Abstract
Background
Acute ischemic stroke due to tandem occlusive lesions of the anterior circulation involves an intracranial large vessel occlusion as well as a concurrent occlusion or high-grade stenosis of the proximal carotid system. The vast majority of proximal lesions in tandem occlusive cases involve the extracranial internal carotid artery, although the lesion can theoretically exist anywhere along the carotid artery pathway, including the common carotid ostium.
Summary
To the best of our knowledge, only 1 report describes common carotid artery ostial lesions in the setting of acute ischemic stroke due to tandem occlusions, in which the authors describe an anterograde treatment paradigm. We present the first 2 cases of acute ischemic stroke secondary to common carotid ostial disease with tandem intracranial occlusion, treated with intracranial thrombectomy followed by subsequent staged balloon-mounted stenting of the common carotid ostium. We review the pathophysiology of tandem occlusions, the controversy surrounding treatment techniques, and various approaches used in the treatment of ostial occlusive lesions.
Key Message
In certain situations where acute carotid stenting is not safe or technically possible, immediate intracranial thrombectomy with a subsequent staged balloon-mounted stenting of the ostial lesion may be a reasonable and safe option.
Keywords: Acute ischemic stroke, Carotid artery stenosis, Mechanical thrombectomy, Stents, Stent retriever, Stroke, Tandem
Introduction
Acute ischemic stroke (AIS) due to tandem occlusive lesions involves acute dissection or ulcerated rupture of an unstable proximal atherosclerotic plaque causing stenosis/occlusion and subsequent embolic intracranial large vessel occlusion (LVO) [1, 2]. While the vast majority of cases of proximal carotid occlusive disease involve the extracranial internal carotid artery (ICA), the common carotid artery (CCA) ostium is rarely implicated in cerebral ischemic events [3]. Data on the endovascular management of common carotid ostial lesions is exceedingly scarce, and has mostly been described outside of the setting of AIS and emergent intracranial LVO. To the best of our knowledge, only 1 report describes CCA ostial lesions in the setting of AIS due to tandem occlusions, in which the authors describe an anterograde treatment paradigm [3]. We present the first 2 cases of AIS secondary to CCA ostial disease with tandem distal intracranial LVO treated with thrombectomy, followed by staged retrograde ostial stenting. We review the pathophysiology of tandem occlusions, the controversy surrounding treatment techniques, and various approaches used in the treatment of ostial occlusive lesions. This study received Institutional Review Board approval. In addition, all patients provided written consent to participate in all research-related activities.
Clinical Presentation
Case 1
A 62-year-old male presented to the emergency room after waking up with a left MCA syndrome. On arrival, he demonstrated a National Institutes of Health Stroke Scale (NIHSS) score of 20. Noncontrast CT of the head showed an Alberta stroke program early CT (ASPECT) score of 7. A CT angiogram demonstrated nonopacification of the entire left CCA with reconstitution of the left ICA and a tandem occlusion of the left ICA terminus (Fig. 1a). Intravenous alteplase (tPA) was withheld due to the time of onset, and the patient was taken as an emergency to the angiography suite. Digital subtraction angiography (DSA) confirmed the CCA ostial disease and tandem intracranial occlusion (Fig. 1b). Despite the ostial lesion, a Penumbra Neuron MAX 088 guide catheter (Penumbra, Alameda, CA, USA) was able to traverse the CCA ostium over a 0.035-inch glidewire (without the need for angioplasty), and was positioned in the cervical ICA. Emergency mechanical thrombectomy was successfully performed using a Solitaire FR 6 × 30 mm stent retriever (eV3/Covidien, Irvine, CA, USA), with continuous machine aspiration through a Penumbra 5MAX ACE68 reperfusion catheter (Fig. 1c). After TICI-3 recanalization was achieved, attention was turned towards the CCA ostium. Over a microcatheter maintaining CCA access, the Neuron MAX guide catheter was retracted proximally to the CCA ostium, and angiography confirmed a 90% left CCA ostial stenosis. The microcatheter was then exchanged for an Aviator Plus Rx Angioplasty Balloon, 5 × 40 mm (Cordis Neurovascular, Milpitas, CA, USA), and balloon angioplasty was performed with improvement of stenosis to 30% (Fig. 1d).
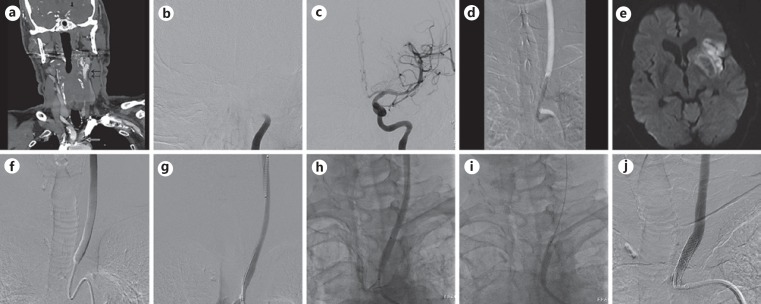
Fig. 1.
a CT angiogram demonstrating high-grade stenosis of the left common carotid artery (CCA) ostium with distal CCA reconstitution and tandem occlusion of the intracranial ICA. Digital subtraction angiogram (DSA) showing occlusion of the intracranial ICA (b) and subsequent postthrombectomy DSA showing TICI-3 recanalization (c). d Road map image of left CCA ostium showing postthrombectomy balloon angioplasty of CCA ostial stenosis with improvement of stenosis to 30%. e MRI of the brain, diffusion-weighted image sequence showing minimal infarct involving the left insula and operculum as well as left putaminal hemorrhage. f Repeat DSA on day 15 showing rebound stenosis to preangioplasty severity of 90%. Note heterogeneity of contrast opacification indicating anterograde flow restriction. With the guide catheter in the aortic arch, an Omnilink 6 × 29 mm balloon-mounted stent was tracked over a 0.035-inch stiff guidewire into the left CCA (g), and then retracted proximally across the ostium (h). i Fluoroscopic image of balloon inflation with stent deployment. j Poststenting DSA showing CCA ostial stent with resolution of stenosis.
A 24-h follow-up MRI of the brain showed minimal scattered infarct in the left insula and frontal operculum as well as left putaminal petechial hemorrhage (Fig. 1e). Follow-up CT angiogram of the chest on day 10 showed rebound stenosis of the left CCA origin. Once the cerebral hemorrhage had stabilized, the patient was treated with aspirin and Plavix (Bristol-Meyers Squibb, New York, NY, USA) for 5 days.
Repeat DSA on day 15 confirmed the rebound stenosis of the left CCA origin to the preangioplasty severity of 90%, with delayed anterograde flow (Fig. 1f), and thus the decision was made to proceed with CCA ostial stenting. Transfemoral access was gained and over a 0.035-inch glidewire, a Cook 5-Fr, 125-cm Berenstein catheter (Cook, Bloomington, IN, USA) was used to traverse the CCA ostial lesion. Over this system, a Penumbra Neuron MAX 088 guide catheter was positioned in the distal CCA. The 0.035-inch glidewire was replaced with a stiff 0.035-inch Terumo Glidewire Advantage exchange length wire (Terumo, Somerset, NJ, USA). The diagnostic catheter was exchanged for an Omnilink 6 × 29 mm balloon-mounted stent (Abbott, Santa Clara, CA, USA) (Fig. 1g). The Penumbra Neuron MAX 088 guide catheter was retracted to unsheathe the stent, and the stent was appropriately positioned across the CCA ostial lesion (Fig. 1h). The balloon was inflated to subnominal pressure to deploy the stent (Fig. 1i), and then removed. Follow-up DSA showed resolution of stenosis and restoration of normal anterograde flow (Fig. 1j). The patient continued treatment with dual antiplatelet therapy, and at the 3-month follow-up demonstrated an NIHSS score of 3.
Case 2
A 50-year-old woman with a history of prior right ICA dissection treated with aspirin presented to the emergency room 2 h after acute onset of left MCA syndrome. On arrival, she demonstrated an NIHSS score of 15 and noncontrast CT of the head showed an ASPECT score of 10. A CT angiogram showed high-grade stenosis of the left CCA origin as well as tandem occlusion of the M1 segment of the left MCA. Intravenous tPA was administered, and subsequent mechanical thrombectomy was successfully performed using a Solitaire FR 6 × 30 mm stent retriever with machine aspiration through a Penumbra 5MAX ACE68 reperfusion catheter, achieving TICI-3 recanalization (Fig. 2a, b).
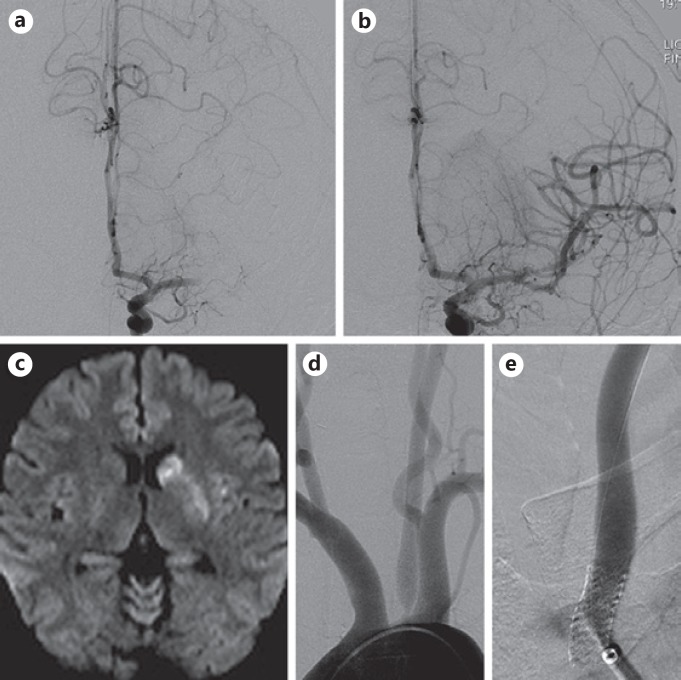
Fig. 2.
DSA showing occlusion of the left middle cerebral artery (a) and subsequent postthrombectomy DSA showing TICI-3 recanalization (b). c MRI of the brain, diffusion-weighted image sequence showing minimal infarct involving the head of the left caudate nucleus and putamen. DSA of the aortic arch showing high-grade stenosis of the left CCA ostium (d) and poststenting DSA showing resolution of stenosis (e).
A follow-up MRI of the brain showed minimal infarct in the left basal ganglia without parenchymal hemorrhage (Fig. 2c). Twenty-four hours after tPA administration, dual antiplatelet therapy with aspirin and Plavix was initiated. The source of the cerebral occlusion was determined to be secondary to embolic artery-to-artery phenomenon from a highly unstable left CCA ostial stenosis.
After 5 days of dual antiplatelet therapy, DSA re-demonstrated the high-grade stenosis of the left CCA ostium with delayed anterograde flow (Fig. 2d). Through a 6-Fr Flexor Shuttle Select guide catheter (Cook Medical, Bloomington, IN, USA) positioned in the aortic arch, a Cook 5-Fr, 125-cm Berenstein catheter was used to select the left CCA. A 0.014-inch Hi-Torque Balance exchange length guidewire (Abbott) was positioned in the left CCA as a buddy wire, and the diagnostic catheter was removed. An 8-mm Angioguard embolic protection device (EPD) (Cordis Neurovascular) was tracked across the CCA ostial lesion and deployed in the mid-left CCA, and the Hi-Torque Balance buddy wire was removed. An Aviator Plus RX Angioplasty Balloon, 4 × 40 mm, was tracked over the EPD monorail wire and across the CCA ostium, and balloon angioplasty was performed. Then, a 7 × 18 mm Herculink Elite balloon-mounted stent (Abbott) was tracked over the EPD monorail wire and subnominal balloon pressure inflation deployed the stent across the left CCA ostium (Fig. 2e). The patient continued treatment with dual antiplatelet therapy, and at the 4-month follow-up had returned to baseline condition without neurological deficit.
Discussion
Approximately 10–25% of patients with intracranial LVO will have a concomitant proximal extracranial occlusion and, conversely, 50% of patients with proximal extracranial occlusion will have a distal intracranial LVO [4]. AIS in patients with tandem lesions carries a poor prognosis with only 2–12% of patients achieving favorable clinical outcome [5]. Intravenous thrombolysis and other combined approaches have limited success in this subgroup of patients [4, 6]. Accordingly, the MR CLEAN, ESCAPE, and REVASCAT trials, which included 32.3, 17, and 18.6% of patients with acute tandem occlusions, respectively, showed treatment effect in favor of thrombectomy compared to medical management [7, 8, 9].
Although the proximal lesion in cases of tandem occlusions usually involves the extracranial ICA, the CCA ostium is rarely implicated, and data on the management of CCA ostial lesions is exceedingly scarce. Only 1 report describes CCA ostial lesions in the setting of tandem occlusions causing AIS, in which the authors describe an anterograde treatment paradigm [3]. Until this report, CCA ostial lesions had only been reported in isolation and in elective cases, and not in the setting of tandem lesions or AIS (Table 1) [10, 11, 12, 13, 14, 15, 16]. We present the first cases of AIS secondary to CCA ostial disease with tandem intracranial LVO, treated with emergent thrombectomy followed by staged retrograde ostial stenting.
Table 1.
All reported cases of transfemoral common carotid artery ostial stenting
| Report, year | Patients, n | Type of stent | Embolic protection | Periprocedural complications |
|---|---|---|---|---|
| Chio [11], 2003 | 9 | All BMS | 0 | UATD |
| Kadkhodayan [10], 2009 | 8 | 7 SES, 1 BMS | 0 | 1 TIA |
| Usman [13], 2010 | 4 | All BMS | 4a | 0 |
| Cam [14], 2012 | 17 | All BMS | 16/17 | 1 TIA |
| Dumont [12], 2013 | 14 | All BMS | 0 | 1 TIA |
| Weiner [3], 2016 | 2 | All BMS | 0 | 0 |
BMS, balloon-mounted stent; SES, self-expanding stent; TIA, transient ischemic attack; UATD, unable to determine.
Embolic protection device or surgical occlusion of distal common carotid artery.
In the treatment of tandem occlusions, controversy exists regarding which lesion to treat first, and consensus does not exist on management guidelines [2, 17, 18, 19, 20]. Advantages of proximal recanalization first include subsequent access to the intracranial LVO with larger guide catheters, collateral restoration, theoretical reduction of further embolization, and in some cases, spontaneous distal recanalization [2, 17, 18, 19]. On the other hand, advantages of the retrograde/reverse approach include prioritizing the critical intracranial thrombectomy step, quicker restoration of cerebral blood flow, and the possibility of reducing final cerebral infarction volume [19]. Controversy also exists regarding balloon angioplasty alone versus carotid stent placement for the treatment of the proximal lesion [2, 19, 21, 22]. The benefit of angioplasty alone includes restoring anterograde flow with a less invasive procedure. Disadvantages of angioplasty alone include the possibility of “rebound” re-stenosis or re-occlusion compared to definitive mechanical stabilization. This debate is similar to that of proximal common iliac artery stenosis, where balloon angioplasty at first seemed to be promising, only to disappoint later [16]. However, emergent stenting necessitates prophylactic antiplatelet medication, and also risks hyperperfusion syndrome and cerebral hemorrhage, issues that are further complicated in patients who have received intravenous tPA or in whom the cerebral infarct volume is unknown [23, 24]. While recent large registries of AIS have shown no significant difference in symptomatic hemorrhage rates after acute stenting in AIS patients presenting with tandem occlusions, other reports suggest an intracerebral hemorrhage rate as high as 20% [25, 26].
In our first case, after performing the critical intracranial thrombectomy, we were hopeful that balloon angioplasty would temporarily stabilize the ostial lesion until a time more suitable for prophylactic antiplatelet medication. Given the patient's extended time of presentation, moderate ASPECT score, and the unknown concurrent infarct volume, this turned out to be a foregone conclusion that fortunately transpired. In retrospect, given the patient's subsequent cerebral hemorrhage (hemorrhagic infarction type 2, per the European Cooperative Acute Stroke Study – ECASS II), perhaps the avoidance of acute antiplatelet medication prevented a potentially devastating hemorrhagic transformation [27]. In our second case, acute stenting of the proximal lesion was deferred in order to avoid antiplatelet medication given the recent tPA administration. Similarly to the first case, once a subsequent MRI confirmed a minimal infarct volume, preprocedural antiplatelet medication could be safely administered at a decreased risk of hemorrhagic transformation.
Surgical treatment of CCA ostial lesions has been performed with bypass surgery (subclavian-carotid, carotid-carotid), although this procedure requires thoracotomy or median sternotomy and carries a perioperative mortality of approximately 6% [12]. Thus, endovascular approaches have become increasingly preferable. While most extracranial ICA lesions are treated with self-expanding stents, the majority of the recent literature on CCA ostial intervention describes the use of balloon-mounted stents (Table 1), perhaps due to the greater amount of precision required to avoid excessive herniation into the parent vessel. However, the use of balloon-mounted stents, which are typically designed on 0.035-inch wire systems, may preclude use of EPDs, which are designed on 0.014-inch wire systems. EPDs have been shown to reduce intracranial embolism by greater than 50% when used in cervical ICA intervention [28].
Recent reports on stenting of the CCA ostium employ techniques similar to those used in our first case [3, 12]. In brief, a diagnostic catheter is used to select the involved CCA, carefully traversing the ostial lesion. If unable to initially cross the ostium, prestent balloon dilatation is performed using monorail angioplasty balloons on either 0.035-inch or 0.014-inch systems. A stiff 0.035-inch exchange length wire is advanced through the diagnostic catheter. Over this system, a large guide sheath (either Cook Shuttle or Neuron MAX guide catheter) is tracked across the lesion. The diagnostic catheter is subsequently exchanged for a 0.035-inch balloon-mounted stent. The guide catheter is then withdrawn to “unsheath” the constrained stent. The balloon-mounted stent is then retracted across the ostial lesion and inflated to subnominal pressure, deployed with a slight overhang into the aortic arch. In cases in which CCA ostial stenting is performed on 0.014-inch wire systems, EPDs may be implemented. In these cases, the involved CCA is selected either through a diagnostic catheter or primarily from the guide catheter, with a 0.014-inch buddy wire. An EPD is then placed alongside the 0.014-inch buddy wire and a balloon-mounted stent is subsequently deployed only on the EPD wire, jailing the 0.014-inch buddy wire. After capturing the EPD, the buddy wire is removed. Some operators choose to use 0.035-inch balloon-mounted stents over the 0.014-inch EPD monorail wire. In order to avoid the potential of lack of support using this technique, Cam et al. [14] describe an innovative technique of deploying a 0.035-inch balloon-mounted stent over a 0.014-inch EPD wire adjacent to a 0.014-inch buddy wire in their report on 17 cases of CCA ostial stenting. Our second patient was treated with a 0.014-inch-system Herculink Elite balloon-mounted stent, which can be deployed over an EPD. However, this stent is only available in diameters of 4–7 mm, which may not be appropriate for many CCA ostial lesions.
Upon review of the scarce literature regarding CCA ostial stenting, most of which cases were performed without the use of EPDs, embolic phenomena have been limited to 3 transient ischemic attacks in 54 patients (5%) [3, 10, 11, 12, 13, 14, 15, 16]. This low incidence may be secondary to flow arrest at the time of balloon deployment [12]. In accordance with the previous CCA ostial stent data, neither of our patients suffered periprocedural embolic complications, regardless of the use of an EPD.
Conclusion
We present 2 cases of AIS secondary to tandem CCA ostial disease and distal intracranial LVO. In certain situations where acute CCA ostial stenting is not safe or technically possible, immediate intracranial thrombectomy with subsequent staged balloon-mounted stenting of the ostial lesion may be a reasonable and safe option.
Disclosure Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. All authors have no personal or institutional financial interest in drugs, materials, or devices described in this submission.
References
- 1.Kim YS, Garami Z, Mikulik R, Molina CA, Alexandrov AV. Early recanalization rates and clinical outcomes in patients with tandem internal carotid artery/middle cerebral artery occlusion and isolated middle cerebral artery occlusion. Stroke. 2005;36:869–871. doi: 10.1161/01.STR.0000160007.57787.4c. [DOI] [PubMed] [Google Scholar]
- 2.Spiotta AM, Lena J, Vargas J, Hawk H, Turner RD, Chaudry MI, et al. Proximal to distal approach in the treatment of tandem occlusions causing an acute stroke. J Neurointerv Surg. 2015;7:164–169. doi: 10.1136/neurintsurg-2013-011040. [DOI] [PubMed] [Google Scholar]
- 3.Weiner GM, Feroze R, Panczykowski DM, Aghaebrahim A, Ares W, Agarwal N, et al. Endovascular treatment of tandem common carotid artery origin and distal intracranial occlusion in acute ischemic stroke. World Neurosurg. 2017;97:360–365. doi: 10.1016/j.wneu.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Christou I, Felberg RA, Demchuk AM, Burgin WS, Malkoff M, Grotta JC, et al. Intravenous tissue plasminogen activator and flow improvement in acute ischemic stroke patients with internal carotid artery occlusion. J Neuroimaging. 2002;12:119–123. doi: 10.1111/j.1552-6569.2002.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 5.Rubiera M, Ribo M, Delgado-Mederos R, Santamarina E, Delgado P, Montaner J, et al. Tandem internal carotid artery/middle cerebral artery occlusion: an independent predictor of poor outcome after systemic thrombolysis. Stroke. 2006;37:2301–2305. doi: 10.1161/01.STR.0000237070.80133.1d. [DOI] [PubMed] [Google Scholar]
- 6.Lekoubou A, Cho TH, Nighoghossian N, Kumako V, Derex L, Trouillas P, et al. Combined intravenous recombinant-tissular plasminogen activator and endovascular treatment of spontaneous occlusive internal carotid dissection with tandem intracranial artery occlusion. Eur Neurol. 2010;63:211–214. doi: 10.1159/000278248. [DOI] [PubMed] [Google Scholar]
- 7.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 8.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 9.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 10.Kadkhodayan Y, Moran CJ, Derdeyn CP, Cross DT., 3rd Outcomes of angioplasty and stenting at the common carotid origin. Surg Neurol. 2009;72:451–455; discussion 455. doi: 10.1016/j.surneu.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Chio FL, Jr, Liu MW, Khan MA, Iyer SS, Roubin GS. Effectiveness of elective stenting of common carotid artery lesions in preventing stroke. Am J Cardiol. 2003;92:1135–1137. doi: 10.1016/j.amjcard.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Dumont TM, Eller JL, Mokin M, Snyder KV, Hopkins LN, Levy EI, et al. Transfemoral endovascular treatment of atherosclerotic stenotic lesions of the left common carotid artery ostium: case series and review of the literature. J Neurointerv Surg. 2013;5:539–542. doi: 10.1136/neurintsurg-2012-010523. [DOI] [PubMed] [Google Scholar]
- 13.Usman AA, Resnick SA, Benzuly KH, Beohar N, Eskandari MK. Late stent fractures after endoluminal treatment of ostial supraaortic trunk arterial occlusive lesions. J Vasc Interv Radiol. 2010;21:1364–1369. doi: 10.1016/j.jvir.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Cam A, Muhammad KI, Shishehbor MH, Bajzer CT, Kapadia SR. Technique and outcome of ostial common carotid artery stenting: a single centre experience. EuroIntervention. 2012;7:1210–1215. doi: 10.4244/EIJV7I10A193. [DOI] [PubMed] [Google Scholar]
- 15.Peterson BG, Resnick SA, Morasch MD, Hassoun HT, Eskandari MK. Aortic arch vessel stenting: a single-center experience using cerebral protection. Archives of Surgery (Chicago, IL: 1960) 2006;141:560–563. doi: 10.1001/archsurg.141.6.560. discussion 3–4. [DOI] [PubMed] [Google Scholar]
- 16.Queral LA, Criado FJ. The treatment of focal aortic arch branch lesions with Palmaz stents. J Vasc Surg. 1996;23:368–375. doi: 10.1016/s0741-5214(96)70282-3. [DOI] [PubMed] [Google Scholar]
- 17.Malik AM, Vora NA, Lin R, Zaidi SF, Aleu A, Jankowitz BT, et al. Endovascular treatment of tandem extracranial/intracranial anterior circulation occlusions: preliminary single-center experience. Stroke. 2011;42:1653–1657. doi: 10.1161/STROKEAHA.110.595520. [DOI] [PubMed] [Google Scholar]
- 18.Mpotsaris A, Bussmeyer M, Buchner H, Weber W. Clinical outcome of neurointerventional emergency treatment of extra- or intracranial tandem occlusions in acute major stroke: antegrade approach with wallstent and solitaire stent retriever. Clin Neuroradiol. 2013;23:207–215. doi: 10.1007/s00062-013-0197-y. [DOI] [PubMed] [Google Scholar]
- 19.Rangel-Castilla L, Rajah GB, Shakir HJ, Shallwani H, Gandhi S, Davies JM, et al. Management of acute ischemic stroke due to tandem occlusion: should endovascular recanalization of the extracranial or intracranial occlusive lesion be done first? Neurosurg Focus. 2017;42:E16. doi: 10.3171/2017.1.FOCUS16500. [DOI] [PubMed] [Google Scholar]
- 20.Al-Mufti F, Amuluru K, Manning NW, Khan I, Peeling L, Gandhi CD, et al. Emergent carotid stenting and intra-arterial abciximab in acute ischemic stroke due to tandem occlusion. Br J Neurosurg. 2017;31:573–579. doi: 10.1080/02688697.2017.1297377. [DOI] [PubMed] [Google Scholar]
- 21.Behme D, Molina CA, Selim MH, Ribo M. Emergent carotid stenting after thrombectomy in patients with tandem lesions. Stroke. 2017;48:1126–1128. doi: 10.1161/STROKEAHA.117.016182. [DOI] [PubMed] [Google Scholar]
- 22.Stampfl S, Ringleb PA, Mohlenbruch M, Hametner C, Herweh C, Pham M, et al. Emergency cervical internal carotid artery stenting in combination with intracranial thrombectomy in acute stroke. AJNR Am J Neuroradiol. 2014;35:741–746. doi: 10.3174/ajnr.A3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behme D, Mpotsaris A, Zeyen P, Psychogios MN, Kowoll A, Maurer CJ, et al. Emergency stenting of the extracranial internal carotid artery in combination with anterior circulation thrombectomy in acute ischemic stroke: a retrospective multicenter study. AJNR Am J Neuroradiol. 2015;36:2340–2345. doi: 10.3174/ajnr.A4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozdemir O, Bussiere M, Leung A, Gulka I, Lee D, Chan R, et al. Intra-arterial thrombolysis of occluded middle cerebral artery by use of collateral pathways in patients with tandem cervical carotid artery/middle cerebral artery occlusion. AJNR Am J Neuroradiol. 2008;29:1596–1600. doi: 10.3174/ajnr.A1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorado L, Castano C, Millan M, Aleu A, de la Ossa NP, Gomis M, et al. Hemorrhagic risk of emergent endovascular treatment plus stenting in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22:1326–1331. doi: 10.1016/j.jstrokecerebrovasdis.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Mueller-Kronast NH, Zaidat OO, Froehler MT, Jahan R, Aziz-Sultan MA, Klucznik RP, et al. Systematic evaluation of patients treated with neurothrombectomy devices for acute ischemic stroke: primary results of the STRATIS registry. Stroke. 2017;48:2760–2768. doi: 10.1161/STROKEAHA.117.016456. [DOI] [PubMed] [Google Scholar]
- 27.Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32:438–441. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- 28.Mousa AY, Campbell JE, Aburahma AF, Bates MC. Current update of cerebral embolic protection devices. J Vasc Surg. 2012;56:1429–1437. doi: 10.1016/j.jvs.2012.05.077. [DOI] [PubMed] [Google Scholar]