Abstract
How robotics could help shape the future of surgical care
For 20 years Intuitive Surgical’s da Vinci® system has held the monopoly in minimally invasive robotic surgery. Restrictive patenting, a well-developed marketing strategy and a high-quality product have protected the company’s leading market share.1 However, owing to the nuances of US patenting law, many of Intuitive Surgical’s earliest patents will be expiring in the next couple of years. With such a shift in backdrop, many of Intuitive Surgical’s competitors (from medical and industrial robotic backgrounds) have initiated robotic programmes – some of which are available for clinical use now. The next section of the review will focus on new and developing robotic systems in the field of minimally invasive surgery (Table 1), single-site surgery (Table 2), natural orifice transluminal endoscopic surgery (NOTES) and non-minimally invasive robotic systems (Table 3).
Table 1.
Novel robotic systems in minimally invasive surgery
| Device | Developer | Special features | Clinical use |
|---|---|---|---|
| Telelap ALF-X/Senhance surgical system | TransEntrix Inc. (Morrisville, US) | 3 separate cart mounted robotic arms, 3D-HD ready with glasses laparoscopic handles with haptic force feedback, eye-tracking camera | CE approval 2006, FDA approval 2017, gynaecology, colorectal, limited cardiothoracics |
| Versius | CMR Surgical (UK) | 5 light weight portable separate cart mounted robotic arms, 3D HD ready monitor with glasses, haptic feedback | Cadaveric and animal studies, anticipated CE approval in 2018. Gynaecology, upper GI, renal, colorectal. |
| MiroSure | Medtronic (Dublin, Ireland), DLR (Germany) | 3–5 lightweight arms attached to operating table, impedance mode, Autopointer, 3D-HD ready with 3D glasses, haptic feedback | Development stage, no FDA/CE approval, minimally invasive surgery |
| REVO-I | Meere Company (South Korea) | 4 robotic arms with single Cart, open 3D/HD control console | Korean ministry of food and drug safety approval 2017. human trials underway, gynaecology, urology, general surgery |
| M7 Telerobotic surgical system/Verb Surgical | Stanford Research International Robotics. Licencing from Verb Surgical | 2 lightweight robotic arms (15kg) with 7 d.o.f., auditory, visual, tactile feedback. Motion compensation | In development, no FDA/CE approval, minimally invasive surgery |
Table 2.
Robotic systems in single port surgery and natural orifice transluminal endoscopic surgery (NOTES)
| Device | Developer | Features | Clinical use |
|---|---|---|---|
| SurgiBot | Transenterix (US) | Bedside control unit, 3D-HD ready with 3D glasses | Single port MIS, FDA rejected, seeking CFDA approval |
| da Vinci® SP1098 platformw | Intuitive Surgical (US) | 3D HD ready articulated camera, 25mm access port, compatible with da Vinci® Xi side cart | Single port minimally invasive surgery, no FDA/CE approval, human pre-clinical studies |
| SPORT | Titan medical (US) | Open console, 3D/HD ready, 25mm single port | Single port minimally invasive surgery, no FDA/CE approval, pre-clinical trials underway |
| MIVR | Virtual Incision (US) | 2 robotic arms, miniaturised motor units housed within the arms themselves, reduced extracorporeal footprint | Single port minimally invasive surgery No FDA/CE approval, human trials conducted |
| MASTER | EndoMaster Pte LTD | 2 robotic arms incorporated in to videoscope/endoscope, haptic feedback, grasper and electrocautery function | NOTES no FDA/CE approval, human clinical trials 2012 |
Table 3.
Novel non-minimally invasive robotic systems
| Device | Developer | Features | Clinical use |
|---|---|---|---|
| Avicenna Roboflex | ELMED (Turkey) | Robotic FURS hand piece manipulator, open surgeon console, increased deflexion range | Retrograde intrarenal surgery, CE approval, human trials, FDA approval pending |
| Medical Microinstruments | Medical Microinstruments (Italy) | World’s smallest robotic articulating wrist, 3mm outer diameter | Microsurgery, preclinical trials underway |
| Preceyes surgical system | Preceyes BV (Eindenhoven, Holland) | Single robotic bedside arm capable of <10micron precision, Intuitive bedside control unit | Vitreoretinal eye surgery, human trials underway, no FDA/CE approval |
Novel and developmental systems in minimally invasive surgery
Senhance Surgical Robotic System
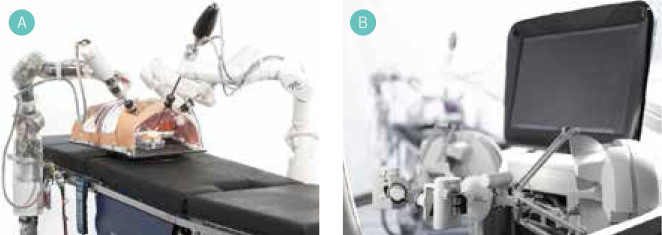
TransEnterix’s (US) Senhance Surgical Robotic System (Fig 1), which was originally developed by the robotic division of Italian healthcare company SOFAR (Italy) and formerly branded the Telelap ALF-X, represents the only competition to the da Vinci® system that has both CE approval for use in Europe and FDA approval. The distinguishing features of this system include an open remote control console capable of 3D-HD through use of 3D glasses, an eye-tracking system for camera control, three laparoscopic robotic arms on separate carts (as opposed the single cart seen in the da Vinci® system), laparoscopic handles with six degrees of freedom to control laparoscopic robotic instruments and, most impressively, haptic force feedback to the surgeon provided through the laparoscopic handles.2 In Europe the system is licensed for use in abdominal, pelvic and thoracic procedures, excluding cardiac surgery. To date clinical trials have shown the Senhance system to be safe and efficacious in minimally invasive surgery. However, trials are from a single centre, involve small patient numbers and include only gynaeco-logical and colorectal procedures.3,4 Animal studies have been successfully conducted in porcine partial nephrectomy and bovine pulmonary lobectomy.2,5
Figure 1.
Senhance Surgical system – Single robotic arm units and open control system. (Image credit TransEnterix Surgical Inc)
Versius
Versius (Fig 2) is developed by the UK-based CMR Surgical and is designed for use in gynaecology, upper GI surgery, colorectal and urology. The system consists of an open control console with 3D-HD monitor necessitating 3D glasses and up to 5 collaborative lightweight individually mounted robotic arms controlled at an open control console providing haptic feedback to the operating surgeon. There is a strong focus on the light-weight compact nature of the robotic arms, which provides seven degrees of freedom and allows for easy set-up and portability across theatres. At present preliminary animal studies and cadaveric trials have taken place; however, the company is anticipated to file for approval for clinical use in 2018.6
Figure 2.
Versius surgical system. (Image credit CMR Surgical)
MiroSure
MiroSure (Fig 3) stemmed from research into telesurgery onboard spacecraft and was developed by the Robotic and Mechatronic wing of the German Aerospace Centre (DLR).7 The licence for commercial use of MiroSure was sold to Medtronic (Dublin, Ireland) in 2013. Medtronic are currently developing a new device incorporating many of the elements of MiroSure. The key features of Miro-Sure system include up to five minimally invasive robot-assisted (MIRO) arms that, unlike the other systems described, can attach to the operating table rails and represent an interesting development allowing operating table repositioning without robot undocking or interrupting the surgery. Each MIRO arm weighs 10kg, provides 7 degrees freedom of movement and uses a variety of laparoscopic instruments. The system contains a 3D-HD monitor with 3D glasses and the control mechanism provides haptic feedback. This system contains some truly unique features. First, the MIRO arms contain an ‘impedance-controlled mode’ whereby the assistant can reposition the robotic arms without altering the position and orientation of the end effecter, which enables surgery to continue uninterrupted and allows quick access to the patient.8 Second, an AutoPointer is included that projects an image onto the patient to guide optimal port placement for efficient kinematics and collision avoidance.9 DLR’s ultimate aim is to operate on the beating heart, with motion compensation providing automatic co-ordination of end effectors in synchronicity with systole/diastole providing the surgeon with a static image of the heart. This feature remains in the early research stage but demonstrates an interesting future application of MiroSure. Currently Medtronic are developing their tenth prototype and the robotic system is expected to launch in India first, followed by the US in 2018. There is currently no FDA/CE approval in place.
Figure 3.
MiroSure robotic system a) Table-mounted robotic arms b) Open 3D-HD control console. (Image credit DLR, CC-BY 3.0)
REVO-I
The MSR-5000 REVO-I Robotic Surgical System was developed by South Korean Meere Company and introduced in 2015.10 The system includes an open 3D-HD control system requiring 3D glasses and a 4-arm single mounted cart that is analogous in layout to that of the da Vinci® SI. Currently there is no haptic feedback but it is reported that Meere Company are integrating this technology in their newest model.11 To date, more than 20 pre-clinical animal studies have been conducted with the Revo-I and the South Korean Ministry of Food and Drug Safety granted approval in August 2017 for use in human clinical trials.
Verb Surgical
Verb Surgical was founded in 2015 from a collaboration between Ethicon Endo-Surgery (a division of Johnson & Johnson) and Verily (formerly Google Life sciences). The company remains secretive about their future robotic system. However, their goal is both public and lofty, ie to create ‘Surgery 4.0’, which transcends the current paradigm of master–slave surgery and incorporates increased robotic autonomy and machine learning.12 Verb Surgical is licensing robotic technology from Stanford Research International Robotics (SRI), which began development of the M7 Telerobotic surgical system back in 1998. The key features of this system include 2 robotic arms with 7 degrees of freedom, a lightweight system weighing 15kg, visual, auditory and tactile feedback to the surgeon, and compensation for external movements such as those caused by turbulence on a plane or moving vehicle.13 This system was principally developed for long-distance telesurgery (particularly in the battlefield) and trials have been conducted both 60 feet underwater and on-board the NASA C-9 aircraft, simulating surgery in zero gravity. Verb Surgical’s future system is shrouded in corporate secrecy but, given the considerable financial backing and expertise from the companies involved, the potential is exciting. We currently lack specific details but Verb’s upcoming system may incorporate technology from SRI’s M7 surgical system.
Single-port surgery
Robotic laparo-endoscopic single site (R-LESS) surgery aims to improve upon the benefits of conventional multiport robotic surgery by decreasing the number of surgical incisions leading to improved cosmesis; reduced recovery time, reduction in postoperative pain and decreased postoperative incisional herni.14 There are disadvantages to decreasing the number of ports in use – principally poor ergonomics, loss of triangulation, instrument clashing, limited tissue retraction and lack of space for surgical assistant.15 To date, the experience of R-LESS has involved modification of existing robotic systems for use with single-access ports, specialised instruments such as Intuitive Surgical’s semi-rigid curved VeSPA instruments, and development of new software for left–right control swapping to negate issues with triangulation.14 These modifications compromise on the functionality of the robotic systems. However, there are several purpose-built single-site robotic systems in development and these are discussed in the next section (Table 2).
da Vinci® SP 1098 surgical system
The Intuitive Surgical da Vinci® SP 1098 platform (Fig 4) has been developed specifically for use in R-LESS since 2014. The system’s unique features include access through a 25mm single port; introduction of 3 6mm articulated arms with surgical instrument end effectors; 8mm articulating 3D-HD camera; slim-lined single-arm platform compatible with da Vinci® Xi side cart; and a new clutch system on the control console to enable control of the articulating instruments separate to control of the platform single arm. Of vital importance is the re-introduction of Intuitive Surgical’s endo-wrist technology, which was lost in the VeSPA instruments, and an extra ‘elbow-joint’ allowing the instrument to ‘fan out’ once through the port, reproducing the conventional multi-port robotic set-up.15 Although Intuitive Surgical gained FDA approval for single-port surgery in urology in 2014, it decided not to market and instead focus on developing the purpose-built SP 1098 platform. This system is yet to receive CE/FDA approval and remains in the development stage. Two human pre-clinical trials have taken place involving five patients in R-LESS radical/partial nephrectomy and three patients in R-LESS radical perineal prostatectomy. Both studies concluded that R-LESS with the SP 1098 platform is feasible but will require further investigation to compare with conventional multi-port robotic surgery.
Figure 4.

Intuitive Surgical SP 1098 single port articulated endoscope and instruments. (Image credit Rassweiler et al, 201725)
Surgibot
Surgibot was developed by TranEnterix (US), who upgraded on the SPIDER16 platform used in non-robotic laparo-endoscopic single-site surgery by adding a robotic arm to control the laparoscopic instruments. It represents a unique system because the control resides within the sterile field at the patient’s bedside sterile field. The platform includes two laparoscopic handles manipulating two articulated instruments inserted through a single port. The camera is 3D-HD ready requiring the surgeon to wear 3D glasses to view an open cart mounted monitor. The FDA rejected Transenterix’s Surgibot in 2016 and since then the company has focused on Asian markets by establishing a sales agreement with Chinese Great Belief International Limited for production and sale of the system in China. To date, the system does not have approval from the China Food Drug Administration and its use has been limited to porcine models in cholecystectomy.17
SPORT
The Single Port Orifice Robotic Technology surgical system (SPORT) (Fig 5) is developed by Titan Medical (US) for use in multi-quadrant R-LESS. It incorporates an open surgeon control console with 3D-HD touchscreen viewing monitor to control 2 articulating arms attached to a manoeuvrable patient cart designed for a 25mm single port. A unique feature is the disposable end effectors, which may reduce cost. FDA approval is awaited but pre-clinical trials in single-port prostatectomy began in February 2018.18
Figure 5.

Single Port Orifice Robotic Technology surgical system a) Open 3D-HD workstation b) single port articulated endoscope and instruments. (Images credit Titan Medical Inc)
Miniature in vivo robot
The miniature in vivo robot surgical system (MIVO) developed by Virtual Incision (US) and the University of Nebraska’s medical centre represents a unique development on previously described R-LESS systems as the bulk of the robotic system resides within the abdominal cavity during surgery. The system includes a central rod attached to two triple-jointed robotic arms with replaceable miniaturised end effectors capable of four degrees of freedom. The central rod is inserted through a 3.5cm incision and can be rotated allowing access to all quadrants of the abdomen. The advantage of this system is that the motor units are housed within the arms themselves, thereby significantly reducing the extracorporeal footprint and allowing easy access to the patients.19 Virtual Incision will focus on general surgery procedures. Currently there is no FDA approval but Virtual Incision has recently gained funding to apply. There has been one human trial conducted in 2016 in Paraguay for a colonic resection to assess feasibility of the system and Virtual Incision plan to commercialise soon.
Robotic Natural Orifice Transluminal Endoscopic Surgery (R-NOTES)
NOTES demonstrates a novel approach to minimally invasive surgery – breaking from the traditional minimally invasive approach of gaining access to the surgical site through abdominal incisions and instead using the body’s natural orifices to enable scarless hernia-free surgery. The NOTES technique has been in development for many years – the first reported transgastric endoscopic appendicectomy was performed in 2004 and the technique has propagated. Initially, the transgastric route was preferred; however, transvaginal, transcolonic, transcystic and transoesophageal techniques have been described with increasing proficiency. Similarly, the number of operations has increased to include challenging resections inside abdominal cavity, such as distal pancreatectomy via transvaginal/transcolonic route.20 The technique has not been adopted as universally as expected and this is because of the extremely challenging surgical nature of the technique, which has prompted the development of robotic systems to shorten the learning curve (Table 2).
MASTER
The Master and Slave Transluminal Endoscopic Robot (Fig 6) is under development by Endomaster Pte Ltd. The system consists of a flexible endoscope incorporating a videoscope, two robotically controlled effector arms with grasping and electrocautery function and seven degrees-of-freedom movement.21 A surgeon at the master control unit manipulates the haptic feedback-enabled end effector instruments; however, the endoscope requires human control and coordination with a second operator. Initial animal trials successfully performed endoscopic submucosal stomach dissection and transgastric hepatic wedge resection in porcine models with comparable operative time to traditional methods.21 Human trials have been conducted in India and Singapore on five patients undergoing successful MASTER-assisted resection of early gastrointestinal tumours with no complications and early discharge.22 The MASTER system currently has no FDA/CE approval but EndoMaster have received Series B funding for further development and commercialisation of the system.
Figure 6.

Master–Slave Transluminal Endoscopic Robot (MASTER) for us in R-NOTES. (Image credit Endomaster Pte Ltd)
Other R-NOTES/endoluminal systems
There are a number of flexible endoscopes with robotically controlled instruments including the ISIS-Scope (Karl Storz), Endomina (Endo Tools), Scorpion Shaped endoscopic robot (Kyushu University), and the Viacath system (Hansen Medical).23 Although the majority of these systems are developed for use in gastroenterology and thus beyond the remit of this review, it is likely that with improving R-NOTES technology the traditional boundaries between conventional resection surgery and endoluminal approaches may become less obvious.
Novel non-minimally invasive robotic systems
The majority of focus and publicity on robotic surgery has been in minimally invasive surgery; however, there are several interesting robotic developments in non-minimally invasive surgery-focused specialties. These systems are described in Table 3.
Avicenna Roboflex
A noteworthy robotic system that inhabits its own subcategory is the Avicienna Roboflex (Fig 7) for use in endourology. It was developed by Turkish company ELMED to facilitate robotic flexible ureterenoscopy for treatment of nephrolithiasis. Conventional hand-operated flexible ureterenoscopy has some disadvantages for the surgeon, ie poor ergonomics and exposure to ionizing radiation. A survey of 160 endo-urologists found that 64.2% complained of orthopaedic problems, predominantly relating to the back (38.1%) and neck (27.6%).24 The Roboflex system includes a flexible ureteroscope hand-piece manipulator unit and a surgeon-control console with two joysticks to control the flexible end of the ureteroscope. The control unit has a touchscreen for adjustment of the laser fibre and control of irrigation inflow. The joystick controllers are ergonomically superior to conventional hand-unit control and the control console can be position far away from the radiation source. A human study of 132 patients undergoing traditional flexible ureterenoscopy vs robotic ureterenoscopy with the Roboflex system found comparable procedure time and stone-free rates.25 The system gained CE approval for use in Europe in 2013 but FDA approval is still pending.
Figure 7.
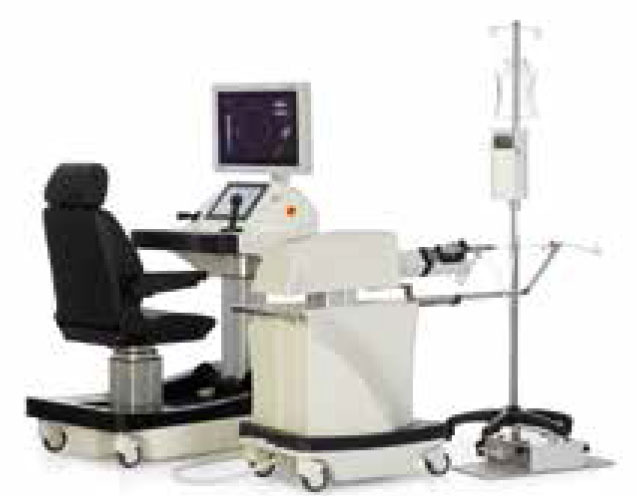
Avicenna Roboflex. Open robotic control console and robotic flexible ureterenoscope. (Image credit Elmed Medical Systems Inc.)
Medical Microinstruments
Medical Microinstruments (MMI) (Fig 8) is an Italian robotic company producing a robotic platform for reconstructive microsurgery. The system uses 2 tiny robotic arms with articulated robotic wrists of 3mm in outer diameter and 150micron width robotic scissors, allowing for easy manipulation of 12.0 suture. The surgeon views the operating field through a microscope and uses a separate control unit with scaled-down tremor-free manipulation of the instruments.
Figure 8.
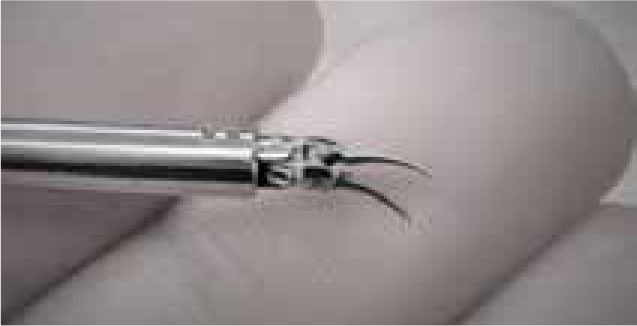
Robotic microinstrument. (Image credit Medical Microinstruments)
Preclinical work including robotic 0.35mm vascular anastomosis has recently been conducted26 and it is expected that robotic surgery will enable super-microsurgery for lymph vessel anastomosis and micro-neurosurgery in the near future.27
Preceyes surgical system
Dutch company Preceyes BV is developing the Preceyes surgical system (Fig 9) for use in vitreoretinal eye surgery. The system gains access to the eye through a 1mm incision and operates at a precision level of 10 microns, which is far superior to that achievable by any unaided ophthalmologist. The operator views the surgical site through a microscope and the robotic joystick is placed at the patient’s bedside, translating and scaling down movements to the micron level. The world’s first human robotic eye surgery using the Preceyes system was performed in Oxford in 2016 and currently the Robotic Retinal Dissection Device Trial (NCT0305 2881) is underway at the University of Oxford, with an expected completion date April 2018.28 The system does not have CE/FDA approval.
Figure 9.
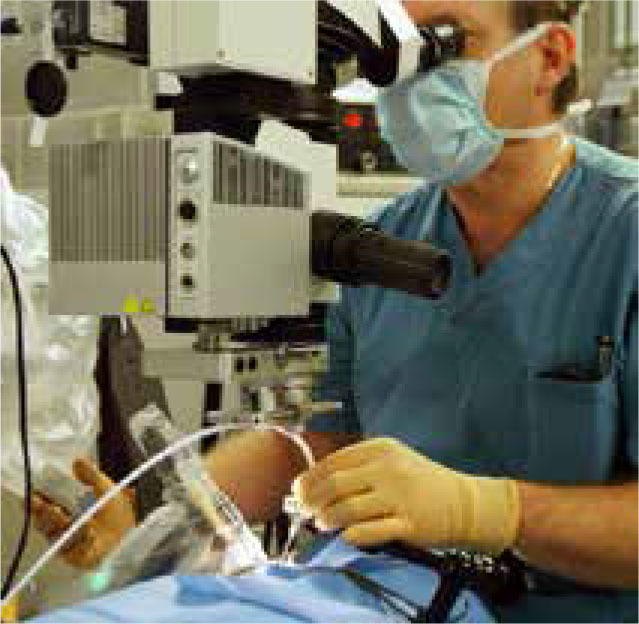
Preceyes microscope and robotic arm. (Image credit Preceyes BV)
Advances in haptic feedback
An often-cited criticism of robotic surgery is the lack of haptic feedback to the surgeon. Haptic feedback is described as a combination of force/kinaesthetic feedback and tactile/cutaneous feedback. Force feedback is useful in assessing the tension or pressure across tissue or suture, whereas tactile feedback provides information on local tissue properties such as compliance or viscosity. In minimally invasive surgery, haptic feedback is attenuated owing to the long shaft of a laparoscopic instrument. In robotic surgery all haptic feedback is lost owing to the dissociation of surgeon to end effector by the robotic system. Studies have shown that absence of haptic feedback can lead to both excessive and inadequate forces being applied to tissue during robotic surgery, which lead to increased tissue injury and inappropriate suture handling.29,30
Advantages of haptic feedback
It seems intuitive that increased sensory feedback should be beneficial to the surgeon. However, many experienced robotic surgeons compensate for the lack of haptic feedback by becoming highly attuned to visual cues such as tissue deformation to act as surrogates for tension and force. Ortmaier et al demonstrated that haptic feedback significantly reduced errors in a telerobotic dissection task.31 The study was corroborated by Wagner et al,32 reporting a significant decreased level of force applied to tissue and a decrease in number of inadvertent incursions into sensitive structures during a simulation involving a force-feedback enabled platform. The majority of trials investigating haptic feedback and RS are ex vivo using surgical models, there are just a handful of clinical trials. Amirabdollahian et al33 conducted a rigorous literature review of haptic feedback-mediated surgery and found only 2 out of the 74 included studies were conducted on patients. One of these studies included the aforementioned haptic feedback-enabled MAKO surgical system and reported increased accuracy of implant placement in unicompartmental arthroplasty.34 The advantages of tactile feedback, as opposed to force feedback, are not easy to quantify. A limited number of studies have considered the benefit of tactile sensors in palpation exercises; for example, palpating a lung for the presence of a tumour35 or ‘feeling’ for gallstones within a gallbladder using laparoscopic instruments.36 The number of experimental studies assessing the benefit of tactile feedback is limited in robotic surgery and there are no human clinical trials published at the time of writing.
Current status and research
At present, the Senhance surgical system and MAKO RIO surgical system (Table 4) are the only commercially available surgical robotic systems providing haptic feedback. There have been no clinical studies comparing the benefits of haptic feedback versus no haptic feedback in these systems. The majority of robotic systems in development (Table 1) include haptic feedback and it seems this will be the benchmark for future systems. The latest da Vinci® Xi platform does not incorporate haptic feedback and there is no indication from Intuitive Surgical on whether future systems will include this feature. Several recent studies have made interesting modifications to the da Vinci® system to add haptic feedback functionality. Pacchieroitti et al37 provide tactile feedback to the surgeon by implanting a BiotTac tactile Sensor onto the effector end of the da Vinci® robotic laparoscopic instrument. The information is relayed to the surgeon through a custom fingertip feedback device mounted to a da Vinci® master controller providing cutaneous feedback to the surgeon’s index-finger tip. This modification significantly improved task performance in a simulated palpation exercise. Mahvash et al38 customised a da Vinci® instrument arm to provide direct force feedback to the controller and reported a significant improvement in palpating hardened nodules in soft-tissue models.
Table 4.
Currently commercially available robotic systems
| System | Developer | Special Features | Clinical Use |
|---|---|---|---|
| Da Vinci® Xi 4th generation | Intuitive Surgical (Sunnyvale, US) | 4 arms on single cart, 8mm instruments, finger-tip control endo-wrist with 7 d.o.f, closed 3D-HD console | Minimally invasive surgery FDA/CE approved Worldwide use |
| Mako RIO surgical system | Stryker orthopaedics (Kalamazoo, US) | Haptic feedback, single cart robotic arm, milling device | Orthopaedics, FDA approval for hip and partial/total knee arthroplasty in 2008 |
| Navio surgical system | Smith and Nephew (US) | Handheld semi-active handheld device, guides milling based on preoperative imaging, live tracking | Orthopaedics, FDA approval 2012 for total and partial knee arthroplasty |
| Mazor Renaissance | Mazor Robotics (Israel) | CT image analysis with fluoroscopic registration, mounted guide for 1.5mm precision of intervention | Orthopaedics, FDA approval 2011 |
| Sensei X robotic system | Hansen Medical (US) | Remote workstation, Mechanically driven catheter delivery | Cardiac intervention, FDA approval 2007 |
| Niobe Magnetic Navigation system | Sterotaxis (USA) | Remote work station, magnetically driven catheter delivery | Cardiac intervention, FDA approval 2003 |
| Amigo Remote Catheter system | Catheter Robotics | Remote control, mechanically driven catheter delivery | Cardiac intervention, FDA approval 2012 |
| Magellan Robotic system | Hansen medical (US) | Remote workstation, mechanically driven catheter delivery | Peripheral vascular intervention, FDA approval 2012 |
| Corpath 200 System | Corindus Vascular Robotics | Remote workstation, mechanically driven catheter delivery | Cardiac intervention, FDA Approval 2012 |
Sensory substitution
In order to avoid the difficulties encountered in relaying force and tactile information directly to the surgeon’s hands, many studies have focused on ‘sensory substitution’ providing haptic information though auditory or graphical cues.39 Reilly et al40 evaluated the benefit of employing visual force feedback (VFF) on robotic surgical knot tying using a da Vinci® robotic system. Strain gauges were implemented into the instruments to measure bending forces and a graphical overlay displayed ideal suture tension in a non-intrusive manner. The study reported significantly fewer suture breakages and lower peak force across suture with VFF.
Virtual fixtures
An interesting application of haptic feedback is the creation of ‘virtual fixtures’ using software to create ‘no-go’ areas and preventing robotic instruments from entering into sensitive tissue. The MAKO RIO system uses virtual fixtures to enable milling of bone to a preoperative plan and disables milling outside of pre-set boundaries. Such an application could easily be applied to minimally invasive surgery to avoid inadvertent injury to major vessels or adjacent organs. One recent study conducted resection of glioma in 18 patients using the NeuroArm robot and reported that the creation of a surgical corridor using virtual fixtures increased the safety and performance of the operation.41
It is problematic to add haptic feedback to an existing robotic system owing to issues with size, weight, biocompatibility, sterility and cost. Thus if the da Vinci® system is to gain haptic feedback it will require significant re-design of the robotic arms and control system. There is intense research focus in this area, which is beginning to translate into clinical practice with novel systems adopting force feedback. The future role of tactile feedback is difficult to assess, given that the research is still in its infancy and the technical difficulty of adding a tactile sensor to an instrument without diminishing its functionality. Haptic feedback is an area where Intuitive Surgical’s rivals may gain a competitive advantage in the surgical robotics market and it is likely to represent the ‘gold standard’ in future robotic surgery.
Advances in nanorobotics
Nanotechnology is a rapidly developing field in medicine and surgery with huge potential. Owing to the recent realisation of several technological milestones, it is anticipated that nanotechnology will in the not too distant future translate from research into clinical practice. Nanorobotics relate to synthetic devices in the size range of 1 to 100nm and microrobotics are those sub-1mm in size. For point of reference, a strand of DNA is 2nm wide and a red blood cell is 7,000nm wide.42 Although many of the robotic systems described earlier comprise large complex rigid devices with invasive patient-robot interface, nanotechnology provides the opportunity to operate at the cellular level with greater precision, efficiency and with unfettered access to all organ systems. This review will focus specifically on nanorobotics in surgery and will not provide a general overview of nanotechnology because the subject is too vast.
Nanorobotic challenges
In order to reach a level of research maturity whereby nanotechnology can be used in clinical practice, several technological milestones have been achieved and these will be outlined first before going on to discuss future applications. To reach target sites and navigate the human body, nanorobots need efficient propulsion mechanisms capable of traversing large blood vessels, capillaries and intra-cellular compartments. Many strategies have been developed involving micromotors that create the energy-required ether from local chemical substrates or external sources such as magnetic, ultrasound, thermal and electrical energy. Solovev et al43 describe a microtubule containing a catalyst for chemical fuel to self-propel through air bubble ejection similar to a ‘microjet’ and capable of speeds of 2 micrometres per second. Ghosh et al44 describe the creation of 200nm-wide nanopropellors, akin to bacterial flagella, powered and directed by externally applied magnetic fields. Martel provides an excellent review on the benefits of coupling nanotechnology with natural microorganisms, harnessing certain bacteria’s innate propulsion mechanism and negating the need for development of novel locomotive strategies.45
After power and locomotion, the second challenge with regard to development of nanorobots is the ability to control and direct the nanorobots within the human body. Early work by Weibel et al46 described a novel ex vivo method of nanorobot control by combing polystyrene beads to a flagellated algae and inducing directional movement via phototaxis from an LED light source. The first description of in vivo directional controlled objects involved the manipulation of a 1.5mm chrome sphere within the carotid artery of a live pig, achieving peak velocity speeds of up to 11.1cm/s. This study used a conventional magnetic resonance imaging (MRI) scanner found in many radiology departments and represented a shift towards the currently used MRI-based navigational systems.47 More recently, Octomag (an electromagnetic navigation system) was used to direct a microrobot to puncture the retinal vessels of a chicken embryo.48 A possible application for this system is in retinal vein cannulation and injection of thrombolysis for retinal vein occlusion.
The development of tiny self-propelling robots capable of harvesting energy from their surroundings or from external energy sources has given the scientific community a glimpse of future therapeutic opportunities. The next part of the review will detail future clinical applications of nanorobots in surgery.
Nanosurgery
It is not inconceivable that within our lifetime nanorobots will be able to enter the human body through injection into vessels, target a particular site either autonomously or surgeon-controlled, manipulate pathological tissue with nano-instruments, and feed back information to the surgeon before biodegrading without a trace. Many of the necessary nanometre-sized surgical instruments have already been produced. Kirson et al49 describe a vibrating micropipette less than a micrometre in diameter used to cleave off denrites from their neuronal cell bodies without damaging the cell. Leong et al50 detail the fabrication of untethered ‘micrograbbers’ that are remotely triggered in response to temperature or chemicals and are capable of capturing tissue from hard-to-reach areas for use in biopsy, for example. Kagan et al51 describe ultrasound-triggered ballistic missile-like nanorobots that are capable of damaging pathological tissue by penetrating deep into it at impressive speeds of up to six metres per second. On an even smaller scale, Srivastava discusses ‘microdiggers’ that drill into individual target cells thereby allowing for highly specific single-cell surgery.52 Analogous to the advancements seen in conventional surgery with the development of increasingly specialised surgical instruments, these nano-instruments will enable exciting developments in nanosurgery.
Targeted oncological therapy
A disadvantage of systemic chemotherapy is global toxicity damaging healthy non-cancerous cells and limiting the therapeutic dose that can be given. Nanorobots have the potential to target cancerous cells and release chemotherapeutic drug ‘pay-loads’ providing targeted therapy. Balasubramanian et al53 developed a self-propelling nanorobot with a functional cancer-specific antibody unit able to search out and bind to pancreatic cancer cells in vitro. Pouponneau et al54 conducted the first in vivo pre-clinical study involving rabbits as a model for treatment of hepatocellular carcinoma. They demonstrated the ability to steer nanorobots to a specific area within the liver using magnetic resonance navigation, followed by selective embolisation of hepatic vasculature and deposition of chemotherapeutic doxorubicin within the hepatic tissue. More recently in 2018, Li et al55 described an in vivo study demonstrating autonomous deposition of thrombin by a DNA nanorobot in response to a tumour vessel marker. This process facilitated chemo-embolisation, which lead to increased survival, tumour size reduction and decreased metastasis in mice melanoma models. It is postulated that nanorobots have the potential to deliver more than just drugs to cancer cells and may in the future target tumour cells with brachytherapy, stem cells or even heat in the form of thermo-ablative therapy.56
Many of the described studies so far include in vitro proof-of-concept research and, although it is easy to get carried away with another ‘Holy Grail’ medical innovation, there are currently no human clinical trials involving nanorobots. Thus, it remains to be seen whether nanrobotics can fulfil its promising potential.
Conclusion
Throughout the past two decades, there has been tremendous change with the advent and proliferation of robotic surgery. In the field of minimally invasive surgery, this landscape has been dominated by one platform, ie Intuitive Surgical’s da Vinci® surgical system. This review has outlined the current status of robotic surgery and provided detailed description of currently commercially available and novel robotic systems in development. These future systems may shift the current paradigm to create a more competitive market, leading to a subsequent decrease in cost and greater availability of robotic surgery to all. The impact on training and comparative research with the likely increased uptake of a variety of heterogeneous robotic systems is difficult to assess at present. However, it seems improbable that robotic manufactures will co-operate with each other to standardise certain aspects of their systems, given what is at stake commercially. Despite these challenges, the development and proliferation of novel robotic systems pushes the boundaries of what is possible with robotic surgery.
This review details the current advances in haptic feedback, which is a field on the verge of translating from the research arena into clinical practice and consequently physically re-connecting robotic surgeons with their patients to the benefit of both. In contrast, nanotechnology remains firmly in the experimental stage, with promising early proof-of-concept studies but little in the way of rigorous Level 1 evidence. It will be interesting to see how this novel technology adapts and propagates, as it draws parallels with the birth of robotic surgery in the ideals it promotes, ie better surgical outcome through miniaturisation, increased autonomy and reduced patient intrusiveness. Whatever the future holds, it is certain that robotic surgery will continue to play an important role.
References
- 1.Rassweiler JJ, Autorino R, Klein J et al. Future of robotic surgery in urology. BJU Int 2017; : 822–841. [DOI] [PubMed] [Google Scholar]
- 2.Bozzini G, Gidaro S, Taverna G. Robotassisted laparoscopic partial nephrectomy with the ALF-X robot on pig models. Eur Urol 2016; (2): 376–377. [DOI] [PubMed] [Google Scholar]
- 3.Spinelli A, David G, Gidaro S et al. First Experience in Colorectal Surgery with a new Robotic Platform with Haptic Feedback. Colorectal Dis 2017. September 14 doi: 10.1111/codi.13882 [DOI] [PubMed] [Google Scholar]
- 4.Gueli Alletti S, Rossitto C, Cianci S et al. The Senhance™ surgical robotic system (“Senhance”) for total hysterectomy in obese patients: a pilot study. J Robot Surg 2017. June 17 doi: 10.1007/s11701-017-0718-9 [DOI] [PubMed] [Google Scholar]
- 5.Lococo A, Larocca V, Marino F et al. experimental robotic pulmonary lobectomy with the TELELAP/ALFX system in the ovine model. Surg Innov 2015; : 252–256. [DOI] [PubMed] [Google Scholar]
- 6.CMR Surgical Press kit 2017. https://cmrsurgical.com/press-kit/ (cited April 2018).
- 7.Hagn U, Konietschke R, Tobergte A et al. DLR MiroSurge: a versatile system for research in endoscopic telesurgery. Int J Comput Assist Radiol Surg 2010; : 183–193. [DOI] [PubMed] [Google Scholar]
- 8.Konietschke R, Hagn U, Nickl M et al. The DLR MiroSurge – A Robotic System for Surgery. 2009 IEEE International Conference on Robotics and Automation Kobe International Conference Center Kobe, Japan, 2009. http://www.robotic.de/fileadmin/robotic/konietschke/Publications/ICRA_2009_Abstract_Konietschke_Miro_0477_CameraReady.pdf (cited April 2018). [Google Scholar]
- 9.Konietschke R, Knoferle A, Hirzinger G. The AutoPointer: ‘A new augmented-reality device for transfer of planning data into the operating room’. In Proceedings of the 21st International Congress and Exhibition of Computer Assisted Radiology and Surgery (CARS) Berlin, Germany: 2007. [Google Scholar]
- 10.Rao PP. Robotic surgery: new robots and finally some real competition! World J Urol 2018; : 537–541. [DOI] [PubMed] [Google Scholar]
- 11.Lim JH, Woo Jung Lee WJ, Dong Won Park DW et al. Robotic cholecystectomy using Revo-i Model MSR-5000, the newly developed Korean robotic surgical system: a preclinical study. Surg Endosc 2017; : 3,391–3,397. [DOI] [PubMed] [Google Scholar]
- 12.Khateeb OM. Democratizing Surgery Part 1: What Verb Surgical Is Creating. https://www.linkedin.com/pulse/democratizing-surgeryhow-verb-surgical-invented-new-category (cited April 2018).
- 13.Henry B, Glenn Novarro G. Peering Behind The Veil of Secrecy In Surgical Robotics & 2016 Market Outlook. www.rbcinsight.com/WM/Share/ResearchViewer/?SSS_522561613B1ADBDE80B721B19D8A2330 (cited April 2018).
- 14.Nelson RJ, Sai J, Chavali S et al. Current status of robotic single-port surgery. Urol Ann 2017; : 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haber GP, White MA, Autorino R et al. Novel robotic da Vinci instruments for laparoendoscopic single-site surgery. Urology 2010; : 1,279–1,282. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez D, Maurice M, Kaou JH. Robotic perineal radical prostatectomy and pelvic lymph node dissection using a purpose-built single-port robotic platform. BJU Int 2016; : 829–833. [DOI] [PubMed] [Google Scholar]
- 17.Pryor AD, Tushar JR, DiBernardo LR. Singleport cholecystectomy with the TransEnterix SPIDER: simple and safe. Surg Endosc 2010; : 917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters BS, Armijo PR, Krause C et al. Review of emerging surgical robotic technology. Surg Endosc 2018; : 1,636–1,655. [DOI] [PubMed] [Google Scholar]
- 19.Titan Medical World’s first single-port prostatectomy using Titan Medical’s Surgical system completed in pre-clinical setting. February 2018. https://titanmedicalinc.com/worlds-first-single-port-prostatectomyusing-titan-medicals-sport-surgical-systemcompleted-preclinical-setting/ (cited April 2018).
- 20.Wortman TD. Design, analysis, and testing of in vivo surgical robots. Department of Mechanical Engineering, University of Nebraska; 2010. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?referer=https://www.google.co.uk/&httpsredir=1&article=1029&context=mechengdiss (cited April 2018). [Google Scholar]
- 21.Ryou M, Fong DG, Pai RD et al. Dual-port distal pancreatectomy using a prototype endoscope and endoscopic stapler: a natural orifice transluminal endoscopic surgery (NOTES) survival study in a porcine model. Endoscopy 2007; : 881–887. [DOI] [PubMed] [Google Scholar]
- 22.Sun Z, Ang RY, Lim EW et al. Enhancement of a masterslave robotic system for natural orifice transluminal endoscopic surgery. Ann Acad Med Singapore 2011; : 223–230. [PubMed] [Google Scholar]
- 23.Phee SJ, Reddy N, Chiu PW et al. Robotassisted endoscopic submucosal dissection is effective in treating patients with early-stage gastric neoplasia. Clin Gastroenterol Hepatol 2012; : 1,117–1,121. [DOI] [PubMed] [Google Scholar]
- 24.Po B, Yeung M, Wai P, Chiu Y. Application of robotics in gastrointestinal endoscopy: A review. World J Gastroenterol 2016; : 1,811–1,825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elkoushy MA, Andonian S. Prevalence of orthopedic complaints among endourologists and their compliance with radiation safety measures. J Endourol 2011; : 1,609– 1,613. [DOI] [PubMed] [Google Scholar]
- 26.Geavlete P, Saglam R, Georgescu D et al. Robotic flexible ureteroscopy versus classic flexible ureteroscopy in renal stones: the initial Romanian experience. Chirurgia (Bucur) 2016; ; 326–329. [PubMed] [Google Scholar]
- 27.Innocenti M. A New Robotic Platform Devoted to Microsurgery: Preliminary Report. Podium presentation at Congress of World Society for Reconstructive Microsurgery. Italy, 2016. [Google Scholar]
- 28.Struk S, Qassemyar Q, Leymarie N et al. The ongoing emergence of robotics in plastic and reconstructive surgery. Ann Chir Plast Esthet 2018; : 105–112. [DOI] [PubMed] [Google Scholar]
- 29.Clinicaltrial.gov Robotic Retinal Dissection Device Trial. https://clinicaltrials.gov/ct2/show/NCT03052881 (cited April 2018).
- 30.Akinbiyi T, Reiley CE, Saha S et al. Dynamic augmented reality for sensory substitution in robot-assisted surgical systems. Conf Proc IEEE Eng Med Biol Soc 2006; : 567–570. [DOI] [PubMed] [Google Scholar]
- 31.Okamura AM. Haptic feedback in robotassisted minimally invasive surgery. Curr Opin Urol 2009; : 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortmaier T, Deml B, Kuebler B et al. Robotassisted force feedback surgery. Ferre M, Buss M, Aracil R et al., Advances in Telerobotics, Springer Tracts in Advanced Robotics (STAR) Vol 31 New York: Springer; 2007. 341–358. [Google Scholar]
- 33.Wagner CR, Stylopoulos N, Jackson PG, Howe RD. The benefit of force feedback in surgery: examination of blunt dissection. Presence: Teleoperators Virtual Environ 2007; : 252–262. [Google Scholar]
- 34.Amirabdollahian F, Livatino S, Vahedi B et al. Prevalence of haptic feedback in robotmediated surgery: a systematic review of literature. J Robot Surg 2018; : 11–25. [DOI] [PubMed] [Google Scholar]
- 35.Roche M. Robotic-assisted unicompartmental knee arthroplasty: The MAKO experience. Clin Sports Med 2014; : 123–132. [DOI] [PubMed] [Google Scholar]
- 36.Eltaib MEH, Hewit JR. Tactile sensing technology for minimal access surgery–a review. Mechatronics 2003; : 1,163– 1,177. [Google Scholar]
- 37.Matsumoto S, Ooshima R, Kobayashi K et al. A tactile sensor for laparoscopic cholecystectomy. Surg Endosc 1997; (9): 939–941. [DOI] [PubMed] [Google Scholar]
- 38.Pacchierotti C, Prattichizzo D, Kuchenbecker KJ. Cutaneous feedback of fingertip deformation and vibration for palpation in robotic surgery. IEEE Trans Biomed Eng 2015; (2): 278–287. [DOI] [PubMed] [Google Scholar]
- 39.Mohsen M, Gwilliam J, Agarwal R et al. Force-feedback surgical teleoperator: controller design and palpation experiment. Conference: Haptic Interfaces For Virtual Environment And Teleoperator Systems, 2008. Symposium on Haptics, 2008 https://www.researchgate.net/publication/4325116_Force-Feedback_Surgical_Teleoperator_Controller_Design_and_Palpation_Experiments (cited April 2018). [Google Scholar]
- 40.Kitagawa M, Dokko D, Okamura AM, Yuh DD. Effect of sensory substitution on suture manipulation forces for robotic surgical systems. J Thorac Cardiovasc Surg 2005; : 151–158. [DOI] [PubMed] [Google Scholar]
- 41.Reiley CE, Akinbiyi T, Burschka D, Chang D, Okamura A, Yuh D. Effects of visual force feedback on robot-assisted surgical task performance. J Thorac Cardiovasc Surg 2008; : 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutherland GR, Maddahi Y, Gan LS et al. Robotics in the neurosurgical treatment of glioma. Surg Neurol Int 2015; : S1–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saadeh Y, Dinesh Vyas D. Nanorobotic applications in medicine: current proposals and designs. Am J Robot Surg 2014; : 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solovev AA, Mei Y, Bermúdez Ureña E et al. Catalytic microtubular jet engines self-propelled by accumulated gas bubbles. Small 2009; : 1,688–1,692. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh A, Fischer P. Controlled propulsion of artificial magnetic nanostructured propellers. Nano Lett 2009; : 2,243–2,245. [DOI] [PubMed] [Google Scholar]
- 46.Martel S. Swimming microorganisms acting as nanorobots versus artificial nanorobotic agents: A perspective view from an historical retrospective on the future of medical nanorobotics in the largest known threedimensional biomicrofluidic networks. Biomicrofluidics 2016; : 021301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weibel DB, Garstecki P, Ryan D et al. Microoxen: Microorganisms to move microscale loads. National Acad Sciences 2005; : 11,963–11,967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martel S, Mathieu J-B, Felfoul O et al. Automatic navigation of an untethered device in the artery of a living animal using a conventional clinical magnetic resonance imaging system. Appl Phys Lett 2007; : 1141105 https://doi.org/10.1063/1.2713229 [Google Scholar]
- 49.Kummer MP, Abbott J, Kratochvil BE et al. OctoMag: An electromagnetic system for 5-DOF wireless micromanipulation. IEEE Transactions on Robotics 2010; : 1,006–1,017. [Google Scholar]
- 50.Kirson ED, Yaari Y. A novel technique for microdissection of neuronal processes. J Neurosci Methods 2000; : 119–122. [DOI] [PubMed] [Google Scholar]
- 51.Leong TG, Randall CL, Benson BR et al. Tetherless thermobiochemically actuated microgrippers. Proc Natl Acad Sci U S A 2009; : 703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kagan D, Benchimol MJ, Claussen JC et al. Acoustic droplet vaporization and propulsion of perfluorocarbon-loaded microbullets for targeted tissue penetration and deformation. Angew Chem Int Ed Engl 2012; : 7,519–7,522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Srivastava SK, Medina-Sánchez M, Koch B et al. Medibots: dual-action biogenic microdaggers for single-cell surgery and drug release. Adv Mat 2016; : 832–837. [DOI] [PubMed] [Google Scholar]
- 54.Balasubramanian S, Kagan D, Hu CM et al. Micromachine-enabled capture and isolation of cancer cells in complex media. Angew Chem Int Ed Engl 2011; : 4,161–4,164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pouponneau P, Leroux JC, Soulez G et al. Co-encapsulation of magnetic nanoparticles and doxorubicin into biodegradable microcarriers for deep tissue targeting by vascular MRI navigation. Biomaterials 2011; : 3,481–3,486. [DOI] [PubMed] [Google Scholar]
- 56.Li S. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nature Biotechnol 2018; : 258–364. [DOI] [PubMed] [Google Scholar]
- 57.Nelson BJ, Kaliakatsos IK, Abbott JJ. Microrobots for Minimally Invasive Medicine. Annu Rev Biomed Eng 2010; : 55–85. [DOI] [PubMed] [Google Scholar]