Abstract
Background
Modern case series often focus on emphasizing low complication rates, “safety,” and “efficacy.” Although patients may not suffer significant or obviously apparent neurological complications, many lessons are buried in the “no complications” cohort.
Methods
The junior author's prospectively maintained caselog was reviewed over a 1-year period for both symptomatic and “minor”/technical complications of neurointerventional cases, the latter referring to an intraprocedural inability to treat a lesion, suboptimal result, or potentially morbid angiographic occurrence/finding that did not result in permanent neurological morbidity – neurointerventional “near morbidity” (NNM).
Results
Of 602 treatments performed over the reviewed period, 163 were interventional neuroendovascular procedures. The most common neuroendovascular procedure performed was stroke thrombectomy (67 cases). Major neurological complications, defined as symptomatic stroke or hemorrhage, occurred in 7 cases (4%). NNM, consisting of instructive, technical issues arose in an additional 9 cases that did not result in neurological morbidity (6%). Overall, in 20/163 cases (12%), there were either major neurological complications, NNM, groin complications, or major medical complications.
Conclusions
“Minor”/technical complications – NNM – can be as instructive and illustrative as major complications despite not resulting in permanent morbidity. In reviewing case series, particularly early in one's career, these cases should be highlighted.
Keywords: Endovascular procedure, Complications, Aneurysm, Arteriovenous malformation, Arteriovenous fistula, Stroke
Introduction
Most case series in the neurosurgical and neuroendovascular literature are single-center retrospective studies. Self-adjudicated technical success rates are often quite high, and varying definitions of “complications” may yield artifactually low complication rates, all in the spirit of concluding that a treatment approach is “safe and effective.” Unfortunately, encouraging and touting vanishingly low complication rates can bury the most instructive elements of a case series. Technical, “minor,” or “transient” complications – “near morbidity” – may harbor the most instructive lessons in treating a particular disease entity. These are often “nonreportable” occurrences that may not meet the criteria for primary or even secondary reporting outcomes. Studies presenting rates of “permanent neurological morbidity” or reviews of nationalized inpatient registries for complications will bury these instructive treasures. Humility among the great surgeons and interventionalists of our time is an important model to respect and emulate throughout one's neurosurgical career [1, 2, 3, 4, 5]. To complement and incite the spirit of those articles, this paper reviews the junior author's endovascular experience over the past year, focusing on “technical,” neurointerventional “near morbidity” (NNM) as a means to highlight its occurrence, mitigate future occurrences, and illustrate with greater external validity to the younger neurovascular community.
Methods
The junior author's (B.A.G.) prospectively maintained caselog was reviewed across all cases from July 2016 through July 2017. Interventional neuroendovascular procedures were reviewed for angiographic results, permanent neurological complications, groin complications, medical complications, and most importantly NNM, documented prospectively by the junior author and adjudicated by the senior authors. NNM referred to an intraprocedural inability to treat a particular lesion, suboptimal result, or an avoidable, potentially morbid angiographic occurrence/finding that did not result in permanent neurological morbidity.
Results
Over a 1-year period, the junior author treated 602 cases. Excluding diagnostic angiographic procedures and neurosurgical operating room cases, 163 were interventional neuroendovascular procedures (Table 1). The most common neuroendovascular procedure performed was stroke thrombectomy (67 cases). Aneurysm or arteriovenous shunt embolization was performed in 44 cases. Treatment of vasospasm was performed in 34 cases; in 9, angioplasty was performed. Elective stent deployment was performed in 13 cases (8 carotid, 2 vertebral, 3 intracranial), and epistaxis or tumor embolization was performed in 5 cases. Major neurological complications defined as symptomatic stroke or hemorrhage occurred in 7 cases (4%). Instructive, technical issues arose in an additional 9 cases and did not result in neurological morbidity – NNM (additional 6% of cases). There were 2 additional cases of significant groin complications: 1 dissection and 1 major groin hematoma, the latter requiring resuscitation and transfusion. One patient had both a technical complication and a groin complication (femoral pseudoaneurysm requiring thrombin injection). Among 37 outpatients undergoing elective procedures, 2 additional patients (5%) suffered major medical complications postoperatively. One suffered clinically significant urosepsis requiring re admission and another suffered from urosepsis and recurrent pneumonia. Overall, in 20/163 cases (12%), there were either major neurological complications, NNM, groin complications, or major medical complications.
Table 1.
Summary of interventional procedures performed
| Case | n | Angiographic result | Major complication | NNM/groin/other complication | Overall complications |
|---|---|---|---|---|---|
| Stroke thrombectomy | 65 | 59/67 TICI 2b/3 | 3 perforation; 1 reperfusion bleed | 1 major groin hematoma | 5/67 |
| Aneurysm coiling +/– balloon/stent | 23 | 8/23 R1; 13/23 pseudoR1; 5/23 R2; 10/23 R3 | 1 stroke/coil prolapse | 1 coil prolapse; 2 major medical complications | 4/23 |
| Aneurysm flow diversion | 6 | 1 in-stent stenosis | none | 1 temporary vessel occlusion | 1/6 |
| AVM embolization | 7 (1 spinal) | 2/2 occluded | 1 stroke | 1 OR delay; 1 asymptomatic perforation | 3/6 |
| AVF embolization | 8 (2 spinal) | 5/8 initially occluded; 7/8 finally occluded | 1 stroke | 1 CFA dissection | 2/8 |
| Carotid occlusion | 2 | widely patent carotid | none | both cases needed assistance | 2/2 |
| Carotid stenosis1 | 6 | widely patent carotid | none | none | none |
| Vertebral stenosis | 2 | widely patent vertebral artery | none | none | none |
| Intracranial stenosis/occlusion | 3 | 1 widely patent vessel; 2 stenotic vessels | none | 2 poor angiographic results (1 also had femoral pseudoaneurysm) | 2/3 |
| Vasospasm intra-arterial vasodilator | 25 | N/A | none | none | none |
| Vasospasm angioplasty | 9 | all improved vessel caliber/flow | none | one temporary PCA occlusion | 1/9 |
| Tumor/epistaxis embolization | 5 | N/A | none | none | none |
Five additional carotid stents for stenosis (n = 3)/occlusion (n = 2) during stroke; no complications.
AVF, arteriovenous fistula; AVM, arteriovenous malformation; CFA, common femoral artery; N/A, not applicable; NNM, neurointerventional “near morbidity”; OR, operating room; PCA, posterior cerebral artery; pseudoR1, pseudo-Raymond 1 (delayed filling of aneurysm dome); R1, Raymond 1 (complete occlusion); R2, Raymond 2 (neck remnant); R3, Raymond 3 (dome remnant); TICI, Thrombolysis in Cerebral Infarction.
Neurointerventional “Near Morbidity”
NNM occurred in 9/163 (6%) cases. A summary of these cases along with major complications is provided in Table 2, illustrating their similarity. In 2 cases of recurrently symptomatic, cervical carotid occlusions referred for revascularization, the junior author was unable to cross the occlusion. In both cases, with assistance from his partners, the occlusion was subsequently crossed, and revascularization occurred successfully without complication. The remaining 7 cases represent potentially avoidable NNM (78%).
Table 2.
Summary of major complications and NNM
| Complication | Approach | Alternative approach |
|---|---|---|
| 3 perforations during stroke thrombectomy | TA | stentriever/smaller reperfusion catheter |
| 1 coil prolapse/stroke | TA | coil likely oversized relative to size of aneurysm |
| 1 cervical AVM nontarget embolization | TA | avoid short vertebral pedicles for embolization |
| 1 AVF nontarget embolization | TA | aggressive transarterial embolization of CCF; consider better transvenous routes/direct puncture |
| 1 coil prolapse/asymptomatic1 | TA | overly aggressive coiling of unruptured aneurysm |
| Temporary PICA occlusion after pipeline1 | TA | accept in-stent stenosis |
| PCA occlusion during vasospasm treatment1 | TA | more proximal intra-arterial therapy, cross lesion more reliably/use a stentriever initially |
| OR delay for AVM embolization1 | TA | ruptured AVM with clot goes to the OR |
| Perforation during AVM embolization1 | TA | recognize limitations in adjunctive embolization of AVMs for surgery |
| Reperfusion hemorrhage | − | − |
| Unable to cross carotid occlusion1 | TT | − |
| Poor angiographic result for intracranial stenosis1 | TT | utilize higher radial force coronary stent |
NNM cases.
AVF, arteriovenous fistula; AVM, arteriovenous malformation; CCF, carotid cavernous fistula; NNM, neurointerventional “near morbidity”; OR, operating room; PCA, posterior cerebral artery; PICA, posterior inferior cerebellar artery; TA, too aggressive; TT, too timid.
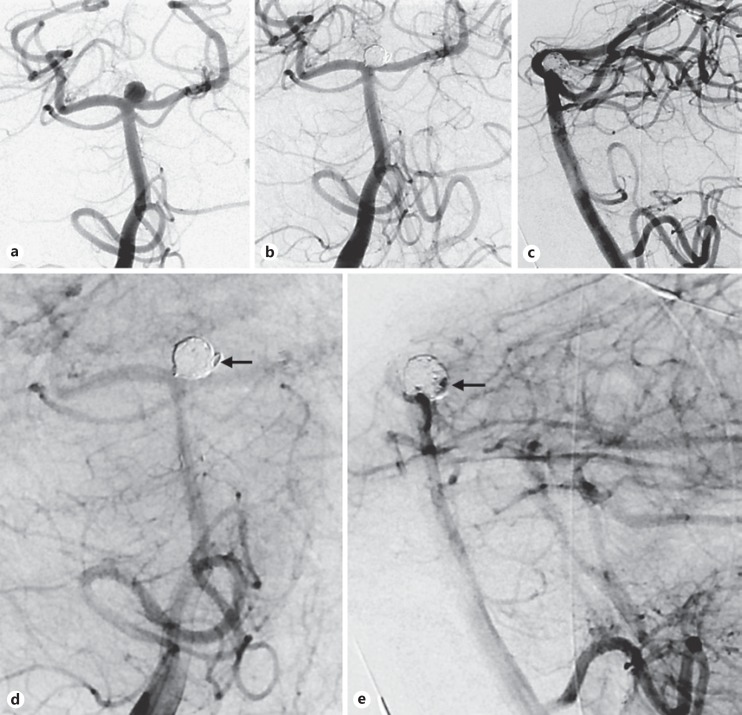
In 2 cases, a suboptimal angiographic result was achieved after utilizing a dedicated intracranial stent to treat symptomatic intracranial stenoses. Despite initially modest angiographic results, significant restenosis occurred in both cases requiring angioplasty. Along with his senior authors' institutional preference, the junior author now defers to the utilization of balloon-mounted coronary stents for intracranial stenting, which exert a higher radial force and potentially superior wall apposition (Fig. 1).
Fig. 1.
Anteroposterior view of a recurrently symptomatic severe left M1 stenosis despite maximal medical therapy (a). In the context of significant proximal vessel tortuosity, a 150-cm microcatheter was used to transverse the lesion and thus a dedicated intracranial stent was deployed to treat the lesion (b, anteroposterior view after stenting). The patient did have a femoral pseudoaneurysm post procedure. Three months later, severe in-stent stenosis was seen (c, anteroposterior view of left internal carotid artery injection). Utilizing a stiff Amplatz wire, carotid tortuosity was straightened and a shorter, less compliant coronary system was used for successful angioplasty. Illustrative pre- (d) and postangiographic views (e) after utilizing a balloon-mounted coronary stent for symptomatic M1 stenosis later in this series.
Remaining NNM arose as a result of a retrospectively assessed, over-aggressive technique. In 1 case, the last coil placed in a 6-mm unruptured anterior communicating artery aneurysm resulted in significant, unraveling coil prolapse into the parent artery upon removal of the microcatheter, requiring an intracranial stent to tack the coil against the vessel wall (Fig. 2). This case reinforces the importance of respecting a satisfactory angiographic result without “overfilling” an aneurysm and also reinforces the potential utility of keeping an adjunctive balloon inflated during microcatheter removal. In another case of pipeline embolization of a large vertebral aneurysm, balloon angioplasty of in-stent stenosis resulted in what was fortunately asymptomatic and transient occlusion of the posterior inferior cerebellar artery. Tolerance of tolerable, non-flow-limiting in-stent stenosis in this case and not performing angioplasty may have mitigated putting the patient at risk of sustaining an infarct. In another case of symptomatic posterior cerebral artery vasospasm (thalamic), aggressive attempts to pass a wire/microcatheter across the P1 segment for infusion of a vasodilator and possible angioplasty resulted in complete spasm and occlusion of the posterior cere bral artery. This was fortunately angiographically treated by the temporary deployment of a stentriever into the spastic vessel, providing instruction on the pitfalls of aggressive microcatheterization of small-caliber vessels. One potential silver lining is the discovery of a “bail out” technique when severe vasospasm cannot be crossed (Fig. 3).
Fig. 2.
Herniation and unraveling of a small, final coil into the A2 after coiling an anterior communicating artery aneurysm (a). The coil microcatheter was used to traverse beyond the coil and deploy an intracranial stent (b, post stenting).
Fig. 3.
A patient with symptomatic posterior circulation and right P1/2 vasospasm was treated via superselective infusion of a vasodilator (a, lateral view of vertebral injection showing the most severe element of P2 stenosis, arrow). This control angiographic run demonstrates a subsequent, paradoxical complete P2 occlusion (b, anteroposterior view of left vertebral artery injection). A microcatheter was brought up to the occlusion and a stentriever was pushed/deployed into the occlusion, resulting in sustained reperfusion (c, anteroposterior view of left vertebral artery injection with stent deployed, arrow). This control run demonstrated wide patency of the P2/3 (d, lateral view of vertebral injection).
In 1 case of a ruptured cerebellar arteriovenous malformation (AVM), aggressive embolization was pursued, potentially delaying decompression in the operating room, and in another case of an unruptured AVM, overzealous catheterization of a small feeder on the underside of the nidus resulted in a perforation that resulted in asymptomatic, cortical subarachnoid hemorrhage. Adjunctive embolization of operable AVMs should be limited to “low-risk” pedicles.
In 1 case, during embolization of a cervical AVM, Onyx was not easily visualized refluxing from a short vertebral artery pedicle into the parent vessel. The Onyx was retrieved; however, P2 embolization occurred, resulting in a radiographic posterior cerebral artery stroke. This case reinforces near “occult” reflux that can occur in a small-caliber vertebral branch that is not visualized until it is in the parent artery, encouraging conservatism in the context of poor distal purchase in these small-caliber branches (Fig. 4). In addition, although the patient did not suffer obvious neurological sequelae from the procedure in the context of being a poor-grade subarachnoid hemorrhage, there was computed tomography evidence of an obvious P2 infarct and as such, the case was of course classified as a major complication, not NNM.
Fig. 4.
This 76-year-old patient presented as a grade IV subarachnoid hemorrhage from a cervical arteriovenous malformation (a, right vertebral artery angiogram, anteroposterior view). This superselective run of a feeding artery pedicle demonstrates the microcatheter position prior to Onyx reflux (b, anteroposterior view; the arrow denotes the interface of branch vessel and vertebral artery, the star denotes microcatheter tip). This control angiographic run demonstrates Onyx in the right vertebral artery (c, anteroposterior view of right vertebral artery injection). The Onyx was retrieved from the vertebral artery using a stentriever, and remaining arterial pedicles of the arteriovenous malformation were embolized. A final angiographic run demonstrated no residual arteriovenous shunting but nonocclusive Onyx in the left P2b/ambient segment (d, anteroposterior view; the arrow denotes Onyx).
Discussion
The evolution of modern case series has been toward higher volumes and lower complications in the spirit of concluding that a device or treatment approach is “safe” and “effective” [6, 7]. While these series may suggest technical prowess of the treating practitioners, there is often much that can be gleaned from patients that do not suffer obvious or “permanent” complications. In the spirit of prior, candid evaluations of individual case series and complications, we hope to use this series to illustrate with potentially unique external validity procedural complications with a focus on NNM.
Beauty Is in the Eye of the Beholder
Self-adjudicated angiographic outcomes are obviously subject to considerable bias. After endovascular coiling, the endovascular practitioner is faced with a challenging test of angiographic occlusion: high-magnification digital subtraction angiography, often with the patient heparinized on antiplatelet therapy. The interventionalist is faced with the overbearing threat that microsurgical clipping “always” completely obliterates the aneurysm. Should another coil be forced in? While the obliteration rates after microsurgical clipping of cerebral aneurysms are higher overall than those of endovascular treatment, this fact should be accepted before the patients undergo treatment of their aneurysm. It will not change during the coiling. It is important to emphasize the reality that grossly reported rates of “complete occlusion” after clipping nearing 100% are likely artifactual as a result of the use of noninvasive, less precise imaging to assess occlusion (computed tomography angiography) or historically poorer-quality angiography. Kallmes et al. [8] eloquently described the pitfalls and potential dangers of striving for “complete” endovascular occlusion in their recent commentary about “why complete occlusion may be a complete disaster.”
In this early experience, true Raymond 1 occlusion of coiled aneurysms was achieved in a minority of patients. Nevertheless, no patients suffered rupture of their aneurysms after treatment, and no patients with 6-month angiographic follow-up needed retreatment. The term “pseudo-Raymond 1” is introduced and illustrated (Fig. 5) to reinforce potential subjectivity that can be introduced in self-adjudicated angiographic analyses.
Fig. 5.
This irregular basilar apex aneurysm with a daughter dome was treated via stent-assisted coiling (a, right vertebral artery anteroposterior-like working view). These selected images of working angle views during the arterial phase demonstrate no filling of the aneurysm (b, c). However, sluggish filling of the dome is seen in the venous phase (magnified working views, d and e), a “pseudo-Raymond 1” angiographic result that was classified as Raymond 3.
In 2 cases of fistula embolization, the procedure was ceased with known partial occlusion of the fistula. The decision in both cases was based on the facts that the symptomatic outflow into the superior ophthalmic vein was obliterated and that the radiation dose would soon exceed the tolerable limit of the treating physician. In both cases, planned second-stage embolization was not performed as the small residual fistula thrombosed (Fig. 6).
Fig. 6.
This symptomatic direct carotid-cavernous fistula (a, lateral view of right internal carotid injection) was treated via transvenous coil embolization. The superior ophthalmic vein was obliterated, followed by near total obliteration of the ipsilateral cavernous sinus with continued shunting primarily into the contralateral cavernous sinus (b, anteroposterior view of left common carotid artery injection post embolization). Having obliterated the “symptomatic vein” and having reached an upper tolerable limit of radiation dose, the procedure was ceased with the plan to stage the following month. Follow-up angiography 1 month later demonstrated serendipitous, complete thrombosis of the fistula and also better demonstrated the culprit cavernous aneurysm (arrow) to be treated via flow diversion (c, lateral view of left internal carotid artery injection).
Perusal of NNM in this series nicely demonstrates the pitfalls of overly aggressive approaches in pursuit of the “perfect” angiographic result. Overly aggressive coiling of an aneurysm led to coil prolapse, unraveling, and use of an intracranial stent in one illustrated case (Fig. 2). In another case, angioplasty of in-stent stenosis after pipeline embolization led to transient occlusion of a major arterial branch that fortunately spontaneously reopened without resultant stroke. In another case, overly aggressive microcatheterization of a serpentine AVM pedicle led to a small perforation that fortunately only resulted in small subarachnoid hemorrhage. This adjunctive, noncurative procedure could have resulted in mortality as a result of this perforation.
Know Your Limits
In reviewing the nature of complications through this series, it is clear that the majority were a result of overly aggressive rather than overly timid technique. Seeking the “perfect” angiographic result is an obvious catalyst for such technique, as already reviewed. It is interesting to note that NNM blends easily with the major complications reviewed (Table 2). The lessons learned are similar, if not more valuable, and the cases are equally illustrative. Importantly, the overall 6% rate of NNM is low and inflates considerably when more complex interventional procedures (aneurysms/atrioventricular shunts) are evaluated. The junior author's experience was largely comprised of stroke thrombectomies and intra-arterial vasodilator cases for vasospasm (n = 90 cases), cases likely less prone to resultant major morbidity or NNM.
With a Little Help from My Friends
“Getting out of trouble” and even avoiding “getting into trouble” is a battle that one need not fight on one's own, particularly in high-volume, experienced centers with resourceful partners. Complex aneurysms and flow diversion cases were initially evaluated with diagnostic angiography alone, with treatment staged in unruptured cases to meticulously evaluate angiography, size anticipated constructs, and potentially review the approach with endovascular partners. In 2 technical complication cases, senior partners were able to cross a carotid occlusion without difficulty or complication. At our institution, the stroke neurology service harbors an aggressive stance toward symptomatic carotid occlusion; as such, endovascular practitioners have uniquely considerable experience crossing challenging, chronic calcified occlusions. In recognizing this unique experience, a low threshold was maintained in seeking their assistance. In contrast, in 2 cases of challenging intracranial stenoses/occlusions, soliciting their input may have led to consideration of higher radial force coronary stents, potentially improving initial and long-term angiographic results (Fig. 1) [9, 10].
Study Limitations
This study is limited by the fact that all authors were not present in all cases and thus perceived instances of NNM by selected authors may not have been captured, resulting in an underestimate of the true frequency of NNM. The illustrative cases lack matched controls; as such, conclusions regarding potential outcomes are speculative.
Conclusions
NNM is a relatively common intraprocedural occurrence that should be scrutinized and noted to mitigate the risk of potential major complications through one's practice. In reviewing major complications and NNM, the authors wish to reinforce the following important “lessons learned” from this case series: (1) A suboptimal angiographic result may be a clinically excellent and well-rewarded result. (2) Self-adjudicated complication rates can be as high or as low as the reviewer wishes them to be. (3) NNM can be as illustrative and instructive as “major” complications or those resulting in “permanent” morbidity. (4) Resourceful partners are a unique asset; the junior author was fortunate to have practiced in the environment he was afforded.
Disclosure Statement
T.G. Jovin is a consultant for Neuravi, Codman Neurovascular, Stryker (PI DAWN; unpaid), Fundacio Ictus (PI REVASCAT; unpaid), and holds stock in Anaconda, Silk Road, and Blockade. B.T. Jankowitz is a consultant for Medtronic.
Funding Sources
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contributions
Drafting of the article: B.A. Gross. Acquisition of data/data analysis: B.A. Gross. Review and revision of the article prior to submission: all authors. Study supervision: B.A. Gross.
References
- 1.Barrow DL. Intraoperative misadventures: complication avoidance and management in aneurysm surgery. Clin Neurosurg. 2011;58:93–109. doi: 10.1227/neu.0b013e3182275574. [DOI] [PubMed] [Google Scholar]
- 2.Batjer HH, Duckworth EA. Selected Drake teachings: an affectionate look back and a look forward - the Charles G. Drake lecture: 2006. Neurosurgery. 2009;65:360–371. doi: 10.1227/01.NEU.0000345651.91532.6B. [DOI] [PubMed] [Google Scholar]
- 3.Batjer HH, Samson DS. Causes of morbidity and mortality from surgery of aneurysms of the distal basilar artery. Neurosurgery. 1989;25:904–915. doi: 10.1097/00006123-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Drake CG. Giant intracranial aneurysms: experience with surgical treatment in 174 patients. Clin Neurosurg. 1979;26:12–95. doi: 10.1093/neurosurgery/26.cn_suppl_1.12. [DOI] [PubMed] [Google Scholar]
- 5.Hauck EF, Wohlfeld B, Welch BG, White JA, Samson D. Clipping of very large or giant unruptured intracranial aneurysms in the anterior circulation: an outcome study. J Neurosurg. 2008;109:1012–1018. doi: 10.3171/JNS.2008.109.12.1012. [DOI] [PubMed] [Google Scholar]
- 6.Binning MJ, Maxwell CR, Stofko D, Zerr M, Maghazehe K, Liebman K, et al. Carotid artery angioplasty and stenting without distal embolic protection. Neurosurgery. 2017;80:60–64. doi: 10.1227/NEU.0000000000001367. [DOI] [PubMed] [Google Scholar]
- 7.Iosif C, Mendes GA, Saleme S, Ponomarjova S, Silveira EP, Caire F, et al. Endovascular transvenous cure for ruptured brain arteriovenous malformations in complex cases with high Spetzler-Martin grades. J Neurosurg. 2015;122:1229–1239. doi: 10.3171/2014.9.JNS141714. [DOI] [PubMed] [Google Scholar]
- 8.Kallmes DF, Fiorella D, Brinjikji W, Derdeyn CP. Patients, not pictures: why complete occlusion may be a complete disaster. J Neurointerv Surg. 2017;9:720–721. doi: 10.1136/neurintsurg-2017-013165. [DOI] [PubMed] [Google Scholar]
- 9.Gupta R, Al-Ali F, Thomas AJ, Horowitz MB, Barrow T, Vora NA, et al. Safety, feasibility and short-term follow-up of drug-eluting stent placement in the intracranial and extracranial circulation. Stroke. 2006;37:2562–2566. doi: 10.1161/01.STR.0000242481.38262.7b. [DOI] [PubMed] [Google Scholar]
- 10.Jiang WJ, Cheng-Ching E, Abou-Chebl A, Zaidat OO, Jovin TG, Kalia J, et al. Multicenter analysis of stenting in symptomatic intracranial atherosclerosis. Neurosurgery. 2012;70:25–30. doi: 10.1227/NEU.0b013e31822d274d. [DOI] [PubMed] [Google Scholar]