Abstract
A review of the current status of robotic surgery use in paediatrics
Paediatric robotic surgery offers unique challenges within this rapidly advancing field. The financial costs forproviding this advanced technology are significant for what are essentially low-volume complex cases. There has been a slow rate of uptake within most paediatric surgical centres around the world.due to both to finance as well difficulties associated with equipment primarily designed for adults. Consequently there has been a slow rate of uptake within most paediatric surgical centres around the world. The ergonomics required for the da Vinci® master–slave-type platform currently challenge the small working space in very small children. Only two centres in the UK currently offer robotic surgery for children.* This article aims to review the current state of robotics within the field of paediatric surgery and surgical subspecialties.
Paediatric urology
Urology has arguably seen the greatest uptake of robotic surgery within paediatrics. One of the first uses of robotics in children was a (dismembered Hynes-Anderson) pyeloplasty for pelviureteric junction obstruction (PUJO), because the ureterico-pelvic anastomosis was a significant technical challenge using conventional laparoscopic surgery.1
Robotic-assisted pyeloplasty (RAP)
A meta-analysis of RAP in 2014 examined 12 retrospective studies comparing open pyeloplasty (OP) with RAP and laparoscopic pyeloplasty (LP) with RAP.2 Darzi’s group found a tendency towards better RAP success rates in comparison with LP; although when compared with OP, RAP was equivalent. There was no difference in rates of re-operation and complications between groups. However, OP was statistically significantly faster and cheaper than RAP – although it resulted in a significantly longer hospital stay (+1 day). The studies included were limited by retrospective technique, variable methods of group selection and inconsistent comparison of outcomes, which limit their own conclusions. Subsequent to this, two large multi-centre comparison of OP, LP and RAP3,4 support Cundy’s findings that RAP demonstrated a high success rate and resulted in a significantly shorter hospital stay.3,4 There was also some evidence that there is a lower rate of complication amongst RAP. However, Chan et al’s larger study found no difference in rates of complications.
Ureteral re-implantation5
The standard operative approach for ureteral re-implantation is via an open approach,5 although there is some evidence of comparable success in RAUR. Kasturi et al achieved VUR resolution with a robotic approach in 99.3% of cases6 and this has been supported in a comparison study finding equivalence of RAUR (97%) with open technique (100%),7 as well as in a case matched series that also examined an intra-vesical technique.8 Marchini et al conclude that there is no significant difference in postoperative complications; however, it is worth noting all significant complications – urinary retention, bladder leak and ureteral leak are within the robotic arm of their comparison. A higher rate of bladder spasm and haematuria in the open group account for an overall finding of no difference in complication rates.8 One RAUR was readmitted with a ureteric leak.6 In line with other minimally invasive surgery, there does appear to be a reduced length of stay and postoperative pain.7,9
Ureteroureterostomy
Ureteroureterostomy is performed in a number of indications as the primary procedure (duplex systems with an upper pole ectopic ureter, obstructed ureterocele, etc) and there are several case series reporting successful robotic-assisted ureteroureterostomy.10–12 Most recently, a small comparison of robotic-assisted ureteroureterostomy with open ureteroureterostomy concluded: ‘Operative times and complication rates were comparable with slightly shorter length of hospitalisation in robotic cases.’13
Mitrofanoff and reconstructive bladder surgery
The appendicovesicostomy (Mitrofanoff) has been established as feasible to perform with robotic assistance by a number of case series and in 2015 Grimsby et al compared the open and robotic approaches. They found no difference in rates of complication (26% vs 29%); however, the severity of complications in the robotic group was clinically greater 7.6% (Clavien>3). Furthermore, rates of continence were just 90% when compared with 97% in the open group.14 Similar results are seen in larger multi-centre study of reconstructive surgeries involving Mitrofanoff.15 Grimsby et al attribute these issues to technical adaptation of the operation to robotic platform. Other complex reconstructive surgery has also been examined in a useful, small comparison of open and robotic bladder augmentation (and associated procedures) by Murphy et al, who found similar functional outcomes and rates of complication but with significantly reduced length of stay.16
Those surgeries that must access the pelvis and therefore have a narrow field may well suit a robotic approach
Miscellaneous
Paediatric urology procedures without a reconstructive element have adapted well to traditional laparoscopic approach; nevertheless, there is extensive literature documenting the use of robotics in hemi-nephrectomy17 and nephroureterectomy.18 Those surgeries that must access the pelvis and therefore have a narrow field may well suit a robotic approach. Indeed, there are reports of robotic-assisted excision of bladder diverticulum,19 urachal cyst excision,20 excision of posterior urethral diverticulae,21 prostatic utricles,22 seminal vesicle cyst,23 and varicocele.24
Paediatric general surgery
As in adult surgery, inguinal hernia repairs are commonplace in paediatrics, although they are performed usually through a smaller open inguinal incision. The paediatric laparoscopic hernia repair is also far less involved than its adult counterpart and does not use a mesh, making robotic assistance an unnecessary technical addition in its current format. Other more complex procedures have been carried out robotically.
Fundoplication
A meta-analysis in 2014 reporting outcomes of 297 children25 found that despite a tendency towards conversion to open surgery in the laparoscopic fundoplication (LF) group (6.1% vs 3%), there was no significant difference in postoperative complications (RF 8.9% vs 8% LF) found. In one study the most common complication in the RF and LF was a tight wrap, requiring dilatation (8% and 6%), whereas in the open series wound infections were more common (4%). The meta-analysis was limited as most studies were retrospective with non-synchronous selection of controls, but perhaps most significantly limited by their absence of long-term follow-up of success.
Figure 1.
Paediatric robotic procedure
Figure 2.
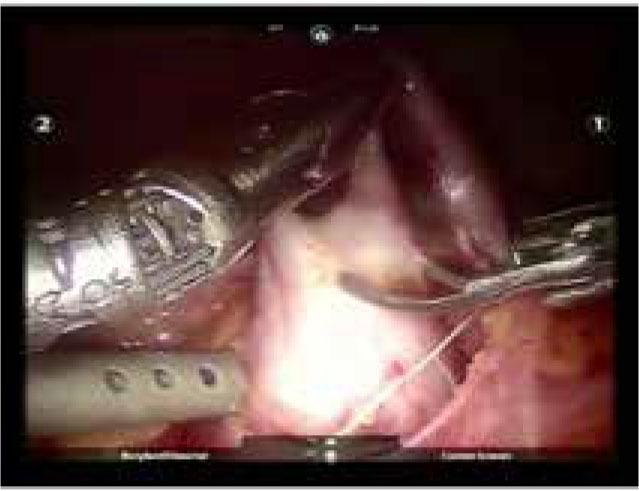
Robotic pyeloplasty (Courtesy of Nisha Rahman, Chelsea and Westminster NHS Foundation Trust)
Hepatobiliary surgery
HPB surgery in children inevitably involves intricate and demanding MIS procedures. Choledochal cyst excision and reconstructive Roux-en-Y hepaticoenterostomy are technically complex and, with the exception of centres in South East Asia,26 open procedures are still relatively prevalent. The laparoscopic technique often involves extending the umbilical incision to allow extra-corporeal anastomosis. Meehan et al describe a robotic approach outlining how additional degrees of freedom offered by the robot conferred a real advantage;27 a view shared by others with experience in the area.28 This approach has also been repeated in small infants (<10kg) (although they use an extracorporeal anastomosis)29 and by the same group in a larger series;30 within this series they converted 19% of their cases, although only 1 patient had any complications. A similar rate of conversion is also seen in another case series,31 which also used extracorporeal anastomosis for the Roux-en-Y loop. Recent evidence,32 however, suggests that laparoscopic Kasai portoenterostomies may have significantly worse outcomes than an open approach. This may reduce enthusiasm for further robotic work.
Miscellaneous
Robotic-assisted cholecystectomies and splenectomies are relatively prevalent in the literature.33–38 However, all authors emphasise that – although these are useful training opportunities in the robot platform – neither robotic-assisted splenectomy nor cholecystectomy seem to offer additional benefit over the laparoscopic approach. Indeed, there is no comparative research in the field.
There are also case reports and series that document a diverse array of successful robotic general and gynaecological surgery such as robotic-assisted diaphragmatic hernia repair,39,40 Heller’s cardiomyotomy for achalasia,41,42 duodenojejunostomy for SMA syndrome,43 repair of duodenal atresia,44 anorectal pull-through for anorectal malformations,38 ovarian cystectomies and salpingo/oophorectomies.45 Further study is needed to assess whether these procedures are indeed effective and whether they confer any benefit above traditional minimally invasive surgery (MIS).
Paediatric cardiothoracic surgery
Cardiac
The surgical interruption of a patent ductus arteriosus (PDA) in robot-assisted thoracoscopic surgery (RATS) vs video-assisted thoracoscopic surgery (VATS) has been described.46 The authors, however, concluded that the VATS procedure is relatively straightforward and that no advantage is offered by RATS, especially allowing for increased complexity of setup and cost.46 One study since examined robotic cardiac surgery specifically in children,47 involving the division of congenital vascular rings, and found RATS comparable but did not offer further advantage over VATS.
Mediastinal
RATS has limited further examination in current literature. The largest series reports on 11 cases including mediastinal cyst excision, diaphragmatic hernia repair, Heller’s myotomy, oesophagoplasty and oesophageal atresia repair via RATS.48 There were several conversions to open surgery in neonatal patients.48 The small neonatal thorax represents the greatest obstacle in adapting the large 5 or 8mm instruments of most robotic platforms into paediatric surgery, and the authors conclude that RATS seems only appropriate in patients with a weight >20kg.
Paediatric oncological surgery
Despite widespread use of MIS in adult oncological surgery and in non-oncological paediatric surgery, open surgery is the usual standard of care for resection of paediatric solid tumours. Paediatric oncological MIS and robotic assistance is a relatively recent development that is lacking high-level evidence,49,50 although there is a wide range of case literature.
Thoracic
Anecdotally, the robot seems well adapted to intricate mediastinal dissection and has been used in the excision of left ventricular myxoma51 and removal of complex massive oesophageal leiomyoma.52 There is support for the robot’s applicability to the mediastinum in a larger case series.53 There is also a relatively large case series that demonstrated in neurogenic chest tumours: ‘Resection [R0] can be as complete as an open procedure without having to complicate the operative technique in the same operating time.’ And the authors felt that ‘the surgeon has a better visualisation of the tumour and its anatomic connections’.54
Abdominal
There are mostly individual case reports for robotic-assisted abdominal oncological surgery in children. Excision of juvenile cystic adenomyoma,55 a radical cystoprostatectomy,55 partial nephrectomy for RCC,57 retroperitoneal lymph node dissection58 and partial adrenalectomy for pheochromocytoma.59 A common theme discussed by many of the authors is of the suitability of the robotic approach to extended lymph node dissection; indeed, one asserts that ‘vision was excellent throughout the procedure, which is very important during dissection and not always the case during open surgery.’56 The solitary case series supports these claims and the authors experienced no complications and achieved R0 resection in all.60
There is some dispute as to whether the fundamental oncological principles of no tumour spillage and total resection of tumour margins can be adhered to by robotic-assisted surgery; a specific concern being the lack of haptics having an impact on the surgeon’s ability to differentiate cancerous from healthy tissue. However, it has been noted that ‘the loss of tactile feedback is, in our opinion, very well compensated for by the excellent optical system’.56 Cancer patients are necessarily followed up for recurrence and only prospective long-term studies of robot resections can give assurances of robotic adherence to oncological principles.
Paediatric neurosurgery
Computer technology has long been used in neurosurgery, specifically in ‘image-guided’ surgery and surgical planning. This has been applied to robotics in the form of the robotised stereotactic assistant (‘ROSA’).61 Robotic-assisted paediatric neurosurgical procedures are sparsely reported, although there is a recent large case series of an array of procedures, making up 128 cases. The authors demonstrated a high rate of success (97.7%) and low rate of complications (3.9%) and no incidences of permanent neurological deficit.61 Such results in a diverse case series is encouraging for the safety and utility of robotic assisted paediatric neurosurgery. Indeed, as is noted in previous case reports, ‘precision of movement millimetre resolution make robotic tools highly attractive in the treatment of intracranial lesions’.62
The future of robotics in paediatric surgery
With advancing technology and the demand for more compact robotic platforms, the future for robotic surgery will no doubt result in a reduction of instrument size and an improvement in haptic feedback. This puts the paediatric patient – in particular, the newborn – at the forefront. Reconstructive surgery such as oesophageal and intestinal anastomosis, all of which require a delicate and more magnified approach will benefit enormously from these advances. The financial restraints that exist in public health systems currently restrict the advancement and training of many in robotics. With a more expanded competitive market, this should improve the landscape but in the interim the paediatric and neonatal patient must be at the forefront of research into the future of robotic surgery.
Footnotes
Leeds General Infirmary and Chelsea and Westminster Healthcare NHS Foundation Trust.
References
- 1.Peters CA. Robotically assisted paediatric pyeloplasty: Cutting edge or expensive toy? BJU International 2004; (9): 1,214–1,215. [DOI] [PubMed] [Google Scholar]
- 2.Cundy TP, Harling L, Hughes-Hallett A et al. Meta-analysis of robot-assisted vs conventional laparoscopic and open pyeloplasty in children. BJU International 2014; (4): 582–594. [DOI] [PubMed] [Google Scholar]
- 3.Silay MS, Spinoit AF, Undre S et al. Global minimally invasive pyeloplasty study in children: Results from the Pediatric Urology Expert Group of the European Association of Urology Young Academic Urologists working party. J Pediatr Urol 2016; (4). [DOI] [PubMed] [Google Scholar]
- 4.Chan YY, Durbin-Johnson B, Sturm RM, Kurzrock EA. Outcomes after pediatric open, laparoscopic, and robotic pyeloplasty at academic institutions. J Pediatr Urol 2017; (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jodal U, Smellie JM, Lax H, Hoyer PF. Ten-year results of randomized treatment of children with severe vesicoureteral reflux. Final report of the International Reflux Study in Children. Pediatr Nephrol 2006; (6): 785–792. [DOI] [PubMed] [Google Scholar]
- 6.Kasturi S, Sehgal SS, Christman MS et al. Prospective long-term analysis of nervesparing extravesical robotic-assisted laparoscopic ureteral reimplantation. Urology 2012; (3): 680–683. [DOI] [PubMed] [Google Scholar]
- 7.Smith RP, Oliver JL, Peters CA. Pediatric robotic extravesical ureteral reimplantation: comparison with open surgery. Journal of Urology 2011; (5): 1,876–1,881. [DOI] [PubMed] [Google Scholar]
- 8.Marchini GS, Hong YK, Minnillo BJ et al. Robotic assisted laparoscopic ureteral reimplantation in children: case matched comparative study with open surgical approach. Journal of Urology 2011; (5): 1,870–1,875. [DOI] [PubMed] [Google Scholar]
- 9.Harel M, Herbst KW, Silvis R et al. Objective pain assessment after ureteral reimplantation: Comparison of open versus robotic approach. J Pediatr Urol 2015; (2). [DOI] [PubMed] [Google Scholar]
- 10.Bansal D, Cost NG, Bean CM et al. Infant robot-assisted laparoscopic upper urinary tract reconstructive surgery. J Pediatr Urol 2014; (5): 869–874. [DOI] [PubMed] [Google Scholar]
- 11.Leavitt DA, Rambachan A, Haberman K, DeMarco R et al. Robot-assisted laparoscopic ipsilateral ureteroureterostomy for ectopic ureters in children: description of technique. J Endourol 2012; (10): 1,279–1,283. [DOI] [PubMed] [Google Scholar]
- 12.Yee DS, Shanberg AM. Robotic-assisted laparoscopic ureteroureterostomy in an adolescent with an obstructed upper pole system and crossed renal ectopia with fusion. Urology 2006; (3): 673.e5–7. [DOI] [PubMed] [Google Scholar]
- 13.Lee NG, Corbett ST, Cobb K et al. Bi-institutional comparison of robotassisted laparoscopic versus open ureteroureterostomy in the pediatric population. J Endourol 2015; (11): 1,237–1,241. [DOI] [PubMed] [Google Scholar]
- 14.Grimsby GM, Jacobs MA, Gargollo PC. Comparison of complications of robot-assisted laparoscopic and open appendicovesicostomy in children. Journal of Urology 2015; (3): 772–776. [DOI] [PubMed] [Google Scholar]
- 15.Gundeti MS, Petravick ME, Pariser JJ et al. A multi-institutional study of perioperative and functional outcomes for pediatric robotic-assisted laparoscopic Mitrofanoff appendicovesicostomy. J Paedr Urol 2016; (6). [DOI] [PubMed] [Google Scholar]
- 16.Murthy P, Cohn JA, Selig RB, Gundeti MS. Robot-assisted laparoscopic augmentation ileocystoplasty and mitrofanoff appendicovesicostomy in children: updated interim results. European Urology 2015; (6): 1,069–1,075. [DOI] [PubMed] [Google Scholar]
- 17.Herz D, DaJusta D, Ching C, McLeod D. Segmental arterial mapping during pediatric robot-assisted laparoscopic heminephrectomy: A descriptive series. J Pediatr Urol 2016; (4): 266.e1–6. [DOI] [PubMed] [Google Scholar]
- 18.Bansal D, Cost NG, Bean CM et al. Comparison of pediatric robotic-assisted laparoscopic nephroureterectomy and laparoendoscopic single-site nephroureterectomy. Urology 2014; (2): 438–442. [DOI] [PubMed] [Google Scholar]
- 19.Christman MS, Casale P. Robot-assisted bladder diverticulectomy in the pediatric population. J Endourol 2012; (10): 1,296–1,300. [DOI] [PubMed] [Google Scholar]
- 20.Rivera M, Granberg CF, Tollefson MK. Robotic-assisted laparoscopic surgery of urachal anomalies: a single-center experience. J Laparoendosc Adv Surg Tech A 2015; (4): 291–294. [DOI] [PubMed] [Google Scholar]
- 21.Alsowayan O, Almodhen F, Alshammari A. Minimally invasive surgical approach to treat posterior urethral diverticulum. Urol Ann 2015; (2): 273–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goruppi I, Avolio L, Romano P et al. Roboticassisted surgery for excision of an enlarged prostatic utricle. Int J Surg Case Rep 2015; : 94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore CD, Erhard MJ, Dahm P. Robotassisted excision of seminal vesicle cyst associated with ipsilateral renal agenesis. J Endourol 2007; (7): 776–779. [DOI] [PubMed] [Google Scholar]
- 24.Hidalgo-Tamola J, Sorensen MD, Bice JB, Lendvay TS. Pediatric robot-assisted laparoscopic varicocelectomy. J Endourol 2009; (8): 1,297–1,300. [DOI] [PubMed] [Google Scholar]
- 25.Cundy TP, Harling L, Marcus HJ et al. Meta analysis of robot-assisted versus conventional laparoscopic fundoplication in children. Journal of Pediatric Surgery 2014; (4): 646–652. [DOI] [PubMed] [Google Scholar]
- 26.Liem NT, Pham HD, Dung LA et al. Early and intermediate outcomes of laparoscopic surgery for choledochal cysts with 400 patients. J Laparoendosc Adv Surg Tech A 2012; (6): 599–603. [DOI] [PubMed] [Google Scholar]
- 27.Meehan JJ, Elliott S, Sandler A. The robotic approach to complex hepatobiliary anomalies in children: preliminary report. J Pediatr Surg 2007; (12): 2,110–2,114. [DOI] [PubMed] [Google Scholar]
- 28.Woo R, Le D, Albanese CT, Kim SS. Robotassisted laparoscopic resection of a type I choledochal cyst in a child. J Laparoendosc Adv Surg Tech A 2006; (2): 179–183. [DOI] [PubMed] [Google Scholar]
- 29.Dawrant MJ, Najmaldin AS, Alizai NK. Robot-assisted resection of choledochal cysts and hepaticojejunostomy in children less than 10 kg. J Pediatr Surg 2010; (12): 2,364–2,368. [DOI] [PubMed] [Google Scholar]
- 30.Alizai NK, Dawrant MJ, Najmaldin AS. Robotassisted resection of choledochal cysts and hepaticojejunostomy in children. Pediatr Surg Int 2014; (3): 291–294. [DOI] [PubMed] [Google Scholar]
- 31.Chang EY, Hong YJ, Chang HK et al. Lessons and tips from the experience of pediatric robotic choledochal cyst resection. J Laparoendosc Adv Surg Tech A 2012; (6): 609–614. [DOI] [PubMed] [Google Scholar]
- 32.Hussain MH, Alizai N, Patel B. Outcomes of laparoscopic Kasai portoenterostomy for biliary atresia: A systematic review. Journal of Pediatric Surgery 2017; (2): 264–267. [DOI] [PubMed] [Google Scholar]
- 33.Cundy TP, Shetty K, Clark et al. The first decade of robotic surgery in children. Journal of Pediatric Surgery 2013; (4): 858–865. [DOI] [PubMed] [Google Scholar]
- 34.Mahida JB, Cooper JN, Herz D et al. Utilization and costs associated with robotic surgery in children. Journal of Surgical Research 2015; (1): 169–176. [DOI] [PubMed] [Google Scholar]
- 35.Meehan JJ, Sandler A. Pediatric robotic surgery: A single-institutional review of the first 100 consecutive cases. Surgical Endoscopy and Other Interventional Techniques 2008; (1): 177–182. [DOI] [PubMed] [Google Scholar]
- 36.Klein MD, Langenburg SE, Lelli JL et al. Pediatric robotic surgery: Lessons from a clinical experience. J Laparoendosc Adv Surg Tech A 2007; (2): 265–271. [DOI] [PubMed] [Google Scholar]
- 37.Alqahtani A, Albassam A, Zamakhshary M et al. Robot-assisted pediatric surgery: how far can we go? World Journal of Surgery 2010; (5): 975–978. [DOI] [PubMed] [Google Scholar]
- 38.Al-Bassam A. Robotic-assisted surgery in children: advantages and limitations. J Robot Surg 2010; (1): 19–22. [DOI] [PubMed] [Google Scholar]
- 39.Meehan JJ, Sandler A. Robotic repair of a Bochdalek congenital diaphragmatic hernia in a small neonate: robotic advantages and limitations. J Pediatr Surg 2007; (10): 1,757–1,760. [DOI] [PubMed] [Google Scholar]
- 40.Meehan JJ, Torres JE. Robotic repair of Morgagni congenital diaphragmatic hernia in an infant. J Robot Surg 2008; (2): 97–9. [DOI] [PubMed] [Google Scholar]
- 41.Chaer RA, Jacobsen G, Elli F, Harris J, Goldstein A, Horgan S. Roboticassisted laparoscopic pediatric Heller’s cardiomyotomy: initial case report. J Laparoendosc Adv Surg Tech A 2004; (5): 270–3. [DOI] [PubMed] [Google Scholar]
- 42.Altokhais T, Mandora H, Al-Qahtani A, Al-Bassam A. Robot-assisted Heller’s myotomy for achalasia in children. Comput Assist Surg 2016; (1): 127–131. [DOI] [PubMed] [Google Scholar]
- 43.Bütter A, Jayaraman S, Schlachta C. Robotic duodenojejunostomy for superior mesenteric artery syndrome in a teenager. J Robot Surg 2010; (4): 265–269. [DOI] [PubMed] [Google Scholar]
- 44.Meehan JJ. Robotic repair of congenital duodenal atresia: a case report. J Pediatr Surg 2007; (7): E31–3. [DOI] [PubMed] [Google Scholar]
- 45.Nakib G, Calcaterra V, Scorletti F et al. Robotic assisted surgery in pediatric gynecology: promising innovation in mini invasive surgical procedures. J Pediatr Adolesc Gynecol 2013; (1): e5–7. [DOI] [PubMed] [Google Scholar]
- 46.Le Bret E, Papadatos S, Folliguet T et al. Interruption of patent ductus arteriosus in children: Robotically assisted versus videothoracoscopic surgery. Journal of Thoracic and Cardiovascular Surgery 2002; (5): 973–976. [DOI] [PubMed] [Google Scholar]
- 47.Mihaljevic T, Cannon JW, del Nido PJ. Robotically assisted division of a vascular ring in children. Journal of Thoracic and Cardiovascular Surgery 2003; (5): 1,163–1,164. [DOI] [PubMed] [Google Scholar]
- 48.Ballouhey Q, Villemagne T, Cros J et al. Assessment of paediatric thoracic robotic surgery. Interactive Cardiovascular and Thoracic Surgery 2015; (3): 300–303. [DOI] [PubMed] [Google Scholar]
- 49.van Dalen EC, de Lijster MS, Leijssen LGJ et al. Minimally invasive surgery versus open surgery for the treatment of solid abdominal and thoracic neoplasms in children. Cochrane Database of Systematic Reviews 2015(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan KW, Lee KH, Tam YH, Yeung CK. Minimal invasive surgery in pediatric solid tumors. J Laparoendosc Adv Surg Tech A 2007; (6): 817–820. [DOI] [PubMed] [Google Scholar]
- 51.Hassan M, Smith JM. Robotic assisted excision of a left ventricular myxoma. Interactive Cardiovascular and Thoracic Surgery 2012; (1): 113–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeUgarte DA, Teitelbaum D, Hirschl RB, Geiger JD. Robotic extirpation of complex massive esophageal leiomyoma. J Laparoendosc Adv Surg Tech A 2008; (2): 286–289. [DOI] [PubMed] [Google Scholar]
- 53.Meehan JJ, Sandler AD. Robotic resection of mediastinal masses in children. J Laparoendosc Adv Surg Tech A 2008; (1): 114–119. [DOI] [PubMed] [Google Scholar]
- 54.Lacreuse I, Valla JS, de Lagausie P et al. Thoracoscopic resection of neurogenic tumors in children. Journal of Pediatric Surgery 2007; (10): 1,725–1,728. [DOI] [PubMed] [Google Scholar]
- 55.Akar ME, Leezer KH, Yalcinkaya TM. Robotassisted laparoscopic management of a case with juvenile cystic adenomyoma. Fertility and Sterility 2010; (3): E55–E6. [DOI] [PubMed] [Google Scholar]
- 56.Anderberg M, Backman T, Annerstedt M. Robot-assisted radical cystoprostatectomy in a small child with rhabdomyosarcoma: a case report. J Robot Surg 2008; (2): 101–103. [DOI] [PubMed] [Google Scholar]
- 57.Cost NG, Geller JI, DeFoor WR et al. A robotic-assisted laparoscopic approach for pediatric renal cell carcinoma allows for both nephron-sparing surgery and extended lymph node dissection. Journal of Pediatric Surgery 2012; (10): 1,946–1,950. [DOI] [PubMed] [Google Scholar]
- 58.Cost NG, DaJusta DG, Granberg CF et al. Robot-assisted laparoscopic retroperitoneal lymph node dissection in an adolescent population. Journal of Endourology 2012; (6): 635–640. [DOI] [PubMed] [Google Scholar]
- 59.Rogers CG, Blatt AM, Miles GE et al. Concurrent robotic partial adrenalectomy and extra-adrenal pheochromocytoma resection in a pediatric patient with von Hippel-Lindau disease. Journal of Endourology 2008; (7): 1,501–1,503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meignan P, Ballouhey Q, Lejeune J et al. Robotic-assisted laparoscopic surgery for pediatric tumors: a bicenter experience. J Robot Surg 2017. [DOI] [PubMed] [Google Scholar]
- 61.De Benedictis A, Trezza A, Carai A et al. Robot-assisted procedures in pediatric neurosurgery. Neurosurgical Focus 2017; (5). [DOI] [PubMed] [Google Scholar]
- 62.Vougioukas VI, Hubbe U, Hochmuth A et al. Perspectives and limitations of image-guided neurosurgery in pediatric patients. Childs Nervous System 2003; (12): 783–791. [DOI] [PubMed] [Google Scholar]


