Abstract
Robotic surgery is safe and feasible offering many potential advantages to the colorectal surgeon
Laparoscopic surgery is increasingly being adopted as routine care in colorectal cancer surgery, and its application in emergency colorectal surgery is becoming more widespread. In cancer surgery, the acceptance of laparoscopic techniques is based upon their proven ability to reduce length of hospital stay and decrease postoperative complications while achieving equivalent oncological outcomes to open surgery. Robotic surgery has not seen the same adoption as laparoscopic surgery and is yet to have a proven benefit that would outweigh its significant cost and longer operating time. Although there is clear evidence that robotic surgery is safe and effective, it is the lack of proven clinical benefit and the increased costs – compared with laparoscopic colorectal surgery – that has limited its mainstream use. This situation may change as the robotics market opens up to more competition.
Advantages of robotic colorectal surgery
The application of robotics in colorectal surgery is theoretically appealing – particularly in technically challenging situations, such as dissection in a narrow male pelvis or the obese patient. The da Vinci® Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, US) was developed to compensate for the technical limitations of laparoscopic surgery. Although primarily aimed at cardiac surgery, it subsequently found niche applications in several other disciplines where laparoscopy was particularly challenging or laparoscopic skills were limited.
In colorectal surgery, the robotic approach offers particular advantages in situations that are technically challenging, including low rectal dissection, intracorporeal suturing in rectopexy, and high-vessel ligation and intracorporeal anastomosis in right hemicolectomy. Surgical precision is augmented by the robotic digital platform – with capabilities for tremor reduction, image magnification, and a stable camera platform, which should lead to less tissue trauma and lower intraoperative blood loss. Ultimately, the combined technological advantages of the robot should lead to a lower conversion rate to open surgery, meaning that more patients will benefit from minimally invasive surgery. With particular reference to rectal cancer surgery, there have been criticisms that the laparoscopic approach fails to yield oncological outcomes equivalent to open surgery, and this might be an area where the robot can add additional benefit. The contrary arguments are that robotic systems are expensive, the lack of haptic feedback makes operations more difficult, and they are associated with significant learning curves.
The first robotic colectomies were reported in 2002 by Weber et al for benign disease (caecal and sigmoid diverticulitis),1 by Hashizume et al for malignant disease,2 and the first proctectomy was reported by Giulianotti et al in 2003.3 Indications for robotic surgery are much the same as those for laparoscopic surgery, as are the relative contraindications – namely emergency surgery, previous abdominal surgery with extensive adhesions, and cardiovascular or respiratory disease preventing safe pneumoperitoneum.
Several systematic and retrospective reviews have summarised the literature and the benefits of robotic surgery compared with laparoscopic surgery. These are shown in Table 1. The reviews collectively conclude that robotic surgery is safe and efficacious, with equivalent oncological outcomes and complication rates, but operative times are significantly longer.4 Length of stay has been shown to be shorter with lower rates of conversion to open surgery for pelvic surgery.4,5 Although there are higher costs associated with robotic surgery,6,7 they could be offset by decreased length of stay.
Table 1.
Summary of systematic reviews comparing robotic with laparoscopic colorectal surgery (LOS = length of stay; LN = lymph nodes)
| Author | Study | Number of patients Robotic vs lap | Operation time | Costs | LOS | Conversion to open | Blood loss | Oncologic Outcome (LN retrieval) | Leak rate | Morbidity/mortality | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trinh et al8 | Systematic review | 920 vs 3,422 | 38.859 minutes longer | n/a | = | Higher – 1.78 times | Lower (14.17ml) | = | = | = | Safe and efficacious |
| Bhama et al4 | Retrospective review | Abdomen: 299 vs 7,790 and pelvis: 331 vs 2,057 | Significantly longer | Significantly shorter | Lower for pelvic surgery but equivalent for abdominal surgery | = | = | ||||
| Tam et al5 | Retrospective review | 2,735 minimally invasive operations studied | Significantly shorter | Lower with robotic – significant for rectal resections | = | = | |||||
| Tyler et al6 | Retrospective review | Significantly higher | = | = | |||||||
| Halabi et al | Retrospective review | Higher | = | Higher | = | = |
The three main colorectal operations where robotic surgery has found most use are:
Right hemicolectomy
Rectal cancer surgery
Ventral mesh rectopexy.
This review will consider the impact of robotic surgery in these operations.
Robotic right hemicolectomy
The use of the robot in right hemicolectomy has been advocated as an ideal training application to familiarise the surgeon with the system,9,10 although these reports probably preceded the wider dissemination of high central ligation of the right colic vessels. Other potential advantages include easier intracorporeal anastomosis with the ability to avoid an umbilical specimen extraction site and so reduce the risk of incisional herniation.
With particular reference to rectal cancer surgery, there have been criticisms that the laparoscopic approach fails to yield oncological outcomes equivalent to open surgery
Potential advantages of robotic right hemicolectomy
Intracorporeal anastomosis
In laparoscopic surgery, the ileocolic anastomosis is commonly performed extracorporeally, with the bowel exteriorised through a mini-laparotomy incision. It has been proposed by a number of studies that there are advantages to performing an intracorporeal anastomosis.
A prospective case-control study in 60 patients who underwent laparoscopic right hemicolectomy in a single centre compared intracorporeal (IC) anastomosis (35 patients) to extracorporeal (EC) anastomosis (25 patients).11 They found that the number of lymph nodes removed was higher in the IC group (21 vs 14; p=0.03). Return to bowel function was significantly earlier in the IC group, with similar complication rates in both groups (14% IC; 16% EC; p=0.89).
A non-systematic review focussed on EC versus IC anastomosis in laparoscopic right hemicolectomy.12 The authors concluded that intracorporeal anastomosis could reduce surgical site infection and postoperative pain, with reduction in the size of the extraction site. Hellen et al prospectively collected data on 80 patients who underwent laparoscopic right hemicolectomy and demonstrated the incision length to be significantly shorter (4cm vs 5cm; p= 0.004) in the intracorporeal group (23 patients) compared with the extracorporeal group (57 patients).13 A retrospective study by Fabouzzi et al looked at 100 laparoscopic right hemicolectomies, 50 with intracorporeal anastomosis and 50 with extracorporeal anastomosis and found that the intracorporeal group had less postoperative pain, earlier return of bowel function and shorter mean hospital stay (5 days vs 7 days, p< 0.05).14
There is therefore some evidence to suggest an advantage for intracorporeal anastomosis, at least in laparoscopic right hemicolectomy. When the ileocolic anastomosis is performed intracorporeally, there is less need to mobilise the transverse colon fully and the extraction site can be smaller. This in turn could lead to an earlier return to bowel function, as well as reduced surgical site infection and incisional hernia rates.
Intracorporeal anastomosis is challenging when performed laparoscopically but is relatively straightforward with the robot, owing to the three-dimensional view and the endo-wrist movements that facilitate intracorporeal suturing. A prospective case series of 20 cancer patients undergoing robotic right colectomy at a single centre demonstrated that robotic intracorporeal anastomosis was safe and feasible with acceptable short-term oncological outcomes.15 A further prospective case series of 52 patients (with both benign and malignant indications for colectomy) also concluded that robotic right colectomy with intracorporeal anastomosis was safe and feasible.16
A retrospective review studied shortterm and long-term outcomes after robotic right colectomy with intracorporeal anastomosis (n=89) and laparoscopic right colectomy with extracorporeal anastomosis (n=135). They found that significantly less blood loss occurred in the robotic group (median=20ml, range 5 to 300ml) than in the laparoscopic group (median 50ml, range 10 to 500ml), (p< 0.001). The incision length was significantly shorter in the robotic group (median 4cm, range 2.5 to 7cm) than the laparoscopic group (median 5cm, range 3 to 11cm), (p< 0.0001). This was associated with a lower incisional hernia rate at mean follow-up of 33 months – robotic 0% compared with laparoscopic 7.1%. Smaller extraction sites can be achieved with intracorporeal anastomosis and the extraction site does not have to be a midline incision; it can be placed off the midline, reducing the risk of incisional hernias and providing better cosmesis. The results of this study should be treated with caution, given that it compared two different anastomotic techniques; the ideal study would compare laparoscopic intracorporeal anastomosis with robotic intracorporeal anastomosis.
A case-control study in cancer patients by Morpurgo et al 18 compared robotic intracorporeal anastomosis (n=48) with laparoscopic extracorporeal anastomosis (n=48). They demonstrated earlier recovery of bowel function (day of first bowel movement 3 ± 1 days in the robotic group (RG) compared with 4.0 ± 1.2 days in the laparoscopic group (LG); (p< 0.05), decreased hospital stay (RG: 7.5 ± 2.0 days vs. LG: 9.0 ± 3.2 days; p< 0.05), lower incisional hernia rate (RG: 0 vs LG: 4), and lower anastomotic leak rate (RG: 0 vs LG 4). They concluded that intracorporeal robotic anastomosis allows a faster recovery than laparoscopic surgery with extracorporeal anastomosis.
Ultimately, a larger randomised study or prospective registry is needed to clarify any such advantage from robotic surgery.
Enable surgeons to establish learning curve to progress to robotic rectal cancer surgery
It has been suggested that robotic surgery has a relatively quick learning curve, particularly for surgeons already proficient in the laparoscopic technique, and that this might favour its ease of adoption into clinical practice.
Raimondi et al2 studied the utility of a right colectomy as a learning procedure at the start of a robotic training programme; they hypothesised that right colectomy contains all the technical steps necessary to acquire basic abilities in robotic surgery. They analysed the first 23 consecutive robotic right colectomies that were performed, using the CUSUM method to evaluate the learning curve. The mean overall time was 265.3 min (180–320 min), docking time was 7 min (5–12 min), console time was 205.9 min (145–260 min), and anastomotic time was 43.6 min (25–60 min). Two learning phases were identified: the ‘starting phase’ and ‘consolidation phase’, with significant differences between the two phases in terms of operative time. Based on their observed differences they concluded that robotic right colectomy was a suitable training procedure.
Witkiewicz et al10 also suggested that right hemicolectomy could be a training procedure in robotic colorectal surgery, allowing surgeons to progress up the learning curve. Parisi et al20 reviewed prospective data on 108 patients undergoing robotic right hemicolectomy with intracorporeal anastomosis and performed CUSUM and risk-adjusted CUSUM analysis to assess the learning curve. They found three phases; the initial learning period (1st to 44th case), phase 2, the consolidation period (45th to 90th case) and phase 3, the mastery period (91st to 108th case). They noted that operation time, conversion to open surgery and the number of harvested lymph nodes significantly improved through the three learning phases. They concluded that although it is oncologically safe to use robotic right hemicolectomy as a training procedure, performance improves after 44 procedures. Therefore, it was suggested that benign diseases should be treated initially by novice robotic surgeons and to reserve oncological resection until after 44 cases. This study was limited by the fact that a single surgeon at a single institution performed all of the included surgical procedures, which decreases the external validity of the results.
Complete mesocolic excision (CME)
Complete mesocolic excision with central vascular ligation for right-sided colon cancer is suggested to offer superior oncological outcomes and a possible survival advantage when compared with standard D 2 lymphadenectomy.21 It has not yet been widely adopted laparoscopically, despite a number of single centre studies demonstrating its feasibility and safety22,23 with acceptable oncological and postoperative outcomes. The technical advantages of the robot might provide more precise dissection of the central vascular structures and so facilitate a more radical resection. However, there has been slow adoption of the robotic CME for right-sided colon cancer and any potential benefit remains unproven.24
Limitations of robotic right hemicolectomy
The limitations of robotic right hemicolectomy are its significantly increased cost, longer operating times and failure to demonstrate patient benefits over laparoscopic surgery, as illustrated in Table 2.
Table 2.
Studies on Robotic vs Laparoscopic Right Hemicolectomy (EBL = estimated blood loss, OO = oncological outcome/LN retrieval, ‘=’ = equivalent outcomes)
| Study | Study type | No. of pts robotic is lap | Operation time | Costs | LOS | Conversion to open | EBL | OO | Postoperative complications | |
|---|---|---|---|---|---|---|---|---|---|---|
| Leak rate | Morbidity/mortality | |||||||||
| Xu et al25 | Systematic review and meta-analysis | 234 vs 415 | Longer operative times by 48.24 minutes (p<0.00001) | (p= 0.12) | = (p=0.48) | Lower (18.79mls) (p= 0.0002) | = (p=0.28) | Significantly lower overall complication rate (p=0.02), faster return of bowel function (p<0.00001) | ||
| Petrucciani et al26 | Meta-analysis of six studies | 168 vs 348 | Longer with robotic | = | = | = | = | = | ||
| Park et al27 | Single institution Randomised clinical trial | 195 vs 130 minutes (p<0.001) | Significantly higher $8,714 vs $5,110 | = | = | = | = | |||
| De Souza et al19 | Single institution randomised controlled trial | 40 vs 135 | Longer | higher | = | = | = | = | = | |
| Deutsch et al 201628 | Retrospective review | 79 vs 92 | 135 min vs 140 min | = | ||||||
| Witkiewicz et al10 | Retrospective review | Longer | Higher | |||||||
Xu et al25 carried out the most recent meta-analysis comparing robotic with laparoscopic right hemicolectomy. They included seven studies, six of which were non-randomised controlled trials and one was a randomised controlled trial. These studies involved 649 patients in total, 234 in the robotic group and 415 in the laparoscopic group. The results showed that robotic right colectomy had longer operative times, lower estimated blood losses, shorter hospital stays, lower overall postoperative complications, and a significantly faster bowel function recovery. Although blood loss has been shown to be lower, this is often by a margin that is not clinically relevant, eg 19mls.
Operating time is influenced by set-up time, docking time, learning curve, and the type of anastomosis. In right hemicolectomy, it may be necessary to use either a hybrid technique or repositioning of the robotic cart if older systems are used – with the da Vinci Xi system this is not necessary. Re-positioning of the robotic cart or robotic arms can add to the overall operative time. The set-up time was excluded from all the seven studies included in the Xu’s meta-analysis. Operative time was reported to be longer in robotic right colectomy than in laparoscopic right colectomy in most of the studies, except for the study by Deutsch et al.28
There is significant heterogeneity in the reporting of operative time, owing to inclusion of different pathologies (diverticular disease/Crohn’s/cancer/polyps), as well as differing surgical techniques, eg anastomotic construction. The surgeon’s learning curve also has a significant impact on operating time and, as experience increases, docking and operative times will decrease – although paradoxically overall operative time may increase as more challenging cases are taken on.
Although robotic assistance for right hemicolectomy is unproven, further large registry studies organised through the European Society of Coloproctology may help to define its future role.
Robotic surgery for rectal cancer
Total mesorectal excision (TME) is the standard of care for rectal cancer and involves removal of the primary cancer along with the whole mesorectum in an intact oncological package. Laparoscopic TME for rectal cancer has been shown to be feasible, although technically challenging – especially in obese, male patients or in patients with a narrow pelvis. The robotic platform could potentially offer benefits during the pelvic dissection, particularly with regard to autonomic nerve preservation, thus limiting the disabling postoperative complications of sexual and urinary dysfunction. While theoretically this seems likely, it has yet to be proven. Laparoscopic TME surgery has also faced much criticism and controversy with regard to oncological outcomes; two large multicentre non-inferiority RCTs (ALaCaRT29 & ACOSOH Z605130 trials) have failed to demonstrate non-inferiority of laparoscopic compared with open surgery using a composite primary end-point for oncological adequacy of surgical resection, including CRM (circumferential resection margin) positivity, distal resection margin clearance, and pathological grade of the TME specimen. Similar difficulties have been encountered in attempts to prove that robotic surgery offers an oncological advantage to laparoscopic surgery for rectal cancer, with several studies failing to show a difference31–33 (see Table 3).
Table 3.
Studies on robotic rectal cancer surgery (taken from Cheng et al35)
| Study | Study type | Comparisons | Patients (N) | Endpoints | Results |
|---|---|---|---|---|---|
| Speicher et al31 | 2010-2011 national cancer database review | Robotic vs laparoscopic vs open low anterior resection | 956 RS, 5,447 LS, 9,872 OS | Conversion rate, LN retrieval, margin status, 30-day mortality, readmission, LOS | RS and LS were associated with shorter length of stay than OS (p<0.001) and lower conversion rates (9.5% vs 17.5%; p<0.001 No significant difference in all other endpoints |
| Lee et al (2015) | 2008-2015 systematic review and meta-analysis | Robotic vs laparoscopic resection for rectal cancer | 1,043 robotic, 1,181 laparoscopic | Conversion rate, time to oral diet and bowel function, EBL, operating time, complications, number of LNs, urologic and sexual function | RS was associated with lower conversion rates (RR 0.28, 95% CI 0.15 to 0.54; p<0.001), and decreased time to flatus (MD -0.13 days, -0.25 to 0.01; p=0.03, shorter time to flatus, improved urologic and sexual function (MD -2.82, -4.78 to -0.87; p=0.02); but longer operating times (MD 49.97 min, 20.45 to 79.52; p<0.001) |
| Liao et al (2017) | Meta-analysis | Robotic vs open resection for rectal cancer | 498 robotic, 576 open | Operative time, EBL, intraoperative transfusion, anastomotic leak, wound infection, time to flatus, LOS, number of LNs, margin positivity, DFS, overall survival | RS was associated with longer operative time (MD 55.76, 29.31 to 82.22; p<0.0001), lower EBL (MD -139.98, -159.11 to -120.86; p<0.00001), less intraoperative transfusion requirements (OR 0.52, 0.28 to 0.99; p=0.05), shorter LOS (MD -2.10, -3.47 to -0.73; p=0.003), shorter time to flatus (MD -0.97, -1.06 to -0.88, p<0.00001). No significant differences in anastomotic leak, wound infection, number of LNs, margin status, DFS, or OS No significant differences in complications (OR 1.00, 0.75 to 1.32), DRM (MD 0.17, -0.14 to 0.48), or DFS (HR 0.84, 0.53 to 1.35) |
| Zhang et al | Meta-analysis | Robotic vs laparoscopic resection for colorectal cancer | 1,466 robotic, 1,852 laparoscopic | Conversion rates, EBL, LOS, operative times, complication rates, number of LNs, DRM, total hospitalisation costs | RS was associated with lower conversion rates (RR 0.52, 0.33 to 0.81; heterogeneity: p=0.426, I2=2.2%) EBL and LOS were lower in the RS group but there was significant heterogeneity between the groups (p=0.000) No differences in complication rates (RR 1.04, 0.91 to 1.18), operation times, number of LNs, positive resection margins (SMD -0.01, -0.10 to 0.08), or costs (SMD 1.23, 1.01 to 1.45) |
| D’Annibale et al36 | Single centre, retrospective | Robotic vs laparoscopic total mesorectal excision | 50 robotic, 50 laparoscopic | Operative time, conversion rates, LOS, morbidity, anastomotic leak rate, urinary and sexual function, number of LNs, CRM, DRM | RS was associated with lower conversion rates (0 vs 6; p=0.011) and better CRM (p=0.022). Among sexually active people, RS had significant improvement in sexual function after one year when compared with LS (100% vs 47%; p=0.045) No differences were seen in DRM, time to diet, LOS, morbidity, number of LNs, anastomotic leak rates |
| Kim et al37 | Single centre, prospective cohort | Robotic vs laparoscopic total mesorectal excision | 30 robotic, 39 laparoscopic | Voiding and sexual function at 1, 6, and 12 months after surgery | RS was associated with earlier recovery of voiding (p=0.036) at three months |
| Park et al (2011) | Single centre, prospective cohort | Robotic vs laparoscopic vs open resection for rectal cancer | 52 robotic, 123 laparoscopic, 88 open | Operating time, LOS, methods of specimen extraction, number of LNs, CRM, DRM, complication rates | Laparoscopy had shorter operating time than open and robotic surgery (158.1 min vs 233.8 min and 232.2 min; p<0.001); robotic and laparoscopic surgery had shorter LOS than open (10.4 d and 9.8 d vs 12.8 d; p<0.001) No differences were seen in complication rates, CRM, or DRM |
| Kang et al (2013) | Single centre prospective cohort | Robotic vs laparoscopic vs open resection for rectal cancer | 165 robotic, 165 laparoscopic, 165 open | Time to flatus, LOS, voiding and sexual function, CRM, two-year DFS | RS and LS were associated with shorter time to flatus (2.2 (SD 1.1), 2.4 (1.2) vs 3.0 (1.4); p<0.001) and shorter LOS (10.8 (5.5), 13.5 (9.2) vs 16.0 (8.6); p<0.001) RS was associated with better voiding (p=0.034), decreased wound infection (p=0.050) and better quality TME (p=0.034) compared with open surgery No differences were seen in two-year DFS |
| Valverde et al (2017) | Single centre retrospective cohort | Robotic vs laparoscopic sphincter saving resection for rectal cancer | 65 robotic, 65 laparoscopic | Conversion rates, morbidity, LOS, quality of TME, CRM, and DRM | RS was associated with lower conversion rate (p=0.044) No differences were seen in quality of TME, resection margins, LOS, or morbidity |
| Feroci et al (2016) | Single centre retrospective | Robotic total mesorectal excision | 53 robotic, 58 laparoscopic | Conversion rates, morbidity, LOS, number of LNs, DRM, three-year overall survival and DFS | RS was associated with shorter LOS (p<0.001), higher number of LNs (p<0.001), longer DRM (p<0.001) No differences were seen in DFS or OS |
| Cho et al (2015) | Retrospective case matched study from Yonsei Colorectal Cancer Database | Robotic total mesorectal excision | 278 robotic, 278 laparoscopic | Conversion rates, LOS, time to flatus, number of LNs, CRM, complications, five-year survival, DFS, local recurrence rates | RS was associated with longer operative times (p<0.001) No differences were seen in complications, survival rates, or disease outcomes |
| Baik et al (2009) | Single centre prospectively randomised | Robotic low anterior resection | 56 robotic, 57 laparoscopic | Operative times, conversion rates, complication rates, TME quality, CRM involvement | RS was associated with lower complication rates (p=0.025), decreased LOS (p=0.001), fewer days to soft diet (p=0.008), and reduced conversion rates (p=0.013) No differences were seen in CRM |
| Somashekhar et al (2015) | Single centre prospective randomised | Robotic vs open resection for rectal cancer | 25 robotic, 25 open | Operative times, conversion rates, LOS, EBL, DRM, quality TME, number of LNs | RS was associated with longer operative times (p<0.001), decreased EBL (p<0.001), better quality TME (p<0.001), decreased LOS (p<0.001) |
| de Jesus et al (2016) | Single centre retrospective | Robotic vs laparoscopic vs open total mesorectal excision | 59 robotic, 41 laparoscopic, 200 open | CRM, number of LNs | No differences were seen in CRM involvement or length |
Abbreviations: CRM=circumferential resection margin; d=days; DFS=disease-free survival; DRM=distal resection margin; EBL=estimated blood loss; HR=hazard ratio; LN=lymph node; LOS=length of stay; LS=laparoscopic surgery; MD=mean difference; OR=odds ratio; OS=open surgery;
RR=risk ratio; RS=robotic surgery; SD=standard deviation; SMD=standard mean difference; TME=total mesorectal excision.
Potential advantages of robotic surgery for rectal cancer
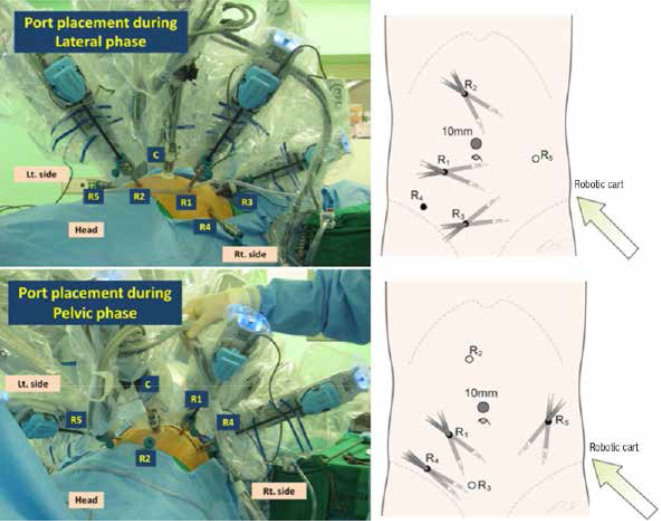
Rectal cancer surgery requires precise pelvic dissection, which can be technically challenging with the straight instruments and limited retraction provided by laparoscopic surgery. In comparison, robotic assistance offers a stable camera platform with three-dimensional operative field and articulating instruments that potentially aid better visualisation and more accurate dissection, with improved rectal retraction and less crowding of instruments in a narrow pelvis. Fig 1 demonstrates a typical set-up with port placement for robotic rectal cancer surgery using the da Vinci® S and Si systems.
Figure 1.
Placement of ports for totally robotic rectal surgery (taken from Park et al34)
The potential advantages of robotic surgery for rectal cancer are:
Lower conversion rates to open surgery
Better oncological outcomes
Nerve preservation.
A recent review on colorectal robotics summarised the evidence for robotic rectal cancer surgery35 – see Table 3.
Lower conversion rates to open surgery
Conversion to open surgery reflects a failure of the laparoscopic technique, which may be due to technical issues or patient factors (adhesions, anatomical variations etc). In the MRC CLASSIC trial, mortality and morbidity rates were highest in rectal cancer patients who were converted from laparoscopic to open surgery.38 A large US national database review analysing 16,275 patients from the National Cancer Database who underwent laparoscopic (n= 5,447), robotic (n=956) or open (n= 9,872) low anterior resection found that robotic surgery was associated with lower rates of conversion to open surgery compared with laparoscopic surgery (p< 0.001), indicating a possible benefit for robotic assistance.31
A meta-analysis by Scarpinata et al, comparing robotic against laparoscopic surgery for rectal cancer, concluded that the robotic approach was associated with increased cost and operating time, but with lower conversion rates (1–7.3% compared with 3–22%), regardless of the surgeon’s experience.39 The authors suggest that the robotic approach was particularly beneficial in difficult cases, such as previous abdominal surgery, lower rectal cancers and previous chemoradiation therapy. Another meta-analysis of 24 studies, including 2 RCTs with 3,318 patients, found a significantly lower rate of conversion in the robotic group.33 A US retrospective database study also demonstrated significantly lower conversion rates to open surgery in robotic rectal cancer surgery (12% for robotic vs 29% for laparoscopic [p<0.0001]).40
Lower conversion rates have a cost implication in that conversion to open surgery significantly increases the costs associated with minimally invasive colorectal surgery. It has been suggested that the lower rates of conversion in robotic colorectal operations may offset some of the excess costs attributable to robotics.41
Oncological outcomes with robotic rectal cancer surgery
Much interest has been focu sed on the quality of the TME specimen in rectal cancer surgery and its effect on the risk of local recurrence. There has been some concern about the higher rate of circumferential resection margin (CRM) positivity in laparoscopic low rectal cancer surgery, as evidenced in the CLASICC trial (12.4% laparoscopic vs 6.3% open)38 and more recently the COLOR II trial (10% laparoscopic vs 10% open).42
Several non-randomised, single-centre studies have reported favourable oncological outcomes following robotic rectal cancer surgery.36,43,44 Baik et al in 2013 reported 3-year overall and 3-year disease-free survival rates of 93.1% and 79.2% respectively.43 CRM positivity rate was 5.7% and the 3-year cumulative incidence of local recurrence was 3.6%. D’Annibale et al reported the oncological outcomes of robotic TME surgery in a single centre in Italy.36 In 100 patients, 50 of whom underwent robotic TME and 50 laparoscopic TME, the CRM was <2mm in 6 laparoscopic patients compared to none in the robotic group (p=0.022). This negative resection margin rate was thought to reflect better visualisation and ergonomics with the robot. The mean number of harvested lymph nodes was greater for robotic TME (robotic: 16.5 ± 7.1 vs laparoscopic: 13.8 ± 6.7), but this was not significant (p=0.073). The authors concluded that robotic TME was oncologically safe and showed better results than laparoscopic TME in terms of CRM positivity.
A prospective analysis of 64 consecutive rectal cancer patients treated with robotic TME in a single centre in California reported a negative CRM in all specimens.44 None of the patients developed isolated local recurrence. The 3-year overall and disease-free survival rates were 96.2% and 73.7%, respectively, which is comparable to the laparoscopic literature.45
A multicentric study based in 3 centres examined 143 patients undergoing robotic rectal cancer surgery and reported remarkable short-term clinical outcomes.46 The 3-year survival rate was 97%, and no isolated local recurrences were found at mean follow-up of 17.4 months. This study involved the extensive use of chemoradiation so results should be interpreted with caution; however, they suggest that robotic surgery is likely to improve the quality of TME relating to laparoscopic surgery.
Although these data are excellent (summarised in Table 4), translation of the results to a western population has to be guarded by different patient characteristics (eg body mass index) and treatment protocol (eg use of chemoradiotherapy). No study has shown a significant difference in terms of the number of retrieved lymph nodes,31 indicating that robotic and laparoscopic approaches produce a resection of similar radicality.
Table 4.
Oncological outcomes of robotic surgery (adapted from Park and Kim34)
| Study | Operation type | Survival | Recurrence | CRM involvement |
|---|---|---|---|---|
| Baik et al43 | Robot | 3yr DFS: 79.2% 3yr OS: 93.1% | 3 yr incidence of local/systemic recurrence: 3.6%/17.6% | 21/370 (5.7%) |
| D’Annibale et al36 | Robot | n/a | n/a | 0/50 |
| Baek et al44 | Robot | 3yr DFS: 73.7% 3yr OS: 96.2% | Total recurrence at mean follow up of 20.2 months: 6/64 (0.09%) | 0/64 |
| Pigazzi et al46 | Robot | 3yr DFS: 77.6% 3yr OS: 97% | Total recurrence at mean follow up of 17.4 months: 13/143 (0.09%) | 1/143 (0.7%) |
Nerve preservation with robotic rectal cancer surgery
Injury to the autonomic nerves along the pelvic side wall, and in close proximity to the prostate in the male, may readily occur during pelvic dissection, leading to sexual and urinary dysfunction (Fig 2). This is a source of comorbidity following abdominoperineal resection and low anterior resection and has a significant impact on quality of life. Several studies have highlighted concerns about bladder and sexual dysfunction following laparoscopic rectal cancer surgery.48 A retrospective review with 80 patients undergoing either robotic or laparoscopic anterior resection showed significantly quicker return of sexual function in the robotic group as judged by the International Index of Erectile Function.49 In a retrospective cohort study by D’Annibale et al sexual function at one year post resection was completely restored in 100% of sexually active patients in the robotic group, compared with only 43% of sexually active patients in the laparoscopic group.36 These results are depicted in Fig 3. With regard to long term outcomes, a single-centre cohort study failed to identify any significant difference in sexual function at 1 year post surgery, but did find earlier return of urinary function 3 months after surgery in the robotic group compared to the laparoscopic group (p=0.036).37
Figure 2.
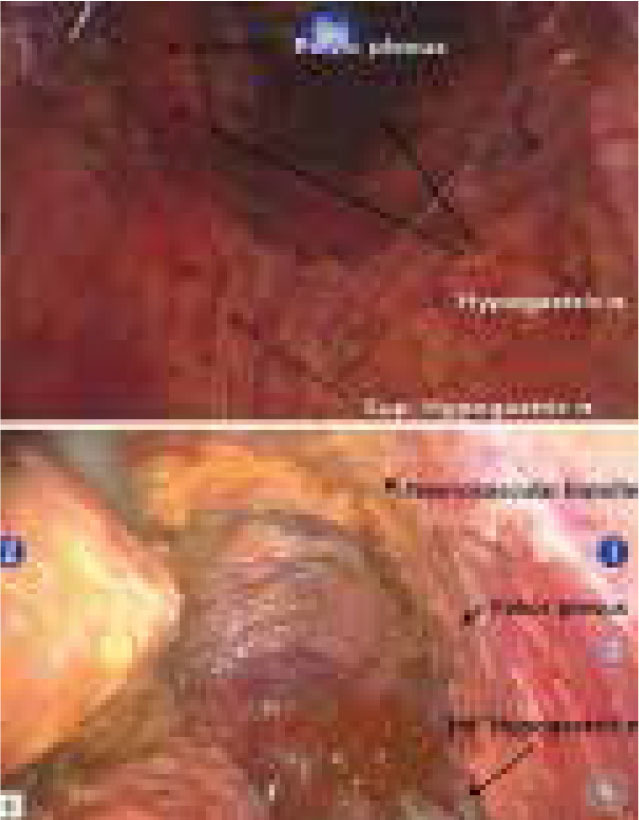
Nerves associated with sexual and urinary function that are at risk of damage during rectal cancer surgery (taken from Park and Kim34)
Figure 3.
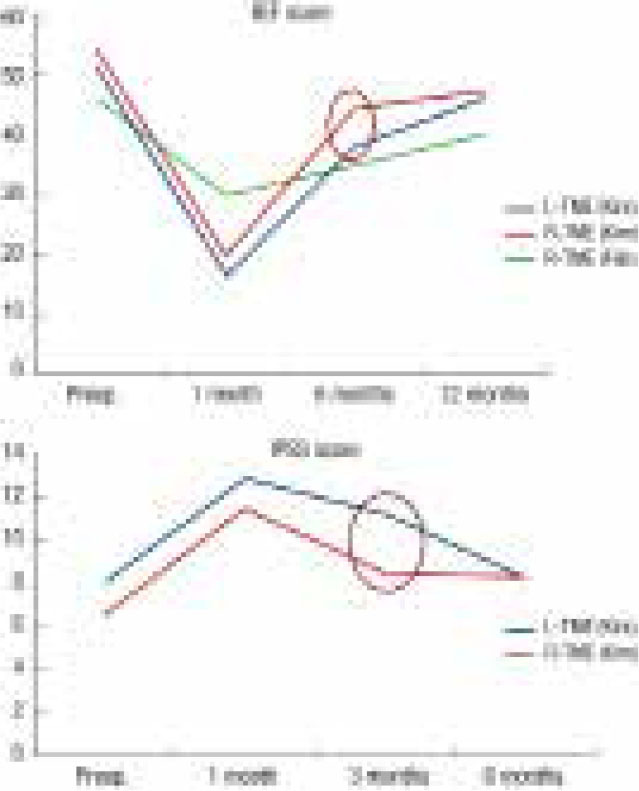
Earlier recovery of sexual and urinary function following robotic rectal cancer surgery compared to laparoscopic37
In comparison to the above single-centre studies, the recently reported MRC/NIHR ROLARR randomised controlled trial failed to show a benefit in terms of bladder and sexual function with the robot as compared with the laparoscopic approach.32 A deterioration in function was noted in both groups, more marked for sexual function and in males, following rectal cancer resection, but with similar rates of dysfunction noted in both the robotic and laparoscopic groups at six months follow-up. This probably reflects the high standard of surgery and rigour of data collection in ROLARR, together with a more generalisable result in terms of multicentre research collaboration.
Limitations of robotic surgery for rectal cancer
Failure to demonstrate benefit compared with laparoscopic surgery
Several studies have failed to demonstrated significant differences between robotic and laparoscopic rectal cancer surgery in terms of short-term outcomes, including rates of resection margin positivity, number of harvested lymph nodes, hospital stay, readmission rates, and 30-day mortality (Table 3).31
The MRC/NIHR ROLARR trial is the first large, multicentre, prospective randomised study comparing the robotic approach with standard laparoscopy for the curative resection of rectal cancer.32 A total of 471 patients from 29 centres in 10 countries were randomised into 2 groups: 234 patients undergoing conventional laparoscopic proctectomy and 237 undergoing robotic proctectomy.
The initial findings demonstrated that the primary outcome of conversion to open procedure did not differ significantly between the 2 arms (12.2% conventional laparoscopic vs 8.1% robotic-assisted laparoscopic surgery).32 Subgroup analysis found an improvement in conversion rates in robotic vs laparoscopic surgery in males (odds ratio [OR]: 0.455; 95% CI 0.21, 0.99), patients with low rectal cancers (OR: 0.49, 95% CI 0.21, 1.120), and obese patients (OR: 0.58, 95%CI 0.21, 1.60), but because this was a subgroup analysis statistical significance could not be concluded. However, the trend to reduced conversion in these technically challenging groups of patients does hint at a potential benefit for the robot that warrants further large-scale evaluation.
The secondary outcomes of the ROLARR trial were 30-day morbidity and mortality, CRM positivity, 3-year local recurrence rates, disease-free and overall survival rates, and sexual and urinary complications, with no significant differences demonstrated between the 2 groups.
Although ROLARR has not shown an obvious benefit for robotic surgery, in keeping with the data from smaller, single-centre studies, it has highlighted certain patient groups that may benefit from the robotic approach.
Increased rate of iatrogenic complications
In robotic surgery, there is loss of haptic feedback that means that the surgeon must rely solely on visual cues to interpret tissue-handling. This is aided by the immersive 3D overview the surgeon experiences with robotic surgery, but vibration, pressure or shearing forces are not always apparent. This is most easily seen when performing robotic suturing – inexperienced surgeons will frequently break a suture through excessive tension applied by the robotic arms. It is also not uncommon for the robotic arms to collide, particularly if ports are placed poorly and with the surgeon position in a remote location without easy visualisation of the patient. Although robotic surgery has repeatedly been shown to be safe, with a similar complications to laparoscopic surgery, the occasional report such as that by Yeo et al has described higher rates of iatrogenic complications with robotic surgery (OR = 1.73).50
In conclusion, robotic rectal cancer surgery has been taken on globally in the absence of any rigorous data to support its benefit in terms of improved patient outcomes, and on the basis of the current evidence it is difficult to justify its routine use in rectal cancer surgery, with the possible exception of the most challenging cases. However, with the introduction of more robotic systems, this situation is likely to change – in particular if they can be made to compete with laparoscopic surgery in terms of cost-effectiveness.
Robotic ventral mesh rectopexy (RVMR)
The minimally invasive surgical approach to pelvic organ prolapse is technically challenging, requiring careful dissection, preservation of vital structures and intracorporeal suturing of a mesh to the rectum deep in the pelvis. Robotic assistance in such procedures appears to be a natural extension of its current applications.
Only three studies have been published reporting the complication rates of robotic ventral mesh rectopexy (see Table 5). A meta-analysis of these three studies demonstrated that compared to laparoscopic ventral mesh rectopexy, robotic surgery showed a non-significant minimal advantage in terms of intra- and postoperative complications.51 A randomised controlled trial (RCT) comparing the two techniques failed to show a significant difference in terms of complication rates.52
Table 5.
Complication rates of RVMR, taken from van Iersel et al56
Regarding functional outcomes after robotic ventral mesh rectopexy (RVMR), a significant improvement has been reported in obstructed defecation symptoms after RVMR,54 which is similar to that reported in the literature for laparoscopic ventral mesh rectopexy. Recurrence rates have also been shown to be similar between the two techniques.
From the limited amount of data available, it appears that robotic mesh rectopexy is a safe procedure with acceptable recurrence rates, although the number of reported cases are small and no data are available on cost-effectiveness. The robot might be justified in this application as an aid for surgeons with limited experience of intra-corporeal suturing or as a training procedure before progressing on to more advanced procedures, such as anterior resection.
Cost of robotic colorectal surgery
The biggest criticism of robotic surgery, and the cause of greatest controversy, is the cost. Currently, the da Vinci® surgical system is the only surgical robot on the market, although several other robotic systems will follow soon. The da Vinci® system comes with a high capital cost, ranging from £1.5m to £2m. In addition to the initial purchase costs, there are annual maintenance costs and the costs for robotic instruments to consider.
Tyler et al6 reported an increase in cost of USD$3,424 for robotic surgery compared with laparoscopic surgery for the entire hospital encounter. They further reported no benefits in terms of length of stay reduction or decreased postoperative complications and therefore concluded that a critical appraisal of its benefits was warranted before advocating its use. Halabi et al7 also arrived at the same conclusion, stating that robotic surgery led to greater costs without any associated advantages to justify its cost.
The costs of robotic procedures are also inflated by the longer operating time as compared with laparoscopic procedures, which is a consistent feature in most of the reported literature. It is possible that with increasing experience, operating times may be reduced,39 but this is difficult to quantify given the complexity of the robotic learning curve. Typically, a triphasic learning curve is described, with an initial reduction in operating time as the surgeon becomes acquainted with the system, followed by an increase in operating time as more challenging cases are undertaken, and finally a second reduction in operating time to a plateau phase as the surgeon reaches proficiency. Some studies have suggested that the initial learning phase with the robot in rectal cancer surgery is around 20–40 cases, with proficiency gained after 41–50 cases;57 operative times and costs were significantly reduced in the experienced phase as compared with the initial learning curve.
Costs are also affected by length of stay, complication rates and readmission rates but data from the ROLARR study, which analysed direct and indirect costs of robotic surgery, showed that overall robotic rectal cancer resection was around £1,000 more expensive than laparoscopic surgery. With minimal benefit in terms of quality of life, this translated into an incremental cost-effectiveness ratio of around £70,000/QALY, which is not cost-effective in terms of the National Institute for Health and Care Excellence willingness to pay threshold of £20,000–£30,000 per QALY.32
Although it is generally accepted that current robotic systems are more expensive than conventional laparoscopy, and not cost-effective, there are several systems coming through to market that will challenge this situation through frugal design and innovative marketing strategies.
This is the challenge for the future – making robotics cost-effective. It is not an insurmountable proposition to reduce the cost of robotic systems and instruments, and as the commercial market opens up there is an inevitability that this will happen. It is then likely that we will see the true potential of robotic assistance being realised.
Conclusion
There is little doubt that robotic assistance extends the capabilities of the surgeon by improving the operative image and providing increased precision through articulating instruments and digital enhancement. But, for robotics to become a mainstream part of colorectal surgery, it is equally important that it produces patient benefits and can be shown to be cost-effective when compared with laparoscopic surgery. To date, the evidence does not support the widespread application of robots in colorectal surgery, but it does hint at certain patient groups and applications that could benefit if the technology was affordable.
References
- 1.Weber PA, Merola S, Wasielewski A, Ballantyne GH. Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum 2002; (12): 1,689–1,694; discussion 95–96. [DOI] [PubMed] [Google Scholar]
- 2.Hashizume M, Shimada M, Tomikawa M et al. . Early experiences of endoscopic procedures in general surgery assisted by a computer-enhanced surgical system. Surg Endosc 2002; (8): 1,187–1,191. [DOI] [PubMed] [Google Scholar]
- 3.Giulianotti PC, Coratti A, Angelini M et al. . Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003; (7): 777–784. [DOI] [PubMed] [Google Scholar]
- 4.Bhama AR, Obias V, Welch KB et al. . A comparison of laparoscopic and robotic colorectal surgery outcomes using the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. Surg Endosc 2016; (4): 1,576–1,584. [DOI] [PubMed] [Google Scholar]
- 5.Tam MS, Kaoutzanis C, Mullard AJ et al. . A population-based study comparing laparoscopic and robotic outcomes in colorectal surgery. Surg Endosc 2016; (2): 455–463. [DOI] [PubMed] [Google Scholar]
- 6.Tyler JA, Fox JP, Desai MM et al. . Outcomes and costs associated with robotic colectomy in the minimally invasive era. Dis Colon Rectum 2013; (4): 458–466. [DOI] [PubMed] [Google Scholar]
- 7.Halabi WJ, Kang CY, Jafari MD et al. . Roboticassisted colorectal surgery in the United States: a nationwide analysis of trends and outcomes. World J Surg 2013; (12): 2,782–2,790. [DOI] [PubMed] [Google Scholar]
- 8.Trinh BB, Jackson NR, Hauch AT et al. . Robotic versus laparoscopic colorectal surgery. JSLS 2014; (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raimondi P, Marchegiani F, Cieri M et al. . Is right colectomy a complete learning procedure for a robotic surgical program? J Robot Surg 2018; (1): 147–155. [DOI] [PubMed] [Google Scholar]
- 10.Witkiewicz W, Zawadzki M, Rząca M et al. . Robot-assisted right colectomy: surgical technique and review of the literature. Wideochir Inne Tech Maloinwazyjne 2013; (3): 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaves JA, Idoate CP, Fons JB et al. . A case-control study of extracorporeal versus intracorporeal anastomosis in patients subjected to right laparoscopic hemicolectomy. Cir Esp 2011; (1): 24–30. [DOI] [PubMed] [Google Scholar]
- 12.Stein SA, Bergamaschi R. Extracorporeal versus intracorporeal ileocolic anastomosis. Tech Coloproctol 2013; Suppl 1: S35–39. [DOI] [PubMed] [Google Scholar]
- 13.Hellan M, Anderson C, Pigazzi A. Extracorporeal versus intracorporeal anastomosis for laparoscopic right hemicolectomy. JSLS 2009; (3): 312–317. [PMC free article] [PubMed] [Google Scholar]
- 14.Fabozzi M, Allieta R, Brachet Contul R et al. . Comparison of short- and medium-term results between laparoscopically assisted and totally laparoscopic right hemicolectomy: a case-control study. Surg Endosc 2010; (9): 2,085–2,091. [DOI] [PubMed] [Google Scholar]
- 15.Trastulli S, Desiderio J, Farinacci F, Ricci F, Listorti C, Cirocchi R et al. . Robotic right colectomy for cancer with intracorporeal anastomosis: short-term outcomes from a single institution. Int J Colorectal Dis 2013; (6): 807–814. [DOI] [PubMed] [Google Scholar]
- 16.Lujan HJ, Molano A, Burgos A et al. . Robotic right colectomy with intracorporeal anastomosis: experience with consecutive cases. J Laparoendosc Advs Surg Tech A 2015; (2): 117–122. [DOI] [PubMed] [Google Scholar]
- 17.Lujan HJ, Plasencia G, Rivera BX et al. . Advantages of robotic right colectomy with intracorporeal anastomosis. Surg Laparosc Endosc Percutan Tech 2018; (1): 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morpurgo E, Contardo T, Molaro R et al. . Robotic-assisted intracorporeal anastomosis versus extracorporeal anastomosis in laparoscopic right hemicolectomy for cancer: a case control study. J Laparoendosc Advs Surg Tech A 2013; (5): 414–417. [DOI] [PubMed] [Google Scholar]
- 19.deSouza AL, Prasad LM, Park JJ et al. . Robotic assistance in right hemicolectomy: is there a role? Dis Colon Rectum 2010; (7): 1,000–1,006. [DOI] [PubMed] [Google Scholar]
- 20.Parisi A, Scrucca L, Desiderio J et al. . Robotic right hemicolectomy: Analysis of 108 consecutive procedures and multidimensional assessment of the learning curve. Surg Oncol 2017; (1): 28–36. [DOI] [PubMed] [Google Scholar]
- 21.West NP, Hohenberger W, Weber K et al. . Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 2010; (2): 272–278. [DOI] [PubMed] [Google Scholar]
- 22.Huang JL, Wei HB, Fang JF et al. . Comparison of laparoscopic versus open complete mesocolic excision for right colon cancer. Int J Surg 2015; (Pt A): 12–17. [DOI] [PubMed] [Google Scholar]
- 23.Bae SU, Saklani AP, Lim DR et al. . Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann Surg Oncol 2014; (7): 2,288–2,294. [DOI] [PubMed] [Google Scholar]
- 24.Ozben V, Aytac E, Atasoy D et al. . Totally robotic complete mesocolic excision for right-sided colon cancer. J Robot Surg 2018. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Li J, Sun Y, Li Z et al. . Robotic versus laparoscopic right colectomy: a metaanalysis. World J Surg Oncol 2014; : 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrucciani N, Sirimarco D, Nigri GR et al. . Robotic right colectomy: A worthwhile procedure? Results of a meta-analysis of trials comparing robotic versus laparoscopic right colectomy. J Minim Access Surg 2015; (1): 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JS, Choi GS, Park SY et al. . Randomised clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg 2012; (9): 1,219–1,226. [DOI] [PubMed] [Google Scholar]
- 28.Deutsch GB, Sathyanarayana SA, Gunabushanam V et al. . Robotic vs. laparoscopic colorectal surgery: an institutional experience. Surg Endosc 2012; (4): 956–963. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson AR, Solomon MJ, Lumley JW et al. . Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: The ALaCaRT randomised clinical trial. JAMA 2015; (13): 1,356–1,363. [DOI] [PubMed] [Google Scholar]
- 30.Fleshman J, Branda M, Sargent DJ et al. . Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: The ACOSOG Z6051 randomised clinical trial. JAMA 2015; (13): 1,346–1,355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Speicher PJ, Englum BR, Ganapathi AM et al. . Robotic low anterior resection for rectal cancer: a national perspective on shortterm oncologic outcomes. Ann Surg 2015; (6): 1040–1,045. [DOI] [PubMed] [Google Scholar]
- 32.Jayne D, Pigazzi A, Marshall H et al. . Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: The ROLARR randomised clinical trial. JAMA 2017; (16): 1,569–1,580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Wei Z, Bie M et al. . Robot-assisted versus laparoscopic-assisted surgery for colorectal cancer: a meta-analysis. Surg Endosc 2016; (12): 5,601–5,614. [DOI] [PubMed] [Google Scholar]
- 34.Park S, Kim NK. The role of robotic surgery for rectal cancer: overcoming technical challenges in laparoscopic surgery by advanced techniques. J Korean Med Sci 2015; (7): 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng CL, Rezac C. The role of robotics in colorectal surgery. BMJ 2018; : j5304. [DOI] [PubMed] [Google Scholar]
- 36.D’Annibale A, Pernazza G, Monsellato I et al. . Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc 2013; (6): 1,887–1,895. [DOI] [PubMed] [Google Scholar]
- 37.Kim JY, Kim NK, Lee KY et al. . A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol 2012; (8): 2,485–2,493. [DOI] [PubMed] [Google Scholar]
- 38.Guillou PJ, Quirke P, Thorpe H et al. . Shortterm endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005; (9,472): 1,718–1,726. [DOI] [PubMed] [Google Scholar]
- 39.Scarpinata R, Aly EH. Does robotic rectal cancer surgery offer improved early postoperative outcomes? Dis Colon Rectum 2013; (2): 253–262. [DOI] [PubMed] [Google Scholar]
- 40.Ackerman SJ, Daniel S, Baik R et al. . Comparison of complication and conversion rates between robotic-assisted and laparoscopic rectal resection for rectal cancer: which patients and providers could benefit most from robotic-assisted surgery? J Med Econ 2018; (3): 254–261. [DOI] [PubMed] [Google Scholar]
- 41.Cleary RK, Mullard AJ, Ferraro J, Regenbogen SE. The cost of conversion in robotic and laparoscopic colorectal surgery. Surg Endosc 2018; (3): 1,515–1,524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Pas MH, Haglind E, Cuesta MA et al. . Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013; (3): 210–218. [DOI] [PubMed] [Google Scholar]
- 43.Baik SH, Kim NK, Lim DR et al. . Oncologic outcomes and perioperative clinicopathologic results after robot-assisted tumor-specific mesorectal excision for rectal cancer. Ann Surg Oncol 2013; (8): 2,625–2,632. [DOI] [PubMed] [Google Scholar]
- 44.Baek JH, McKenzie S, Garcia-Aguilar J, Pigazzi A. Oncologic outcomes of robotic-assisted total mesorectal excision for the treatment of rectal cancer. Ann Surg 2010; (5): 882–886. [DOI] [PubMed] [Google Scholar]
- 45.Jayne DG, Thorpe HC, Copeland J et al. . Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 2010; (11): 1,638– 1,645. [DOI] [PubMed] [Google Scholar]
- 46.Pigazzi A, Luca F, Patriti A et al. . Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol 2010; (6): 1,614–1,620. [DOI] [PubMed] [Google Scholar]
- 47.Baik SH, Gincherman M, Mutch MG et al. . Laparoscopic vs open resection for patients with rectal cancer: comparison of perioperative outcomes and long-term survival. Dis Colon Rectum 2011; (1): 6–14. [DOI] [PubMed] [Google Scholar]
- 48.Jayne DG, Brown JM, Thorpe H et al. . Bladder and sexual function following resection for rectal cancer in a randomised clinical trial of laparoscopic versus open technique. Br J Surg 2005; (9): 1,124–1,132. [DOI] [PubMed] [Google Scholar]
- 49.Park SY, Choi GS, Park JS et al. . Shortterm clinical outcome of robot-assisted intersphincteric resection for low rectal cancer: a retrospective comparison with conventional laparoscopy. Surg Endosc 2013; (1): 48–55. [DOI] [PubMed] [Google Scholar]
- 50.Yeo HL, Isaacs AJ, Abelson JS et al. . Comparison of open, laparoscopic, and robotic colectomies using a large national database: outcomes and trends related to surgery center volume. Dis Colon Rectum 2016; (6): 535–542. [DOI] [PubMed] [Google Scholar]
- 51.Rondelli F, Balzarotti R, Villa F et al. . Is robotassisted laparoscopic right colectomy more effective than the conventional laparoscopic procedure? A meta-analysis of short-term outcomes. Int J Surg 2015; : 75–82. [DOI] [PubMed] [Google Scholar]
- 52.Mäkelä-Kaikkonen J, Rautio T et al. . Robotassisted vs laparoscopic ventral rectopexy for external or internal rectal prolapse and enterocele: a randomised controlled trial. Colorectal Dis 2016; (10): 1,010–1,015. [DOI] [PubMed] [Google Scholar]
- 53.Wong MT, Meurette G, Rigaud J et al. . Robotic versus laparoscopic rectopexy for complex rectocele: a prospective comparison of shortterm outcomes. Dis Colon Rectum 2011; (3): 342–346. [DOI] [PubMed] [Google Scholar]
- 54.Mantoo S, Podevin J, Regenet N et al. . Is robotic-assisted ventral mesh rectopexy superior to laparoscopic ventral mesh rectopexy in the management of obstructed defaecation? Colorectal Dis 2013; (8): e469–475. [DOI] [PubMed] [Google Scholar]
- 55.Mäkelä-Kaikkonen J, Rautio T et al. . Roboticassisted and laparoscopic ventral rectopexy in the treatment of rectal prolapse: a matched-pairs study of operative details and complications. Tech Coloproctol 2014; (2): 151–155. [DOI] [PubMed] [Google Scholar]
- 56.van Iersel JJ, Paulides TJ, Verheijen PM et al. . Current status of laparoscopic and robotic ventral mesh rectopexy for external and internal rectal prolapse. World J Gastroenterol 2016; (21): 4,977–4,987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morelli L, Guadagni S, Lorenzoni V et al. . Robot-assisted versus laparoscopic rectal resection for cancer in a single surgeon’s experience: a cost analysis covering the initial 50 robotic cases with the da Vinci Si. Int J Colorectal Dis 2016; (9): 1,639–1,648. [DOI] [PubMed] [Google Scholar]