Abstract
Objective
To identify risk factors for surgical site infection (SSI) in patients who had undergone lumbar spinal surgery.
Methods
Studies published in PubMed, Web of Science, and Embase were systematically reviewed to determine risk factors for SSI following lumbar spinal surgery. Results are expressed as risk ratios (RRs) with 95% CIs and weighted mean difference (WMD) with 95% CI. A fixed-effect or random-effect model was used to pool the estimates according to heterogeneity among the studies included.
Results
Sixteen studies involving 13,393 patients were included in this meta-analysis. Pooled estimates suggested that diabetes (RR 2.19, 95% CI 1.43–3.36; P<0.001), obesity (RR 2.87, 95% CI 1.62–5.09; P<0.001), BMI (WMD 1.32 kg/m2, 95% CI 0.39–2.25; P=0.006), prolonged operating time (WMD 24.96 minutes, 95% CI 14.77–35.15; P<0.001), prolonged hospital stay (WMD 2.07 days, 95% CI 0.28–3.87; P=0.024), hypertension (RR 1.28, 95% CI 1.08–1.52; P=0.005), and previous surgery (RR 2.06, 95% CI 1.39–3.06; P<0.001) were independent risk factors for SSI in patients who had undergone lumbar spine surgery. Current smoking (RR 0.89, 95% CI 0.75–1.06; P=0.178), American Society of Anesthesiologists grade >2 (RR 2.63, 95% CI 0.84–8.27; P=0.098), increased age (WMD 1.43 years, 95% CI −1.15 to 4.02; P=0.278), COPD (RR 1.21, 95% CI 0.68–2.17; P=0.521), cardiovascular disease (RR 1.63, 95% CI 0.40–6.70; P=0.495), rheumatoid arthritis (RR 1.76, 95% CI 0.53–5.90; P=0.359), and osteoporosis (RR 1.91, 95% CI 0.79–4.63; P=0.152) were not risk factors for postoperative SSI.
Conclusion
Our results identified several important factors that increased the risk of postoperative SSI. Knowing these risk factors, surgeons could adequately analyze and evaluate risk factors in patients and then develop prevention measurements to reduce the rate of SSI.
Keywords: lumbar spinal surgery, surgical site infection, risk factors, meta-analysis
Introduction
Surgical site infection (SSI) is one of the most serious complications following lumbar spine surgery during the early postoperative stage. SSI rates have been reported to be 0.7%–12.0%.1,2 Despite several interventions in clinical practice, including the use of prophylactic antibiotics, improvements in surgical techniques, and postoperative care, SSI continues to affect patients after lumbar surgery.3,4 SSI usually requires surgical debridement, which leads to higher postoperative morbidity and mortality.5–7 This would increase the duration of hospital stay, reoperation rates, and additional treatment costs.5–7 Therefore, determining risk factors for postoperative SSI and seeking methods to reduce SSI rates are very necessary.
There have been several studies to investigate postoperative SSI risk factors, such as increased age,8 obesity,9,10 diabetes,8,10 smoking,10 previous infection,11 prolonged operating time,12 prolonged hospital stay,13 and admission from a health care facility.14 However, the results of these studies were inconsistent. In order to systematically assess the most important risk factors for SSI following lumbar spinal surgery, we conducted this meta-analysis. Based upon identified risk factors, we can deduce preventive strategies to reduce the risk for SSI, thereby decreasing the morbidity, mortality, and health care costs.
Methods
Search strategy
This meta-analysis was performed according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) statement.15 We did a comprehensive search on PubMed, Embase, and Web of Science from their inception to May 11, 2018. Search items were ((“lumbosacral region” [MeSH terms] OR (“lumbosacral” [all fields] AND “region” [all fields]) OR “lumbosacral region” [all fields] OR “lumbar” [all fields]) AND (“surgery” [subheading] OR “surgery” [all fields] OR “surgical procedures, operative” [MeSH terms] OR (“surgical” [all fields] AND “procedures” [all fields] AND “operative” [all fields]) OR “operative surgical procedures” [all fields] OR “surgery” [all fields] OR “general surgery” [MeSH terms] OR (“general” [all fields] AND “surgery” [all fields]) OR “general surgery” [all fields])) AND (“surgical wound infection” [MeSH terms] OR (“surgical” [all fields] AND “wound” [all fields] AND “infection” [all fields]) OR “surgical wound infection” [all fields] OR (“surgical” [all fields] AND “site” [all fields] AND “infection” [all fields]) OR “surgical site infection” [all fields]) AND (“risk factors” [MeSH terms] OR (“risk” [all fields] AND “factors” [all fields]) OR “risk factors” [all fields] OR (“risk” [all fields] AND “factor” [all fields]) OR “risk factor” [all fields]). There was no limitation on language or publication type. Moreover, we also manually searched the references of the studies and reviews included to identify other potentially eligible studies.
Inclusion criteria
Two independent investigators performed the literature search, literature review (title/abstract review, full-text review, and included eligible studies). Any disagreement between them was resolved by discussion and consensus. All studies that investigated risk factors for postoperative SSI after lumbar spinal surgery were considered eligible for data analysis. We included the studies that met inclusion criteria of randomized controlled trial, cohort study, or case–control study, adult patients who had undergone lumbar spinal surgery, and presence of risk factors for postoperative SSI.
Data extraction and quality assessment
Two independent investigators performed the data extraction. Data extracted included country of study, number of patients in SSI group and non-SSI group, baseline characteristics, and outcomes. We used the modified Newcastle–Ottawa Scale (NOS)16 to evaluate the quality of observational studies (cohort study, case–control study). This method consists of three items: patient selection, comparability of experimental and control groups, and assessment of outcomes of interest.16 The total score is 9, and higher scores indicate better quality. Any study is considered of high quality if the NOS score is >5 points.16
Statistical analysis
Dichotomous variables are expressed as RRs with 95% CIs and continuous variables weighted mean difference (WMD) with 95% CIs. We used a fixed-effect model (Mantel–Haenszel method)17 or random-effect model (DerSimonian–Laird method)18 to pool all data according to heterogeneity across the included studies. Heterogeneity among the studies was assessed using the I2 statistic,19 where I2>50% was considered substantial heterogeneity.19 When significant heterogeneity was identified, sensitivity analysis was performed to explore the potential source of heterogeneity. Publication bias was evaluated by Begg’s20 and Egger’s21 test. We considered P<0.05 statistically significant, except where otherwise specified. All statistical analyses were performed using Stata version 12.0 (StataCorp, College Station, TX, USA).
Results
Study identification and selection
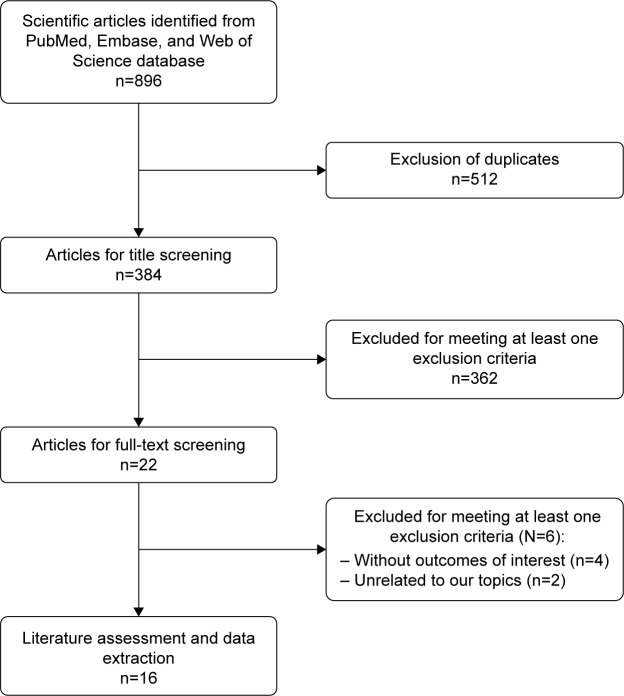
Figure 1 shows the article-screening and -selection process for inclusion in this study. The initial search yielded 896 studies. Of these, 512 were excluded for duplicate records and 362 excluded after the review of title/abstract. Then, 22 studies were left for full-text review. Among these, six were excluded: four for not providing eligible data,22–25 and two that were unrelated to our topic.26,27 Finally, 16 studies28–43 met the inclusion criteria and were included in this meta-analysis.
Figure 1.
Eligibility of studies for inclusion in meta-analysis.
Study characteristics and quality assessment
The main characteristics of included studies are presented in Table 1. These were published between 2003 and 2018. The total sample size was 13,393, of which 704 were in the SSI group and 12,689 the non-SSI group. Among these studies, nine28,31,32,35–37,40,41,43 were conducted in the US, two in China,33,42 and one each in South Korea,29 Norway,30 Japan,34 Brazil,38 and the Netherlands.39 Most studies were performed with a retrospective case–control design, except three, which were prospective34,38 or retrospective41 cohort design. All patients had undergone lumbar fusion surgery or posterior lumbar spinal surgery. NOS scores ranged from 5 to 7, which indicated that these studies were of high quality.
Table 1.
Baseline characteristics of patients in trials included
| Study | Country | Design | SSI group | Non-SSI group | Surgery | NOS s core |
|---|---|---|---|---|---|---|
|
| ||||||
| Lim et al28 | USA | Retrospective case–control | 173 | 3,180 | Single-level lumbar fusion surgery | 7 |
| Kim et al29 | South Korea | Retrospective case–control | 30 | 1,801 | Posterior lumbar interbody fusion | 7 |
| Habiba et al30 | Norway | Retrospective case–control | 40 | 1,732 | Lumbar disc herniation without laminectomy or fusion | 7 |
| Koutsoumbelis et al31 | USA | Retrospective case–control | 84 | 168 | Posterior lumbar instrumented arthrodesis | 5 |
| Lee et al32 | USA | Retrospective case–control | 15 | 134 | Lumbar spine surgery | 5 |
| Liu et al33 | China | Retrospective case–control | 64 | 192 | Posterior lumbar spinal surgery | 6 |
| Ogihara et al34 | Japan | Prospective cohort study | 24 | 2,712 | Posterior lumbar spinal surgery | 7 |
| Chaichana et al35 | USA | Retrospective case–control | 37 | 780 | Posterior instrumented lumbar fusion | 5 |
| Mehta et al36 | USA | Retrospective case–control | 24 | 274 | Lumbar spinal fusion | 7 |
| Petilon et al37 | USA | Propensity score-matched case–control study | 30 | 30 | Instrumented lumbar spinal fusion | 6 |
| Falavigna et al38 | Brazil | Prospective cohort study | 13 | 39 | Lumbar arthrodesis | 7 |
| Schimmel et al39 | the Netherlands | Retrospective case–control | 36 | 135 | Lumbar spinal fusion | 5 |
| Chen et al40 | USA | Retrospective case–control | 30 | 165 | Lumbar spinal fusion | 6 |
| Blam et al41 | USA | Retrospective cohort | 24 | 232 | Lumbar spinal fusion | 7 |
| Lai et al42 | China | Retrospective case–control | 26 | 897 | Lumbar spine surgery | 6 |
| Haleem et al43 | USA | Retrospective case–control | 54 | 218 | Lumbar spine surgery | 6 |
Abbreviations: NOS, Newcastle–Ottawa Scale; SSI, surgical site infection.
Risk factors
Sex
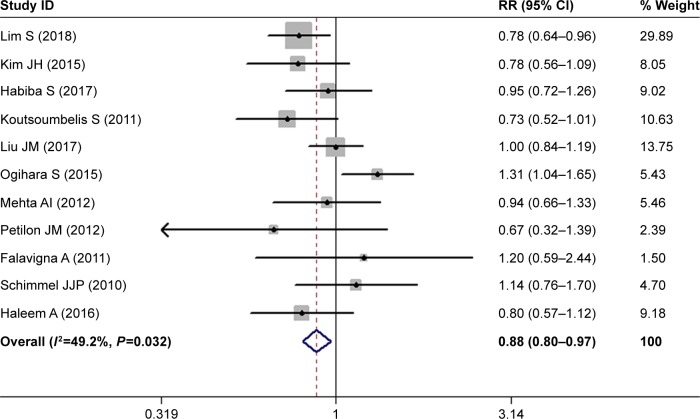
The most important risk factors for SSI are presented in Table 2. Eleven studies investigated the relationship between sex and postoperative SSI.28–31,33,34,36–39,43 The pooled estimate showed that males had a significantly lower risk of developing postoperative SSI compared with females (RR 0.88, 95% CI 0.80–0.97; P=0.008; Figure 2). There was no significant heterogeneity among the studies (I2=49.2%, P=0.032).
Table 2.
Pooled estimates of RR (WMD)a obtained from meta-analysis of risk factors of SSI following lumbar spine surgery
| RR | 95% CI | P-value | |
|---|---|---|---|
|
| |||
| Male sex | 0.88 | 0.80–0.97 | 0.008 |
| Diabetes | 2.19 | 1.43–3.36 | <0.001 |
| Current smoking | 0.89 | 0.75–1.06 | 0.178 |
| ASA grade >II | 2.63 | 0.84–8.27 | 0.098 |
| Obesity | 2.87 | 1.62–5.09 | <0.001 |
| Increased agea | 1.43 | −1.15 to 4.02 | 0.2777 |
| BMIa | 1.32 | 0.39–2.25 | 0.006 |
| Duration of surgerya | 24.96 | 14.77–35.15 | <0.001 |
| Duration of hospital staya | 2.07 | 0.28–3.87 | 0.024 |
| Estimated blood lossa | 106.90 | −65.14 to 278.53 | 0.224 |
| COPD | 1.21 | 0.68–2.17 | 0.521 |
| Hypertension | 1.28 | 1.08–1.52 | 0.005 |
| Cardiovascular disease | 1.63 | 0.40–6.70 | 0.495 |
| Rheumatoid arthritis | 1.76 | 0.53–5.90 | 0.359 |
| Osteoporosis | 1.91 | 0.79–4.63 | 0.152 |
| Allogeneic blood transfusion | 1.39 | 0.59–3.27 | 0.457 |
| Previous surgery | 2.06 | 1.39–3.06 | <0.001 |
| Implanted instrument | 1.41 | 1.19–1.66 | 0.533 |
| Sleep apnea | 1.00 | 0.18–5.35 | 1.00 |
| Hypercholesterolemia | 1.07 | 0.70–1.62 | 0.764 |
Note:
Results expressed as WMD with 95% CI.
Abbreviations: WMD, weighted mean difference; SSI, surgical site infection; ASA, American Society of Anesthesiologists; BMI, body-mass index.
Figure 2.
Forest plot showing the relationship between male sex and postoperative surgical site infection.
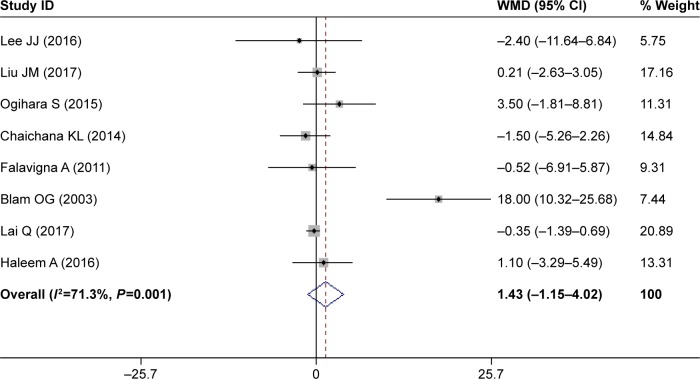
Increased age
Eight studies assessed the relationship between increased age and postoperative SSI.32–35,38,41–43 The pooled result suggested that patients with SSI were older than those without (WMD 1.43 years, 95% CI 1.15–4.02; Figure 3); however, this difference was not significant (P=0.2777). This indicated that increased age was not a significant risk factor for SSI.
Figure 3.
Forest plot showing relationship between increased age and postoperative surgical site infection.
Note: Weights are from random-effects analysis.
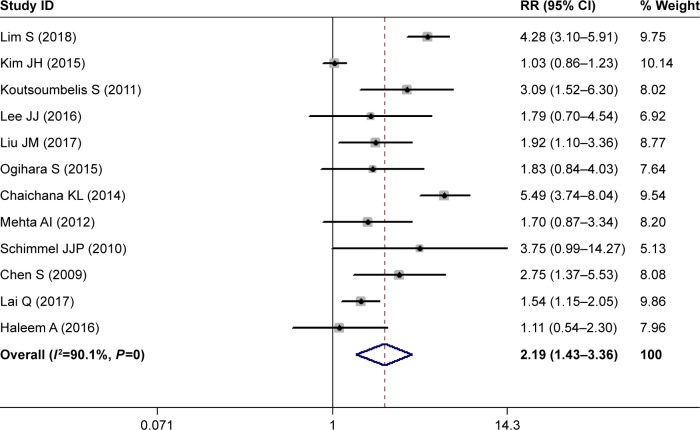
Diabetes
Twelve studies investigated the relationship between diabetes and postoperative SSI.28,29,31–36,39,40,42,43 The pooled estimate suggested that diabetes patients had a 2.19-fold increased risk of developing SSI compared with those without diabetes (RR 2.19, 95% CI 1.43–3.36; P<0.00; Figure 4). Heterogeneity was significant (I2=90.1%, P<0.001), and thus we conducted sensitivity analysis. When we excluded a study with a relatively small sample (n=149),32 the pooled estimate of the remaining studies did not change substantially (RR 2.23, 95% CI 1.71–3.43; P<0.001), but heterogeneity was still present (I2=89.5%, P<0.001). Furthermore, we excluded studies one at a time, and overall estimates changed slightly, but heterogeneity was still observed.
Figure 4.
Forest plot showing the relationship between diabetes and postoperative surgical site infection.
Note: Weights are from random-effects analysis.
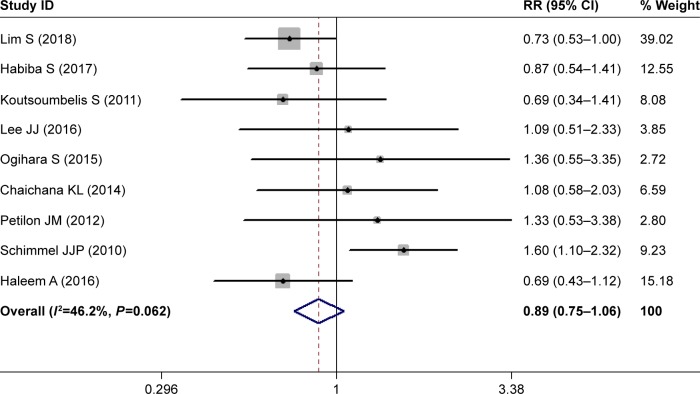
Current smoking
Nine studies investigated the relationship between current smoking and postoperative SSI.28,30–32,34,35,37,39,43 Pooled estimates showed that current smokers had a comparable rate of postoperative SSI than nonsmokers (RR 0.89, 95% CI 0.75–1.06; P=0.178; Figure 5). This indicated that current smoking did not increase the risk of postoperative SSI in patients with lumbar spine surgery. Heterogeneity was not significant (I2=46.2%, P=0.062).
Figure 5.
Forest plot showing the relationship between current smoking and postoperative surgical site infection.
Obesity
Six studies investigated the relationship between obesity and postoperative SSI.30–32,35,36,43 The pooled result showed that obesity patients had a 2.87-fold increased risk of SSI than those of normal weight (RR 2.87, 95% CI 1.62–5.09; P<0.001). This indicated that obesity was a significant risk for SSI. There was no significant heterogeneity among the studies (I2=43.4%, P=0.078).
ASA grade >2
Four studies investigated the relationship between American Society of Anesthesiologists (ASA) grade and postoperative SSI.28,30,34,43 Pooled estimates suggested that patients with ASA grade >2 had a similar rate of postoperative SSI compared with those with ASA grade 1–2 (RR 2.63, 95% CI 0.84–8.27; P=0.098). This demonstrated that ASA grade >2 did not increase the risk of postoperative SSI. Heterogeneity was not significant (I2=45.6%, P=0.073).
BMI
Nine studies investigated the relationship between body-mass index (BMI) and postoperative SSI.30,32,34,36,37,39,41–43 Pooled estimates suggested that patients with high BMI values had a higher risk of developing SSI than those with normal BMI (WMD 1.32 kg/m2, 95% CI 0.39–2.25; P=0.006). This indicated that BMI was a significant risk factor for postoperative SSI. Heterogeneity was not significant (I2=48.3%, P=0.067).
Duration of surgery
Ten studies investigated the relationship between duration of surgery and postoperative SSI.29–33,37,39,41–43 Pooled estimates suggested that patients with longer surgeries were more likely to develop SSI (WMD 24.96 minutes, 95% CI 14.77–35.15; P<0.001). This indicated that prolonged surgery was an (I2=33.9%, P=0.267).
Duration of hospital stay
Five studies investigated the relationship between duration of hospital stay and postoperative SSI.30,31,35,37,41 Pooled estimates suggested that patients with longer hospital stay had a higher risk of SSI (WMD 2.07 days, 95% CI 0.28–3.87; P=0.024). This indicated that prolonged hospital stays increased the risk of SSI. Heterogeneity was not significant (I2=29.6%, P=0.384).
Estimated blood loss
Four studies investigated the relationship between estimated blood loss and postoperative SSI.33,37,41,42 The pooled result showed that patients with greater blood loss had a higher risk of SSI (WMD 106.9 mL, 95% CI 65.14–278.53); however, this was not significant (P=0.224). This indicated that increased blood loss was not a significant risk factor for SSI in patients who had undergone lumbar spine surgery. Heterogeneity was not significant (I2=44.2%, P=0.0698).
Chronic obstructive pulmonary disease
Five studies investigated the relationship between COPD and postoperative SSI.28,31,39,42,43 Pooled estimates suggested that patients with COPD had a similar rate of SSI as those without (RR 1.21, 95% CI 0.68–2.17; P=0.521). This indicated that COPD was not a significant risk factor for SSI in patients who had undergone lumbar spine surgery. Heterogeneity was not significant (I2=13.8%, P=0.292).
Publication bias
Assessment of publication bias using Begg’s and Egger’s tests showed that there was no potential publication bias across the included studies (Egger’s test, P=0.473; Begg’s test, P=0.527).
Discussion
The present study was a meta-analysis of eligible studies with the objective of identifying risk factors for SSI following lumbar spinal surgery. Our study suggested that female sex, diabetes, obesity, BMI, pronged operation time, prolonged hospital stay, hypertension, and previous surgery were risk factors for SSI in patients who had undergone lumbar spinal surgery, whereas, current smoking, ASA grade >2, increased age, COPD, cardiovascular disease, rheumatoid arthritis, and osteoporosis were not.
To the best of our knowledge, this is the first comprehensive meta-analysis to investigate risk factors for SSI in patients who have undergone lumbar spinal surgery. Our study indicated that patients with diabetes had a 2.19-fold increased risk of developing postoperative SSI compared with those without. Findings from the present study were consistent with most of the studies included, except three,29,32,34 which found that diabetes was not a risk factor for SSI. Lee et al32 retrospectively analyzed 149 adult patients who had undergone lumbar spine surgery with a midline posterior approach. Among these patients, 15 experienced postoperative SSI and 134 had no infection.32 The prevalence of patients with diabetes in the SSI and non-SSI groups was 26.7% (four of 15) and 14.9% (20 of 134), respectively, which were not significant (P=0.249).32 Similarly, Kim et al29 undertook a review of a case series to identify risk factors for SSI in posterior lumbar interbody fusion, and they also reported a negative relationship between diabetes and SSI. In that study, 80% (24 of 30) of patients in the SSI group had diabetes compared with 77.8% (1,401 of 1,801) in non-SSI group.29 However, in another retrospective study of 2,715 patients investigating risk factors for SSI following posterior lumber spinal surgery, the authors suggested that diabetes was an independent risk factor for SSI.33 The rate of diabetes in SSI and control groups was 25% (16 of 64) and 13% (25 of 192), respectively, which demonstrated that diabetes patients were at higher risk of developing SSI.33 The inconsistent results of these three studies are difficult for us to explain, since they all had large samples and used multivariate logistic regression analyses to reduce the influences of selection bias in retrospective studies.
In the present study, we found that obesity was a significant risk factor for SSI in patients who had undergone lumbar spinal surgery. These results were in line with previous studies.31,32,36 Koutsoumbelis et al collected 3,218 patients who had undergone posterior lumbar instrumented arthrodesis,31 and found that 42.9% (36 of 84) of them who developed SSI had obesity compared with 7.1% (12 of 168) of patients who had no SSI.31 The OR for obesity was 9.75 (95% CI 4.70–20.21, P<0.001), indicating that patients with obesity had 9.75-fold increased risk of developing SSI than those without. Consistent with these results, Lee et al32 reported that obesity was associated with a 4.09-fold increased risk of SSI (OR 4.09, 95% CI 1.32–12.7; P=0.015). In that study, the obesity rate in the SSI and non-SSI groups was 66.7% (ten of 15) and 32.8% (44 of 134), respectively, which indicated that obese patients were more likely to develop SSI than normal patients.32 When obese patients are undergoing surgery, it is necessary to cut through a large amount of oily liquid. The surgical incision is filled with sterile gauze, and bacteria can become embedded in the incision.42 This increases the risk of infection. Moreover, previous studies2,44 have demonstrated that BMI is a risk factor for postoperative complications: when BMI is increased by 5 kg/m2, the risk of postoperative SSI is accordingly increased by 10%.
Consistently with prior studies, prolonged operations were significantly associated with postoperative SSI.29,31,33 Kim et al29 analyzed 1,831 patients who had undergone posterior lumbar interbody fusion, and found that SSI patients had had longer surgery than those in the non-SSI group. In that study, operation times in SSI and non-SSI groups were 195.3 minutes and 177.1 minutes (P=0.008), respectively,29 suggesting that prolonged surgery increased the risk of SSI. Similar results were found in another study, which assessed risk factors for SSI among patients with posterior lumbar instrumented arthrodesis.31 In that study, the duration of surgery in SSI and non-SSI groups was 373.1±167.1 minutes and 291.6±130.7 minutes, respectively.31 The difference between them was significant (P<0.001), which confirmed the role of prolonged surgery in postoperative SSI. However, in another case–control study,37 a negative relationship was found between duration of surgery and SSI. In that study, the authors performed a propensity-score-matched case–control study of 60 patients who had undergone instrumented lumbar fusion.37 The operating time for SSI patients was less (259.27 minutes) than non-SSI patients (288.17), and the difference between them was not significant (P=0.298).37 The negative result might be explained by the small sample.
Previous surgery was another risk factor for SSI, and this result was comparable to previous studies.35,39 Ogihara et al performed prospective multicenter surveillance to determine the risk factors for SSI in adult patients who had undergone lumbar spinal surgery.34 They enrolled 2,736 patients, and 24 (0.9%) developed SSI.34 The prevalence of patients who had had previous surgery in deep SSI and nondeep SSI groups was 29.2% and 15.5%, respectively, suggesting that previous surgery was an increased risk for postoperative SSI.34 Chaichana et al performed a study with 817 consecutive cases, and found previous surgery was associated with 2.994-fold increased risk of SSI (RR 2.994, 95% CI 1.26–9.35; P=0.009).35 It was assumed that patients who had had previous lumbar spine surgery typically had longer surgeries, which increased procedural complexity and propensity for durotomies, thereby increasing the risk of SSI.45
Limitations
This study has several potential limitations. First, in some outcomes, substantial heterogeneity was identified among the included studies. Despite sensitivity analysis being performed to detect potential sources of heterogeneity, no valuable information was found. Second, most of the studies were conducted with a retrospective design, and their results might be biased by the inherent disadvantages. This may have had a potential impact on our pooled estimates.
Conclusion
Our study indicates that female sex, diabetes, obesity, BMI, prolonged operation, prolonged hospital stay, hypertension, and previous surgery are independent risk factors for SSI following lumbar spine surgery, whereas, current smoking, ASA grade >2, increased age, COPD, cardiovascular disease, rheumatoid arthritis, and osteoporosis are not. Knowing these risk factors, surgeons could adequately analyze and evaluate risk factors in patients, and then develop prevention measurements to reduce the rate of SSI.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fei Q, Li J, Lin J, et al. Risk Factors for Surgical Site Infection After Spinal Surgery: A Meta-Analysis. World Neurosurg. 2016;95:507–515. doi: 10.1016/j.wneu.2015.05.059. [DOI] [PubMed] [Google Scholar]
- 2.Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008;90(1):62–69. doi: 10.2106/JBJS.F.01515. [DOI] [PubMed] [Google Scholar]
- 3.Wang TY, Back AG, Hompe E, Wall K, Gottfried ON. Impact of surgical site infection and surgical debridement on lumbar arthrodesis: A single-institution analysis of incidence and risk factors. J Clin Neurosci. 2017;39:164–169. doi: 10.1016/j.jocn.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Pull Ter Gunne AF, Mohamed AS, Skolasky RL, van Laarhoven CJ, Cohen DB. The presentation, incidence, etiology, and treatment of surgical site infections after spinal surgery. Spine. 2010;35(13):1323–1328. doi: 10.1097/BRS.0b013e3181bcde61. [DOI] [PubMed] [Google Scholar]
- 5.Calderone RR, Garland DE, Capen DA, Oster H. Cost of medical care for postoperative spinal infections. Orthop Clin North Am. 1996;27(1):171–182. [PubMed] [Google Scholar]
- 6.de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387–397. doi: 10.1016/j.ajic.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Veeravagu A, Patil CG, Lad SP, Boakye M. Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine. 2009;34(17):1869–1872. doi: 10.1097/BRS.0b013e3181adc989. [DOI] [PubMed] [Google Scholar]
- 8.Satake K, Kanemura T, Matsumoto A, Yamaguchi H, Ishikawa Y. Predisposing factors for surgical site infection of spinal instrumentation surgery for diabetes patients. Eur Spine J. 2013;22(8):1854–1858. doi: 10.1007/s00586-013-2783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang J, Teng Y, Fan Z, Khan S, Xia Y. Does obesity affect the surgical outcome and complication rates of spinal surgery? A meta-analysis. Clin Orthop Relat Res. 2014;472(3):968–975. doi: 10.1007/s11999-013-3346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng F, Cao J, Meng X. Risk factors for surgical site infections following spinal surgery. J Clin Neurosci. 2015;22(12):1862–1866. doi: 10.1016/j.jocn.2015.03.065. [DOI] [PubMed] [Google Scholar]
- 11.Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine. 2005;30(12):1460–1465. doi: 10.1097/01.brs.0000166532.58227.4f. [DOI] [PubMed] [Google Scholar]
- 12.Sasso RC, Garrido BJ. Postoperative spinal wound infections. J Am Acad Orthop Surg. 2008;16(6):330–337. doi: 10.5435/00124635-200806000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326(5):281–286. doi: 10.1056/NEJM199201303260501. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Singletary R, Schmader K, Anderson DJ, Bolognesi M, Kaye KS. Surgical site infection in the elderly following orthopaedic surgery. Risk factors and outcomes. J Bone Joint Surg Am. 2006;88(8):1705–1712. doi: 10.2106/JBJS.E.01156. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells G, Shea B, O’Connell D, Peterson J, Welch V. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses; 3rd Symposium on Systematic Reviews: Beyond the Basics; Jul 3–5. 2000; Oxford UK. [Google Scholar]
- 17.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 18.Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim BD, Hsu WK, de Oliveira GS, Saha S, Kim JY. Operative duration as an independent risk factor for postoperative complications in single-level lumbar fusion: an analysis of 4588 surgical cases. Spine. 2014;39(6):510–520. doi: 10.1097/BRS.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 23.Klemencsics I, Lazary A, Szoverfi Z, Bozsodi A, Eltes P, Varga PP. Risk factors for surgical site infection in elective routine degenerative lumbar surgeries. Spine J. 2016;16(11):1377–1383. doi: 10.1016/j.spinee.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Tsubouchi N, Fujibayashi S, Otsuki B, et al. Risk factors for implant removal after spinal surgical site infection. Eur Spine J. 2017 Sep 14; doi: 10.1007/s00586-017-5294-1. Epub. [DOI] [PubMed] [Google Scholar]
- 25.Asomugha EU, Miller JA, Mclain RF. Surgical Site Infections in Posterior Lumbar Surgery: A Controlled-Cohort Study of Epidural Steroid Paste. Spine. 2017;42(1):63–69. doi: 10.1097/BRS.0000000000001668. [DOI] [PubMed] [Google Scholar]
- 26.Golubovsky JL, Ilyas H, Chen J, Tanenbaum JE, Mroz TE, Steinmetz MP. Risk factors and associated complications for postoperative urinary retention after lumbar surgery for lumbar spinal stenosis. Spine J. 2018;18(9):1533–1539. doi: 10.1016/j.spinee.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Glassman S, Carreon LY, Andersen M, et al. Predictors of Hospital Readmission and Surgical Site Infection in the United States, Denmark, and Japan: Is Risk Stratification a Universal Language? Spine. 2017;42(17):1311–1315. doi: 10.1097/BRS.0000000000002082. [DOI] [PubMed] [Google Scholar]
- 28.Lim S, Edelstein AI, Patel AA, Kim BD, Kim JYS, Jys K. Risk Factors for Postoperative Infections After Single-Level Lumbar Fusion Surgery. Spine. 2018;43(3):215–222. doi: 10.1097/BRS.0000000000000608. [DOI] [PubMed] [Google Scholar]
- 29.Kim JH, Ahn DK, Kim JW, Kim GW. Particular Features of Surgical Site Infection in Posterior Lumbar Interbody Fusion. Clin Orthop Surg. 2015;7(3):337–343. doi: 10.4055/cios.2015.7.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Habiba S, Nygaard ØP, Brox JI, Hellum C, Austevoll IM, Solberg TK. Risk factors for surgical site infections among 1,772 patients operated on for lumbar disc herniation: a multicentre observational registry-based study. Acta Neurochir. 2017;159(6):1113–1118. doi: 10.1007/s00701-017-3184-2. [DOI] [PubMed] [Google Scholar]
- 31.Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2011;93(17):1627–1633. doi: 10.2106/JBJS.J.00039. [DOI] [PubMed] [Google Scholar]
- 32.Lee JJ, Odeh KI, Holcombe SA, et al. Fat Thickness as a Risk Factor for Infection in Lumbar Spine Surgery. Orthopedics. 2016;39(6):e1124–e1128. doi: 10.3928/01477447-20160819-05. [DOI] [PubMed] [Google Scholar]
- 33.Liu JM, Deng HL, Chen XY, et al. Risk Factors for Surgical Site Infection After Posterior Lumbar Spinal Surgery. Spine. 2018;43(10):732–737. doi: 10.1097/BRS.0000000000002419. [DOI] [PubMed] [Google Scholar]
- 34.Ogihara S, Yamazaki T, Maruyama T, et al. Prospective multicenter surveillance and risk factor analysis of deep surgical site infection after posterior thoracic and/or lumbar spinal surgery in adults. J Orthop Sci. 2015;20(1):71–77. doi: 10.1007/s00776-014-0669-1. [DOI] [PubMed] [Google Scholar]
- 35.Chaichana KL, Bydon M, Santiago-Dieppa DR, et al. Risk of infection following posterior instrumented lumbar fusion for degenerative spine disease in 817 consecutive cases. J Neurosurg Spine. 2014;20(1):45–52. doi: 10.3171/2013.10.SPINE1364. [DOI] [PubMed] [Google Scholar]
- 36.Mehta AI, Babu R, Karikari IO, et al. 2012 Young Investigator Award winner: The distribution of body mass as a significant risk factor for lumbar spinal fusion postoperative infections. Spine. 2012;37(19):1652–1656. doi: 10.1097/BRS.0b013e318241b186. [DOI] [PubMed] [Google Scholar]
- 37.Petilon JM, Glassman SD, Dimar JR, Carreon LY. Clinical outcomes after lumbar fusion complicated by deep wound infection: a case–control study. Spine. 2012;37(16):1370–1374. doi: 10.1097/BRS.0b013e31824a4d93. [DOI] [PubMed] [Google Scholar]
- 38.Falavigna A, Righesso O, Traynelis VC, Teles AR, da Silva PG. Effect of deep wound infection following lumbar arthrodesis for degenerative disc disease on long-term outcome: a prospective study: clinical article. J Neurosurg Spine. 2011;15(4):399–403. doi: 10.3171/2011.5.SPINE10825. [DOI] [PubMed] [Google Scholar]
- 39.Schimmel JJ, Horsting PP, de Kleuver M, Wonders G, van Limbeek J. Risk factors for deep surgical site infections after spinal fusion. Eur Spine J. 2010;19(10):1711–1719. doi: 10.1007/s00586-010-1421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen S, Anderson MV, Cheng WK, Wongworawat MD. Diabetes associated with increased surgical site infections in spinal arthrodesis. Clin Orthop Relat Res. 2009;467(7):1670–1673. doi: 10.1007/s11999-009-0740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blam OG, Vaccaro AR, Vanichkachorn JS, et al. Risk factors for surgical site infection in the patient with spinal injury. Spine. 2003;28(13):1475–1480. doi: 10.1097/01.BRS.0000067109.23914.0A. [DOI] [PubMed] [Google Scholar]
- 42.Lai Q, Song Q, Guo R, et al. Risk factors for acute surgical site infections after lumbar surgery: a retrospective study. J Orthop Surg Res. 2017;12(1):116. doi: 10.1186/s13018-017-0612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haleem A, Chiang HY, Vodela R, et al. Risk Factors for Surgical Site Infections Following Adult Spine Operations. Infect Control Hosp Epidemiol. 2016;37(12):1458–1467. doi: 10.1017/ice.2016.193. [DOI] [PubMed] [Google Scholar]
- 44.Collins I, Wilson-Macdonald J, Chami G, et al. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J. 2008;17(3):445–450. doi: 10.1007/s00586-007-0559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wimmer C, Gluch H, Franzreb M, Ogon M. Predisposing factors for infection in spine surgery: a survey of 850 spinal procedures. J Spinal Disord. 1998;11(2):124–128. [PubMed] [Google Scholar]