Salmonella-specific virulence regulators. By monitoring real-time promoter kinetics, we reveal that SpvA has the ability to impart negative feedback on spvABCD expression without affecting spvR expression. Together, our data suggest that the SpvA protein counteracts the positive feedback loop imposed by SpvR, and could thus be responsible for dampening spvABCD expression and coordinating virulence protein production in time. The results presented here yield new insights in the intriguing regulation of the spv operon and adds this operon to the growing list of virulence factors exhibiting marked expression heterogeneity in S. typhimurium.
SALMONELLA species comprise important Gram-negative intestinal pathogens with the capacity to survive and multiply intracellularly in various mammals and, depending on the serovar, can cause two major clinical syndromes in humans: typhoid fever, which is a systemic and potentially fatal invasive disease affecting multiple organs in the body, and nontyphoid gastroenteritis, which causes a usually self-limiting diarrheal disease (Coburn et al. 2007; McQuiston et al. 2008; Fabrega and Vila 2013). The serovar Salmonella typhimurium is commonly isolated in gastroenteritis disease outbreaks and harbors a wide range of virulence factors that are typically located on laterally acquired genetic elements such as Salmonella pathogenicity islands (SPIs) (large genomic regions containing multiple virulence factors) and virulence plasmids (Rhen and Dorman 2005; Hébrard et al. 2011; Sterzenbach et al. 2013).
As such, SPI-1 is the best characterized pathogenicity island and is mainly important for the intestinal phase of S. typhimurium infection (Lee et al. 1992; Mills et al. 1995; Phoebe Lostroh and Lee 2001). The genes on this island encode several effector proteins that are secreted through a type three secretion system (TTSS) (termed TTSS-1 and encoded within SPI-1) into the intestinal epithelial cells to facilitate invasion and subsequent internalization of Salmonella (Rhen and Dorman 2005; Morgan 2007). The SPI-2 island of S. typhimurium encodes its own secretion system (TTSS-2), and different effectors facilitating survival and replication inside epithelial cells and macrophages (Hensel et al. 1995; Ochman et al. 1996; Figueira and Holden 2012). Regulation of gene expression from both islands is extremely complex and involves a network of interacting transcriptional regulators that are responsive to a combination of environmental and intracellular signals (Deiwick et al. 1999; Worley et al. 2000; Brown et al. 2005; Bustamante et al. 2008; Fass and Groisman 2009; Saini et al. 2010; Sturm et al. 2011).
S. typhimurium also harbors the pSLT virulence plasmid of ∼90 kb that contains a highly conserved ca. 8 kb region of five genes (spvRABCD) that have been reported to be implicated in intracellular survival and growth (Gulig and Doyle 1993; Libby et al. 1997; Cano et al. 2001) and killing of (mostly) macrophages (Guilloteau et al. 1996; Libby et al. 2000). The SpvB, SpvC, and SpvD proteins have unequivocally been proven to be virulence factors with specific roles in infected host cells (Tezcan-Merdol et al. 2005; Browne et al. 2008; Mazurkiewicz et al. 2008; Haneda et al. 2012; Rolhion et al. 2016) and are thought to be exported outside the cell mainly through the TTSS-2 (Browne et al. 2002; Gotoh et al. 2003; Mazurkiewicz et al. 2008). In particular, SpvB has been characterized as an (ADP-ribosyl)transferase, activating host cell actin degradation and thereby mediating cytotoxicity in macrophages and virulence in mice (Mazurkiewicz et al. 2008), while SpvC was found to exhibit phosphothreonine lyase activity on host mitogen-activated protein kinases (MAPKs), leading to attenuation of the intestinal inflammatory response, which is thought to be important during systemic infection of S. typhimurium (Otto et al. 2000). Recently, the function and crystal structure of the SpvD protein were elucidated and it was found that SpvD acts as a cysteine protease, which downregulates proinflammatory responses by indirectly inhibiting NF-κB regulated promoters and by doing so contributes to the systemic growth of S. typhimurium in mice (Grabe et al. 2016; Rolhion et al. 2016). In contrast, little is known about the SpvA protein and it is yet unclear if it is involved in Salmonella virulence (Roudier et al. 1992; Rotger and Casadesus 1999). While many global regulators can influence spv expression (Fang et al. 1992; Kowarz et al. 1994; O’Byrne and Dorman 1994a,b; Robbe-Saule et al. 1997; Marshall et al. 1999; Mangan et al. 2006), the spvR gene that is located directly upstream of the spvABCD operon is thought to be transcribed separately (Robbe-Saule et al. 1997; Wilson and Gulig 1998) and encodes the DNA-binding SpvR protein, which acts as an essential activator of both spvR and the spvABCD genes and is absolutely required for spv-mediated virulence in vivo (Grob and Guiney 1996; Guilloteau et al. 1996; Sheehan and Dorman 1998).
Apart from the timely expression of virulence factors, Salmonella appears to exploit population heterogeneity as an important aspect of its virulence as well (Stewart and Cookson 2012; Ackermann 2015). In fact, Salmonella benefits from the creation of phenotypically heterogeneous subpopulations to enable bet-hedging and division-of-labor strategies that are indispensable for proper colonization of its host and for the survival in nonhost environments (Ackermann et al. 2008; Arnoldini et al. 2014; MacKenzie et al. 2015). Most notably in this context, the regulatory architecture of the SPI-1 gene circuit results in the bistable expression of the SPI-1 genes that gives rise to a subpopulation of SPI-1–expressing cells that produce the costly TTSS-1 components to invade the gut tissue and elicit gut inflammation, and of cells that do not express SPI-1 and manage to outcompete the gastrointestinal microbiota by respiring inflammation specific compounds (Hautefort et al. 2003; Saini et al. 2010; Sturm et al. 2011; Diard et al. 2013; Bäumler and Sperandio 2016). As opposed to SPI-1 expression, the expression pattern of the SPI-2 genes does not exhibit a clear bifurcation in ON and OFF cells, but rather corresponds to an inducible expression pattern where the whole population turns on SPI-2 gene expression when making the transition from extracellular to intracellular environments (Hautefort et al. 2003; Laughlin et al. 2014).
In this study, we used random transposition of a promoterless yfp gene (encoding the yellow fluorescent protein) to examine single-cell level gene expression from the S. typhimurium pSLT plasmid and found that the spv operon exhibits a bimodal expression pattern with only a subset of the population actively expressing the spv genes. We confirmed the indispensable role of SpvR in spv expression and extended its function with respect to the bimodal expression of the spvABCD genes. Moreover, we identified the SpvA protein as an important regulator of spv expression and provide evidence that SpvA is necessary in obtaining coordinated spvR and spvABCD expression.
Materials and Methods
Strains and growth conditions
Bacterial strains, phages, and plasmids used throughout this study are listed in Supplemental Material, Tables S1 and S2. S. typhimurium LT2 was used for transposon mutagenesis of pSLT and the more virulent S. typhimurium ATCC14028s strain was used for all subsequent experiments involving spv regulation. For culturing bacteria, lysogeny broth (LB; Sambrook and Russell 2001) medium was standardly used either as broth or as agar plates after the addition of 1.5% (for spreading plates) or 0.7% (for soft-agar plates) agar. Cultures were grown in LB broth for 16–20 hr at 37° under well-aerated conditions (200 rpm on an orbital shaker) to reach stationary phase. Exponential phase cultures were in turn prepared by diluting stationary phase cultures 1/100 or 1/1000 in prewarmed broth, and allowing further incubation at 37° until an optical density at 630 nm (OD = 630) of 0.4–0.6 was reached. In indicated cases, intracellular salts medium (ISM) (Headley and Payne 1990), containing 1% glycerol as a carbon source and with a pH of 6, was used. Finally, the transposon mutagenesis screen was performed using AB minimal medium supplemented with 0.2% glucose as a carbon source (Clark and Maaløe 1967) (http://openwetware.org/wiki/AB_medium).
When appropriate, the following chemicals (Applichem, Darmstadt, Germany) were added to the growth medium at the indicated final concentrations: ampicillin (100 μg/ml; Ap100), chloramphenicol (30 μg/ml; Cm30), kanamycin (50 μg/ml; Km50), tetracycline (20 μg/ml; Tc20), anhydrotetracycline (aTc; different concentrations), and L-arabinose (0.2%).
Phages were propagated on S. typhimurium LT2 or ATCC14028s as plaques in LB soft-agar or as lysates in LB broth as described previously (Davis et al. 1980). Phage stocks were filter sterilized with 0.2 μm filters (Thermo Fisher Scientific, Aalst, Belgium) and chloroform was added to maintain sterility. Generalized transduction was performed with phage P22 HT105/1 int-201 as described previously (Schmieger 1972; Davis et al. 1980). This mutant is unable to integrate into the host chromosome as a prophage due to the lack of integrase activity.
Construction of the Tn5-mVenus transposon
The Tn5-mVenus transposon was largely designed in silico and obtained from a DNA synthesis company (GenScript). For its use, the Tn5-mVenus transposon was cut out of its cloning vector (pUC57) using the PvuII restriction enzyme and ligated bluntly in the backbone of the pBAM1-GFP plasmid (Martínez-García et al. 2011). The pBAM1-GFP plasmid had been cut as well with PvuII (thereby removing its gfp gene) and the backbone was extracted from an agarose gel using the GeneJet Gel Extraction Kit (Thermo Fisher Scientific). The ligation mix was electroporated to an Escherichia coli S17-1λpir strain and ApR colonies were PCR verified for the integration of the Tn5-mVenus transposon and subsequently sequenced.
Next, a sequence verified clone was used to incorporate a neomycin phosphotransferase (npt) cassette, conferring KmR, and a PCR-based strategy was followed. The pBAM1-Tn5-mVenus backbone was amplified using an outward PCR, with primers containing 5′ BamHI restriction enzyme sites and subsequently cut with BamHI. In parallel, the npt cassette was PCR amplified from the pKD4 plasmid (Datsenko and Wanner 2000) using primers with a 5′ BamHI restriction enzyme site, and cut with BamHI. This fragment was used for sticky end ligation in the BamHI-treated pBAM1-Tn5-mVenus PCR fragment and the ligation mixture was electroporated to E. coli S17-1λpir, selecting on KmR. Resistant colonies were picked up and sequence verified. The complete sequence of this transposon can be provided upon request.
Transposon mutagenesis of pSLT using Tn5-mVenus and screening
Suicide delivery of the Tn5-mVenus transposon was accomplished through mating of donor and acceptor strains (Martínez-García et al. 2011). More specifically, the donor (E. coli S17-1λpir strain bearing the pBAM1-Tn5-mVenus plasmid) and the acceptor (S. typhimurium LT2 finO::TetRA) were grown overnight with the appropriate antibiotics. Deletion of the pSLT-borne finO gene increases conjugation efficiency (Camacho and Casadesus 2002) and was necessary to avoid enrichment of pSLT finO mutants in the second conjugation step. Next, cells were washed with 10 mM MgSO4 and four times concentrated. Then, 100 µl of donor and 100 µl of acceptor were thoroughly mixed in 5 ml of 10 mM MgSO4 and further concentrated onto a filter disk (0.45 µm pore, 47 mm diameter; Pall). The filter was subsequently incubated on an LB agar plate for 2–4 hr at 30°, after which it was transferred to 5 ml of a 10 mM MgSO4 solution and intensely vortexed to resuspend the cells. Finally, cells were plated out on AB minimal medium plates containing kanamycin to counter-select for the donor strain in mating. The occurrence of plasmid integrants, instead of the desired transposon mutants, was checked through streaking out on Ap100 (backbone marker of pBAM1), and it was found that mutant libraries contained 1–10% false positives. This value is acceptable due to the large sizes of the constructed libraries. The specificity for pSLT mutants was accomplished through a second conjugation step. The constructed library of ∼13,000 Tn5-mVenus mutants was pooled together and used as a donor library in a mating with the S. typhimurium LT2 mrr::Cm ΔpSLT recipient strain, after which the mixture was plated on AB minimal medium containing kanamycin and chloramphenicol. Using this protocol, chromosomal transposon insertions present in the donor library were efficiently separated from the insertions in pSLT that could be collected in the final recipient. The latter collection was subsequently used for screening for fluorescent mutants.
The mutant library was screened using both fluorescence microscopy and fluorescence-activated cell sorting (FACS). In the first approach, 1720 mutants were manually screened using fluorescence microscopy and the retrieved fluorescent mutants were stored at −80°. An alternative approach was adopted where the remaining pSLT mutants were pooled together and subjected to one round of FACS to enrich for mVenus-expressing mutants. These mutants were validated using fluorescence microscopy and stored at −80°. Finally, all transposon insertions yielding fluorescent mutants were transferred to clean background strains using generalized transduction and their insertion location was determined.
Mapping of transposon insertions
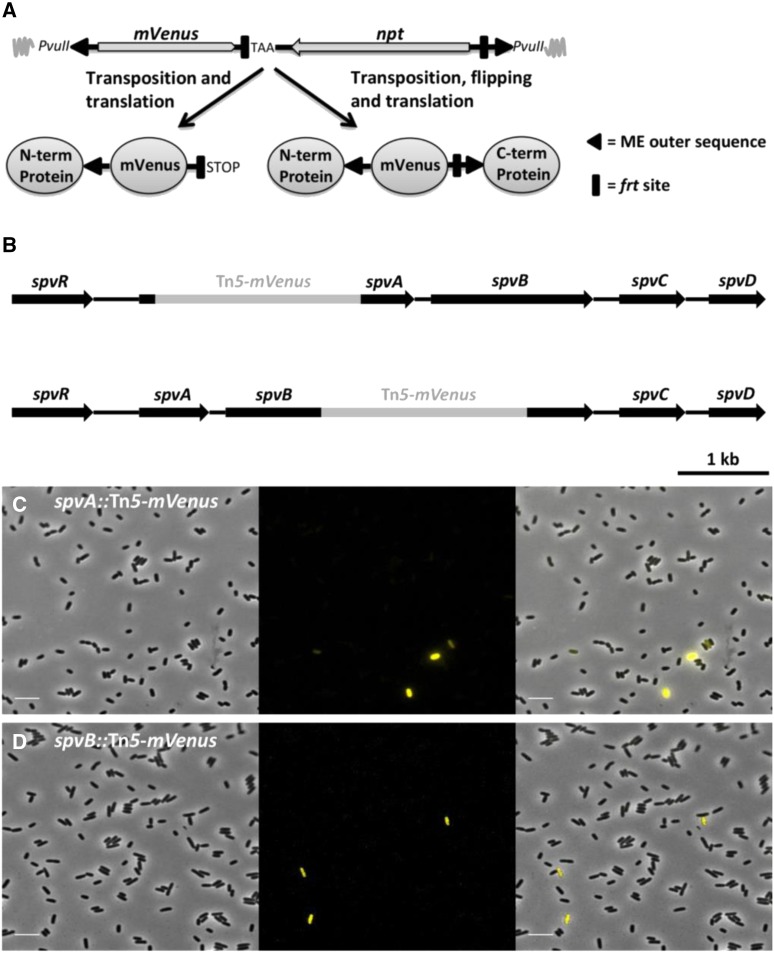
Mapping of the transposon insertions was performed in analogy with the method used by Kwon and Ricke (2000). First, 20 μl of linker1 (350 ng/μl) (Table S3) was added to 18 μl of phosphorylated linker2 (350 ng/μl) and heated for 2 min at 95°, after which the mixture was left to cool down and allow annealing of the linkers (Y-linker). Genomic DNA of transposon mutants was extracted via phenol:chloroform extraction (Wilson 2001) and completely digested with NlaIII (Thermo Fisher Scientific). The digested DNA was purified with the GeneJet PCR Purification kit (Thermo Fisher Scientific) and ∼40 μg was ligated to 1 μg of the Y-linker with 1 μl of T4 DNA ligase (1 unit/μl; Thermo Fisher Scientific) in a final volume of 20 μl. After overnight incubation at 22° the reaction mixture was heated at 65° for 10 min to denature the ligase. A total of 2 μl of this mixture was used as DNA template in a PCR mixture together with a primer specific to the transposon (Tn5-mVenus_up_out), a primer specific to the Y-linker (Y-linker primer) and a Taq polymerase (DreamTaq DNA polymerase; Thermo Fisher Scientific). The PCR products were purified, sequenced (EZ-seq; Macrogen, Amsterdam, The Netherlands) and the exact position of the transposon was determined using a basic local alignment search tool (BLAST). In the spvA::Tn5-mVenus mutant the transposon was inserted 177 bp downstream of the +1 transcription start site, while in the spvB::Tn5-mVenus mutant, the transposon was inserted 1047 bp downstream of the transcription start site.
Construction of bacterial mutants
All S. typhimurium mutants were constructed via a λ-red–mediated homologous recombination approach (Datsenko and Wanner 2000). The temperature-sensitive pKD46 plasmid was routinely used to provide the λ-red genes under arabinose inducible control and strains containing the plasmid were typically grown to exponential phase at 30°, whereupon arabinose was added for an extra 30 min. Subsequently the cells were made electrocompetent, by keeping them at 4° and washing them three times with Milli-Q water, and the PCR product was electroporated, after which the cells were left to resuscitate for a couple of hours at 37°. Cells were plated out at 37° (to cure them from pKD46) on LB medium complemented with an appropriate antibiotic to select for recombinants, and validated through PCR with primer pairs flanking the homologous region. Correct integration of PCR products was further verified by sequencing (EZ-seq; Macrogen). All primer sequences for constructing the bacterial mutants with a detailed description are listed in Table S3. The antibiotic cassette was usually flanked by frt sites and could thus be flipped out by providing cells with the site-specific Flp recombinase (Cherepanov and Wackernagel 1995). Cells were first electroporated with the temperature-sensitive pCP20 plasmid, constitutively expressing the Flp recombinase, and flipping was verified using PCR and sequencing. The pCP20 plasmid was cured by growing the strains at 37° and loss of pCP20 was verified by streaking the cells on Ap100-containing medium. All strains containing multiple fluorescence markers were constructed through sequential recombination steps and all the different loci targeted in the final strain were PCR-validated and sequence-verified. The rpoS deletion was first constructed in a S. typhimurium ATCC14028s background using λ-red–mediated homologous recombination, and subsequently introduced in the relevant spvA reporter strains using generalized transduction with P22 HT105/1 int-201.
The pSLT-spvA::Tn5-mVenus plasmid was transferred from Salmonella to E. coli MG1655 cat-lacI via conjugation and conjugants were selected on LB medium containing both Cm30 (to counter-select the donor strain) and Km50.
FACS, time-lapse fluorescence microscopy, and image analysis
The pooled pSLT::Tn5-mVenus library was sorted by a FACS (BD influx cell sorter) to enrich for mVenus-expressing mutants. A 488 nm excitation laser in combination with a 530/40 nm emission filter was used, and the ±0.1% most mVenus fluorescent clones were sorted and plated out on AB minimal medium containing Km50.
Fluorescence microscopy was further used to screen for fluorescent transposon mutagenesis mutants. All fluorescence microscopy and time-lapse fluorescence microscopy experiments were performed with a temperature-controlled (Okolab, Ottaviano, Italy), Ti-Eclipse inverted microscope (Nikon, Champigny-sur-Marne, France) equipped with a TI-CT-E motorized condenser, a YFP filter (Ex 500/24 nm, DM 520 nm, Em 542/27 nm), a DAPI filter (Ex 377/50 nm, DM 409 nm, Em 447/60), a GFP filter (Ex 473/30, Dm 495, Em 520/35), an mCherry filter (Ex 562/40, Dm 593, Em 647/75), and a CoolSnap HQ2 FireWire CCD-camera.
For imaging, cells were grown to midlog or stationary phase and placed between LB or ISM agarose pads and a cover glass, essentially as described previously (Cenens et al. 2013), and incubated at 37°. Images were acquired using NIS-Elements (Nikon) and resulting pictures were further handled with open-source software ImageJ (downloaded from http://rsbweb.nih.gov/ij/). Detailed image analysis was performed using the open source MicrobeTracker software (Sliusarenko et al. 2011) and the cell meshes generated by this software were used to measure the average cellular fluorescence. The average cellular fluorescence was determined by dividing the integrated pixel intensities of individual cells (after background subtraction) by their respective areas. Appropriate bins for the average cellular fluorescence were chosen by visual inspection of spv ON and spv OFF cells. In short, for every experiment, a threshold was set to separate ON cells from OFF cells and this was based on inspecting single-cell fluorescence intensities in the image and visually defining them as ON or OFF.
Data availability
Strains and plasmids are available upon request. File S1 contains supplemental figures and tables. File M1 contains Movie 1. File M2 contains Movie 2. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6976106.
Results
The spvABCD operon is expressed in a bimodal fashion
A random transposon mutagenesis approach using a modified Tn5 transposon (termed Tn5-mVenus and encoding the YFP variant mVenus; Figure 1A) was used to specifically target the pSLT virulence plasmid of S. typhimurium LT2 in an effort to better characterize its encoded functions. The Tn5-mVenus transposon has the potential of creating C-terminal mVenus translational fusions when randomly inserted into a gene in the correct orientation and reading frame, thereby allowing one to visualize protein expression and/or localization patterns using fluorescence microscopy. During our screening procedure, two transposon mutants were picked up that showed marked expression heterogeneity, with only a small subpopulation of cells actively expressing the mVenus protein (termed ON cells throughout the manuscript) compared to a majority of nonfluorescent siblings (OFF cells) (Figure 1, C and D). Interestingly, the transposon insertions could be mapped to the spvA (i.e., the spvA::Tn5-mVenus mutant, yielding the SpvA_59::mVenus C-terminal protein fusion) and spvB (i.e., the spvB::Tn5-mVenus mutant, yielding the SpvB_349::mVenus C-terminal protein fusion) genes, which are part of the well-studied pSLT-borne spvABCD virulence operon (Figure 1B). Although both the function and the regulation of the spv genes has been studied extensively over the past 25 years, this is, to the best of our knowledge, the first time these genes are being reported to have a bimodal expression pattern.
Figure 1.
Bimodal expression of spvA and spvB in S. typhimurium LT2. (A) Schematic of the constructed Tn5-mVenus transposon (total size of 2248 bp) as integrated in the pBAM1 plasmid and the different fluorescent protein fusions it is able to generate after in frame transposition. The mVenus gene lacks its natural stop codon but a stop codon has been incorporated right after the left frt site. The PvuII restriction enzyme sites were used for cloning the transposon in the pBAM1 plasmid. Image not drawn to scale. (B) Schematic showing the exact insertion position of the Tn5-mVenus transposon into the spvA gene, yielding the SpvA_59::mVenus C-terminal fusion protein (top scheme), and spvB gene, yielding the SpvB_349::mVenus C-terminal fusion protein (bottom scheme). (C) Representative image of the spvA::Tn5-mVenus mutant yielding the SpvA_59::mVenus C-terminal protein grown to stationary phase in AB minimal medium. (D) Representative image of the spvB::Tn5-mVenus mutant yielding the SpvB_349::mVenus C-terminal protein fusion grown to stationary phase in AB minimal medium. The pixel intensities in the different frames are not comparable and the images are merely a qualitative illustration of the bimodal expression of spvA and spvB. The image panels represent the phase contrast channel, the YFP fluorescent channel, and the two channels merged. Bar, 5 µm.
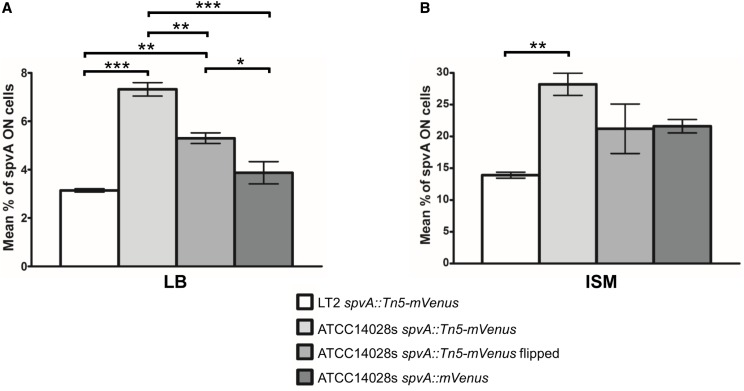
Since the S. typhimurium LT2 strain is known to be virulence attenuated (Wilmes-Riesenberg et al. 1997), the transposon insertions were transduced to the S. typhimurium ATCC14028s strain, which has retained its lethality in mice and its ability to survive in murine macrophages (Jarvik et al. 2010). Comparison of the frequency of spvA ON cells in S. typhimurium LT2 and S. typhimurium ATCC14028s revealed a significant difference between both strains of respectively 3.2% spvA ON cells vs. 7.3% spvA ON cells (Figure 2A and Figure S1A, i and ii) in LB medium. In addition to the difference in frequency, the fluorescence intensity of spvA ON cells (measured as the average cellular fluorescence, described in the Materials and Methods section) in S. typhimurium LT2 was also lower than that in S. typhimurium ATCC14028s (Figure S1A, v; white and light gray bars). S. typhimurium LT2 has been shown to have decreased RpoS protein levels due to a rare UUG start codon in the rpoS gene (Wilmes-Riesenberg et al. 1997), and the spv operon has been shown to be regulated by RpoS (Norel et al. 1992; Kowarz et al. 1994). To address if RpoS has an influence on the frequency of spvA ON cells, the rpoS allele was knocked out in both the LT2 and ATCC14028s spvA::Tn5-mVenus strains. It was found that knocking out rpoS completely abrogated spvA expression for both strains in LB (Figure S2), suggesting that different RpoS levels between LT2 and ATCC14028s might be influencing the frequency of spvA ON cells. Since these observations indicate that proper spv expression is better studied in the S. typhimurium ATCC14028s strain, all strains mentioned throughout the rest of this article were constructed in this background.
Figure 2.
The proportion of spvA ON cells in populations of the indicated strains grown to stationary phase in LB (A) or ISM (B). Cells of the corresponding populations were analyzed with fluorescence microscopy and binned as ON when their mVenus fluorescence exceeded 10 A.U., with this cut-off being based on visual inspection of the raw microscopy images. The data show the mean proportion of spvA ON cells in the total population with the corresponding SEM from three biological replicates. The number of cells quantified for every biological replicate of each strain in both growth media was in the range of 800–2200 cells. One-way ANOVA, followed by Tukey’s honest significant difference test, was performed on the two separate data sets to test for statistically significant differences between the strains (* P < 0.05, ** P < 0.01, *** P < 0.001).
It has previously been described that spvABCD expression is more pronounced in ISM, which mimics the intracellular environment of mammalian cells and has been used routinely to study spv regulation (Wilson et al. 1997; Wilson and Gulig 1998). Indeed, when a population of the S. typhimurium ATCC14028s spvA::Tn5-mVenus transposon mutant was grown to stationary phase in ISM, the frequency of spvA ON cells significantly increased when compared to LB medium (28.2% spvA ON cells vs. 7.3% spvA ON cells, respectively; Figure 2, one-way ANOVA followed by Tukey’s honest significant difference test was performed on the whole data set to compare the mean percentage of spvA ON cells of each strain in both media and statistical significance was found for every strain on the P < 0.01 significance level), although the ON cells showed on average a higher fluorescence signal when originating in LB medium (Figure S1, A and B, v). The outcome of the spv bimodal response thus strongly depends on environmental inputs.
To ensure that the bimodal response of the transposon mutants was not an artifact of the transposon mutagenesis or screening protocol, an S. typhimurium ATCC14028s reporter strain was de novo constructed in which the mVenus fluorescent protein was translationally fused C-terminally to the SpvA protein (spvA::mVenus). The resulting strain was grown in both LB and ISM and monitored under the microscope, and again a clear bimodal expression pattern could be observed (Figure S1, A and B, iv), confirming the phenotype observed with our transposon insertion mutants. It was noticed, however, that the fluorescence intensity of the spvA signal (Figure S1, A and B, v) and the proportion of spvA ON cells (Figure 2) was considerably lower in the S. typhimurium ATCC14028s spvA::mVenus strain when compared to the S. typhimurium ATCC14028s spvA::Tn5-mVenus transposon mutant (respectively, 3.9% vs. 7.3% spvA ON cells in LB and 21.6% vs. 28.2% spvA ON cells in ISM), and this observation is further elaborated upon below.
Next, we translationally fused the mCherry fluorescent protein to the C-terminus of SpvC in the S. typhimurium ATCC14028s spvB::Tn5-mVenus strain and observed a bimodal expression pattern for SpvC (Figure S3A). Furthermore, all spvC ON cells were also spvB ON, suggesting that the entire spvABCD operon is simultaneously expressed in a bimodal fashion.
The pSLT plasmid can be conjugated to E. coli as well (Ahmer et al. 1999), and upon conjugating the spvA::Tn5-mVenus encoding pSLT plasmid to E. coli K-12 MG1655, it was observed that spvA expression in this background was also bimodal and that both burst frequency and intensity compared very well to Salmonella burst frequency and intensity (Figure 1C and Figure S3, B and C). This latter observation suggests that bimodality of the spv operon is insulated from the regulation imposed by Salmonella-specific virulence regulators.
SpvR determines the frequency of spvA ON cells and is itself expressed in a bimodal fashion
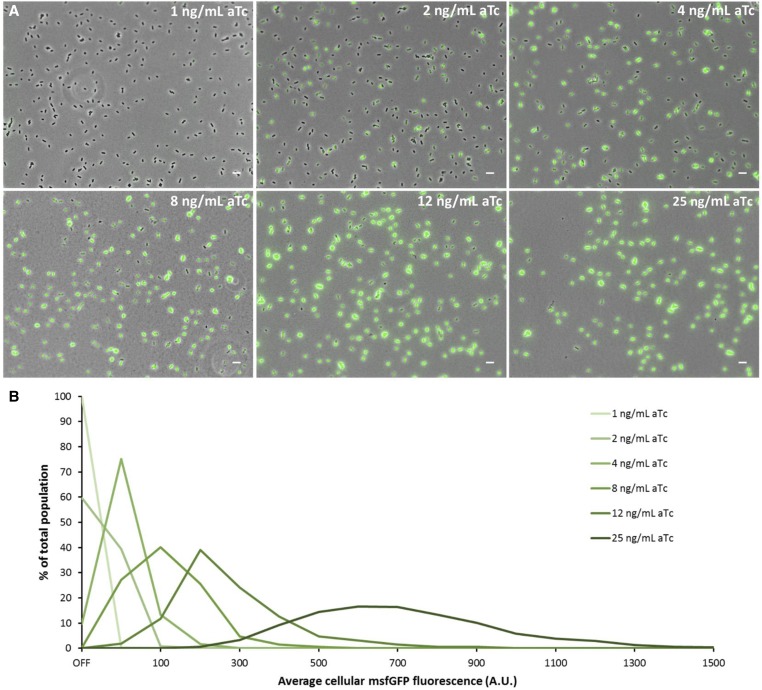
Since SpvR constitutes the essential activator protein of the spvABCD operon, we aimed to examine its effect on the observed bimodality as well. In this regard, a S. typhimurium ATCC14028s strain was constructed in which the spvR promoter was replaced by the synthetic PLtetO-1 promoter (Lutz and Bujard 1997), resulting in aTc-inducible expression of spvR, and the msfGFP gene was inserted transcriptionally directly downstream of the 3′ end of spvA. Using this strain (PLtetO-1-spvR_spvA-msfGFP), a clear range of aTc concentrations could be distinguished, yielding populations with only spvA OFF cells (<2 ng/ml aTc), a bimodal regime with both spvA ON and OFF cells (2–8 ng/ml aTc), and finally populations with only spvA ON cells (>8 ng/ml aTc) (Figure 3). In the bimodal regime, the proportion of spvA ON cells increased from ca. 40% at 2 ng/ml aTc to ca. 90% at 4 ng/ml aTc and reaching ca. 99% at 8 ng/ml aTc, and the average cellular fluorescence intensity of the spvA ON cells increased accordingly (Figure 3B). Together, this confirms the essential role of SpvR in spvA expression and further shows that a certain threshold concentration of SpvR should be reached to fully induce spvA expression, and when SpvR concentrations fluctuate around this threshold, the population can bifurcate into an spvA ON and spvA OFF population.
Figure 3.
SpvR determines the proportion of spvA ON cells and the intensity of spvA expression. (A) Representative images (overlay of phase contrast and GFP channels) of the S. typhimurium ATCC14028s PLtetO-1-spvR_spvA-msfGFP strain grown overnight in ISM containing 1 ng/ml aTc, 2 ng/ml aTc, 4 ng/ml aTc, 8 ng/ml aTc, 12 ng/ml aTc, and 25 ng/ml aTc. (B) Histogram (based on the experiment shown in A) displaying the distribution of the average cellular fluorescence intensity for the different aTc concentrations. The OFF bin in this experiment was set between 0 and 10 A.U., based on visual inspection of the raw microscopy images. The first ON bin was between 10 and 50 A.U. and subsequent bins were defined every 50 A.U. The number of cells (n) used for quantification was as follows: n = 2050 (1 ng/ml aTc), n = 1678 (2 ng/ml aTc), n = 1751 (4 ng/ml aTc), n = 1726 (8 ng/ml aTc), n = 1871 (12 ng/ml aTc), and n = 1615 (25 ng/ml aTc). The experiment was repeated on several independent occasions and similar trends were observed. The pixel intensities of the images in A are not directly comparable with each other and the images are merely a qualitative illustration of the differently sized subpopulations observed. The image panels in A represent the phase contrast channel merged with the GFP fluorescent channel. Bar, 5 µm.
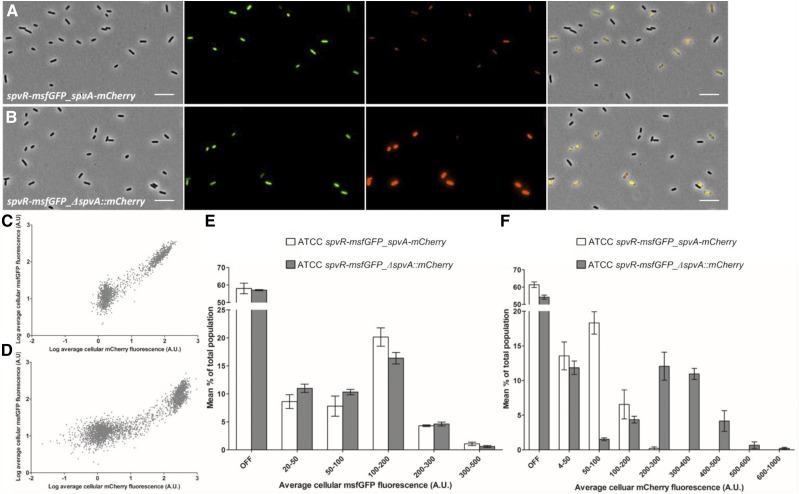
Moreover, these data suggest that the origin of the bimodal expression pattern of spvA lies in the expression of spvR. To test whether spvR is also expressed in a bimodal fashion and overlaps with spvA expression, a new S. typhimurium ATCC14028s strain was constructed in which the msfGFP gene was transcriptionally inserted directly downstream of the spvR gene, while the mCherry gene was transcriptionally inserted directly downstream of the spvA gene (spvR-msfGFP_spvA-mCherry). Expression of spvR was indeed found to be bimodal, and the msfGFP and mCherry signal exhibited a strong positive correlation with two distinct clusters representing the spv OFF and ON populations (R2 = 0.9375; Figure 4, A and C), suggesting that spvR and spvA expression levels are highly interlinked with each other. Furthermore, when monitoring this strain with time-lapse microscopy, it could be observed that PspvR and PspvA bursting were correlated in time as well (Figure 5). Together, the results presented in this section suggest that bimodality of the spvABCD operon is causally preceded by the bimodal expression of SpvR.
Figure 4.
Expression of spvR and spvA is highly correlated. (A) Representative image of the S. typhimurium ATCC14028s spvR-msfGFP_spvA-mCherry strain grown to stationary phase in ISM. (B) Representative image of the S. typhimurium ATCC14028s spvR-msfGFP_ΔspvA::mCherry strain grown to stationary phase in ISM. The pixel intensities in A and B have been adjusted similarly for the msfGFP and mCherry channel to make them visually comparable. The image panels in A and B represent the phase contrast channel, the GFP fluorescent channel, the mCherry fluorescent channel, and the three channels merged. Bar, 5 µm. (C) Scatterplot showing the average cellular mCherry and msfGFP fluorescence of all three biological replicates of the spvR-msfGFP_spvA-mCherry strain. (D) Scatterplot showing the average cellular mCherry and msfGFP fluorescence of all three biological replicates of the spvR-msfGFP_ΔspvA::mCherry strain. (E) Histogram showing the distribution of the average cellular msfGFP fluorescence within populations of the spvR-msfGFP_spvA-mCherry (open bars) and spvR-msfGFP_ΔspvA::mCherry (shaded bars) strains. Averages and the corresponding SEM from three biological replicates are shown. (F) Histogram showing the distribution of average the cellular mCherry fluorescence within populations of the spvR-msfGFP_spvA-mCherry (open bars) and spvR-msfGFP_ΔspvA::mCherry (shaded bars) strains. Averages and the corresponding SEM from three biological replicates are shown. The OFF bin was set between 0 and 20 A.U. (E) and 0–4 A.U. (F), and was based on visual inspection of the raw microscopy images. The number of cells quantified for every biological replicate of every strain was in the range of 560–1130 cells.
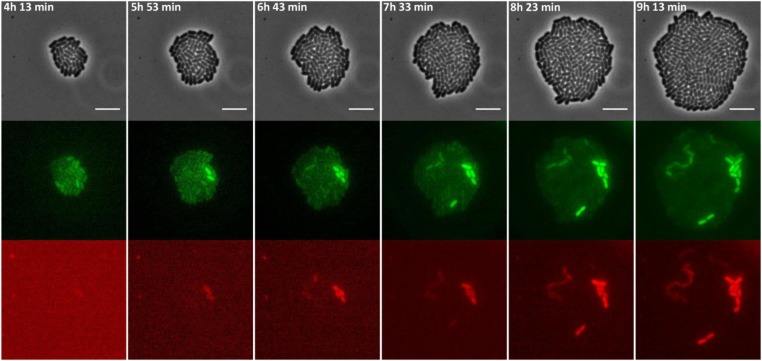
Figure 5.
Images of a time-lapse fluorescence microscopy recording of the growth of a representative microcolony of the S. typhimurium ATCC14028s spvR-msfGFP_spvA-mCherry strain showing highly coordinated PspvR and PspvA bursting. The strain was grown to stationary phase in ISM and subsequently monitored on ISM agarose pads at 37° for the time indicated. The pixel intensities in the different frames are not comparable and the images are merely a qualitative illustration of the bursting process. The image panels represent the phase contrast channel, the GFP fluorescent channel, and the mCherry fluorescent channel. Bar, 5 µm.
Deletion of spvA increases its own promoter activity without affecting spvR expression
When comparing the fluorescence signal from the spvA::Tn5-mVenus allele (in which mVenus is preceded by the first 59 amino acids of SpvA) to the de novo constructed spvA::mVenus allele (in which mVenus is preceded by the full length of SpvA), it was observed that the latter allele caused a decreased amount of spvA ON cells and that these spvA ON cells exhibited a reduced fluorescence intensity, both in LB and ISM (Figure 2 and Figure S1, A and B, v). Furthermore, when flipping out the npt cassette in the spvA::Tn5-mVenus mutant (Figure S1), yielding a strain expressing the SpvA_59::mVenus::SpvA_197 “sandwich” fusion protein (where the mVenus protein is now followed by the remaining 197 C-terminal amino acids of SpvA), again a decreased number of spvA ON cells (Figure 2) with a substantially lower fluorescence intensity compared to the parental spvA::Tn5-mVenus mutant was observed, more closely matching the intensities of the spvA::mVenus allele (Figure S1, v). Together, these preliminary observations suggested that completely abrogating the SpvA protein leads to an increase in spvA expression.
To independently confirm the above observations, a clean knockout strain of spvA was constructed de novo in the S. typhimurium ATCC14028s spvR-msfGFP background where only the first 10 amino acids of SpvA were kept and translationally fused to the mCherry fluorescent protein (ΔspvA::mCherry). The fluorescence intensity of the ON cells in the spvR-msfGFP_ΔspvA::mCherry strain was subsequently compared to the spvR-msfGFP_spvA-mCherry strain and it was observed that the spv ON cells in the ΔspvA strain showed a substantially higher mCherry fluorescence intensity compared to the spv ON cells in the strain containing a functional spvA copy (Figure 4, A and B). Quantification of the average cellular fluorescence intensity of both strains revealed that the proportion of PspvA ON cells (i.e., the mCherry signal) was higher in the spvR-msfGFP_ΔspvA::mCherry strain (38.6% ON cells for spvR-msfGFP_spvA-mCherry strain vs. 45.8% for the spvR-msfGFP_ΔspvA::mCherry strain), and that the average cellular fluorescence intensity of the PspvA ON cells in the ΔspvA strain was substantially higher giving rise to a well-separated, highly fluorescent subpopulation (Figure 4, B, D, and F).
Quantification of the average cellular msfGFP fluorescence of both strains did not show any marked difference in both the proportion of PspvR ON cells or the average cellular msfGFP fluorescence intensity between the two strains (42.0% ON cells for spvR-msfGFP_spvA-mCherry strain vs. 42.9% ON cells for the spvR-msfGFP_ΔspvA::mCherry strain; Figure 4E) and suggests that the increased expression from PspvA in the ΔspvA::mCherry strain is not caused by an increased expression of the SpvR activator. In addition to this, the msfGFP and mCherry signals are still highly positively correlated (R2 = 0.7993; Figure 4D) in the spvR-msfGFP_ΔspvA::mCherry strain, indicating that PspvR and PspvA expression are still intimately coupled. However, when comparing Figure 4, C and D it is clear that the spvR-msfGFP_ΔspvA::mCherry strain reaches higher PspvA expression levels compared to the spvR-msfGFP_spvA-mCherry strain and this for similar PspvR expression levels.
Together, these data confirm that functionally compromising the SpvA protein leads to an increase in spvA promoter activity. At the same time, these results also suggest that the increased expression driven from the spvA promoter is not directly caused by an increased spvR expression, which complicates the interpretation of the ΔspvA phenotype.
Deletion of spvA reveals uncoordinated PspvA and PspvR bursting
To investigate the role of SpvA in altering spvA and spvR expression in more detail, time-lapse fluorescence microscopy was performed on the spvR-msfGFP_ΔspvA::mCherry strain to better characterize the coordination between PspvR and PspvA bursting behavior in time. Interestingly, in this strain, PspvR and PspvA bursting appeared not as tightly coordinated and predictable as in the spvR-msfGFP_spvA::mCherry strain, and different bursting characteristics could be discriminated (Figure 5 vs. Figure 6 and Figure S4). While the scenario in which PspvR bursting is quickly followed by PspvA bursting was still observed (Figure 6, purple arrows), in more than half of the bursting cells, PspvA bursting occurred without an observable PspvR burst preceding it (Figure 6 and Figure S4, white arrows). In those cases, PspvR bursting could usually be observed somewhat later in PspvA ON cells and, interestingly, this PspvR burst seemed necessary to sustain the initial PspvA burst and to fully induce both genes over time (Figure 6 and Figure S4, blue and yellow arrows). As mentioned previously, if no SpvR protein is present, no spvA expression could be observed (Figure 3A) and this might imply that our microscopy set-up is not sensitive enough to detect (smaller) spvR bursts possibly occurring below our camera’s detection limit.
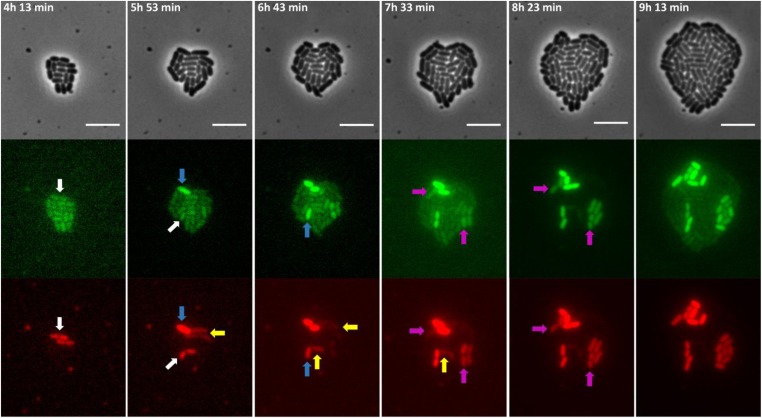
Figure 6.
Images of a time-lapse fluorescence microscopy recording of the growth of a representative microcolony of the S. typhimurium ATCC14028s spvR-msfGFP_ΔspvA::mCherry strain showing heterogeneous and uncoordinated PspvR and PspvA bursting. The strain was grown to stationary phase in ISM and subsequently seeded and monitored on ISM agarose pads at 37° for the time indicated. White arrows indicate cells with an initial PspvA burst but without an observable PspvR burst. Blue arrows indicate cells with a delayed observable PspvR burst leading to a sustained PspvA burst. Yellow arrows indicate cells in which the initial PspvA burst is not sustained in time, likely due to lack of an observable PspvR burst. Purple arrows indicate cells in which PspvA and PspvR bursting is coordinated, reminiscent of the dynamics of the S. typhimurium ATCC14028s spvR-msfGFP_spvA::mCherry strain. The pixel intensities in the different frames are not comparable and the images are merely a qualitative illustration of the bursting process. The image panels represent the phase contrast channel, the GFP fluorescent channel and the mCherry fluorescent channel. Bar, 5 µm.
To further reveal the effect of the SpvA protein on its own expression while trying to minimize potential polar effects in the fluorescent reporter strains, an alternative approach was pursued based on ssrA-mediated tagging of the SpvA and/or msfGFP protein (Andersen et al. 1998). Specific tagging of the C terminus of these proteins with an 11 amino acid ssrA tag (the last three amino acids determine the degradation rate and were LAA in this study) makes them recognizable for intracellular tail-specific proteases, leading to their rapid degradation and thus the possibility to monitor real-time promoter bursting more accurately.
In this regard, a new reporter strain was constructed to assert real-time PspvA bursting. This was achieved through insertion of the msfGFP gene, containing its own ribosome binding site, start codon, and a C-terminal ssrA degradation tag, right after the 3′ end of the spvA gene (i.e., the spvA-msfGFP-LAA strain) (Figure 7A). When monitoring this strain (expressing wild-type SpvA) using time-lapse fluorescence microscopy, short-lived bursting events were observed but only in a limited number of cells (Figure 7A and File M1, Movie 1). Next, we reasoned that if the SpvA protein itself would be actively degraded as well, thus mimicking a functional knockdown of SpvA, then different bursting kinetics should be observed. To test this, a strain was constructed in which, in addition to the msfGFP-LAA part, the ssrA degradation tag was also fused directly to the C terminus of the SpvA protein (i.e., the spvA-LAA-msfGFP-LAA strain), yielding a strain where the SpvA protein is prone to degradation (Figure 7B). Interestingly, when performing time-lapse fluorescence microscopy with this strain and comparing with the spvA-msfGFP-LAA strain, it was observed that bursting events appeared more pronounced, with more cells showing bursting behavior, bursting cells exhibiting higher but more variable burst sizes, and bursting events being sustained for a longer time period (Figure 7B and File M2, Movie 2). The latter was quantified in more detail and it was found that the average burst time of spvA-msfGFP-LAA cells was 56 min, while the average burst time increased significantly to 218 min in spvA-LAA-msfGFP-LAA cells (Figure 7C and Figure S5).
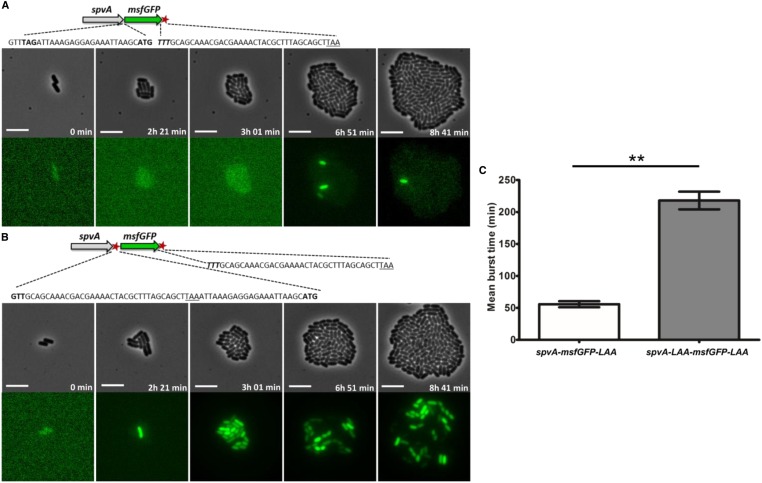
Figure 7.
Images of a time-lapse fluorescence microscopy recording of the growth of a representative microcolony of the (A) S. typhimurium ATCC14028s spvA-msfGFP-LAA and the (B) S. typhimurium ATCC14028s spvA-LAA-msfGFP-LAA strain, showing different PspvA bursting dynamics. (A) Schematic of the S. typhimurium ATCC14028s spvA-msfGFP-LAA strain with the ssrA tag shown as a red asterisk. The junction site between spvA and msfGFP is shown in more detail with the TAG stop codon of SpvA (shown in bold), followed by the ribosome biding site (RBS) and ATG start codon of msfGFP (shown in bold). The 3′ end of msfGFP constitutes of the penultimate TTT codon of msfGFP (shown in bold and in italics), followed by the ssrA degradation tag sequence, and a TAA stop codon (underlined). (B) Schematic of the S. typhimurium ATCC14028s spvA-LAA-msfGFP-LAA strain with the ssrA tags shown as red asterisks. The junction site between spvA and msfGFP is shown in more detail with the penultimate GTT codon of SpvA (shown in bold) followed by the ssrA degradation tag sequence, a TAA stop codon (underlined), and the RBS and ATG start codon of msfGFP (shown in bold). The 3′ end of msfGFP is identical to the one described in A. (C) Mean burst times of the spvA-msfGFP-LAA strain and the spvA-LAA-msfGFP-LAA strain. The burst time was defined as the time in which an individual cell showed an msfGFP signal that exceeded the background levels of OFF cells until this signal faded and returned to background levels again. The mean burst times and SEM for two biological replicates are shown and an unpaired t-test was performed to test for statistical significance (** P < 0.01). The strains were grown to stationary phase in ISM and subsequently seeded and monitored on ISM agarose pads at 37° for the time indicated. The pixel intensities between A and B, and in the different frames, are not comparable and the images are merely a qualitative illustration of the bursting process. The image panels represent the phase contrast channel and the GFP fluorescent channel. Bar, 5 µm.
Together, these results clearly underscore the ability of the SpvA protein to impart regulatory feedback on the spv operon, although further research is required to determine the underlying molecular mechanism and possible contributions of potential polar effects in the engineered reporter strains.
Discussion
The spv operon is an important and conserved virulence trait in most Salmonella enterica strains (with S. typhi, S. paratyphi, and S. sendai being important exceptions) and has been shown to be important for triggering and maintaining systemic disease in mice, and required for causing nontyphoid extraintestinal disease with bacteremia in humans (Rotger and Casadesus 1999; Guiney and Fierer 2011). While the regulation of this operon has been studied extensively over the past several decades, the bimodal expression pattern of these genes has not been reported. Moreover, the single-cell data reported here were derived from strains in which the spv genes are kept in their native genomic context, and this implies that previous work on spv expression, based on population-level assays and often the use of multicopy plasmids, should be interpreted with care and with the concepts of burst frequency (i.e., the number of spv ON cells in the population), burst intensity (i.e., the intensity of spv expression), and burst time (i.e., the duration of being ON) in mind.
Time-lapse fluorescence microscopy analysis revealed that PspvR and PspvA bursting are perfectly coordinated, and that PspvR bursting precedes and is responsible for subsequent PspvA bursting. The observed heterogeneity appears to be supported by the genetic architecture of the spv regulon, which seems to fulfill some key features known to be important for bimodal expression patterns. First of all, the fact that the SpvR protein is an activator of its own expression, thus creating a positive feedback loop, is a hallmark for bimodal expression systems (Ferrell 2002). In addition to this positive feedback loop, the system should display nonlinear kinetics, which in the case of the spv operon is likely accomplished through dimerization of the SpvR protein and subsequent binding on PspvR and PspvA, and cooperative binding of SpvR dimers to two distinct operators site of PspvA (termed the promoter proximal site and the promoter distal site), further amplifying the nonlinear kinetics of this promoter (Grob and Guiney 1996; Sheehan and Dorman 1998). As a result, in cells stochastically expressing a higher number of SpvR molecules, SpvR would be more prone to bind the spvR (establishing the positive feedback loop) and spvA promoter, thereby activating spv expression. So far, it has been shown that the spvR locus contains one dominant promoter with only one SpvR operator site and a putative translational enhancer element within the 5′ spvR coding sequence (Sheehan and Dorman 1998; Robbe-Saule et al. 1999), and future work should address whether spvR bimodality can be attributed solely to these elements or if an (unknown) upstream factor is required as well. In the later context, the observation that the proportion of spv ON cells increases in ISM when compared to LB medium suggests the presence of an extra signal facilitating SpvR in engaging the positive feedback loop, and the existence of such a signal has already been postulated in earlier studies (Robbe-Saule et al. 1997; Wilson et al. 1997; Sheehan and Dorman 1998).
Importantly, when looking at the real-time PspvA bursting events in single cells, short-lived promoter bursts were observed and it seems unlikely that these would be able to sustain a stable spv ON state for multiple generations. The spv genetic circuit thus seems to resemble an excitable system rather than a pure bistable system. In short, excitable systems are known to exhibit an activation pulse after a certain threshold crossing event and are generally driven by positive and negative feedback loops with different time scales (Süel et al. 2006; Eldar and Elowitz 2010; Young et al. 2013; Martins and Locke 2015). Furthermore, the activation pulse causes a transient drift away from the rest state in which the system is temporarily insensitive to further input, but does not fix the system in two stable states, as is the case for bistable systems.
Interestingly, we observed that compromising SpvA function increased the expression directed from PspvA, suggesting that the SpvA protein establishes a negative feedback loop acting on the spvA promoter. The increase in spvA promoter activity is not due to an increased spvR expression and shows that the spvR and spvA promoters, although intimately linked, seem to be uncoupled to a certain extent. This finding seems to contradict other studies where SpvA has been shown to repress spvR expression (Spink et al. 1994; Abe and Kawahara 1995; Wilson and Gulig 1998). In this regard, it should be noted that these studies were performed on the population level (using northern blots, immunoblots, and β-galactosidase assays), and often using multicopy plasmids, which might have skewed these observations. Time-lapse fluorescence microscopy further showed that in an spvA compromised strain, the timing and coordination of PspvR-PspvA bursting was altered, giving rise to a less coordinated, less predictable, and more heterogeneous bursting process. Real-time PspvA bursting in a strain actively degrading the SpvA protein seems to support the latter statement. In this strain bursting cells are abundant and show very pronounced bursting behavior with higher burst sizes and with longer lasting bursts, when compared with the strain having an intact SpvA protein. Although potentiating polar effects in the engineered reporter strains cannot be excluded, the SpvA protein seems to have the ability to control PspvA bursting behavior through a negative feedback loop, counteracting the positive feedback loop SpvR imposes, and so contributes to the excitability of the spv system. It is likely that the SpvR-mediated positive feedback loop and the SpvA-mediated negative feedback loop are indeed working on different time scales, with the positive feedback loop setting in first and leading to expression of the spvABCD operon, after which the SpvA-mediated negative feedback loop kicks in and quickly curbs spvABCD expression. This creates a situation where a subset of cells highly expresses the SpvABCD virulence proteins, and only for a limited amount of time.
It currently remains unclear whether the (potentially bet-hedging) bifurcation in a heterogeneous spv ON and spv OFF population is the actual biological aim of the spv regulon, or rather a side-effect of its genetic wiring. In fact, the SpvR-mediated positive feedback loop could also be viewed as a kind of memory function that is needed to sustain a short activation pulse for a time period lasting longer than the actual activation pulse (as is typically the case for excitable systems which, after a threshold crossing event, are insensitive to further input; Martins and Locke 2015). This implies that the spv operon would always be induced in the niche where all of its inducing agents are present (presumably inside the macrophage) and that the genetic architecture has evolved in a way to provide the right amount of virulence protein for the right amount of time (even when the initial inducing signals are not present anymore). Bifurcation of the population in spv ON and OFF negative cells would then rather be a side-effect of inappropriate or insufficient inducing conditions where spvR activation is occurring on a purely stochastic base.
It is yet unclear how SpvA would exert this negative feedback loop, but considering the fact that the protein does not contain any predicted DNA binding domains and has previously been shown to reside in the outer membrane (although it lacks any typical N-terminal secretion signals) (Valone and Chikami 1991; El-Gedaily et al. 1997), it seems that we can rule out a direct effect on the spvA promoter. More work is needed to further confirm this regulatory feedback, to precisely map the domain(s) responsible for SpvA-mediated repression and to find out how this outer membrane protein exactly causes these changes in spvABCD gene expression. We speculate that other factors are involved and act together with SpvA in tuning the bimodal response of the spv genes, but further research is needed to fully characterize this intricate genetic circuit on the singe-cell level.
The bimodal expression pattern of the spv regulon is another addition to Salmonella’s realm of heterogeneously expressed virulence operons and future work should address how this pattern is integrated into the sophisticated infection strategies of these notorious pathogens.
Acknowledgments
The authors would like to thank William Cenens, Peter Goos, Kristof Vanoirbeek, Catherine Royer, and Marjan van der Woude for helpful suggestions and fruitful discussions; Sander Van Dromme for helping to screen the Tn5-mVenus mutant library; Víctor de Lorenzo for his kind gift of the pBAM1-GFP plasmid; and Johan Paulsson for his kind gift of the pDHL1029 plasmid. This work was supported by doctoral fellowships from the Flemish Agency for Innovation by Science and Technology (IWT-Vlaanderen; to I.P. and S.K.G.) and the Research Foundation - Flanders (FWO-Vlaanderen; to A.C.), and a grant from the Katholieke Universiteit Leuven Research Fund (IDO/10/012). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no conflicts of interest.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6976106.
Communicating editor: J. Lawrence
Literature Cited
- Abe A., Kawahara K., 1995. Transcriptional regulation and promoter sequence of the spvR gene of virulence plasmid pKDSC50 in Salmonella choleraesuis serovar Choleraesuis. FEMS Microbiol. Lett. 129: 225–230. [DOI] [PubMed] [Google Scholar]
- Ackermann M., 2015. A functional perspective on phenotypic heterogeneity in microorganisms. Nat. Rev. Microbiol. 13: 497–508. 10.1038/nrmicro3491 [DOI] [PubMed] [Google Scholar]
- Ackermann M., Stecher B., Freed N. E., Songhet P., Hardt W. D., et al. , 2008. Self-destructive cooperation mediated by phenotypic noise. Nature 454: 987–990. 10.1038/nature07067 [DOI] [PubMed] [Google Scholar]
- Ahmer B. M., Tran M., Heffron F., 1999. The virulence plasmid of Salmonella typhimurium is self-transmissible. J. Bacteriol. 181: 1364–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J. B., Sternberg C., Poulsen L. K., Bjorn S. P., Givskov M., et al. , 1998. New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Appl. Environ. Microbiol. 64: 2240–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoldini M., Vizcarra I. A., Pena-Miller R., Stocker N., Diard M., et al. , 2014. Bistable expression of virulence genes in Salmonella leads to the formation of an antibiotic-tolerant subpopulation. PLoS Biol. 12: e1001928 10.1371/journal.pbio.1001928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumler A. J., Sperandio V., 2016. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535: 85–93. 10.1038/nature18849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. F., Vallance B. A., Coombes B. K., Valdez Y., Coburn B. A., et al. , 2005. Salmonella pathogenicity island 2 is expressed prior to penetrating the intestine. PLoS Pathog. 1: e32 10.1371/journal.ppat.0010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne S. H., Lesnick M. L., Guiney D. G., 2002. Genetic requirements for Salmonella-induced cytopathology in human monocyte-derived macrophages. Infect. Immun. 70: 7126–7135. 10.1128/IAI.70.12.7126-7135.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne S. H., Hasegawa P., Okamoto S., Fierer J., Guiney D. G., 2008. Identification of Salmonella SPI-2 secretion system components required for SpvB-mediated cytotoxicity in macrophages and virulence in mice. FEMS Immunol. Med. Microbiol. 52: 194–201. 10.1111/j.1574-695X.2007.00364.x [DOI] [PubMed] [Google Scholar]
- Bustamante V. H., Martinez L. C., Santana F. J., Knodler L. A., Steele-Mortimer O., et al. , 2008. HilD-mediated transcriptional cross-talk between SPI-1 and SPI-2. Proc. Natl. Acad. Sci. USA 105: 14591–14596. 10.1073/pnas.0801205105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho E. M., Casadesus J., 2002. Conjugal transfer of the virulence plasmid of Salmonella enterica is regulated by the leucine-responsive regulatory protein and DNA adenine methylation. Mol. Microbiol. 44: 1589–1598. 10.1046/j.1365-2958.2002.02981.x [DOI] [PubMed] [Google Scholar]
- Cano D. A., Martinez-Moya M., Pucciarelli M. G., Groisman E. A., Casadesus J., et al. , 2001. Salmonella enterica serovar Typhimurium response involved in attenuation of pathogen intracellular proliferation. Infect. Immun. 69: 6463–6474. 10.1128/IAI.69.10.6463-6474.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenens W., Mebrhatu M. T., Makumi A., Ceyssens P. J., Lavigne R., et al. , 2013. Expression of a novel P22 ORFan gene reveals the phage carrier state in Salmonella Typhimurium. PLoS Genet. 9: e1003269 10.1371/journal.pgen.1003269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherepanov P. P., Wackernagel W., 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158: 9–14. 10.1016/0378-1119(95)00193-A [DOI] [PubMed] [Google Scholar]
- Clark D. J., Maaløe O., 1967. DNA replication and the division cycle in Escherichia coli. J. Mol. Biol. 23: 99–112. 10.1016/S0022-2836(67)80070-6 [DOI] [Google Scholar]
- Coburn B., Grassl G. A., Finlay B. B., 2007. Salmonella, the host and disease: a brief review. Immunol. Cell Biol. 85: 112–118. 10.1038/sj.icb.7100007 [DOI] [PubMed] [Google Scholar]
- Datsenko K. A., Wanner B. L., 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97: 6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, R. W., D. Botstein, and J. R. Roth, 1980 Advanced Bacterial Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Deiwick J., Nikolaus T., Erdogan S., Hensel M., 1999. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol. Microbiol. 31: 1759–1773. 10.1046/j.1365-2958.1999.01312.x [DOI] [PubMed] [Google Scholar]
- Diard M., Garcia V., Maier L., Remus-Emsermann M. N., Regoes R. R., et al. , 2013. Stabilization of cooperative virulence by the expression of an avirulent phenotype. Nature 494: 353–356. 10.1038/nature11913 [DOI] [PubMed] [Google Scholar]
- Eldar A., Elowitz M. B., 2010. Functional roles for noise in genetic circuits. Nature 467: 167–173. 10.1038/nature09326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gedaily A., Paesold G., Krause M., 1997. Expression profile and subcellular location of the plasmid-encoded virulence (Spv) proteins in wild-type Salmonella dublin. Infect. Immun. 65: 3406–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrega A., Vila J., 2013. Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin. Microbiol. Rev. 26: 308–341. 10.1128/CMR.00066-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F. C., Libby S. J., Buchmeier N. A., Loewen P. C., Switala J., et al. , 1992. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc. Natl. Acad. Sci. USA 89: 11978–11982. 10.1073/pnas.89.24.11978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass E., Groisman E. A., 2009. Control of Salmonella pathogenicity island-2 gene expression. Curr. Opin. Microbiol. 12: 199–204. 10.1016/j.mib.2009.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell Jr., J. E., 2002. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14: 140–148. 10.1016/S0955-0674(02)00314-9 [DOI] [PubMed] [Google Scholar]
- Figueira R., Holden D. W., 2012. Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology 158: 1147–1161. 10.1099/mic.0.058115-0 [DOI] [PubMed] [Google Scholar]
- Gotoh H., Okada N., Kim Y. G., Shiraishi K., Hirami N., et al. , 2003. Extracellular secretion of the virulence plasmid-encoded ADP-ribosyltransferase SpvB in Salmonella. Microb. Pathog. 34: 227–238. 10.1016/S0882-4010(03)00034-2 [DOI] [PubMed] [Google Scholar]
- Grabe G. J., Zhang Y., Przydacz M., Rolhion N., Yang Y., et al. , 2016. The Salmonella effector SpvD is a cysteine hydrolase with a serovar-specific polymorphism influencing catalytic activity, suppression of immune responses, and bacterial virulence. J. Biol. Chem. 291: 25853–25863. 10.1074/jbc.M116.752782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob P., Guiney D. G., 1996. In vitro binding of the Salmonella Dublin virulence plasmid regulatory protein SpvR to the promoter regions of spvA and spvR. J. Bacteriol. 178: 1813–1820. 10.1128/jb.178.7.1813-1820.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloteau L. A., Wallis T. S., Gautier A. V., MacIntyre S., Platt D. J., et al. , 1996. The Salmonella virulence plasmid enhances Salmonella-induced lysis of macrophages and influences inflammatory responses. Infect. Immun. 64: 3385–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney D. G., Fierer J., 2011. The role of the spv genes in Salmonella pathogenesis. Front. Microbiol. 2: 129 10.3389/fmicb.2011.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Doyle T. J., 1993. The Salmonella Typhimurium virulence plasmid increases the growth rate of salmonellae in mice. Infect. Immun. 61: 504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneda T., Ishii Y., Shimizu H., Ohshima K., Iida N., et al. , 2012. Salmonella type III effector SpvC, a phosphothreonine lyase, contributes to reduction in inflammatory response during intestinal phase of infection. Cell. Microbiol. 14: 485–499. 10.1111/j.1462-5822.2011.01733.x [DOI] [PubMed] [Google Scholar]
- Hautefort I., Proenca M. J., Hinton J. C., 2003. Single-copy green fluorescent protein gene fusions allow accurate measurement of Salmonella gene expression in vitro and during infection of mammalian cells. Appl. Environ. Microbiol. 69: 7480–7491. 10.1128/AEM.69.12.7480-7491.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley V. L., Payne S. M., 1990. Differential protein expression by Shigella flexneri in intracellular and extracellular environments. Proc. Natl. Acad. Sci. USA 87: 4179–4183. 10.1073/pnas.87.11.4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébrard M., Kroger C., Sivasankaran S. K., Handler K., Hinton J. C., 2011. The challenge of relating gene expression to the virulence of Salmonella enterica serovar Typhimurium. Curr. Opin. Biotechnol. 22: 200–210. 10.1016/j.copbio.2011.02.007 [DOI] [PubMed] [Google Scholar]
- Hensel M., Shea J. E., Gleeson C., Jones M. D., Dalton E., et al. , 1995. Simultaneous identification of bacterial virulence genes by negative selection. Science 269: 400–403. 10.1126/science.7618105 [DOI] [PubMed] [Google Scholar]
- Jarvik T., Smillie C., Groisman E. A., Ochman H., 2010. Short-term signatures of evolutionary change in the Salmonella enterica serovar Typhimurium 14028 genome. J. Bacteriol. 192: 560–567. 10.1128/JB.01233-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowarz L., Coynault C., Robbe-Saule V., Norel F., 1994. The Salmonella Typhimurium katF (rpoS) gene: cloning, nucleotide sequence, and regulation of spvR and spvABCD virulence plasmid genes. J. Bacteriol. 176: 6852–6860. 10.1128/jb.176.22.6852-6860.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y. M., Ricke S. C., 2000. Efficient amplification of multiple transposon-flanking sequences. J. Microbiol. Methods 41: 195–199. 10.1016/S0167-7012(00)00159-7 [DOI] [PubMed] [Google Scholar]
- Laughlin R. C., Knodler L. A., Barhoumi R., Payne H. R., Wu J., et al. , 2014. Spatial segregation of virulence gene expression during acute enteric infection with Salmonella enterica serovar Typhimurium. MBio 5: e00946–13 10.1128/mBio.00946-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. A., Jones B. D., Falkow S., 1992. Identification of a Salmonella Typhimurium invasion locus by selection for hyperinvasive mutants. Proc. Natl. Acad. Sci. USA 89: 1847–1851. 10.1073/pnas.89.5.1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby S. J., Adams L. G., Ficht T. A., Allen C., Whitford H. A., et al. , 1997. The spv genes on the Salmonella Dublin virulence plasmid are required for severe enteritis and systemic infection in the natural host. Infect. Immun. 65: 1786–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby S. J., Lesnick M., Hasegawa P., Weidenhammer E., Guiney D. G., 2000. The Salmonella virulence plasmid spv genes are required for cytopathology in human monocyte-derived macrophages. Cell. Microbiol. 2: 49–58. 10.1046/j.1462-5822.2000.00030.x [DOI] [PubMed] [Google Scholar]
- Lutz R., Bujard H., 1997. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1–I2 regulatory elements. Nucleic Acids Res. 25: 1203–1210. 10.1093/nar/25.6.1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie K. D., Wang Y., Shivak D. J., Wong C. S., Hoffman L. J., et al. , 2015. Bistable expression of CsgD in Salmonella enterica serovar Typhimurium connects virulence to persistence. Infect. Immun. 83: 2312–2326. 10.1128/IAI.00137-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan M. W., Lucchini S., Danino V., Croinin T. O., Hinton J. C., et al. , 2006. The integration host factor (IHF) integrates stationary-phase and virulence gene expression in Salmonella enterica serovar Typhimurium. Mol. Microbiol. 59: 1831–1847. 10.1111/j.1365-2958.2006.05062.x [DOI] [PubMed] [Google Scholar]
- Marshall D. G., Sheehan B. J., Dorman C. J., 1999. A role for the leucine-responsive regulatory protein and integration host factor in the regulation of the Salmonella plasmid virulence (spv) locus in Salmonella Typhimurium. Mol. Microbiol. 34: 134–145. 10.1046/j.1365-2958.1999.01587.x [DOI] [PubMed] [Google Scholar]
- Martínez-García E., Calles B., Arevalo-Rodriguez M., de Lorenzo V., 2011. pBAM1: an all-synthetic genetic tool for analysis and construction of complex bacterial phenotypes. BMC Microbiol. 11: 38 10.1186/1471-2180-11-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins B. M., Locke J. C., 2015. Microbial individuality: how single-cell heterogeneity enables population level strategies. Curr. Opin. Microbiol. 24: 104–112. 10.1016/j.mib.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Mazurkiewicz P., Thomas J., Thompson J. A., Liu M., Arbibe L., et al. , 2008. SpvC is a Salmonella effector with phosphothreonine lyase activity on host mitogen-activated protein kinases. Mol. Microbiol. 67: 1371–1383. 10.1111/j.1365-2958.2008.06134.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston J. R., Herrera-Leon S., Wertheim B. C., Doyle J., Fields P. I., et al. , 2008. Molecular phylogeny of the salmonellae: relationships among Salmonella species and subspecies determined from four housekeeping genes and evidence of lateral gene transfer events. J. Bacteriol. 190: 7060–7067. 10.1128/JB.01552-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. M., Bajaj V., Lee C. A., 1995. A 40 kb chromosomal fragment encoding Salmonella Typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol. Microbiol. 15: 749–759. 10.1111/j.1365-2958.1995.tb02382.x [DOI] [PubMed] [Google Scholar]
- Morgan E., 2007. Salmonella Pathogenicity Islands. Horizon bioscience, Wymondham, United Kingdom. [Google Scholar]
- Norel F., Robbe-Saule V., Popoff M. Y., Coynault C., 1992. The putative sigma factor KatF (RpoS) is required for the transcription of the Salmonella Typhimurium virulence gene spvB in Escherichia coli. FEMS Microbiol. Lett. 78: 271–276. 10.1111/j.1574-6968.1992.tb05580.x [DOI] [PubMed] [Google Scholar]
- O’Byrne C. P., Dorman C. J., 1994a The spv virulence operon of Salmonella Typhimurium LT2 is regulated negatively by the cyclic AMP (cAMP)-cAMP receptor protein system. J. Bacteriol. 176: 905–912. 10.1128/jb.176.3.905-912.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Byrne C. P., Dorman C. J., 1994b Transcription of the Salmonella Typhimurium spv virulence locus is regulated negatively by the nucleoid-associated protein H-NS. FEMS Microbiol. Lett. 121: 99–105. 10.1111/j.1574-6968.1994.tb07082.x [DOI] [PubMed] [Google Scholar]
- Ochman H., Soncini F. C., Solomon F., Groisman E. A., 1996. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc. Natl. Acad. Sci. USA 93: 7800–7804. 10.1073/pnas.93.15.7800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto H., Tezcan-Merdol D., Girisch R., Haag F., Rhen M., et al. , 2000. The spvB gene-product of the Salmonella enterica virulence plasmid is a mono(ADP-ribosyl)transferase. Mol. Microbiol. 37: 1106–1115. 10.1046/j.1365-2958.2000.02064.x [DOI] [PubMed] [Google Scholar]
- Phoebe Lostroh C., Lee C. A., 2001. The Salmonella pathogenicity island-1 type III secretion system. Microbes Infect. 3: 1281–1291. 10.1016/S1286-4579(01)01488-5 [DOI] [PubMed] [Google Scholar]
- Rhen M., Dorman C. J., 2005. Hierarchical gene regulators adapt Salmonella enterica to its host milieus. Int. J. Med. Microbiol. 294: 487–502. 10.1016/j.ijmm.2004.11.004 [DOI] [PubMed] [Google Scholar]
- Robbe-Saule V., Schaeffer F., Kowarz L., Norel F., 1997. Relationships between H-NS, sigma S, SpvR and growth phase in the control of spvR, the regulatory gene of the Salmonella plasmid virulence operon. Mol. Gen. Genet. 256: 333–347. 10.1007/s004380050577 [DOI] [PubMed] [Google Scholar]
- Robbe-Saule V., Kowarz L., Norel F., 1999. A coding segment of the virulence regulatory gene spvR enhances expression of spvR-lacZ and spvR-gfp translational fusions in Salmonella Typhimurium. Mol. Gen. Genet. 261: 472–479. 10.1007/s004380050990 [DOI] [PubMed] [Google Scholar]
- Rolhion N., Furniss R. C., Grabe G., Ryan A., Liu M., et al. , 2016. Inhibition of nuclear transport of NF-ĸB p65 by the Salmonella type III secretion system effector SpvD. PLoS Pathog. 12: e1005653 10.1371/journal.ppat.1005653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotger R., Casadesus J., 1999. The virulence plasmids of Salmonella. Int. Microbiol. 2: 177–184. [PubMed] [Google Scholar]
- Roudier C., Fierer J., Guiney D. G., 1992. Characterization of translation termination mutations in the spv operon of the Salmonella virulence plasmid pSDL2. J. Bacteriol. 174: 6418–6423. 10.1128/jb.174.20.6418-6423.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini S., Ellermeier J. R., Slauch J. M., Rao C. V., 2010. The role of coupled positive feedback in the expression of the SPI1 type three secretion system in Salmonella. PLoS Pathog. 6: e1001025 10.1371/journal.ppat.1001025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Russell R. W., 2001. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Schmieger H., 1972. Phage P22-mutants with increased or decreased transduction abilities. Mol. Gen. Genet. 119: 75–88. 10.1007/BF00270447 [DOI] [PubMed] [Google Scholar]
- Sheehan B. J., Dorman C. J., 1998. In vivo analysis of the interactions of the LysR-like regulator SpvR with the operator sequences of the spvA and spvR virulence genes of Salmonella Typhimurium. Mol. Microbiol. 30: 91–105. 10.1046/j.1365-2958.1998.01041.x [DOI] [PubMed] [Google Scholar]
- Sliusarenko O., Heinritz J., Emonet T., Jacobs-Wagner C., 2011. High-throughput, subpixel precision analysis of bacterial morphogenesis and intracellular spatio-temporal dynamics. Mol. Microbiol. 80: 612–627. 10.1111/j.1365-2958.2011.07579.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink J. M., Pullinger G. D., Wood M. W., Lax A. J., 1994. Regulation of spvR, the positive regulatory gene of Salmonella plasmid virulence genes. FEMS Microbiol. Lett. 116: 113–121. 10.1111/j.1574-6968.1994.tb06684.x [DOI] [PubMed] [Google Scholar]
- Sterzenbach T., Crawford R. W., Winter S. E., Baümler A. J., 2013. Salmonella Virulence Mechanisms and their Genetic Basis, Chap 5. CAB International, Wallingford, United Kingdom: 10.1079/9781845939021.0080 [DOI] [Google Scholar]
- Stewart M. K., Cookson B. T., 2012. Non-genetic diversity shapes infectious capacity and host resistance. Trends Microbiol. 20: 461–466. 10.1016/j.tim.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A., Heinemann M., Arnoldini M., Benecke A., Ackermann M., et al. , 2011. The cost of virulence: retarded growth of Salmonella Typhimurium cells expressing type III secretion system 1. PLoS Pathog. 7: e1002143 10.1371/journal.ppat.1002143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Süel G. M., Garcia-Ojalvo J., Liberman L. M., Elowitz M. B., 2006. An excitable gene regulatory circuit induces transient cellular differentiation. Nature 440: 545–550. 10.1038/nature04588 [DOI] [PubMed] [Google Scholar]
- Tezcan-Merdol D., Engstrand L., Rhen M., 2005. Salmonella enterica SpvB-mediated ADP-ribosylation as an activator for host cell actin degradation. Int. J. Med. Microbiol. 295: 201–212. 10.1016/j.ijmm.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Valone S. E., Chikami G. K., 1991. Characterization of three proteins expressed from the virulence region of plasmid pSDL2 in Salmonella Dublin. Infect. Immun. 59: 3511–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmes-Riesenberg M. R., Foster J. W., Curtiss R., III, 1997. An altered rpoS allele contributes to the avirulence of Salmonella Typhimurium LT2. Infect. Immun. 65: 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. A., Gulig P. A., 1998. Regulation of the spvR gene of the Salmonella Typhimurium virulence plasmid during exponential-phase growth in intracellular salts medium and at stationary phase in L broth. Microbiology 144: 1823–1833. 10.1099/00221287-144-7-1823 [DOI] [PubMed] [Google Scholar]
- Wilson J. A., Doyle T. J., Gulig P. A., 1997. Exponential-phase expression of spvA of the Salmonella Typhimurium virulence plasmid: induction in intracellular salts medium and intracellularly in mice and cultured mammalian cells. Microbiology 143: 3827–3839. 10.1099/00221287-143-12-3827 [DOI] [PubMed] [Google Scholar]
- Wilson K., 2001. Preparation of Genomic DNA From Bacteria in Current Protocols in Molecular Biology. John Wiley & Sons, Inc., New York. [DOI] [PubMed] [Google Scholar]
- Worley M. J., Ching K. H., Heffron F., 2000. Salmonella SsrB activates a global regulon of horizontally acquired genes. Mol. Microbiol. 36: 749–761. 10.1046/j.1365-2958.2000.01902.x [DOI] [PubMed] [Google Scholar]
- Young J. W., Locke J. C., Elowitz M. B., 2013. Rate of environmental change determines stress response specificity. Proc. Natl. Acad. Sci. USA 110: 4140–4145. 10.1073/pnas.1213060110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. File S1 contains supplemental figures and tables. File M1 contains Movie 1. File M2 contains Movie 2. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6976106.