Abstract
Background
Proline-rich/Ca2+-activated tyrosine kinase 2 (PYK2) belongs to the non-receptor tyrosine kinase family, regulates downstream signaling via catalyzing protein phosphorylation. We aimed to investigate clinical significance and mechanisms of PYK2 in colon adenocarcinoma (CAC).
Methods
Real time quantitative PCR and immunohistochemistry staining was used to evaluate the expression of PYK2 in clinical CAC tissues. Its association with clinicopathologic characteristics was analyzed by Chi-square test. Kaplan-Meier univariate survival analysis and multivariate Cox regression analysis were used to identify clinical significance of PYK2 in the overall survival of CAC patients. Transfection of PYK2 were conducted to reveal the underlying mechanism in regulating CAC progression.
Results
We found that PYK2 was upregulated in CAC tissues compared with normal colon tissues on both RNA and protein levels. Higher tissue PYK2 expression level was closely associated with lymph node metastasis. Statistical analyses indicated PYK2 as an independent prognostic biomarker for CAC. Cellular studies demonstrated that PYK2 enhanced the capacities of tumor proliferation and invasion. Moreover, the phosphorylation level of AKT was positively correlated with PYK2 expression, subsequently modulate expression of c-Myc and Cyclin D1, suggesting that PYK2 may promote tumor progression through activating AKT signaling.
Conclusion
High PYK2 in CAC tissues indicate poor prognosis.
Keywords: colon adenocarcinoma, PYK2, prognosis
Introduction
Colon cancer is one of the leading causes of globally cancer-related motility.1,2 Current treating strategies toward colon cancer mainly include surgical resection, radiotherapy, and chemotherapy.3 Although great achievements in surgical approach, more than half of the patients will develop disease metastases, particularly the unresectable liver metastases.4,5 In general, the overall survival of colon cancer was not substantially improved during the past decades.6 From the molecular basis, colon cancer is caused due to multistep processes of aberration accumulation which drives malignant transformation of normal colon cells.7 Nevertheless, the detail genetic and epigenetic changes responsible for the development and progression of colon cancer are still under investigation. Several biomarkers had been reported for predicting clinical prognosis of colon cancer.8 Unfortunately, the clinical sensitivity needs further validation by more clinical trials.9 Therefore, novel biomarkers that are of clinical significance are still in urgent need to help predict patients’ prognosis and develop novel target-specific therapies.
Recently, more and more attentions are focused on the effects of protein posttranslational modifications on tumor development, such as phosphorylation and ubiquitination.10,11 Proline-rich/Ca2+-activated tyrosine kinase 2 (PYK2), encoded by the protein kinase two beta (PTK2B) gene, is a kind of non-receptor protein-tyrosine kinase that catalyze phosphorylation of tyrosine residues.12 PYK2 is also named as focal adhesion kinase 2 (FAK2) due to its critical roles in focal adhesion and similar sequence with FAK1.13 As many other kinases, PYK2 had been reported to participate in cell proliferation and differentiation regulation by targeting its downstream substrates. PYK2 can promote progression of prostate cancer,14 breast cancer,15 small cell lung cancer,16 and hepatocellular carcinoma.17 Moreover, PYK2 was reported to regulate the proliferation of SW480 cells, a kind of colon adenocarcinoma (CAC) cell line.
Despite cellular evidence suggesting a potential implication of PYK2 in cancer progression, its prognostic significance in CAC is unknown. In this study, we first analyzed the mRNA and protein expressions of PYK2 in clinical tumor tissues in a colon cancer patients cohort comprising 87 patients. We found that PYK2 is a novel independent prognostic marker in CAC progression. Furthermore, the mechanistic role of PYK2 in colon cancer cell proliferation and invasion was investigated by functional studies.
Methods
Patients and samples
A total of 87 patients who were diagnosed with primary CAC were enrolled in this study, including 23 females and 64 males. This retrospective cohort was randomly selected from 492 CAC patients from Linyi Central Hospital and Qilu Hospital (Shandong, China) between June 2007 and June 2015. All the patients underwent surgical resection and none of them accepted preoperative drug treatment. Retrospectively collected clinical information included age, tumor location, tumor differentiation, tumor size, invasion depth, and lymph node metastasis. Complete follow-up data were obtained for all CAC patients and were included for overall survival analyses. Besides the formalin-fixed paraffin-embedded (FFPE) tissues, we also collected 16 pairs of freshly resected CAC and adjacent normal colon tissue samples, which were frozen with liquid nitrogen and stored in −80°C until further use. Among the 16 pairs of fresh-frozen samples, one is from TNM stage I, six from TNM stage II, seven from TNM stage III, and two from TNM stage IV. This study was approved by the ethics committee of Qilu Hospital and Linyi Central Hospital, and written informed consents were obtained from all patients.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from fresh-frozen tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription PCR was performed using the SuperScript™ III One-Step RT-PCR System with Platinum™ Taq DNA Polymerase (Thermo Fisher Scientific, Pittsburgh, PA, USA) according to the manufacturer’s instructions. RT-PCR was carried out to quantify transcript levels using Applied Biosystems™ (Thermo Fisher Scientific) as we described before.18 The housekeeping gene GAPDH was used as normalization standard. The primers were as follows:
PYK2 sense: 5′-GGACTATGTGGTGGTGGTGA-3′;
PYK2 antisense: 5′-TCTGCCAGGTCTTTGTTGAG-3′;
c-Myc sense: 5′-AAACACAAACTTGAACAGCTAC-3′;
c-Myc antisense: 5′-ATTTGAGGCAGTTTACATTATGG-3′;
Cyclin D1 sense: 5′-ATGTTCGTGGCCTCTAAGA TGA-3′;
Cyclin D1 antisense: 5′-CAGGTTCCACTTGAGCTT GTTC-3′;
GAPDH sense: 5′-CAACTTTGGCATTGTGGAAGG GCTC-3′;
GAPDH antisense: 5′-GCAGGGATGATGTTCTGG GCAGC-3′.
Immunohistochemistry (IHC) staining and evaluation
Interestingly, there had been evidence that PYK2 may regulate the activation of AKT in HEK293 kidney cell line and cardiomyocytes.19,20 Taking into consideration that AKT was frequently hyperactivated in colon cancers,21,22 we also evaluated the phosphorylation level of AKT and its association with PYK2 expression. IHC staining for PYK2 and pS473-AKT was carried out by using the standard protocols as published before.23 Briefly, 5 µm serial sections were dried at 70°C and then deparaffinized with xylene and rehydrated in alcohol gradients. The microwave antigen retrieval was carried out using citrate buffer (pH 6.0). Subsequently, slides were incubated with the monoclonal PYK2 antibody (1:500 dilution, #610548; BD Biosciences, San Jose, CA, USA) or pS473-AKT antibody (1:500 dilution, #700392; Thermo Fisher Scientific) at 4°C overnight. On the next day, the sections were washed and detected by using poly HRP IgG and DAB substrate. Primary antibodies were replaced with PBS as a negative control.
Stained sections were examined and scored by two independent pathologists. Briefly, slides were observed at 400× magnification and five fields of each section were randomly selected. Staining intensity was divided into four grades as followings: 1 (negative); 2 (weak); 3 (moderate); 4 (strong). The staining percentage was scored as followings: 1 (<25%); 2 (25%–50%); 3 (51%–75%); 4 (>75%). The IHC score was finally determined by multiplying the intensity score with the staining percentage score (range 1–16). In this study, 44 patients were with low PYK2 expression (IHC score <8), and the other 43 patients were with high PYK2 expression (IHC score ≥8) in tumor tissues.
Cell culture and transfection
The human CAC cell line SW480 was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS and 1% penicillin (10,000 U/mL)/streptomycin (10 mg/mL) in a humidified atmosphere at 37°C with 5% CO2.
The full-length coding regions of PYK2 were cloned into pCDNA3.1 vector by Genepharma (Shanghai, China), which was used for overexpressing PYK2 in SW480 cells. Knockdown of PYK2 was achieved by using PYK2-siRNA from Santa Cruz Biotechnology (#sc-36332; Dallas, TX, USA). Both overexpression and siRNA-knockdown were performed with Lipofectamine 2000 reagent (Invitrogen) following the manufacturer’s procedure.24 The transfection efficiencies were tested by Western blot analysis.
Western blot
The cells were lysed in lysis buffer containing 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 0.5% NP-40, and 10 mM NaF. The protein concentration was first determined by a BCA assay kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). Approximately 20 µg protein were then subjected to SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad Laboratories Inc.). After blocked with 5% nonfat milk at room temperature for 1 hour, the membrane was incubated with corresponding primary antibodies (PYK2, AKT-pS473, AKT, c-Myc, Cyclin D1, and β-actin) in 4°C overnight. Immunoblotting was conducted by further incubation with secondary antibodies conjugated to horseradish peroxidase. Immunoreactivity was visualized on X-ray developing film using ECL-Plus detection reagents (Santa Cruz Biotechnology) as described by others.25
Cell Counting Kit-8 (CCK-8) assay
To evaluate the effect of PYK2 on tumor cell proliferation, plasmid or siRNA transfected cells were seeded at 2×104 cells per well in a 96-well plate and cultured in DMEM. At designated time points, cell viability was assessed by a CCK-8 (#CK04-500; Dojindo Molecular Technologies, Rockville, MD, USA) according to the manufacturer’s instructions. Briefly, 10 µL of CCK-8 reagent was added into each well and incubated for 4 hours at 37°C. Absorbance at 450 nm was measured by a microplate reader, and corresponding proliferation curves were plotted.26 All experiments were performed in triplicate and repeated for at least three times.
Cell migration and invasion assays
Transwell assays were performed to evaluate cell invasion capacity using transwell chambers (Corning, Cambridge, MA, USA). The chambers were first pre-coated with 50 µL of 2.5 mg/mL Matrigel (BD Biosciences) and left to polymerize for 4–6 hours at 37°C. Transfected cells were then seeded into the upper chambers with DMEM containing 1% FBS and allowed to invade for 48 hours at 37°C in incubator. The lower chambers were fulfilled with culture medium containing 20% FBS. Two days later, the cells on the membrane were fixed with paraformaldehyde and stained with 0.1% crystal violet. Invasion cells were counted by a microscope from five randomly selected visual fields. Each experiment was performed in triplicate.
Statistical
Statistical analyses were performed with SPSS 24.0 software (SPSS, Inc., Chicago, IL, USA). The associations between PYK2 expression and the clinicopathologic parameters of the CAC patients were evaluated by chi-squared tests. Kaplan– Meier and log-rank test were conducted to identify the risk factors on CAC overall survival. Multivariate analysis was further performed to evaluate their independent prognostic effects. Data from the cellular experiments were presented with mean ± standard error of the mean (SEM), and the statistical differences between groups were determined by Student’s t-tests compared with control groups. Differences were considered statistically significant with P<0.05.
Results
Patients’ information
This retrospective study included 87 CAC patients who underwent curative resections in Linyi Central Hospital and Qilu Hospital (Shandong, China). The median age at the time of diagnosis was 58.0 years. Patients’ clinicopathological information was summarized in Table 1. Most of the patients were males (64/87, 73.6%), only 23 (26.4%) were females. Among the entire cohort, tumors were located in ascending colon for 33 (37.9%) patients, transverse colon for 13 (14.9%) patients, descending or sigmoid colon for 41 (47.1%) patients. Most of the tumors were with poor (17/87, 19.5%) or moderate (66/87, 75.9%) pathological differentiation. Thirty-one (35.6%) of the patients suffered with a tumor size larger than 5.0 cm diameter. As for the T stage based on tumor invasion depth, 36 (41.4%) patients were with T1–T2, while the other 51 (58.6%) patients with T3–T4. Besides T stages, we retrieved the lymph node status, 48 (55.2%) patients were diagnosed with positive lymph nodes, and there was no lymph node metastasis for the other 39 (44.8%) patients at the time of primary surgery.
Table 1.
Correlations between PYK2 level and clinicopathological characteristics
| Variables | Patients
|
PYK2 level
|
P-value | |
|---|---|---|---|---|
| (n=87) | Low (n=44) | High (n=43) | ||
| Gender | 0.759 | |||
| Female | 23 | 11 | 12 | |
| Male | 64 | 33 | 31 | |
| Age (years) | 0.067 | |||
| ≤58 | 41 | 25 | 16 | |
| >58 | 46 | 19 | 27 | |
| Location | 0.253 | |||
| Ascending | 33 | 13 | 20 | |
| Transverse | 13 | 7 | 6 | |
| Descending/sigmoid | 41 | 24 | 17 | |
| Differentiation | 0.525 | |||
| Poor | 17 | 8 | 9 | |
| Moderate | 66 | 35 | 31 | |
| Well | 4 | 1 | 3 | |
| Tumor size (diameter, cm) | 0.230 | |||
| ≤5.0 | 56 | 31 | 25 | |
| >5.0 | 31 | 13 | 18 | |
| Invasion depth | 0.224 | |||
| T1–T2 | 36 | 21 | 15 | |
| T3–T4 | 51 | 23 | 28 | |
| Lymph node metastasis | 0.023a | |||
| Negative | 39 | 25 | 14 | |
| Positive | 48 | 19 | 29 | |
Note:
indicates statistically significant (P<0.05).
Correlation between PYK2 expression and clinicopathological factors
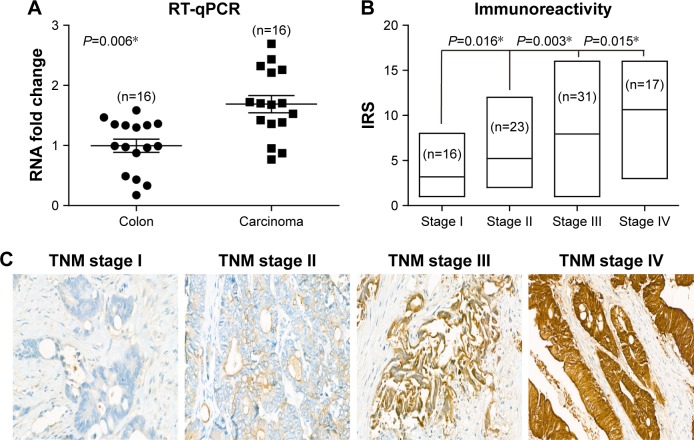
We first tested the RNA levels of PYK2 in both CAC and matched adjacent normal colon tissues, and found CAC possessed a higher PYK2-mRNA level (Figure 1A, P=0.006). Since RT-qPCR data implied a possible involvement of PYK2 in CAC development, we next explored its protein levels in tumor tissues, which demonstrated a positive correlation with patient’s TNM stage (Figure 1B). Besides, the protein level of PYK2 in tumor tissues was evaluated by IHC experiments, which showed a higher level in the tumor tissues with more advanced clinical stage (Figure 1C).
Figure 1.
PYK2 is upregulated in colon adenocarcinoma tissues.
Notes: (A) Real-time PCR quantification of PYK2 mRNA levels in colon adenocarcinoma tissues and matched adjacent nontumoral tissues (n=16). The mRNA expression of PYK2 in cancer tissues was significantly higher than those in nontumoral tissues (P=0.006). (B) Protein expression patterns of PYK2 in tumor tissues with different TNM stages, showing that patients with higher tumor stages were more frequent with higher PYK2 IRS. (C) Representative immunohistochemical results of PYK2 protein expression in colon adenocarcinoma tissues with different TNM stages, showing a higher PYK2 level in advanced tumor stages. Magnification ×400. *P<0.05 by chi-squared test.
Abbreviations: IRS, immunoreactivity score; RT-qPCR, real time quantitative polymerase chain reaction.
According to the cutoff of IHC score described in the “Methods” section, our cohort was divided into two subgroups, namely low or high expression of PYK2. The relationship between PYK2 expression and various clinicopathological factors was analyzed with chi-squared test and presented in Table 1. Accordingly, high expression of PYK2 was significantly associated with positive lymph node metastasis (P=0.023), suggesting PYK2 may be involved in tumor cell invasion of CAC. Moreover, patients with high PYK2 expression seemed to suffer with larger tumor sizes (P=0.230) though did not reach statistical significance.
Prognostic value of PYK2 and other clinicopathological factors
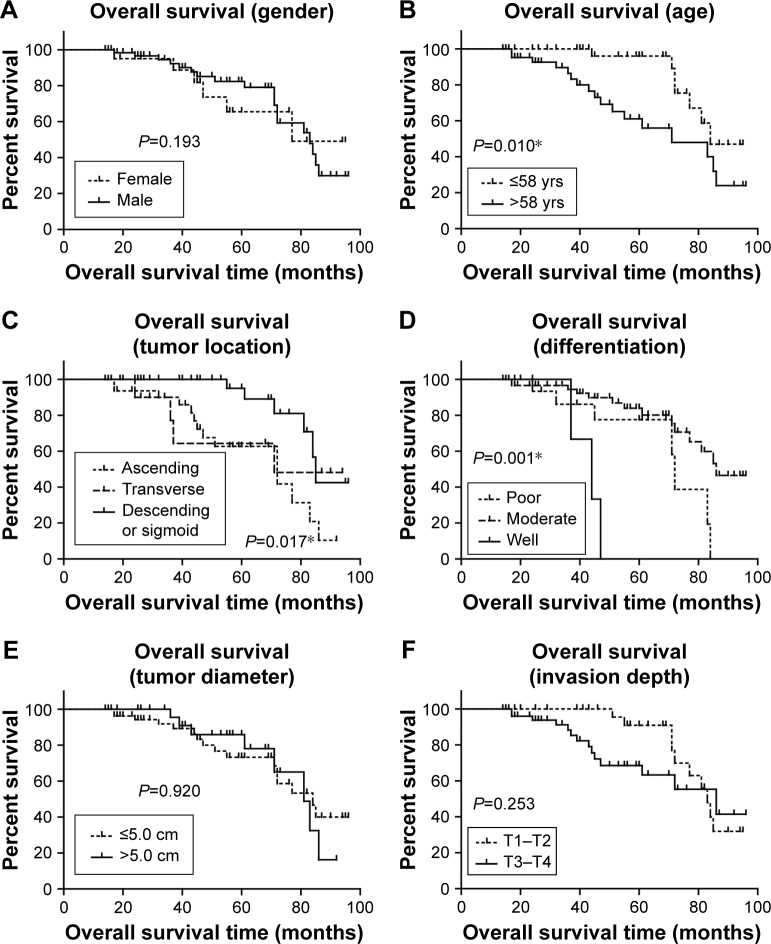
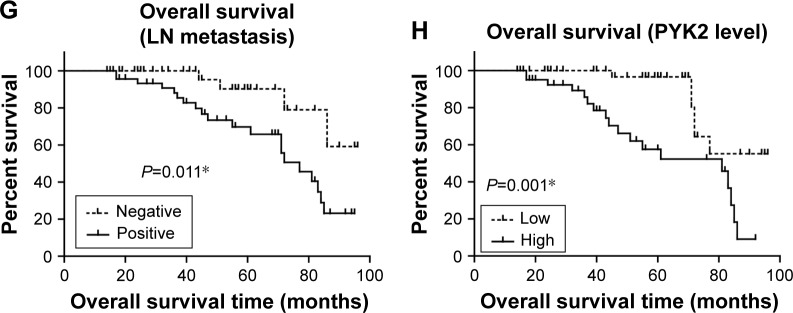
The prognostic value of all these clinicopathological factors including the PYK2 expression level were evaluated by univariate and multivariate analysis. Survival curves were first plotted with Kaplan–Meier method to analyze the correlation between all these factors and overall survival rate (Figure 2). In the study, patients with older ages had lower survival rates (P=0.010, Table 2). Moreover, tumor location (P=0.017), tumor differentiation (P<0.001), and lymph node metastasis (P=0.011) were also demonstrated to be significantly associated with overall survival rates (Table 2). Expression level of PYK2 can also affect clinical outcomes, patients with high tissue PYK2 had poorer overall survival (P=0.001).
Figure 2.
Kaplan–Meier survival analyses of the overall survival of colon adenocarcinoma patients.
Notes: The prognostic effect of each clinicopathological characteristic of colon adenocarcinoma patients was evaluated according to the Kaplan–Meier analysis, including gender (A), age (B), tumor location (C), tumor differentiation (D), tumor diameter (E), tumor invasion depth (F), LN metastasis (G), and PYK2 expression level (H). *P<0.05 by log-rank test.
Abbreviations: LN, lymph node; yrs, years.
Table 2.
Kaplan–Meier survival analyses and log-rank tests
| Variables | Cases (n) | Survival months (Mean ± SD) | 5-year OS (%) | P-value |
|---|---|---|---|---|
| Gender | 0.913 | |||
| Female | 23 | 73.80±6.72 | 65.5 | |
| Male | 64 | 76.11±3.42 | 82.3 | |
| Age (years) | 0.010a | |||
| ≤58 | 41 | 84.19±3.13 | 96.0 | |
| >58 | 46 | 67.29±4.77 | 61.1 | |
| Location | 0.017a | |||
| Ascending | 33 | 64.87±4.62 | 62.7 | |
| Transverse | 13 | 68.52±9.78 | 64.3 | |
| Descending/sigmoid | 41 | 85.07±3.35 | 95.5 | |
| Differentiation | <0.001a | |||
| Poor | 17 | 67.87±5.93 | 77.5 | |
| Moderate | 66 | 80.05±3.45 | 83.9 | |
| Well | 4 | 42.67±2.96 | 0.00 | |
| Tumor size (diameter, cm) | 0.920 | |||
| ≤5.0 | 56 | 74.85±4.05 | 73.3 | |
| >5.0 | 31 | 75.19±4.18 | 85.9 | |
| Invasion depth | 0.253 | |||
| T1–T2 | 36 | 81.34±3.03 | 90.9 | |
| T3–T4 | 51 | 72.19±4.85 | 68.5 | |
| Lymph node metastasis | 0.011a | |||
| Negative | 39 | 86.59±4.02 | 90.2 | |
| Positive | 48 | 69.10±4.05 | 69.7 | |
| PYK2 expression | 0.001a | |||
| Low | 44 | 84.61±3.57 | 96.6 | |
| High | 43 | 64.42±4.49 | 57.6 |
Note:
Statistically significant (P<0.05).
Multivariate analysis was further performed to identify the independent prognostic factors of CAC (Table 3). All factors with P<0.05 in univariate analysis were enrolled into the Cox-regression model for multivariate analysis. Tumors located in ascending colon or transverse colon subgroups were combined for better comparison. Similar combination was conducted for moderate or well-differentiated tumors. According to the Cox model, high expression of PYK2 was identified as an independent unfavorable prognostic factor (HR =3.237, 95% CI =1.124–9.322, P=0.030) in our study. In addition, tumor location (P=0.022) and lymph node metastasis (P=0.031) were also demonstrated to predict poorer outcomes independently.
Table 3.
Multivariate analysis for independent predictive factors of colon adenocarcinoma patients
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| Age (years) | 0.399 | ||
| ≤58 | Reference | ||
| >58 | 1.563 | 0.554–4.415 | |
| Location | 0.022a | ||
| Ascending/transverse | Reference | ||
| Descending/sigmoid | 0.567 | 0.350–0.920 | |
| Differentiation | 0.412 | ||
| Poor | Reference | ||
| Moderate/well | 0.679 | 0.269–1.713 | |
| Lymph node metastasis | 0.031a | ||
| Negative | Reference | ||
| Positive | 3.318 | 1.116–9.860 | |
| PYK2 expression | 0.030a | ||
| Low | Reference | ||
| High | 3.237 | 1.124–9.322 |
Note:
Statistically significant (P<0.05).
PYK2 exerts tumor promoting effects by activating AKT
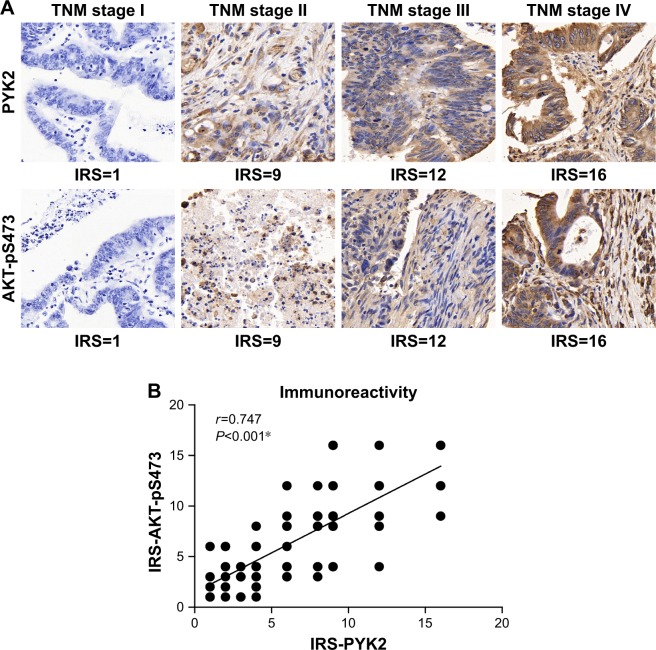
Furthermore, we suspected whether PYK2 participated in CAC progression by regulating AKT signaling. By testing the phosphorylation level of Ser473 of AKT (AKT-pS473), the major phosphorylation site ensuring its activation, we found its similar tendency with PYK2 level (Figure 3A). As expected, statistical analysis identified a significantly positive correlation between PYK2 expression and AKT-S473 phosphorylation in clinical tumor tissues (P<0.001, Figure 3B), indicating the possible role of PYK2 in phosphorylating AKT-S473 in CAC cells.
Figure 3.
Correlation between PYK2 expression and AKT phosphorylation in clinical samples.
Notes: (A) Representative immunohistochemical results of PYK2 protein expression and AKT-pS473 levels in colon adenocarcinoma tissues with different TNM stages. By comparing the two proteins in same samples, we found that advanced tumors showed simultaneously higher immunoreactivity score (IRS) of both PYK2 and AKT-pS473. Magnification ×400. (B) A positive correlation between PYK2 and AKT-pS473 level was observed according to statistical analysis (r=0.747, P<0.001). *P<0.05, Spearman correlation test.
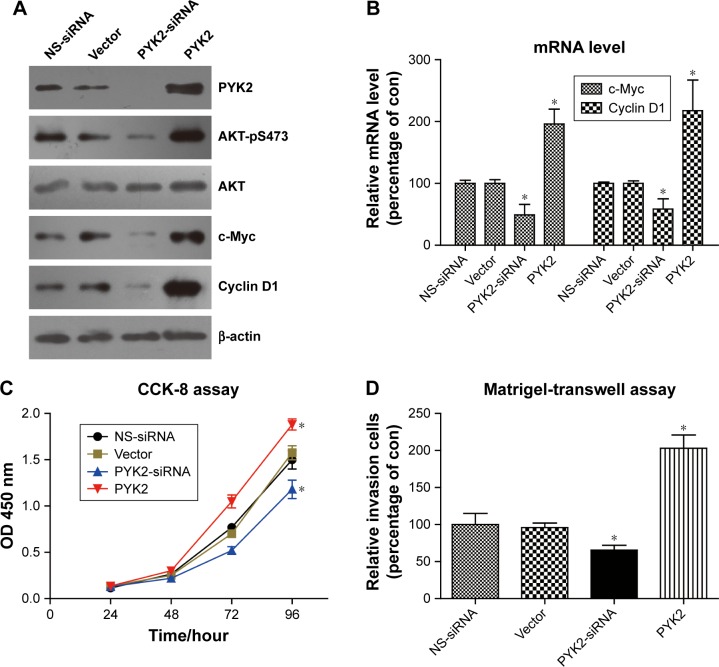
We next performed cellular studies by silencing and overexpressing PYK2 in SW480 CAC cells, respectively. Western blot results verified that the AKT-pS473 level was positively regulated by PYK2 protein expression (Figure 4A). Additionally, the protein expression levels of c-Myc and Cyclin D1 were also affected by PYK2. To better investigate the involvement of AKT in regulating gene transcription of tumor markers, we conducted RT-qPCR experiments to assess their RNA levels. Consistent with the protein alteration, RNA levels of c-Myc and Cyclin D1 were downregulated by PYK2 knockdown, while both were enhanced upon PYK2 overexpression (Figure 4B).
Figure 4.
PYK2 activates AKT and promotes colon cancer cell aggressiveness.
Notes: (A) Using pcDNA3.1 vector or non-specific siRNA (NS-siRNA) as control, SW480 CAC cells were transfected with PYK2-siRNA or PYK2-plasmids, revealing that PYK2 can upregulate the protein phosphorylation level of AKT2. Besides, expression of c-Myc and Cyclin D1 proteins, two well-acknowledged AKT2 downstream effectors, were positively modulated by PYK2 levels. (B) RT-qPCR assay demonstrated that c-Myc and Cyclin D1 were regulated by PYK2 from the transcription levels. Functional analyses showed that overexpression of PYK2 increased cell proliferation (C) and invasion (D). Data were presented as mean ± SEM, *P<0.05 by Student’s t-tests compared with control groups.
Abbreviations: con, control; SEM, standard error of the mean.
Finally, we tested the phenotype effects of PYK2 in modulating CAC progression. Both the proliferation and invasion capacities were enhanced by PYK2 overexpression (Figure 4C and D), whereas silencing PYK2 attenuated these tumor-promoting effects. Our cellular data indicated that PYK2 can promote the CAC progression at least partially through activating AKT and its downstream transcription process.
Discussion
An accurate evaluation of post-operative clinical outcomes would remarkably improve cancer patients’ survival by individualized treatment. Accumulating evidence has revealed the significance of studying tumor-associated proteins underlying the development of colon cancer, not only on recognizing the molecular mechanisms of tumor progression but also on risk identification and therapy selection.
As a non-receptor tyrosine kinase, PYK2 functions downstream of membrane receptors including growth factor receptors, G-protein coupled receptors (GPCRs), and immune receptors.27 It can form signaling complexes by recruiting Src protein and catalyze phosphorylation of phosphatidylinositol 3-kinase.28 Other downstream kinase cascades of PYK2 include ERK1/2 and JNK1 signaling.29 The diversity of PYK2 signaling effectors allows its different functions in cell survival, cell cycle, cell differentiation, etc. According to our data, PYK2 also activates AKT signaling. Interestingly, phosphorylation and activation of AKT is correlated to autophagy regulation in cancer cells. As reported by Wang et al, Akt signaling perhaps mechanistically linked to tumorigenesis and autophagy inhibition in tumor progression.30 The relationship between autophagy and CRC development has been well reviewed recently.31 Briefly, autophagy could either promote tumor survival or cause cancer cell death, depending on the tumor type, tumor stage, and the metabolism condition. Furthermore, the potential cross talk between PYK2 and autophagy via AKT signaling pathway has been reported in prostate cancer cells.32 Therefore, the interplay between PYK2 and AKT in CAC cells may also participate in the autophagy regulation. However, we have to keep in mind that although it can increase the phosphorylation level of AKT-pS473, PYK2 is an intrinsic tyrosine kinase, therefore PYK2-AKT is perhaps not a direct kinase–substrate relationship. The detailed mechanisms of how PYK2 indirectly activate AKT need further experimental investigation.
Anyhow, we revealed the tumor-promoting role of PYK2 in CAC on enhancing cell proliferation and invasion. Our results were consistent with the reported functions of PYK2 in other tumor types such as glioma,33 lung cancer,16 and prostate cancer.14 The upstream signaling of PYK2 on tumor development seems cancer type specific. For example, PYK2 modulates the signaling of androgen receptors and thus promotes growth of prostate cancer cells.34 In breast cancer cells, PYK2 functions by integrating growth factor and cytokine receptors signaling.35 Besides membrane receptors, PYK2 can also sustain endosomal-derived receptor signaling and enhance epithelial-to-mesenchymal transition of breast cancer cells.36 Considering many receptors are dysregulated in colon cancer,37 it is highly likely that PYK2 may also functions downstream of these receptor onco-proteins. Further studies are needed to elucidate the interplays between PYK2 and membrane receptors in colon cancer progression.
The potential of PYK2 in tumor therapy is attracting more attentions due to recent development of PYK2 inhibitors.38,39 Several PYK2 inhibitors had been reported to exert tumor-suppressing role in bone tumors and breast cancer.40–42 For example, the PF-562,271 is a potent, ATP-competitive, reversible inhibitor of PYK2. Although there is no clinical trial studying its therapeutic role, PF-562,271 has been reported to show significant tumor-inhibiting effects in animal models, including glioblastoma, hepatocellular carcinoma, and pancreatic cancer.43–45 Here we provided initial evidence of PYK2 in promoting colon cancer progression, therefore may also direct further exploration toward the effects of PYK2 inhibitors on colon cancer cells.
Conclusion
We for the first time demonstrated that PYK2 in cancer tissue was an independent prognostic biomarker of CAC. Moreover, intracellular high PYK2 level induces cancer cell proliferation and invasion via activating AKT signaling pathways. Our findings could not only expand the understanding of clinical significance of PYK2 in cancer but also help in guiding the individual treatment toward CAC patients.
Acknowledgments
The study was supported by National Natural Science Foundation of China (grant no. 81601668), Shandong Province Major Research and Design Program (grant no. 2017GSF218059), and China Postdoctoral Science Foundation (grant nos. 2017M610167 and 2017T100163).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Hegde SR, Sun W, Lynch JP. Systemic and targeted therapy for advanced colon cancer. Expert Rev Gastroenterol Hepatol. 2008;2(1):135–149. doi: 10.1586/17474124.2.1.135. [DOI] [PubMed] [Google Scholar]
- 3.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 4.van Cutsem E, Nordlinger B, Adam R, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42(14):2212–2221. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Xu Y, Zhang Q, et al. Prognostic significance of TBL1XR1 in predicting liver metastasis for early stage colorectal cancer. Surg Oncol. 2017;26(1):13–20. doi: 10.1016/j.suronc.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Neri E, Faggioni L, Cini L, Bartolozzi C. Colonic polyps: inheritance, susceptibility, risk evaluation, and diagnostic management. Cancer Manag Res. 2010;3:17–24. doi: 10.2147/CMR.S15705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Liu Z, Li K, et al. TBL1XR1 predicts isolated tumor cells and micrometastasis in patients with TNM stage I/II colorectal cancer. J Gastroenterol Hepatol. 2017;32(9):1570–1580. doi: 10.1111/jgh.13749. [DOI] [PubMed] [Google Scholar]
- 8.Coghlin C, Murray GI. Biomarkers of colorectal cancer: recent advances and future challenges. Proteomics Clin Appl. 2015;9(1–2):64–71. doi: 10.1002/prca.201400082. [DOI] [PubMed] [Google Scholar]
- 9.Tan E, Gouvas N, Nicholls RJ, Ziprin P, Xynos E, Tekkis PP. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg Oncol. 2009;18(1):15–24. doi: 10.1016/j.suronc.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Yang X, Li Z, et al. Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progression. Oncotarget. 2017;8(3):4888–4900. doi: 10.18632/oncotarget.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Zhang Q, Li K, et al. Prognostic significance of USP33 in advanced colorectal cancer patients: new insights into β-arrestin-dependent ERK signaling. Oncotarget. 2016;7(49):81223–81240. doi: 10.18632/oncotarget.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383(6600):547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 13.Lev S, Moreno H, Martinez R, et al. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376(6543):737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 14.Iiizumi M, Bandyopadhyay S, Pai SK, et al. RhoC promotes metastasis via activation of the Pyk2 pathway in prostate cancer. Cancer Res. 2008;68(18):7613–7620. doi: 10.1158/0008-5472.CAN-07-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zrihan-Licht S, Fu Y, Settleman J, et al. RAFTK/Pyk2 tyrosine kinase mediates the association of p190 RhoGAP with RasGAP and is involved in breast cancer cell invasion. Oncogene. 2000;19(10):1318–1328. doi: 10.1038/sj.onc.1203422. [DOI] [PubMed] [Google Scholar]
- 16.Roelle S, Grosse R, Buech T, Chubanov V, Gudermann T. Essential role of Pyk2 and Src kinase activation in neuropeptide-induced proliferation of small cell lung cancer cells. Oncogene. 2008;27(12):1737–1748. doi: 10.1038/sj.onc.1210819. [DOI] [PubMed] [Google Scholar]
- 17.Sun CK, Ng KT, Sun BS, et al. The significance of proline-rich tyrosine kinase2 (Pyk2) on hepatocellular carcinoma progression and recurrence. Br J Cancer. 2007;97(1):50–57. doi: 10.1038/sj.bjc.6603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Y-F X, F-J G, Han B, et al. High-mobility group box 1 expression and lymph node metastasis in intrahepatic cholangiocarcinoma. World J Gastroenterol: WJG. 2015;21(11):3256. doi: 10.3748/wjg.v21.i11.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi C-S, Kehrl JH. PYK2 links Gqα and G13α signaling to NF-κB activation. J Biol Chem. 2001;276(34):31845–31850. doi: 10.1074/jbc.M101043200. [DOI] [PubMed] [Google Scholar]
- 20.Guo J, Sabri A, Elouardighi H, Rybin V, Steinberg SF. Alpha1-adrenergic receptors activate AKT via a Pyk2/PDK-1 pathway that is tonically inhibited by novel protein kinase C isoforms in cardiomyocytes. Circ Res. 2006;99(12):1367–1375. doi: 10.1161/01.RES.0000252830.01581.fd. [DOI] [PubMed] [Google Scholar]
- 21.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9(6):667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 22.Itoh N, Semba S, Ito M, Takeda H, Kawata S, Yamakawa M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apop-tosis and tumor progression in human colorectal carcinoma. Cancer. 2002;94(12):3127–3134. doi: 10.1002/cncr.10591. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Xu Y, Zhang Q, et al. Correlations between TBL1XR1 and recurrence of colorectal cancer. Sci Rep. 2017;7(1):44275. doi: 10.1038/srep44275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan C, Liu H-D, Gong Z, et al. Cadmium is a potent inhibitor of PPM phosphatases and targets the M1 binding site. Sci Rep. 2013;3(1):2333. doi: 10.1038/srep02333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Q, Yuan L, Liu D, et al. Hydrogen sulfide attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Pharmacol Res. 2014;84:32–44. doi: 10.1016/j.phrs.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Hou S, du P, Wang P, Wang C, Liu P, Liu H. Significance of MNK1 in prognostic prediction and chemotherapy development of epithelial ovarian cancer. Clin Transl Oncol. 2017;19(9):1107–1116. doi: 10.1007/s12094-017-1646-x. [DOI] [PubMed] [Google Scholar]
- 27.Andreev J, Galisteo ML, Kranenburg O, et al. Src and Pyk2 mediate G-protein-coupled receptor activation of epidermal growth factor receptor (EGFR) but are not required for coupling to the mitogen-activated protein (MAP) kinase signaling cascade. J Biol Chem. 2001;276(23):20130–20135. doi: 10.1074/jbc.M102307200. [DOI] [PubMed] [Google Scholar]
- 28.Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol. 2005;25(16):6889–6898. doi: 10.1128/MCB.25.16.6889-6898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kodama H, Fukuda K, Takahashi E, et al. Selective involvement of p130Cas/Crk/Pyk2/c-Src in endothelin-1-induced JNK activation. Hypertension. 2003;41(6):1372–1379. doi: 10.1161/01.HYP.0000069698.11814.F4. [DOI] [PubMed] [Google Scholar]
- 30.Wang RC, Wei Y, An Z, et al. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science. 2012;338(6109):956–959. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mokarram P, Albokashy M, Zarghooni M, et al. New frontiers in the treatment of colorectal cancer: Autophagy and the unfolded protein response as promising targets. Autophagy. 2017;13(5):781–819. doi: 10.1080/15548627.2017.1290751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conte A, Kisslinger A, Procaccini C, et al. Convergent effects of resveratrol and PYK2 on prostate cells. Int J Mol Sci. 2016;17(9):1542. doi: 10.3390/ijms17091542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipinski CA, Tran NL, Menashi E, et al. The tyrosine kinase pyk2 promotes migration and invasion of glioma cells. Neoplasia. 2005;7(5):435–445. doi: 10.1593/neo.04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsiao Y-H, Huang Y-T, Hung C-Y, Kuo T-C, Luo F-J, Yuan T-C. PYK2 via S6K1 regulates the function of androgen receptors and the growth of prostate cancer cells. Endocr Relat Cancer. 2016;23(8):651–663. doi: 10.1530/ERC-16-0122. [DOI] [PubMed] [Google Scholar]
- 35.Selitrennik M, Lev S. PYK2 integrates growth factor and cytokine receptors signaling and potentiates breast cancer invasion via a positive feedback loop. Oncotarget. 2015;6(26):22214–22226. doi: 10.18632/oncotarget.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma N, Keinan O, Selitrennik M, Karn T, Filipits M, Lev S. PYK2 sustains endosomal-derived receptor signalling and enhances epithelial-to-mesenchymal transition. Nat Commun. 2015;6(1):6064. doi: 10.1038/ncomms7064. [DOI] [PubMed] [Google Scholar]
- 37.Fds Em, Kurtova AV, Harnoss JM, et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature. 2017;543(7647):676–680. doi: 10.1038/nature21713. [DOI] [PubMed] [Google Scholar]
- 38.Meirson T, Samson A, Gil-Henn H. An in silico high-throughput screen identifies potential selective inhibitors for the non-receptor tyrosine kinase Pyk2. Drug Des Devel Ther. 2017;11:1535–1557. doi: 10.2147/DDDT.S136150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ueda H, Abbi S, Zheng C, Guan J-L. Suppression of Pyk2 Kinase and Cellular Activities by Fip200. J Cell Biol. 2000;149(2):423–430. doi: 10.1083/jcb.149.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vultur A, Buettner R, Kowolik C, et al. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol Cancer Ther. 2008;7(5):1185–1194. doi: 10.1158/1535-7163.MCT-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felty Q. Redox sensitive Pyk2 as a target for therapeutics in breast cancer. Front Biosci. 2011;16(1):568–577. doi: 10.2741/3706. [DOI] [PubMed] [Google Scholar]
- 42.Bagi CM, Roberts GW, Andresen CJ. Dual focal adhesion kinase/Pyk2 inhibitor has positive effects on bone tumors: implications for bone metastases. Cancer. 2008;112(10):2313–2321. doi: 10.1002/cncr.23429. [DOI] [PubMed] [Google Scholar]
- 43.Bagi CM, Christensen J, Cohen DP, et al. Sunitinib and PF-562,271 (FAK/Pyk2 inhibitor) effectively block growth and recovery of human hepatocellular carcinoma in a rat xenograft model. Cancer Biol Ther. 2009;8(9):856–865. doi: 10.4161/cbt.8.9.8246. [DOI] [PubMed] [Google Scholar]
- 44.Stokes JB, Adair SJ, Slack-Davis JK, et al. Inhibition of focal adhesion kinase by PF-562,271 inhibits the growth and metastasis of pancreatic cancer concomitant with altering the tumor microenvironment. Mol Cancer Ther. 2011;10(11):2135–2145. doi: 10.1158/1535-7163.MCT-11-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts WG, Ung E, Whalen P, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68(6):1935–1944. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]