Abstract
Background
Anticoagulation is the mainstay treatment for cerebral venous thrombosis (CVT). A subset of patients might deteriorate despite anticoagulation, and in such cases, endovascular therapy is recommended.
Methods
A retrospective review was performed on subjects with CVT from January 2005 to October 2016. The primary outcome was clinical deterioration. Bivariate analysis, multiple logistic regression modeling, and linear discriminant analysis were used to determine a predictive model for deterioration; the results from these models were used to construct a CVT score in order to measure the individual likelihood of deterioration.
Results
We identified 147 subjects with CVT. The majority were treated with anticoagulation (n = 109, 74.15%); 38 (25.85%) were found to have deterioration, 12 (8.16%) of whom underwent endovascular intervention. The most important risk factors of deterioration, per bivariate analysis, included decreased level of consciousness (odds ratio [OR] = 5.76; 95% confidence interval [CI] 2.59–12.77) and papilledema (OR = 4.52; 95% CI 1.55–13.18). The final multivariable model also included CVT location score (number of sinuses involved), oral contraceptive pill use, sodium level, platelet count, and seizure activity on presentation. This model had a predictive ability to identify deterioration of 83.2%, with a sensitivity of 71.4% and a specificity of 76.2%. Patients with a CVT score of ≥5 have at least 50% chance of deterioration.
Conclusions
Decreased mental status, seizure activity, papilledema, number of involved sinuses, as well as sodium level and platelet count are the most important factors in predicting deterioration after CVT. This group may represent a subset of patients in whom early endovascular therapy may be considered.
Keywords: Anticoagulants, Cerebral venous thrombosis, Dural sinus thrombosis, Mechanical thrombectomy, Thrombolysis
Introduction
Cerebral venous thrombosis (CVT) is an unusual form of stroke, with extremely heterogeneous presentation due to significant variability in both the venous architecture and the extent of thrombosis, which can range from headache and elevated intracranial pressure to venous infarction and hemorrhage [1]. It is estimated to constitute approximately 0.5–1% of strokes and usually affects the younger population compared to arterial stroke [2]. The risk factors for CVT can be transient or permanent, including central nervous system or systemic infections, head trauma, drugs with prothrombotic action, genetic conditions, and malignancy [3]. CVT is more common in females taking oral contraceptive pills (OCPs) and in pregnancy [4]. Approximately 13% of patients in the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) had poor neurological outcomes, in terms of disability and death, even after treatment with anticoagulation. In the VENOPORT study [5], cerebral edema with or without seizures and sudden cardiopulmonary arrest were the major causes of death.
Endovascular therapy is currently used in a subset of patients who clinically or radiographically deteriorate despite anticoagulation, and it is considered an effective salvage option with a reasonable safety profile, but not supported by high-level evidence [4, 6, 7]. It is intuitive to hypothesize that earlier intervention is more likely to be technically efficacious since there is less fibrin cross-linking in the clot. At the delayed interval, scarring and mechanical stenosis due to inflammatory changes may make the thrombus unamenable to simple thrombolysis or thrombectomy and may require adjunctive procedures such as sinus stenting [8, 9]. If this high-risk group can be targeted for early intervention, this intervention may be both more effective and avoid permanent neurological injury.
We sought to identify patients who not only had a poor outcome (as has been previously well published [1, 6]), but those patients with CVT who went on to have clinical deterioration. We then wanted to determine whether there were identifiable features at the time of presentation that could predict a high-risk group in which early endovascular therapy might be considered.
Methods
This study was conducted at University of New Mexico Hospitals. A retrospective chart review was performed at our institution to identify subjects with CVT from January 2005 to October 2016. Patients were identified using International Classification of Diseases, 9th revision codes. The diagnosis of CVT was established by computed tomography venography, magnetic resonance venography, or catheter-based angiography. We collected extensive baseline clinical, laboratory, and radiographic data on these subjects from the time of initial admission. Clinical risk factors included altered mental status, Glasgow Coma Scale (GCS) score, seizure activity on presentation, papilledema, and OCP use. Laboratory factors included admission complete blood count, comprehensive metabolic panel, liver function tests, lactate, urine drug screen, and hypercoagulable panel. Radiographic data included location of thrombosis, number of sinus segments involved, ischemic stroke, and presence of hemorrhage. From these data, composite risk factors were also constructed to group similar findings. The first composite risk factor was decreased level of consciousness (LOC) (GCS score < 15, diagnosis of altered mental status at admission, or documented encephalopathy). To standardize the location of thrombus, one point was assigned to each sinus, including superior sagittal sinus, transverse sinus, sigmoid sinus, intracranial jugular vein, straight sinus, deep venous system, or cortical vein. Each location was analyzed both independently and as a composite score (location score).
We defined our primary outcome as a binary composite measure of delayed deterioration which was constructed based on symptomatic worsening due to CVT at follow-up visits, death, repeat emergency room visits after discharge for CVT-related complaints, or need for endovascular intervention. Symptomatic worsening due to CVT included new neurological deficits such as focal weakness or cranial nerve abnormalities, worsening mental status or decrement in GCS score, new or worsening bleeding on neuroimages like midline shift, worsening edema, or mass effect.
We performed bivariate analysis using logistic regression for each independent variable to identify potential risk factors for deterioration. We then performed multiple logistic regression modeling and linear discriminant analysis to determine a predictive model for deterioration using the best subset method which gives maximal accuracy while avoiding overfitting [10, 11]. We used the area under the receiver operating characteristic (ROC) curve as a measure for classification accuracy. The independent variables from this predictive model were used to construct a risk stratification scale, named the CVT score, to give a rough estimate of the individual likelihood of deterioration. Sensitivity and specificity analysis for the predictive model was done. For all significant continuous predictors for deterioration, we identified optimal thresholds by minimizing the Euclidean distance of sensitivity and specificity from the unitary point (1, 1) [12]. Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
General Characteristics
We identified 147 patients who met the inclusion criteria. Most patients were aged > 18 years (76.87%) and females (55.24%). Headache was the most common symptom (n = 98, 66.67%), followed by nausea or vomiting (n = 58, 39.46%), focal weakness (n = 33, 22.45%), seizure (n = 21, 14.29%), and papilledema (n = 16, 10.88%). The mean time to presentation was 8.9 days. The majority of patients were treated with anticoagulation (n = 109, 74.15%). Urine toxicology screen was positive in 14 patients with benzodiazepines and/or amphetamine, though this was not routinely measured on all patients. The patient characteristics are summarized in Table 1.
Table 1.
Patients characteristics
| Characteristics | No deterioration n/N (%) | Deterioration n/N (%) | Total n/N (%) | |
|---|---|---|---|---|
| Age | ||||
| ≤18 years | 24/109 (22.02) | 10/38 (26.32) | 34/147 (23.13) | |
| >18 years | 85/109 (77.98) | 28/38 (73.68) | 113/147 (76.87) | |
| Sex | ||||
| Female | 60/107 (56.07) | 19/36 (52.78) | 79/143 (55.24) | |
| Male | 47/107 (43.93) | 17/36 (47.22) | 64/143 (44.76) | |
| Glasgow Coma Scale score1 | ||||
| 15 | 87/105 (82.86) | 18/36 (50.00) | 105/141 (74.47) | |
| 3–14 | 18/105 (17.14) | 18/36 (50.00) | 36/141 (25.53) | |
| Glasgow Coma Scale score | ||||
| >8 | 98/105 (93.33) | 30/36 (83.33) | 128/141 (90.78) | |
| ≤8 | 7/105 (6.67) | 6/36 (16.67) | 13/141 (9.22) | |
| Hemorrhage on initial head computed tomography | ||||
| Yes | 36/109 (33.03) | 18/38 (47.37) | 54/147 (36.73) | |
| No | 73/109 (66.97) | 20/38 (52.63) | 93/147 (63.27) | |
| Ischemic infarction on initial imaging | ||||
| Yes | 15/107 (14.02) | 10/38 (26.32) | 25/145 (17.24) | |
| No | 92/107 (85.98) | 28/38 (73.68) | 120/145 (82.76) | |
| Seizure activity1 | ||||
| Yes | 11/109 (10.09) | 10/38 (26.32) | 21/147 (14.29) | |
| No | 98/109 (89.91) | 28/38 (73.68) | 126/147 (85.71) | |
| Papilledema1 | ||||
| Yes | 7/109 (6.42) | 9/38 (23.68) | 16/147 (10.88) | |
| No | 102/109 (93.58) | 29/38 (76.32) | 131/147 (89.12) | |
| Location score (number of sinuses involved)1 | ||||
| >3 | 14/108 (12.96) | 13/37 (35.14) | 27/145 (18.62) | |
| ≤3 | 94/108 (87.04) | 24/37 (64.86) | 118/145 (81.38) | |
| Oral contraceptive pill use | ||||
| Yes | 11/109 (10.09) | 1/38 (2.63) | 12/147 (8.16) | |
| No | 98/109 (89.91) | 37/38 (97.37) | 135/147 (91.84) | |
| Mean age, years | 35.65 | 35.32 | 35.56 | |
| Mean sodium level, mEq/L | 140.28 | 138.71 | 139.88 | |
| Mean platelet count, ×109/L | 257.75 | 226.17 | 249.51 | |
Statistically significant according to the χ2 test for independence at the 5% significance level.
Hypercoagulable labs were tested in 81/147 patients, and despite many patients undergoing early testing, positive results were very uncommon. Three out of 25 patients tested had elevated plasma homocystine. Twenty-one out of 81 patients tested had positive lupus-like inhibitor. Factor 5 Leiden mutation was found in 3 out of 75 patients tested. Because of the relatively smaller numbers, these factors were ultimately not considered in the final model.
The location of CVT was as follows: transverse sinus involvement was most common in 100 patients (68.03%), sigmoid sinus in 94 (63.95%), superior sagittal sinus in 53 (36.05%), bilateral transverse sinus in 18 (12.24%), bilateral sigmoid sinus in 4 (2.72%), internal jugular vein in 31 (21.08%), straight sinus in 17 (11.56%), deep venous system in 12 (8.16%), and cortical vein in 13 (8.84%).
Out of 147 patients, 38 (25.85%) were found to have deterioration, 12 (8.16%) of whom required endovascular intervention. The other cases of deterioration were due to clinical worsening [12], death [10], and repeat emergency room visits [13]. Out of 35 patients who had no anticoagulation, 10 deteriorated, but this was not significant (odds ratio [OR] = 1.215; 95% confidence interval [CI] 0.518–2.850). The association between anticoagulation and deterioration is presented in Table 2. Reasons for no anticoagulation included venous hemorrhage on initial images (3 patients), trauma (11 patients), concurrent central nervous system infection (2 patients), gastrointestinal bleeding (2 patients), arteriovenous fistula causing sinus thrombosis (1 patient), as well as underlying malignancy and decision for hospice care and no further treatment (4 patients). Outcomes for the 12 patients with clinical worsening included 6 patients with mild deficits (Rankin scores 1 and 2) and 4 patients with moderate to severe deficits (Rankin scores 3 and 4). As regards mortality, 6 patients were attributed to CVT. One patient died from acute myeloid leukemia, the other patient died due to hemorrhagic shock form gastrointestinal bleeding. The median time of hospitalization was 9.76 days. Fifteen patients developed ≥1 seizure during hospitalization.
Table 2.
Association between anticoagulation and deterioration
| Anticoagulation | Deterioration | ||
|---|---|---|---|
| no | yes | total | |
| No | |||
| Frequency | 25 | 10 | 35 |
| Row percentage | 71.43 | 28.57 | 24.31 |
| Column percentage | 23.36 | 27.03 | |
| Yes | |||
| Frequency | 82 | 27 | 109 |
| Row percentage | 75.23 | 24.77 | 75.69 |
| Column percentage | 76.64 | 72.97 | |
| Total | |||
| Frequency | 107 | 37 | 144 |
| Percentage | 74.31 | 25.69 | 100.00 |
Anticoagulation by deterioration: p = 0.6544 (statistically not significant according to the χ2 test for independence at the 5% significance level).
Patients Treated with Endovascular Therapy
We treated 12 patients with endovascular therapy. Most of them (9/12) were treated with anticoagulation on the day of the diagnosis or on the next day. Indications for endovascular intervention were primarily uncontrolled elevated intracranial pressure with papilledema as well as large venous infarct with hemorrhagic transformation and extensive thrombosis with poor clinical exam. Three patients had venous infarcts and hemorrhagic transformation before endovascular treatment. One patient had thrombosis of the deep cerebral venous system and ultimately died despite initial anticoagulation and chemical thrombolysis with tissue plasminogen activator due to intraventricular hemorrhage and hydrocephalus.
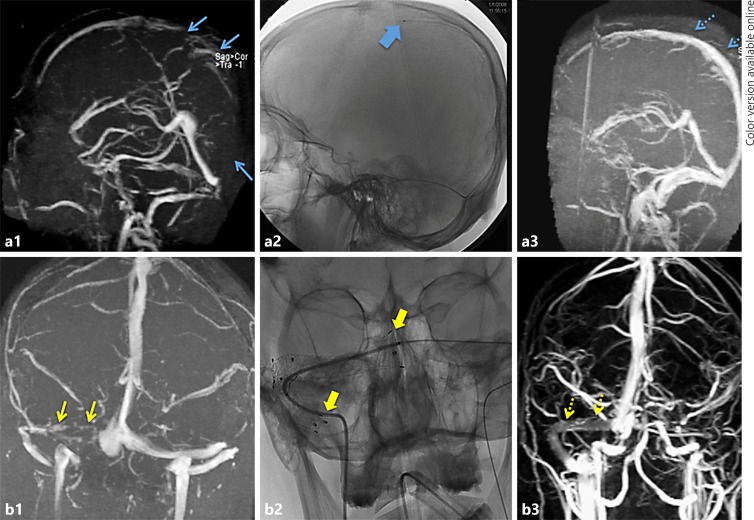
Four patients out of 12 had mechanical thrombectomy (aspiration, stent retriever thrombectomy, or balloon thrombectomy), 5 patients had intra-arterial thrombolysis, 1 patient had thrombolysis with thrombectomy, 1 patient underwent thrombectomy with stenting, and 1 patient had stenting primarily. Seven patients had complete or near complete recanalization, 2 patients had partial recanalization, and 2 patients had no recanalization. Eight patients were discharged home (recovered or with minimal residual symptoms with Rankin scores 0 or 1), 2 patients had moderate or severe deficits (Rankin scores 3 and 4), and 2 patients died. One death was attributed to trauma and coma prior to intervention, the other was due to extensive deep sinus thrombosis (see Fig. 1 for representative cases of early and late intervention).
Fig. 1.
Two patients who underwent endovascular therapy. a A patient with superior sagittal sinus thrombosis on magnetic resonance venography (a1, blue arrows) which extended through the right transverse, sigmoid, and jugular. Due to the extensive thrombosis, the patient was taken for early thrombolysis (a2, microcatheter in the superior sagittal sinus, wide blue arrow.) Tissue plasminogen activator was infused for 2 days with daily angiograms to assess progress. Magnetic resonance venography 3 days later showed near complete recanalization of the entire superior sagittal, transverse, sigmoid, and jugular (a3, dotted blue arrows). b A patient with right transverse and sigmoid thrombosis (b1, yellow arrows.) Despite anticoagulation, the patient developed severe papilledema and bilateral sixth nerve palsies 4 weeks later. Attempted thrombolysis and balloon thrombectomy had been unsuccessful and a transverse sinus stent was placed (b2, wide yellow arrows). The patient's papilledema and sixth nerve palsies reversed rapidly, and 1-year follow-up magnetic resonance venography showed wide patency through the stents (b3, dotted yellow arrows).
Risk Factors for Deterioration
The risk factors for deterioration on bivariate analysis are summarized in Table 3. Significant factors included decreased LOC (OR = 5.76; 95% CI 2.60–12.77), initial GCS score (OR = 4.83; 95% CI 2.11–11.05), papilledema (OR = 4.52; 95% CI 1.55–13.19), seizure activity on presentation (OR = 3.18; 95% CI 1.23–8.26), bilateral transverse sinus thrombosis (OR = 3.45; 95% CI 1.25–9.48), altered mental status (OR = 3.96; 95% CI 1.80–8.71), and location score (OR = 1.35; 95% CI 1.02–1.76).
Table 3.
Bivariate analysis (provides unadjusted ORs)
| Presenting symptoms and risk factors | OR (95% CI) | p value |
|---|---|---|
| Decreased level of consciousness | 5.76 (2.60–12.77) | <0.0001* |
| Initial Glasgow Coma Scale score | 4.83 (2.11–11.05) | 0.0002* |
| Papilledema | 4.52 (1.55–13.19) | 0.006* |
| Altered mental status | 3.96 (1.80–8.71) | 0.001* |
| Bilateral transverse sinus thrombosis | 3.45 (1.25–9.49) | 0.02* |
| Seizure activity | 3.18 (1.23–8.26) | 0.02* |
| Unilateral internal jugular thrombosis | 2.19 (0.94–5.09) | 0.07 |
| Ischemic infarct on neuroimages | 2.19 (0.89–5.42) | 0.09 |
| Bleeding on initial neuroimages | 1.83 (0.86–3.90) | 0.12 |
| Location score (number of sinuses involved) | 1.35 (1.03–1.76) | 0.03* |
| Nausea/vomiting | 1.34 (0.64–2.84) | 0.44 |
| Focal weakness | 1.34 (0.57–3.14) | 0.51 |
| Cortical vein thrombosis | 1.31 (0.38–4.52) | 0.68 |
| Superior sagittal sinus thrombosis | 1.26 (0.58–2.70) | 0.56 |
| Elevated total bilirubin | 1.12 (0.87–1.44) | 0.38 |
| Unilateral sagittal sinus thrombosis | 1.12 (0.51–2.42) | 0.78 |
| Elevated potassium | 1.02 (0.95–1.10) | 0.60 |
| Elevated creatinine | 1.01 (0.62–1.64) | 0.98 |
| Protein S deficiency | 1.00 (1.00–1.02) | 0.84 |
| Platelet count | 1.00 (0.99–1.00) | 0.13 |
| Elevated blood urea nitrogen | 1.00 (0.97–1.02) | 0.74 |
| Elevated lactate | 0.99 (0.77–1.28) | 0.95 |
| Deep venous sinus thrombosis | 0.95 (0.24–3.72) | 0.94 |
| Sodium level | 0.92 (0.82–1.03) | 0.16 |
| Abnormal hypercoagulable panel | 0.89 (0.35–2.24) | 0.80 |
| Sex | 0.88 (0.41–1.87) | 0.73 |
| Straight sinus thrombosis | 0.87 (0.27–2.85) | 0.82 |
| Headache | 0.81 (0.38–1.76) | 0.59 |
| Decreased visual acuity | 0.77 (0.26–2.23) | 0.62 |
| Unilateral transverse sinus thrombosis | 0.74 (0.34–1.62) | 0.46 |
| Cranial sixth nerve palsy | 0.69 (0.12–3.90) | 0.67 |
| Albumin | 0.48 (0.23–1.01) | 0.05 |
| Oral contraceptive pill use | 0.24 (0.03–1.93) | 0.18 |
CI, confidence interval; OR, odds ratio.
Statistically significant (p < 0.05).
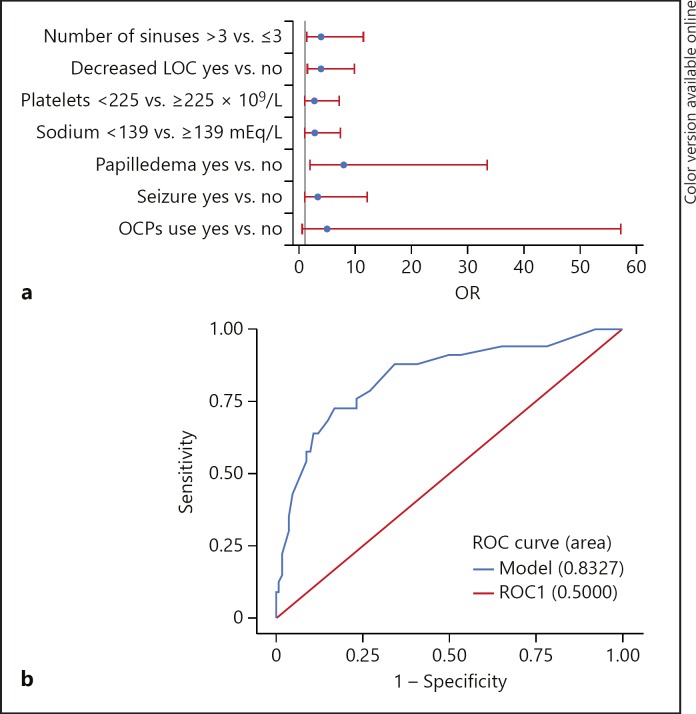
We tested multiple predictive models of deterioration using multivariable logistic regression. For practical use of these models, we calculated the optimum threshold for the continuous variables and then converted them to binary for the multivariable analysis. The selected final model (Fig. 2) included the following seven predictors: number of sinuses > 3 (OR = 3.80; 95% CI 1.27–11.39), decreased LOC (OR = 3.73; 95% CI 1.43–9.76), platelet count < 225 × 109/L (OR = 2.61; 95% CI 0.96–7.09), sodium level < 139 mEq/L (OR = 2.59; 95% CI 0.93–7.19), papilledema (OR = 7.88; 95% CI 1.87–33.33), seizure activity (OR = 3.16; 95% CI 0.83–11.99), and no OCP use (OR = 4.90; 95% CI 0.42–57.19). Though not each of these was individually significant in the model, the combination resulted in a model with good accuracy and discrimination power with 83.2% area under the ROC curve, which decreased with attempted stepwise reduction of variables. A post hoc analysis of linear discrimination confirmed that the final model had a sensitivity of 72.73%, a specificity of 78.79%, a positive predictive value of 53.33%, and a negative predictive value of 89.66%.
Fig. 2.
a ORs with 95% Wald confidence limits from the final multivariable model. b ROC curve for the model. LOC, level of consciousness; OCPs, oral contraceptive pills; OR, odds ratio; ROC, receiver operating characteristic.
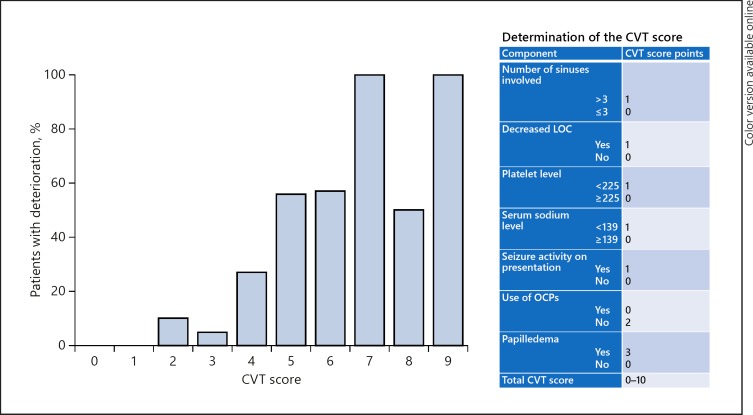
To construct the risk stratification scale (the CVT score) for calculating individual probabilities of deterioration, ORs were calculated for each of the binary predictors from the final model. We assigned one point to ORs around 2.5 (the lowest OR in the model) and multiple points based on multiplicities of 2.5 (see Fig. 3 for the detailed assignment). Ten possible points were assigned for the proposed model. One point was assigned for > 3 sinuses involved, decreased LOC, platelets count < 225 × 109/L, serum sodium level < 139 mEq/L, and seizure activity on presentation. Two points were assigned for no OCP use, and three points were assigned for the presence of papilledema. The individual likelihood of deterioration by CVT score was then calculated based on the existing data set (Fig. 3). This analysis showed that patients with a CVT score of ≥5 have at least 50% chance of deterioration, whereas scores of < 5 demonstrate very low risk of deterioration. A CVT score of 5 seemed to be the optimal prediction threshold to stratify patients for potential to need earlier intervention based on our data.
Fig. 3.
CVT score to predict early deterioration. The proposed score (right) was applied to our data set, revealing these percentages of patients with deterioration. Note that a score of ≥5 results in a ≥50% chance of deterioration versus a much lower risk with a lower score. Though this is a 10-point scale, none of our patients had a score of 10, so none was omitted from this figure. CVT, cerebral venous thrombosis; LOC, level of consciousness; OCPs, oral contraceptive pills.
Discussion
Although CVT is an uncommon stroke in the general population, it can be potentially life-threatening and disproportionately affects younger patients. In the ISCVT, 4.3% of patients died during the acute phase of CVT and 3.4% within 30 days from symptom onset [1]. The incidence of CVT has been reported to be as high as 1.32 per 100 000 person-years, which is higher than previously published [14]. This incidence may be increased at higher elevation such as ours (mean 5,700 feet above sea level) in New Mexico due to relative thrombophilia [15].
CVT presentation is quite variable. Headache, usually unilateral, is the most common presenting symptom in 70–90% of patients and can be the sole complaint [16]. In our study, headache was also the most common symptom, reported in 66.67%. Other commonly reported symptoms include focal neurological deficit in 40.8%, seizure in 30.2%, and altered mental status in 40.2% [4]. The superior sagittal sinus was the most common location (72.2%) in that series. In comparison, we found focal weakness in 22.45% and seizure in 14.29%, while transverse sinus involvement was the most common location (68%) in our series. Mortality occurs in the range of 3–5% [13]. In our cohort, the deaths of 6/147 patients (4.08%) were related to CVT.
The European Federation of Neurological Societies guidelines on the treatment of CVT [17] recommend that patients with CVT and no contraindications for anticoagulation should be treated with anticoagulation. The 2011 American Heart Association/American Stroke Association guidelines state that anticoagulation is reasonable for patients with acute CVT, even in selected patients with intracranial hemorrhage [6]. In our cohort, 109/147 (74.15%) patients were started on anticoagulation. Though we did not investigate the specific reasons for no anticoagulation, this is done in cases where there is a large amount of hemorrhage, need for surgical intervention, or perceived benign nature of the thrombosis by the treating team.
Endovascular techniques for CVT have evolved and new devices have been introduced over the last decade. Different techniques include direct catheter thrombolysis, balloon-assisted thrombectomy, rheolytic catheter thrombectomy, aspiration thrombectomy, and stent retriever thrombectomy [4]. Multimodality treatment is often required, though several centers have reported success with suction aspiration [18]. In our cohort, multimodality treatment was used in 6 patients (2 patients had complete recanalization, 2 had partial recanalization, 1 had no recanalization, and 1 died with extensive deep venous sinus thrombosis). Five patients underwent only intra-arterial thrombolysis, and 1 patient underwent stenting.
In a recent systematic review of 17 studies (235 patients) which evaluated the use of endovascular therapy for CVT [4], the indications for endovascular therapy included failure of systemic anticoagulation, extensive clot burden, cerebral edema, altered mental status, and progressively worsening neurological symptoms. Eighty-two percent (155/189) of their patients were started on anticoagulation prior to intervention. Postoperative complete radiographic resolution of CVT was achieved in 69.0% (147/213) of cases, while partial resolution was achieved in 26.3% (56/213). The Thrombolysis or Anticoagulation for Cerebral Venous Thrombosis trial (NCT01204333) is a closed multicenter, prospective trial comparing the safety and efficacy of endovascular therapy with medical therapy in high-risk patients which unfortunately did not reach recruitment goals to continue enrollment. The investigators defined high risk factors as decreased mental status, hemorrhage, or deep venous thrombosis. In our data, of these only decreased mental status was a significant predictor of deterioration. Our contention is that it is not simply the poor outcome with CVT group that should be targeted, but also patients who start out with a relatively benign exam and then deteriorate. Many patients with poor outcome have a severe problem at admission (such as thalamic infarction with deep thrombosis) and so may not be the optimal group to target for intervention. The concept of early intervention in certain patients, however, does make sense in that delaying thrombectomy until deterioration or failure of anticoagulation has the unwanted effects of (1) allowing the clot to become fibrous and less amenable to simple thrombectomy and (2) potentially allowing for new permanent neurological deficits to develop. The above systematic review also supports this role for earlier intervention in terms of improved odds of good outcome in patients treated early (OR = 0.12; 95% CI 0.02–0.86; p = 0.03).
Based on the model we developed, decreased LOC at admission, platelet count < 225 × 109/L, sodium level < 139 mEq/L, presence of seizure activity, absence of OCP use, and number of sinuses involved > 3 all were important factors in predicting deterioration. Though some of these factors were not initially expected to be significant, we think that they make sense physiologically. Thrombocytosis and hypernatremia are likely reflective of dehydration (a reversible risk factor), and the use of OCPs is similarly reversible, so these would result in less chance of progression. Decreased mental status, more segmental sinuses involved, and seizure are all likely reflective of more severe disease. A CVT score of ≥5 points seemed to be highly predictive of a greater chance of poor outcome. This is a simple scoring system that uses easily accessible data from admission to determine who should be considered for intervention. Prospective assessment is required for refinement and validation of this score; nonetheless, we feel that it at least offers preliminary guidance in decision-making if thrombectomy is being considered.
The limitations of our study include the use of a retrospective cohort with relatively small sample size and the lack of standardized criteria for endovascular therapy. The composite outcome of deterioration we used was intended to capture a variety of patients with deterioration, not just patients with poor outcome, but also makes for a somewhat less defined group compared to a simple poor modified Rankin Scale score or another ordinal scale of outcome. Our goal for doing this was specifically to target patients who have a higher likelihood of salvage, since many patients with poor outcome likely have such a poor prognosis from the onset and may not have a significant opportunity for intervention. It is also possible that some patients with deterioration were missed because there were no specific follow-up requirements, but since our institution is the only tertiary referral center in New Mexico, most patients with delayed deterioration will return to our institution. Finally, there may be other risk factors, particularly within the hypercoagulable panel, that may also be relevant, but as many of these were not routinely obtained at admission, we did not have enough numbers to analyze them as specific risk factors. That said, our model provided a surprisingly high predictive value with the data we were able to incorporate.
Conclusions
Many patients with CVT (up to 25%) are at risk of deterioration despite anticoagulation. Decreased mental status, seizure activity, papilledema, number of involved sinuses, low serum sodium level, and low platelet count are the most important combination of risk factors in predicting deterioration after CVT. We present a prediction score based on these clinical, radiographic, and laboratory data at admission which performed well in our retrospective cohort to predict the status of these patients. We hypothesize that this subgroup would be the optimal target for early venous thrombectomy or thrombolysis. Prospective validation and ultimately trials are required to confirm this hypothesis.
Statement of Ethics
Ethics approval was obtained from the institutional review board of the University of New Mexico School of Medicine.
Disclosure Statement
The authors have no conflicts of interest to disclose. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F, ISCVT Investigators Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) Stroke. 2004;35:664–670. doi: 10.1161/01.STR.0000117571.76197.26. [DOI] [PubMed] [Google Scholar]
- 2.Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352:1791–1798. doi: 10.1056/NEJMra042354. [DOI] [PubMed] [Google Scholar]
- 3.Ferro JM, Canhão P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep. 2014;16:523. doi: 10.1007/s11886-014-0523-2. [DOI] [PubMed] [Google Scholar]
- 4.Ilyas A, Chen CJ, Raper DM, Ding D, Buell T, Mastorakos P, Liu KC. Endovascular mechanical thrombectomy for cerebral venous sinus thrombosis: a systematic review. J Neurointerv Surg. 2017;9:1086–1092. doi: 10.1136/neurintsurg-2016-012938. [DOI] [PubMed] [Google Scholar]
- 5.Ferro JM, Lopes MG, Rosas MJ, Ferro MA, Fontes J, Cerebral Venous Thrombosis Portuguese Collaborative Study Group Long-term prognosis of cerebral vein and dural sinus thrombosis. Results of the VENOPORT study. Cerebrovasc Dis. 2002;13:272–278. doi: 10.1159/000057855. [DOI] [PubMed] [Google Scholar]
- 6.Saposnik G, Barinagarrementeria F, Brown RD, Jr, Bushnell CD, Cucchiara B, Cushman M, deVeber G, Ferro JM, Tsai FY, American Heart Association Stroke Council and the Council on Epidemiology and Prevention Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–1192. doi: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]
- 7.Mammen S, Keshava SN, Moses V, Aaron S, Ahmed M, Chiramel GK, Mani SE, Alexander M. Role of penumbra mechanical thrombectomy device in acute dural sinus thrombosis. Indian J Radiol Imaging. 2017;27:82–87. doi: 10.4103/0971-3026.202956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adachi H, Mineharu Y, Ishikawa T, Imamura H, Yamamoto S, Todo K, Yamagami H, Sakai N. Stenting for acute cerebral venous sinus thrombosis in the superior sagittal sinus. Interv Neuroradiol. 2015;21:719–723. doi: 10.1177/1591019915609120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto N, Kuramoto Y, Shinoda N, Ueno Y. A case of stenting for acute cerebral venous sinus thrombosis in the superior sagittal sinus. Interv Neuroradiol. 2016;22:709–710. doi: 10.1177/1591019916663093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King JE. Running a best-subsets logistic regression: an alternative to stepwise methods. Educ Psychol Meas. 2003;63:392–403. [Google Scholar]
- 11.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 12.Saeed AI, Qeadan F, Sood A, VanderJagt DJ, Mishra SI, Hill DA, Peikert T, Sopori ML. A novel cytokine profile associated with cancer metastasis to mediastinal and hilar lymph nodes identified using fine needle aspiration biopsy - a pilot study. Cytokine. 2017;89:98–104. doi: 10.1016/j.cyto.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Coutinho JM, Zuurbier SM, Stam J. Declining mortality in cerebral venous thrombosis: a systematic review. Stroke. 2014;45:1338–1341. doi: 10.1161/STROKEAHA.113.004666. [DOI] [PubMed] [Google Scholar]
- 14.Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis: a cross-sectional study. Stroke. 2012;43:3375–3377. doi: 10.1161/STROKEAHA.112.671453. [DOI] [PubMed] [Google Scholar]
- 15.Zavanone C, Panebianco M, Yger M, Borden A, Restivo D, Angelini C, Pavone A, Grimod G, Rosso C, Dupont S. Cerebral venous thrombosis at high altitude: a systematic review. Rev Neurol (Paris) 2017;173:189–193. doi: 10.1016/j.neurol.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Wasay M, Kojan S, Dai AI, Bobustuc G, Sheikh Z. Headache in cerebral venous thrombosis: incidence, pattern and location in 200 consecutive patients. J Headache Pain. 2010;11:137–139. doi: 10.1007/s10194-010-0186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Einhäupl K, Stam J, Bousser MG, De Bruijn SF, Ferro JM, Martinelli I, Masuhr F, European Federation of Neurological Societies EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol. 2010;17:1229–1235. doi: 10.1111/j.1468-1331.2010.03011.x. [DOI] [PubMed] [Google Scholar]
- 18.Mokin M, Lopes DK, Binning MJ, Veznedaroglu E, Liebman KM, Arthur AS, Doss VT, Levy EI, Siddiqui AH. Endovascular treatment of cerebral venous thrombosis: contemporary multicenter experience. Interv Neuroradiol. 2015;21:520–526. doi: 10.1177/1591019915583015. [DOI] [PMC free article] [PubMed] [Google Scholar]